Abstract
Purpose of review:
To perform an extensive review of recent literature and provide an update on the current epidemiology, clinical features and management of cryptococcal disease with a focus on the differences between patients depending on their immune status.
Recent findings:
Emerging literature has highlighted the inflammatory pathophysiology and varied manifestations of cryptococcal infections in patients who are apparently healthy but paradoxically have a more critical clinical course compared to their immunosuppressed counterparts.
Summary:
Non-HIV cryptococcal meningitis has greater mortality compared to that seen in HIV patients. Basic science experiments closely analyzing the underlying pathophysiological response to this infection have demonstrated the predominant role of T cell-mediated inflammatory injury in causing worse clinical outcomes. Further studies are needed to define the need for immunosuppressive agents in the treatment of this illness.
Keywords: Cryptococcus, meningitis, HIV, immunity, steroids, ventricular shunt
Introduction
Background
Cryptococcus is an encapsulated yeast causing infections in both immunosuppressed as well as the previously healthy. Infections surged in incidence during the AIDS era and continue to persist in other immunosuppressive conditions including solid organ transplant recipients and those subjected to chemotherapy. [1, 2] Although the incidence of cryptococcal infections within the United States has declined by 70–90% from the 1990s due to anti-retroviral therapy (ART), it remains one of the leading causes of meningitis in Sub – Saharan Africa, despite the availability of ART, currently responsible for 11% of AIDS-related deaths.[3] cases of cryptococcal meningoencephalitis (CM) are diagnosed every year and based on estimates in 2014, it is responsible for causing approximately 181,100 deaths annually with poor survival despite therapy.[4] In addition, due to vaccine-related reductions in bacterial meningitis and persistence of CM in non-HIV-related cases, CM is now the leading cause of non-viral meningitis in the United States, where the mortality rate persists in all host populations at about 20–30% despite therapy.[5] [4]
Cryptococcus is divided into two species, C neoformans and C gattii
C. neoformans and C. gattii are both species that belong to the genus Basidiomycota and vary in distribution, clinical manifestations and the hosts they target. Initially isolated in 1894[6], C. neoformans has a worldwide distribution and is found abundantly in soil contaminated by avian guano. C. gattii is typically associated with tropical/subtropical regions and has been isolated from eucalyptus and other plant species and noted to be a weak plant pathogen of seedlings. 80% of all cryptococcal infections worldwide are caused by C. neoformans, which is usually the culprit in immunocompromised patients with disseminated disease while C. gattii is responsible for the remaining 20%. In recent years, outbreaks of C. gattii have occurred in Vancouver Island, Canada (1999–2002) and the Pacific Northwest (2004–2011), suggesting that C. gattii may have become more prevalent. [5, 6] Although both species are known to affect both the apparently healthy or those with compromised immune systems, the latter is historically known to affect a substantially larger number of apparently immunocompetent patients.[7]
At the 10th International Conference on Cryptococcus and Cryptococcosis (ICCC10) held in Brazil in May 2017, the nomenclature on cryptococcal species was extensively discussed and remains controversial. In a perspective published in 2017, Ferry Hagen et al. proposed that while several species exist, 7 species should be officially recognized; C. neoformans var. grubii, C. neoformans var. neoformans and C. gattii VG1, VGII, VG III, VG IV and VG IV/VGIIIc. They believed this method of identification would stimulate physicians to investigate the phenotypic and genotypic differences between these species further. On the other hand, Kwon-Chung and colleagues argued that the genotypes of 2,606 Cryptococcus strains had been recognized and this genetic diversity may not be encompassed by just 7 species. Instead, they preferred dichotomizing the fungus as a “Cryptococcus neoformans complex” or a “Cryptococcus gattii complex” to avoid confusion. [8, 9]
Life cycle and virulence mechanisms
Both cryptococcal species exhibits a tropism for the brain that is unique to this fungus and is particularly pronounced in more immunosuppressed individuals such as those with HIV/AIDS.[10] Cryptococcus expresses a number of virulence factors important in penetration of the blood brain barrier including urease, metalloprotease and hyaluronic acid as well as an immune-modifying laccase enzyme and copper/iron acquisition factors that potentiate growth within the CNS. [11–13] In a recent study, M.C. Olave et al described how an in vitro infection of a human astrocytoma cell line with C. neoformans and C. gattii demonstrated increased HLA class II expression and intracellular survival of C. neoformans, suggesting that it possesses sophisticated virulence mechanisms to invade and survive within the CNS. [14]
Cryptococcus spores or dessicated yeast cells usually enter the human host via inhalation and can lay dormant for several years within the body.[15] In immunocompetent hosts, they may not produce any symptoms or remain confined to the lung in the form of granulomas. When host immunity is suppressed, these latent organisms may reactivate and cause infection, which can then disseminate to include multiple organs, primarily the brain..[15–17] Unlike bacteria, Cryptococci penetrate the blood brain barrier (BBB) through cortical capillaries rather than the choroid plexus. 3 possible mechanisms of penetration exist: 1) transcellular passage across the endothelial cells or, 2) between endothelial cells by BBB disruption, or 3) a “Trojan horse” mechanism by which the fungus straddles a host monocyte to move across the BBB. [18] Attesting to the last mechanism are the results of a small experiment carried out in vitro, in which a 3-fold increase in brain fungal burden was seen 24 hours after inoculating a mouse with Cryptococcus-infected bone marrow-derived monocytes compared to inoculation with free yeasts alone.[18]
As an opportunistic fungus which has spent most of its evolutionary timespan within the environment, many of its virulence factors have evolved to ensure survival within these external environments which has then been applied by apparent serendipity to the host environment. Unique to Cryptococcus is a polysaccharide capsule, which it wields as armor to protect itself against unfavorable circumstances including an excessively alkaline pH, high carbon dioxide levels, dessication and a paucity of iron.[19] This same capsule enables protection against human immunity by a compacted structure that reduces complement and antibody binding. [20] It is also able to actively secrete multiple virulence factors outside the cell surface within microvesicles and this mechanism has been implicated in its ability to effectively penetrate the blood brain barrier.[21] An example is an extracellular laccase secreted in exosomes [22], which helps the fungus evade free radicals, and may contribute to antifungal resistance.[23]
Immunocompromising conditions associated with Cryptococcus
Apart from HIV, other immune-impairing conditions including prolonged corticosteroid treatment, organ transplant, malignancy, diabetes and sarcoidosis have been linked with cryptococcal infections.[5] Within the realm of immune deficiencies, the most common condition associated with this infection is idiopathic chronic lymphopenia (ICL) followed by auto-antibodies to GMCSF, although it is hypothesized that the presence of ICL exclusively is insufficient to cause infection and a second trigger factor may be required. [24] ICL has been defined as the repeated presence of CD4 + T cells <300cells/cubic mm or of < 20% of total T cells with no evidence of HIV and no other condition that could explain the decreased CD4 count.[25] To add to the list are occasional cases linked to immune deficiencies such as GATA 2 deficiency, Job’s syndrome, CGD and X-linked CD 40 ligand mutations.[26] Within the United States, 13–18% of patients with a diagnosis of cryptococcal meningitis are apparently healthy, but the mortality rate (30–50%) in this population is as high as those who are more immunocompromised. In addition, Cryptococcus presents indolently and typically without fevers within this subset of patients, leading to a delay in diagnosis with its attendant high mortality and residual neurological deficits such as cranial nerve palsies and cognitive impairment.[27] This curious aspect of CM in the previously healthy may be related to macrophage defects leading to absence of production of the pyrogen, TNF-α [28]
Immune activation syndromes associated with cryptococcal infections
In a paper titled “The damage-response framework of microbial pathogenesis and infectious diseases”, Pirofski and Casadevall highlight a principle that an overactive immune response may be as detrimental to a patient as an underactive one.[29] The most well-known example of this in cryptococcal disease is a Cryptococcus – related immune reconstitution syndrome (cIRIS) in AIDS patients that was first described in 2005 when patients with CM who were started on ART were found to have paradoxical worsening of their mental status despite achieving viral and fungal control with improvements in CD4 counts.[30] A variant of cIRIS is unmasking cIRIS, described in cases where a subclinical cryptococcal infection is unmasked during immune reconstitution shortly after starting ART. At the time of HIV diagnosis, before initiation of ART, a more recent study looked at CM in patients with CD4 counts ranging from <50 to >100. As anticipated, the percentage of death occurring in the study arm with CD4 counts < 50 superseded those with CD4 counts 50–99, but there were also more deaths in those with CD4 counts> 100. The latter group was found to present more frequently with altered mental status despite having a 10-fold decrease in fungal burden, pointing to the possible contribution of a dysfunctional immune pathology in this group as well. [31] In addition, an analogous inflammatory response has been observed in transplant recipients, especially if immune suppression is reduced after fungal diagnosis.[32] Most recently, a post-infectious inflammatory response syndrome (PIIRS) was identified in previous healthy patients with refractory disease [28], an unexpected result that runs counter to the expectation of a reduced immune response in patients developing an opportunistic infection such as CM.
Pathophysiology of Immune Activation Syndromes
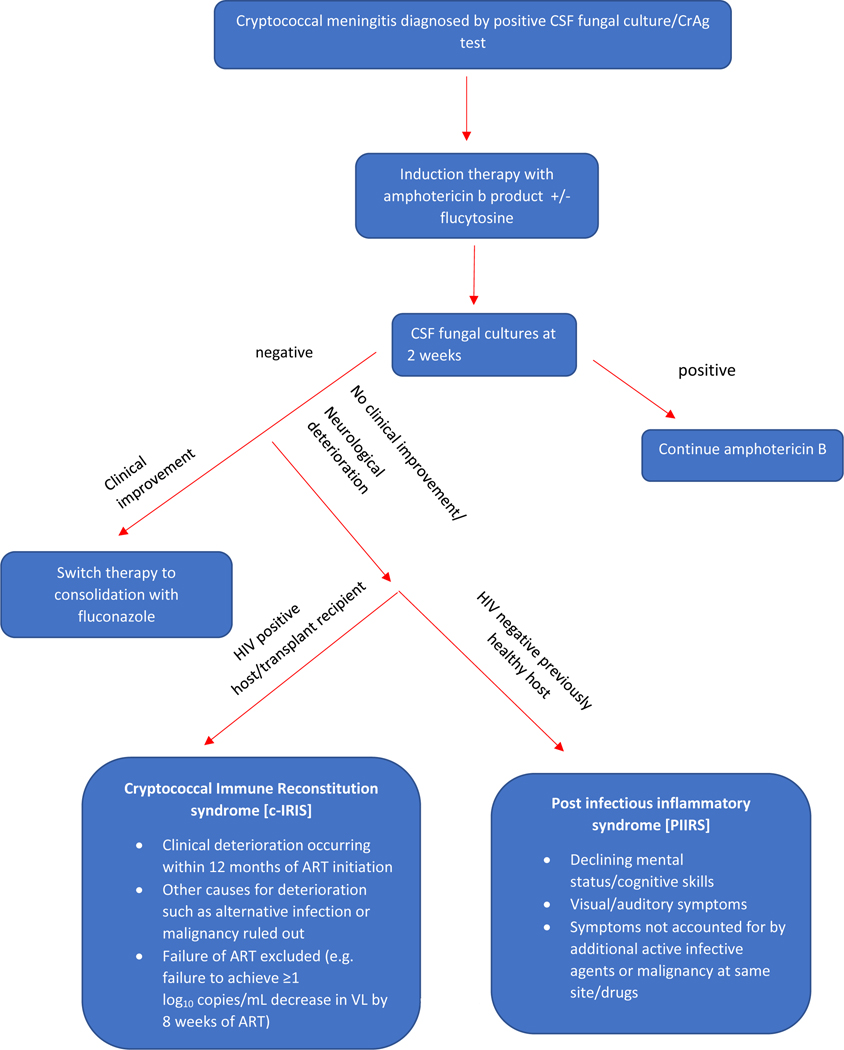
The pathophysiology of immune damage in cryptococcal disease varies between HIV positive and negative individuals. While cIRIS in HIV patients is well established, literature on a similar post-infectious immune response syndrome (PIIRS) described in apparently healthy hosts is nascent. PIIRS can be best defined as a deterioration of mental status and/or audio-visual capacity in an otherwise healthy host despite negative CSF fungal cultures after being optimally treated for cryptococcal meningitis. In both HIV and non-HIV-associated disease, the patient has cleared the active infection after completing an appropriate antifungal regimen (including ART in HIV cases) but deteriorates clinically because of an overly robust immune response against non-viable organisms.(Figure 1) [33] Excess inflammation of CNS infections is particularly problematic within the closed space of the skull where increased pressures have the ability to effect herniation and cranial nerve abnormalities. [34]
Figure 1 –
An algorithmic approach to diagnose patients with inflammatory response syndromes (c-IRIS and PIIRS) after acute cryptococcal meningitis has been successfully treated. CrAg=cryptococcal antigen, ART=antiretroviral therapy, VL= viral load
During an effective immune response, inhaled cryptococcal spores are first recognized by a number of macrophage associated surface receptors including Dectin-1, Mincle, mannose receptor, CD14, and toll-like receptors [26]. This stimulates macrophages to release CCL2, which recruits monocytes and dendritic cells. In turn, these produce pro-inflammatory cytokines such as INF-γ, TNF-α and IL-6 and promote the differentiation of T cells to T helper cells. Activated T helper cells further secrete IFNγ, IL-6, IL-10 and granulocyte-macrophage colony stimulating factor (GM-CSF) which activate and polarize M1 macrophages to further secrete TNF-α and IL 12 to effect successful killing of the fungus. Granulomas, composed of macrophages, CD4 T cells and Cryptococcus-containing multinucleated giant cells, are a sign of controlled infection, although Cryptococcus is reported to survive latently within these granulomas.
Whether a macrophage is activated through the classical or alternative pathway depends on stimulation through Th1 or Th2 cytokines respectively. When compared to alternatively activated M2 macrophages, classically activated M1 macrophages exhibit enhanced fungicidal activity in studies done in vitro. In a recent review describing the pathophysiology of cIRIS, it was hypothesized that a fine, mutually exclusive balance exists between a Th1 and Th2/17 mediated response. A Th2/17 response predominates in the early stages of the disease and with antifungals and delayed ART initiation, the immune balance is restored. However, in cases when ART is started soon after antifungal therapy, a hyperactive compensatory Th1 response follows, leading to increased TNF-α, IFN-γ, IL-6 and G-CSF levels, characteristic of cIRIS. [35].This inflammatory cascade is similar in previously healthy CM patients, as recognized in a study describing 17 patients with severe CNS disease, defined as a deterioration in mental status (Glasgow Coma Scale < 15) despite 6 weeks of optimal antifungal therapy and negative CSF fungal cultures.[28]. A 1000-fold increase in the number of CSF T cells and a lack of Th2 and Th17 cytokines was reported in this study, indicating a dominant T1 mediated inflammatory response. However, there was one significant difference – autopsy and biopsy specimens in this group of patients showed activated tissue M2 macrophages, which failed to phagocytose fungal cells.[28]
Immunophenotyping tests on spinal fluid in patients with PIIRS have demonstrated a CNS-compartmentalized, increased number of HLA DR4 positive CD4 + and CD8+ cells along with NK cells, confirming a predominantly T-cell-mediated injury. [28] This is accompanied by elevated levels of cytokines including soluble IFN-γ, IL-18 and CXCL10. In the study referenced earlier describing the immune pathology in non-HIV cryptococcosis, CSF neurofilament light chain levels (NFL) were assayed. The latter is a neuron specific and sensitive biomarker of axonal damage. Compared to patients with non-CNS disease and healthy donors, those with severe CNS disease were found to have a 10-fold increase in CSF NFL levels, indicating that this was a pathogenic and not a protective immune response.[28]
Clinical findings
Differences at the cellular level may help us understand why clinical features and outcomes in patients vary based on their immune function. In a retrospective study describing 302 patients with varying immune status, HIV-positive patients were the most likely to have CNS involvement, compared to transplant recipients and those previously healthy.[27] Among the different categories the previously healthy was the group associated with highest mortality at 90 days and the reason hypothesized was a delay in diagnosis with a resultant increase in neurological complications including strokes, auditory/visual defects and cognitive dysfunction. [27]
Common presenting signs and symptoms in HIV-associated CM are well documented and include fever, headache, altered mental status, auditory/visual changes and cranial nerve palsies.[7] Previously healthy adults with CM are more likely to present with visual symptoms, auditory problems, altered mental status and seizures. [36] Headache and fever, both hallmark symptoms of meningitis are found to be less prevalent in this latter group. [37, 38] Lack of fever results in a reduced consideration of meningitis in the differential and may be a contributor to late diagnosis, resulting in a more critically ill presentation in this population. In one study, the mean time from symptom development to diagnosis in this group was found to be significantly longer at 81 days compared to just 34 days in the typical immunocompromised hosts.[39](Table 1)
Table 1.
Distinguishing clinical features, CSF and radiological findings in immunocompromised and apparently healthy patients.
| Clinical features | HIV positive/transplant recipients/on chemotherapy | HIV negative, apparently healthy |
|---|---|---|
| Symptoms | ||
| Altered mental status | + | + |
| Fever | ++ | + |
| Headache | + | + |
| Auditory/visual problems | + | ++ |
| Cranial neuropathies | + | + |
| Time from symptom development to diagnosis | ~ 1 month | ~ 1–3 months |
| Neurological complications (neurocognitive deficits, spinal arachnoiditis) | + | ++ |
| Pulmonary involvement | + | + |
| CSF analysis | ||
| Opening pressure | ++ | + |
| CSF pleocytosis | + | ++ |
| Elevated protein | + | ++ |
| Hypoglycorrhachia | + | ++ |
| Higher cryptococcal antigen titers | ++ | + |
| Positive CSF fungal culture | ++ | + |
| Positive fungal blood culture | ++ | + |
| MRI brain findings | ||
| Meningeal enhancement | + | + |
| Hydrocephalus | + | + |
| Cryptococcomas | + | + |
| Ependymitis | + | ++ |
| Choroid plexitis | + | ++ |
| Ischemic infarcts | + | ++ |
(+): present (++): occurs more frequently compared to the other category
The increased mortality associated with HIV-negative cryptococcal disease has recently generated more interest within the academic fraternity to study this group of patients more diligently. According to the large multi-center CINCH (Cryptococcus Infection Network Cohort) study following HIV-negative cryptococcal patients from the time of diagnosis for up to 2 years post diagnosis across 25 hospitals within the United States, it was found that those initially admitted with cognitive deficits had very slow improvement in serial MOCA scores over time and some were not able to return to baseline despite completing a year of therapy.[40, 41] In another cohort of 27 patients at the NIH clinical center who underwent comprehensive neurological assessment from 1 to 4 years after diagnosis, most were found to have scores less than the 16th percentile in all domains except attention when compared to normative test averages. When these patients were compared to age and education matched Alzheimer’s disease patients, they were found to exhibit greater relative deficits within the domains of psychomotor and executive function as well.[42]
Otological manifestations associated with cryptococcal disease have also been described in this population. Cryptococcus is able to invade either the neural or vestibulo-cochlear apparatus to subsequently result in a sensorineural, bilateral and progressive/fluctuating hearing loss. In a small study conducted at the NIH clinical center, 19 out of 29 patients with CM presented with hearing loss which ranged from mild to moderate and was sensorineural in origin. Almost half of these patients also had hearing thresholds below 4Hz, a frequency crucial for hearing normal speech. [43]
One of the neurological sequelae also reported in recent literature is a spinal arachnoiditis, which can be hard to decipher clinically when presenting concomitantly with meningoencephalitis. When involving the lumbar region, it can present with saddle anesthesia, urinary retention, sensory and gait disturbances with a tendency towards asymmetric lower extremity weakness. Patients with arachnoiditis have also been found to have elevated soluble CD27 and NFL levels in their CSF.[44] Some of the independent risk factors for mortality reported in HIV-negative patients include older age, liver or renal disease, diabetes and hematological malignancies treated with chemotherapy. [45] This contrasts with HIV-positive CM where the main independent risk factors for poor outcome are altered mental status, CD4 lymphopenia, high CSF fungal burden and older age at diagnosis.[46]
Laboratory tests
In the same retrospective study alluded to earlier [27], there were also features on CSF analysis that varied according to immune status. While HIV positive patients were found to have higher initial opening pressures and cryptococcal antigen titers as well as more frequent cryptococcal growth from fungal blood and CSF cultures, previously healthy adults were found to have more frequent findings of CSF pleocytosis, elevated protein and hypoglycorrhachia, all indicative of a heightened immune response in these patients. [27, 36] Although hypoglycorrhachia has been associated with microbial meningitis and CSF inflammation in the past, the actual mechanism behind it is unclear and has been attributed to decreased glucose transport across the blood brain barrier and an increase in brain metabolism.[47] CSF pleocytosis has long been used as a measure of neuroinflammation but studies examining inflammatory biomarkers such as soluble CSF CD27(produced by activated T cells) or HLA-DR+ CD4 cells suggest that these may be more accurate markers of intrathecal inflammation compared to CSF WBC as the latter does not indicate the identity or inflammatory activity of the intrathecal cell population. [48] Cell numbers within the CSF represent only the ‘tip of the iceburg’ in a disease which is predominantly within the substance of the brain. Thus, ratios of soluble cytokines to T-cell numbers such as CSF CD27/CD4 cells may better represent the degree of inflammation in a meningoenephalitis such as CM, compared to that of a meningitis-only disease. [48]
The advent of cryptococcal antigen detection techniques has played a major role in the rapid diagnosis of cryptococcal disease. One of the earliest methods was the latex agglutination (LA) technique, which was able to detect the presence of cryptococcal capsular polysaccharide GXM (glucuronoxylomannan) using antibodies raised in rabbits. This was then followed by point-of-care testing with the lateral flow assay (LFA), which used gold-conjugated monoclonal antibodies that targeted all GXM serotypes (A-D).[49] Compared to EIA (Enzyme Immuno - Assay) and LA, the LFA is more rapid, able to quantify cryptococcal antigen titers and has a high sensitivity and specificity (100% and 99.8% respectively in CSF).[49] In fact, the LFA is so sensitive that it can be used as a simple blood test for CM, even in previously healthy patients who typically have low antigen loads. [50]. This is thus an important tool that could improve time-to-diagnosis for a life threatening, though rare disease that presents with little more than a headache to suggest the diagnosis. In contrast, in a recent study from 2018, it was found that molecular techniques such as the FilmArray Meningitis/Encephalitis panel have a poor percent positive agreement for CM (52%) when compared to antigen testing, indicating that the LFA represents the most optimal method to diagnose CM.[51] However, it is important to note that while important for diagnosis, antigen tests do not differentiate live from dead organisms, limiting their ability to assess for treatment responses after diagnosis.
Imaging
Since cryptococcal meningoencephalitis is predominately a compartmentalized intracranial infection, lumbar punctures with measurement of opening pressures are still the standard of care for initial diagnosis and monitoring during the acute phase of the illness. Brain MRI scans are also a reliable modality to evaluate both the degree of anatomic damage as well as a gross measure of inflammation.
Typical MRI brain findings seen in cryptococcal meningitis irrespective of immune status include meningitis, meningoencephalitis, hydrocephalus, enlarged perivascular (Virchow-Robin) spaces, cryptococcomas and disseminated disease. In a case series following 3 patients, cryptococcomas on brain imaging were seen to persist as long as 7 years after initial diagnosis despite clinical resolution, signifying that they do not necessarily represent active infection.[52] Optic nerve edema, ischemic strokes and spinal cord abscesses have also been reported in cIRIS patients.[53]
In non-HIV patients, ependymitis and choroid plexitis have been noted to occur at a greater frequency than in HIV-related CM. A study describing MRI brain findings in HIV negative patients was able to corelate ependymitis with elevated soluble CD27 levels, a marker for T cell mediated intrathecal inflammation while choroid plexitis was found to be a predictor of higher NFL levels, an indicator of axonal damage. [54] The presence of ependymitis and choroiditis is not specific for cryptococcal meningitis and has been associated with several other infections such as tuberculosis, toxoplasmosis, nocardiosis and CMV.[7] However, in the setting of known CM, it is a harbinger of active inflammation and may also predispose to central obstruction of the foramina of Monro, Luschka and Magendie. These findings can help guide the physician regarding the need for therapeutic steroids and ventricular shunting versus serial lumbar punctures to reduce intracranial pressure.[54] Interestingly, unlike other neurologic infections such as neurocysticercosis [55], CM rarely obstructs at the level of the Silvian fissure, likely due to the absence of an adjoining choroid with its inflammatory potential.
Treatment
According to IDSA guidelines, the recommended treatment duration for CM in HIV positive and transplant recipients is 2 weeks of induction therapy with amphotericin and flucytosine followed by 8 weeks of fluconazole. Per the literature, the use of flucytosine in the first 2 weeks of induction therapy has been associated with lower fungal burden and a decreased risk of relapse.[2] Interestingly, as shown in a murine model with disseminated cryptococcal disease, the combination of flucytosine and AMB is reported to retain its superiority over AMB monotherapy even against flucytosine resistant C. neoformans strains. [56] In the non-HIV populations, it is generally preferred to continue induction therapy for at least 4–6 weeks or 2 weeks from negative CSF fungal cultures and before a transition to fluconazole. After the initial 10–12 weeks of treatment, the patient can be transitioned to suppressive-dosed 200 mg fluconazole. The best method to monitor microbiological treatment efficacy is the CSF fungal culture, although in the pre-fluconazole era, 15–20% of previously healthy patients relapsed after a 4 week course of amphotericin B and negative CSF cultures at discharge.[57] While a decrease in cryptococcal antigen titer may suggest improvement, a positive antigen test does not differentiate between live and dead Cryptococcus and significantly lags behind fungal clearance.
The role of steroids is controversial and has been limited to patients with cIRIS, elevated intracranial pressure or patients with pulmonary Cryptococcus having ARDS.[58] In a large study performed in Thailand and a similar landmark study published in 2016, there was no mortality benefit seen with the use of adjunctive corticosteroids in HIV positive patients upon their initial presentation with CM. In fact, the latter study had to be suspended because the dexamethasone group was noted to have higher rates of mortality at 10 weeks and 6 months. Treated patients also had higher rates of disability and adverse events. The reason for this was unclear and could be related to differences in pathophysiology, but steroid use was found to be associated with lower rates of fungal clearance which may explain the poorer outcome. However, the study wasn’t powered for patients who had cIRIS.[59, 60] Moreover, it is noteworthy that the patients included in this study were begun on steroids at the time of diagnosis and received a prolonged course. There is a possibility that outcomes may have been different if patients were started on steroids after fungal clearance was achieved and perhaps for a shorter duration to reduce comorbid infections. Many case reports have highlighted the role of steroid use in cIRIS, especially when accompanied by elevated intracranial pressure. [61]
In the CINCH study which was restricted to non-HIV patients with CM, 80 percent of patients who received steroids after CSF fungal cultures were negative showed an improvement in functionality and outcomes.[40] In another study conducted from 2011 to 2016, it was found that previously shunted HIV negative patients with cryptococcal meningitis and PIIRS had improved functional outcomes when corticosteroids were used as salvage therapy [62]. Other anecdotal reports suggest improvement in CM with refractory disease. [63, 64] This may imply that there is a niche for corticosteroid use to suppress inflammatory responses in these patients. When treating patients with steroids, it is imperative to continue fluconazole and be attentive for recurrence since steroid use is an independent risk factor for infection [65, 66] and may lead to recurrence.
Hydrocephalus in these patients is a concerning finding and requires meticulous management. In the HIV host, hydrocephalus is usually due to blockage at the level of the arachnoid granulations and therefore represents a communicating process.[67] Serial lumbar punctures are essential to relieve intracranial pressure and typically suffice to sustain low pressures. Lumbar drain or ventriculo-peritoneal shunt insertion is only occasionally required. On the other hand, non-HIV patients more commonly have obstructions of the choroid plexus distal to the 4th ventricle and are also more likely to develop hydrocephalus. [7] Ventriculo-peritoneal shunting has been shown to provide sustained relief of neurological symptoms and patients most likely to benefit are those with hydrocephalus, an initial OP > 25 cm H20 and an HIV negative status. [38, 68] Resolution of gait instability and reduced mental acuity, both typical features of hydrocephalus, are more likely if shunting is performed soon after the onset of symptoms and outcomes may be improved by adjunctive corticosteroid therapy.[62, 69]
Pulmonary cryptococcal disease has been associated with a paradoxical cIRIS as well. [70] In these patients, symptoms usually develop between 1 to 10 months after ART initiation. Usual radiographic manifestations include solitary or multiple nodules which can progress to cavitary lesions, pneumonic infiltrates and pleural effusions [71]. CIRIS has also been known to cause extra CNS manifestations such as chorioretinitis, lymphadenitis, ARDS and soft tissue abscesses.[72]
Conclusions and future perspectives
Despite antifungals and effective diagnostic tools, mortality rates due to Cryptococcus have not changed substantially since the advent of amphotericin B reduced mortality from 100% to the current 20–40% in most host populations currently. Moreover, mortality continues to be high even in previously healthy patients who counter intuitively have a worse prognosis and mortality rates comparable to HIV patients. Interestingly, transplant patients tend to have somewhat lower mortalities which may be due to more rapid diagnosis because of their close follow-up [27]. Thus, future frontiers for more effective therapy appear to be to 1) improve the time to diagnosis and 2) identify and manage associated immune inflammatory conditions. Related to the first, advent of the more sensitive laminar flow assay (LFA) has made the diagnosis of CM more facile [73] as a simple blood test may have significant sensitivity in hard-to-diagnose hosts with low antigen loads such as the previously healthy [50]. Immunomodulatory therapy remains an elusive goal and is the subject of active research both in the US and abroad. A particular challenge in resource-limited countries is how to alter immune responses in a cost-effective manner.
Acknowledgement:
This research was supported in part by the Intramural Research Program of the NIH, Grant funding number AI001123 and AI00112.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Seher Anjum and Peter Williamson declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lui G, et al. , Cryptococcosis in apparently immunocompetent patients. QJM, 2006. 99(3): p. 143–51.DOI: 10.1093/qjmed/hcl014. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JE, et al. , A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med, 1979. 301(3): p. 126–31.DOI: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 3.Mirza SA, et al. , The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis, 2003. 36(6): p. 789–94.DOI: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 4.Rajasingham R, et al. , Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis, 2017. 17(8): p. 873–881.DOI: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon-Chung KJ, et al. , Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med, 2014. 4(7): p. a019760DOI: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gast CE, et al. , Azole resistance in Cryptococcus gattii from the Pacific Northwest: Investigation of the role of ERG11. Antimicrob Agents Chemother, 2013. 57(11): p. 5478–85.DOI: 10.1128/AAC.02287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson PR, et al. , Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol, 2017. 13(1): p. 13–24.DOI: 10.1038/nrneurol.2016.167.* An updated review of the epidemiology, immune pathology and clinical management strategies for HIV-associated and HIV-seronegative cryptococcal meningitis patients.
- 8.Hagen F, et al. , Importance of Resolving Fungal Nomenclature: the Case of Multiple Pathogenic Species in the Cryptococcus Genus. mSphere, 2017. 2(4).DOI: 10.1128/mSphere.00238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon-Chung KJ, et al. , The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere, 2017. 2(1).DOI: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, et al. , Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis, 2000. 31(2): p. 499–508.DOI: 10.1086/313992. [DOI] [PubMed] [Google Scholar]
- 11.Vu K, Garcia JA, and Gelli A, Cryptococcal Meningitis and Anti-virulence Therapeutic Strategies. Front Microbiol, 2019. 10: p. 353DOI: 10.3389/fmicb.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterman SR, et al. , Role of CTR4 in the Virulence of Cryptococcus neoformans. MBio, 2012. 3(5).DOI: 10.1128/mBio.00285-12e00285-12 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S and Williamson P, Lessons from cryptococcal laccase: from environmental saprophyte to pathogen. Curr Fungal Infect Rep, 2011. 5: p. 233–44. [Google Scholar]
- 14.Olave MC, et al. , Infective capacity of Cryptococcus neoformans and Cryptococcus gattii in a human astrocytoma cell line. Mycoses, 2017. 60(7): p. 447–453.DOI: 10.1111/myc.12619. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Hermoso D, Janbon G, and Dromer F, Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol, 1999. 37(10): p. 3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, Cryptococcus neoformans: morphogenesis, infection, and evolution. Infect Genet Evol, 2009. 9(4): p. 401–16.DOI: 10.1016/j.meegid.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Saha DC, et al. , Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol, 2007. 14(12): p. 1550–4.DOI: 10.1128/CVI.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlier C, et al. , Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun, 2009. 77(1): p. 120–7.DOI: 10.1128/IAI.01065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo Gde S, et al. , Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS One, 2012. 7(1): p. e29561DOI: 10.1371/journal.pone.0029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Y-D, et al. , A Role for LHC1 in Higher Order Structure and Complement Binding of the Cryptococcus neoformans Capsule. PLoS Pathog, 2014. 10(5): p. e1004037DOI: 10.1371/journal.ppat.1004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, et al. , Virulence Factors Identified by Cryptococcus neoformans Mutant Screen Differentially Modulate Lung Immune Responses and Brain Dissemination. Am J Pathol, 2012. DOI: S0002–9440(12)00487–7 [pii] 10.1016/j.ajpath.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panepinto J, et al. , Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. 2008. [DOI] [PubMed] [Google Scholar]
- 23.Almeida F, Wolf JM, and Casadevall A, Virulence-Associated Enzymes of Cryptococcus neoformans. Eukaryot Cell, 2015. 14(12): p. 1173–85.DOI: 10.1128/EC.00103-15.* A review of newly discovered enzymatic mechanisms through which Cryptococcus neoformans adapts to survive and infect mammalian hosts.
- 24.Panackal AA, et al. , Susceptibility to Cryptococcal Meningoencephalitis Associated With Idiopathic CD4(+) Lymphopenia and Secondary Germline or Acquired Defects. Open Forum Infect Dis, 2017. 4(2): p. ofx082DOI: 10.1093/ofid/ofx082.* Two case studies of patients with Idiopathic CD4+ lymphopenia (ICL) and opportunistic cryptococcal meningoencephalitis infections with an immunological focus on the E57K genetic mutation and acquired granulocyte-macrophage colony stimulating factor autoantibodies.
- 25.Zonios DI, et al. , Cryptococcosis and idiopathic CD4 lymphocytopenia. Medicine (Baltimore), 2007. 86(2): p. 78–92.DOI: 10.1097/md.0b013e31803b52f5. [DOI] [PubMed] [Google Scholar]
- 26.Elsegeiny W, Marr KA, and Williamson PR, Immunology of Cryptococcal Infections: Developing a Rational Approach to Patient Therapy. Front Immunol, 2018. 9: p. 651DOI: 10.3389/fimmu.2018.00651.** New insights on immune activation in HIV-positive and previously healthy patients with cryptococcal meningoencephalitis to update treatment strategies
- 27.Brizendine KD, Baddley JW, and Pappas PG, Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One, 2013. 8(3): p. e60431DOI: 10.1371/journal.pone.0060431.** A retrospective cohort study of 302 patients with cryptococcosis that identifies cryptococcemia, high intracranial pressure, and a non-HIV, non-transplant immune status as predictive markers for mortality.
- 28.Panackal AA, et al. , Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis. PLoS Pathog, 2015. 11(5): p. e1004884DOI: 10.1371/journal.ppat.1004884.** This immunological study higlights unusual findings of strong intrathecal expansion and activation of T cells involved in both innate and adaptive immunity in HIV-negative patients with cryptococcal meningoencephalitis.
- 29.Pirofski LA and Casadevall A, The damage-response framework of microbial pathogenesis and infectious diseases. Adv Exp Med Biol, 2008. 635: p. 135–46.DOI: 10.1007/978-0-387-09550-9_11.** An overview of the damage-response framework explains how an imbalance in the host-microbe interaction can determine variable outcomes in different patients.
- 30.Shelburne SA 3rd and Hamill RJ, The immune reconstitution inflammatory syndrome. AIDS Rev, 2003. 5(2): p. 67–79. [PubMed] [Google Scholar]
- 31.Tugume L, et al. , HIV-Associated Cryptococcal Meningitis Occurring at Relatively Higher CD4 Counts. J Infect Dis, 2019. 219(6): p. 877–883.DOI: 10.1093/infdis/jiy602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legris T, et al. , Immune reconstitution inflammatory syndrome mimicking relapsing cryptococcal meningitis in a renal transplant recipient. Transpl Infect Dis, 2011. 13(3): p. 303–8.DOI: 10.1111/j.1399-3062.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 33.Neal LM, et al. , CD4(+) T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis. MBio, 2017. 8(6).DOI: 10.1128/mBio.01415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panackal AA, et al. , Fighting the Monster: Applying the Host Damage Framework to Human Central Nervous System Infections. MBio, 2016. 7(1).DOI: 10.1128/mBio.01906-15.* This review applies the host damage-response framework to nonviral generalized CNS infections, including cryptococcal meningitis in non-HIV-infected patients.
- 35.Balasko A and Keynan Y, Shedding light on IRIS: from Pathophysiology to Treatment of Cryptococcal Meningitis and Immune Reconstitution Inflammatory Syndrome in HIV-Infected Individuals. HIV Med, 2019. 20(1): p. 1–10.DOI: 10.1111/hiv.12676.** A review of the pathophysiology of HIV-associated cryptococcal immune reconstitution inflammatory syndrome with the consideration of thalidomide as a targeted alternative for type 1 T helper (Th1) cell overcompensation.
- 36.Liao CH, et al. , Different presentations and outcomes between HIV-infected and HIV-uninfected patients with Cryptococcal meningitis. J Microbiol Immunol Infect, 2012. 45(4): p. 296–304.DOI: 10.1016/j.jmii.2011.12.005.* A retrospective study describing potential predictors of outcomes with findings of lower mortality rates in HIV-infected CM compared to non-HIV patients with CM.
- 37.Bratton EW, et al. , Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS One, 2012. 7(8): p. e43582DOI: 10.1371/journal.pone.0043582.* This study compares trends for cryptococcosis in patients with varying immune status with the main finding of an increased prevalence in HIV-uninfected cases.
- 38.Nguyen MH, et al. , Outcomes of central nervous system cryptococcosis vary with host immune function: results from a multi-center, prospective study. J Infect, 2010. 61(5): p. 419–26.DOI: 10.1016/j.jinf.2010.08.004.* This observational study demonstrated significant differences in outcomes for CNS cryptococcosis in relation to host immune status with the main finding of a higher mortality rate in non-immunocompromised patients.
- 39.Pappas PG, Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc, 2013. 124: p. 61–79. [PMC free article] [PubMed] [Google Scholar]
- 40.Marr KA, et al. , A Multicenter, Longitudinal Cohort Study of Cryptococcosis in HIV-Negative People in the United States. Clin Infect Dis, 2019. DOI: 10.1093/cid/ciz193.** This prospective cohort study identified declining Montreal Cognitive Assessment (MoCA) scores and subsequent long-term neurological sequelae in HIV-uninfected patients despite the use of antifungal therapy.
- 41.Spec A, et al. , Cryptococcus Infection Network in Non-Human Immunodeficiency Virus Cohort (CINCH) Study: Initial Report of Treatment and Outcomes. Open Forum Infect Dis, 2016. 3(Suppl 1): p. 122DOI: 10.1093/ofid/ofw194.35. [DOI] [Google Scholar]
- 42.Traino K, HIV-Negative Cryptococcal Meningoencephalitis Results in a Persistent Frontal-Subcortical Syndrome. Nature Scientific Reports 2019(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly A King GA,AAP, Zalewski Chris, Anjum Seher, Bennett John E., Beri Andrea, Kim H. Jeff, Hammoud Dima, Brewer Carmen C., Williamson Peter R., Audiologic and Otologic Complications of Cryptococcal Meningoencephalitis in Non-HIV Previously Healthy Patients. Otology and Neurotology. 2019(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panackal AA, et al. , Spinal Arachnoiditis as a Complication of Cryptococcal Meningoencephalitis in Non-HIV Previously Healthy Adults. Clin Infect Dis, 2017. 64(3): p. 275–283.DOI: 10.1093/cid/ciw739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Halloran JA, Powderly WG, and Spec A, Cryptococcosis today: It is not all about HIV infection. Curr Clin Microbiol Rep, 2017. 4(2): p. 88–95.DOI: 10.1007/s40588-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasquier E, et al. , Long-term Mortality and Disability in Cryptococcal Meningitis: A Systematic Literature Review. Clin Infect Dis, 2018. 66(7): p. 1122–1132.DOI: 10.1093/cid/cix870. [DOI] [PubMed] [Google Scholar]
- 47.Chow E and Troy SB, The differential diagnosis of hypoglycorrhachia in adult patients. Am J Med Sci, 2014. 348(3): p. 186–90.DOI: 10.1097/MAJ.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komori M, et al. , Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann Neurol, 2015. 78(1): p. 3–20.DOI: 10.1002/ana.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nalintya E, Kiggundu R, and Meya D, Evolution of Cryptococcal Antigen Testing: What is new? Curr Fungal Infect Rep, 2016. 10(2): p. 62–67.DOI: 10.1007/s12281-016-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jitmuang A, et al. , Performance of the Cryptococcal Antigen Lateral Flow Assay in Non-HIV-Related Cryptococcosis. J Clin Microbiol, 2016. 54(2): p. 460–3.DOI: 10.1128/JCM.02223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liesman RM, et al. , Evaluation of a Commercial Multiplex Molecular Panel for Diagnosis of Infectious Meningitis and Encephalitis. J Clin Microbiol, 2018. 56(4).DOI: 10.1128/JCM.01927-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hospenthal DR and Bennett JE, Persistence of cryptococcomas on neuroimaging. Clin Infect Dis, 2000. 31(5): p. 1303–6.DOI: 10.1086/317434. [DOI] [PubMed] [Google Scholar]
- 53.Ellis JP, et al. , Ischemic stroke as a complication of cryptococcal meningitis and immune reconstitution inflammatory syndrome: a case report. BMC Infect Dis, 2018. 18(1): p. 520DOI: 10.1186/s12879-018-3386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammoud DA, et al. , Choroid Plexitis and Ependymitis by Magnetic Resonance Imaging are Biomarkers of Neuronal Damage and Inflammation in HIV-negative Cryptococcal Meningoencephalitis. Sci Rep, 2017. 7(1): p. 9184DOI: 10.1038/s41598-017-09694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takayanagui OM and Odashima NS, Clinical aspects of neurocysticercosis. Parasitol Int, 2006. 55 Suppl: p. S111–5.DOI: 10.1016/j.parint.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz P, et al. , Efficacy of amphotericin B in combination with flucytosine against flucytosine-susceptible or flucytosine-resistant isolates of Cryptococcus neoformans during disseminated murine cryptococcosis. Antimicrob Agents Chemother, 2006. 50(1): p. 113–20.DOI: 10.1128/AAC.50.1.113-120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diamond RD and Bennett JE, Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med, 1974. 80(2): p. 176–81. [DOI] [PubMed] [Google Scholar]
- 58.Perfect JR, et al. , Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis, 2010. 50(3): p. 291–322.DOI: 10.1086/649858.* IDSA clinical practice guidelines for cryptococcosis in HIV-infected individuals, organ transplant recipients, and non HIV-infected (non-transplant) patients.
- 59.Beardsley J, et al. , Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N Engl J Med, 2016. 374(6): p. 542–54.DOI: 10.1056/NEJMoa1509024.* This is a double-blind, randomized, placebo-controlled landmark trial of dexamethasone use in HIV-infected patients with cryptococcal meningitis that demonstrated no improvement in outcomes for the experimental group.
- 60.Liu J, et al. , Ventriculoperitoneal shunts in non-HIV cryptococcal meningitis. BMC Neurol, 2018. 18(1): p. 58DOI: 10.1186/s12883-018-1053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tellez RM, et al. , [Cerebral cryptococcosis and immune reconstitution inflammatory syndrome. Case report]. Rev Med Chil, 2018. 146(12): p. 1481–1485.DOI: 10.4067/s0034-98872018001201481. [DOI] [PubMed] [Google Scholar]
- 62.Mehta GU, et al. , Corticosteroids for shunted previously healthy patients with non-HIV cryptococcal meningoencephalitis. J Neurol Neurosurg Psychiatry, 2018. 89(2): p. 219–220.DOI: 10.1136/jnnp-2017-315830.* This letter details the successful use of corticosteroids as well as antifungal therapy to control post-infectious inflammatory response syndrome (PIIRS) and prevent long-term neurological complications in cryptococcal meningitis (CM) patients.
- 63.Lane M, McBride J, and Archer J, Steroid responsive late deterioration in Cryptococcus neoformans variety gattii meningitis. Neurology, 2004. 63(4): p. 713–4. [DOI] [PubMed] [Google Scholar]
- 64.Chen SC, et al. , Antifungal therapy and management of complications of cryptococcosis due to Cryptococcus gattii. Clin Infect Dis, 2013. 57(4): p. 543–51.DOI: 10.1093/cid/cit341. [DOI] [PubMed] [Google Scholar]
- 65.Kwon-Chung KJ and Bennett JE, Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol, 1984. 120(1): p. 123–30. [DOI] [PubMed] [Google Scholar]
- 66.Pyrgos V, et al. , Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS One, 2013. 8(2): p. e56269DOI: 10.1371/journal.pone.0056269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loyse A, et al. , Histopathology of the arachnoid granulations and brain in HIV-associated cryptococcal meningitis: correlation with cerebrospinal fluid pressure. AIDS, 2010. 24(3): p. 405–10.DOI: 10.1097/QAD.0b013e328333c005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graybill JR, et al. , Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis, 2000. 30(1): p. 47–54.DOI: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 69.Park MK, Hospenthal DR, and Bennett JE, Treatment of hydrocephalus secondary to cryptococcal meningitis by use of shunting. Clin Infect Dis, 1999. 28(3): p. 629–33.DOI: 10.1086/515161. [DOI] [PubMed] [Google Scholar]
- 70.Calligaro G, Meintjes G, and Mendelson M, Pulmonary manifestations of the immune reconstitution inflammatory syndrome. Curr Opin Pulm Med, 2011. 17(3): p. 180–8.DOI: 10.1097/MCP.0b013e328344f692. [DOI] [PubMed] [Google Scholar]
- 71.Hu Z, et al. , Radiological characteristics of pulmonary cryptococcosis in HIV-infected patients. PLoS One, 2017. 12(3): p. e0173858DOI: 10.1371/journal.pone.0173858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jenny-Avital ER and Abadi M, Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clin Infect Dis, 2002. 35(12): p. e128–33.DOI: 10.1086/344467. [DOI] [PubMed] [Google Scholar]
- 73.Gates-Hollingsworth MA and Kozel TR, Serotype sensitivity of a lateral flow immunoassay for cryptococcal antigen. Clin Vaccine Immunol, 2013. 20(4): p. 634–5.DOI: 10.1128/CVI.00732-12. [DOI] [PMC free article] [PubMed] [Google Scholar]