Abstract
Tendon injuries increase with age, yet the age-associated changes in tendon properties remain unexplained. Decorin and biglycan are two matrix proteoglycans that play complex roles in regulating tendon formation, maturation, and aging, most notably in extracellular matrix assembly and maintenance. However, the roles of decorin and biglycan have not been temporally isolated in a homeostatic aged context. The goal of this work was to temporally isolate and define the roles of decorin and biglycan in regulating aged murine patellar tendon mechanical properties. We hypothesized that decorin would have a larger influence than biglycan on aged tendon mechanical properties and that biglycan would have an additive role in this regulation. When decorin and biglycan were knocked down in aged tendons, minimal changes in gene expression were observed, implying that these models directly define the roles of decorin and biglycan in regulating tendon mechanical properties. Knockdown of decorin or biglycan led to minimal changes in quasi-static mechanical properties. However, decorin deficiency led to increases in stress relaxation and phase shift that were exacerbated when coupled with biglycan deficiency. This study highlights an important role for decorin, alone and in tandem with biglycan, in regulating aged tendon viscoelastic properties.
Keywords: tendon, biomechanics, decorin, biglycan, aging
Introduction
Tendon is a highly aligned, collagen I-rich tissue that transmits forces from muscle to bone, and when injured, presents a large clinical burden [1–3]. While varied across distinct tendons, there is an overall increase in tendon injury risk with age [1,2]. The cause of this age-associated injury risk remains unknown and is not directly explained by changes in tendon mechanical properties. While some animal studies report decreases in tendon mechanical properties with age [4–7], other studies report moderate or no differences in mechanical properties across ages [8–10]. Age-related mechanical changes (or lack thereof) may be tissue-specific, but dynamic properties, especially dynamic modulus, are affected by age when measured [4,7]. Studies that report no differences in mechanical properties through aging did not analyze dynamic properties, which may explain the discrepancy in conclusions about the effect of aging on tendon mechanical properties. Ex vivo testing of human tendons has also demonstrated uncertainty in the effect of age on mechanical properties. While most mechanical properties are unaffected by age, moderate correlations between decreased mechanical properties and age have been observed [11–13]. These studies measured viscoelastic and quasi-static, but not dynamic, properties. These conflicting results highlight the need for better understanding of the mechanisms by which aging affects tendon function, including dynamic properties.
Decorin and biglycan are two small leucine-rich proteoglycans (SLRPs) present in tendon. Decorin and biglycan share a similar structure, with one and two glycosaminoglycan (GAG) chains attached to a protein core, respectively [14]. Decorin and biglycan expression peaks in mice during early tendon development, but decorin expression is maintained at consistent levels into maturity while biglycan expression tapers off by 10 days of age [15]. Both decorin and biglycan play vital roles in collagen fibrillogenesis during tendon development. The absence of either molecule results in irregular fibril shape and abnormal shifts in fibril diameters [15,16]. These perturbations in fibril structure correspond with changes in tissue-scale mechanical properties, highlighting a role of these SLRPs in regulating tendon mechanical propertiess. While the effect of SLRP knockout on tendon mechanical properties is tissue-specific, in general there is an increase in stress relaxation and a decrease in strain sensitivity with decorin deficiency [17,18] and changes in dynamic modulus with deficiency of decorin or biglycan [19–21]. In addition to the isolated roles of decorin and biglycan in regulating collagen fibrils and tendon mechanical properties, there is evidence that these SLRPs work in tandem to regulate tendon properties. Biglycan expression increases in the absence of decorin [15,22]. The dual knockout of decorin and biglycan results in a more severe fibril phenotype than seen with knockout of either SLRP alone [22,23]. Further, induced knockdown of both decorin and biglycan results in increased stress relaxation and phase shift and decreased stiffness, dynamic modulus, and failure load [24]. These studies demonstrate that decorin and biglycan, both alone and in tandem, play critical roles in regulating tendon function.
Along with the detrimental effects seen during tendon development, decorin and/or biglycan deficiency leads to a differential response in tendon properties through aging. While wild-type (WT) and biglycan-deficient murine patellar tendons exhibit substantial decreases in dynamic modulus and increases in phase shift with age, these changes are mitigated with decorin deficiency [7]. In the murine supraspinatus tendon, biglycan deficiency protected against age-associated decreases in stress relaxation [25]. These studies highlight the complex effect of decorin and biglycan presence in regulating tendon mechanical properties throughout the tissue lifetime. These studies used conventional knockout models in which tendons are SLRP-deficient during development. Given that decorin and biglycan expression peaks during development, it is unknown whether the effect of SLRP deficiency seen in aged tendons is due to persisting differences from tendon development or due to the specific role of decorin and biglycan in aged tendons. Inducible knockdown mouse models allow for the role of SLRPs in regulating tendon mechanics to be temporally isolated and defined in the context of homeostatic aged tendons.
Therefore, the objective of this study was to temporally isolate and define the role of decorin and biglycan in aged murine patellar tendon mechanical properties. To address this objective, three inducible mouse models were used in which decorin (Dcn), biglycan (Bgn), or both SLRPs were knocked down with temporal specificity in aged mice. Mechanical properties and gene expression patterns of patellar tendons were defined to test the hypothesis that decorin knockdown would decrease tendon mechanical properties more than biglycan knockdown. We hypothesized that knockdown of both SLRPs would cause larger mechanical deficiencies than knockdown of either SLRP alone.
Materials and Methods
Mice.
Forty-one female mice with a C57/BL6 Charles River background were used in this study (IACUC approved). WT (n = 6) mice were used as controls (Charles River, Wilmington, MA). Dcnflox/flox, Bgnflox/flox, and Dcnflox/flox/Bgnflox/flox mice were crossed with knockin tamoxifen (TM)-inducible Cre mice (B6.129-Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J, Jackson Labs, Bar Harbor, ME) to achieve mouse lines in which excision of floxed genes is achieved upon TM injection [24]. TM base (T5648, Sigma, St. Louis, MO) in corn oil (C8267, Sigma) was used for injections. All mice were aged until 485 days old, at which time they received three consecutive daily TM injections (4 mg/40 g body weight). The resulting I-Dcn−/− (n = 11), I-Bgn−/− (n = 9), and I-Dcn−/−/Bgn−/− (n = 15) mice were sacrificed 30 days post-TM injection (515 days of age). At the time of sacrifice, right hindlimb tibia-patellar tendon–patella complexes were isolated and flash frozen at −80 °C for gene expression analysis. Left hindlimbs were preserved in the body and frozen at −20 °C for later mechanical analysis.
Viscoelastic Mechanical Testing.
Left limb tibia-patellar tendon–patella complexes were prepared for mechanical testing as previously described [7]. Following gross and fine dissection of the bone-patellar tendon–bone complex, tendon cross-sectional area (CSA) was measured using a custom laser device [26]. Tendons were then stamped into a dog bone shape with a 2 mm biopsy punch to achieve a midlength width of 0.75 mm. Fiducial markers using Verhoeff's stain were applied to the tendon in 1 mm increments starting at the tibial insertion. The insertion was defined as the region between the first and second stain line (0–1 mm from tibial insertion), while the midsubstance was defined as the region between the second and third stain line (1–2 mm from tibial insertion). Tendon CSA was remeasured poststamping, and the tibia was potted in custom three-dimensional-printed fixtures with polymethyl methacrylate. Tendons were then loaded in a 37 °C 1× phosphate buffered saline bath within a tensile testing machine (Instron 5848, Instron, Norwood, MA). Tendons were imaged throughout the mechanical test using an established cross-polarization light setup [27,28]. This setup involves a linear backlight (Dolan-Jenner, Boxborough, MA), rotating polarizer sheets offset by 90 deg (Edmund Optics, Barrington, NJ), and a digital camera (Baxter, Exton, PA). The loading protocol began with ten cycles of preconditioning at 0.25 Hz with an amplitude of 0.5% strain. Tendons were then subjected to stress relaxations at 3%, 4%, and 5% strain magnitude. After loads equilibrated for 600 s, tendons underwent frequency sweeps of ten cycles at 0.1, 1, 5, and 10 Hz with an amplitude of 0.125% strain. Following the 5% stress relaxation, tendons were unloaded for 60 s, followed by a ramp-to-failure at 0.1% strain/s. From each stress relaxation and frequency sweep, percent relaxation, dynamic modulus (E*), and phase shift (δ) were calculated. From the ramp-to-failure and CSA data, stiffness, maximum load, and maximum stress were quantified. Optical tracking of the insertion and midsubstance tendon regions during the ramp-to-failure allowed for calculation of region-specific stiffness using a custom matlab program (matlab, Natick, MA). This stiffness was normalized to CSA for region-specific moduli values. Circular variance of fiber alignment was calculated through the ramp-to-failure using a custom matlab program to measure fiber realignment dynamics in response to load [28].
Gene Expression Analysis.
Patellar tendons were isolated from frozen complexes and thawed in RNAlater-ICE (ThermoFisher, Waltham, MA) for RNA stabilization. Tendon tissue was disrupted in TRIzol (ThermoFisher) with a pestle and was vortexed. RNA was isolated from the supernatant using Direct-zol RNA Microprep kits (Zymo Research, Irvine, CA). RNA was reverse-transcribed using a High Capacity cDNA RT kit (ThermoFisher). The resulting cDNA underwent 15 cycles of pre-amplification with selected Taqman Gene Expression Assays (Fluidigm, San Francisco, CA ThermoFisher). Pre-amplified cDNA was loaded into a Fluidigm 96.96 Dynamic Array™ IFC (Fluidigm) at the Molecular Profiling Facility (University of Pennsylvania). Expression of 48 genes was measured in duplicate. Taqman Assay information for the selected genes can be found in the Supplemental Materials available on the ASME Digital Collection. Rps17 and Abl1 were used as housekeeper genes. ΔCt values for each gene were calculated by subtracting the Ct value from the average Ct value of the housekeeper genes. ΔΔCt values were calculated for each sample by subtracting ΔCt values from those of the WT average.
Statistics.
For all mechanical properties, one-way ANOVAs with Tukey post hoc tests were used to compare values across genotypes. For fiber realignment data, two-way ANOVAs with Tukey post hoc tests were used to compare values across genotypes and strain levels. For gene expression data, principal component analysis (PCA) was performed on ΔΔCt values via ClustVis to identify global patterns in differential expression [29]. One-way ANOVAs with Tukey post hoc tests were used to compare principal component (PC) scores and ΔCt values for all genes across genotypes. Linear regression of selected parameters was run on Prism 7 (GraphPad, San Diego, CA). Significance was set at p ≤ 0.05, and trends were set at p ≤ 0.1.
Results
Mouse Models Demonstrated Effective Knockdown of Targeted Genes.
I-Dcn−/− had a 5.8-fold and I-Dcn−/−/Bgn−/− tendons had a 5.9-fold significant decrease in Dcn expression relative to WT tendons, while Dcn expression was unaffected in I-Bgn−/− tendons (Fig. 1(a)). I-Bgn−/− had a 4.5-fold and I-Dcn−/−/Bgn−/− tendons had a 10.7-fold significant decrease in Bgn expression relative to WT tendons, while Bgn expression was unaffected in I-Dcn−/− tendons (Fig. 1(b)). I-Dcn−/−/Bgn−/− tendons trended toward decreased Bgn expression relative to I-Bgn−/− tendons.
Fig. 1.
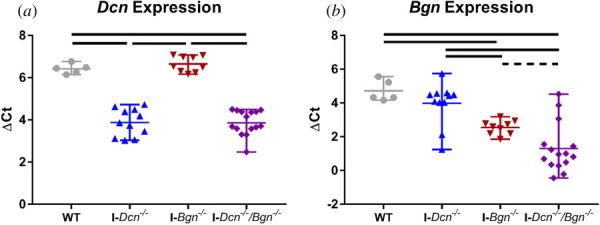
Effective knockdown of targeted genes in aged tendons. Knockdown models demonstrated effective knockdown of Dcn (a) and Bgn (b) in the appropriate genotypes. Solid lines denote p ≤ 0.05, and dotted lines denote p ≤ 0.1.
Principal Component Analysis Revealed Altered Expression Profiles in Decorin-Deficient Tendons.
Principal Component Analysis was run on ΔΔCt values, and the first three principal components accounted for 39.1%, 13.7%, and 11.4% of the overall variance, respectively (Fig. 2(a)). While PC1 scores correlated with high or low abundant genes (e.g., ECM genes versus transcription factors), PC2 scores significantly correlated with standard deviation of ΔΔCt values across all genes (r2 = 0.36, p < 1 × 10−4), indicative of divergence from WT controls. I-Dcn−/− tendons had significantly increased PC2 scores relative to I-Bgn−/− tendons (Fig. 2(b)). I-Dcn−/−/Bgn−/− tendons had significantly increased PC2 scores relative to WT and I-Bgn−/− tendons. A heatmap of ΔΔCt values revealed a subset of I-Dcn−/−/Bgn−/− and I-Dcn−/− tendons with elevated expression of Mmp13, Col2a1, and Acan (Fig. S1, which is available in the Supplemental Materials on the ASME Digital Collection). Mmp13 and Col2a1 had high PC2 loading scores (See Supplemental Material).
Fig. 2.
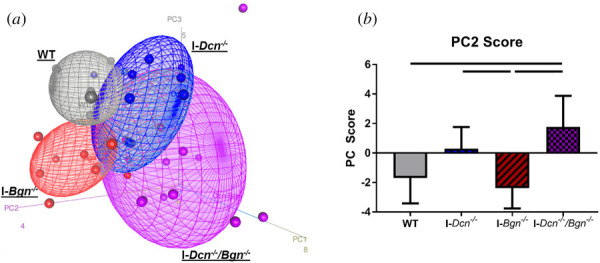
Decorin-deficient aged tendons exhibited array-level differences in gene expression. PCA demonstrated distinct clustering with genotype (a). Quantified PC2 scores revealed that I-Dcn−/−/Bgn−/− tendons had increased PC2 scores relative to WT and I-Bgn−/− tendons, while I-Dcn−/− tendons had increased PC2 scores relative to I-Bgn−/− tendons (b). Solid lines denote p ≤ 0.05.
Individual Gene Expression Was Largely Unaffected by Small Leucine-Rich Proteoglycan Knockdown.
ANOVAs revealed that expression of two genes, Eln and Fmod, was significantly influenced by genotype. While post hoc tests demonstrated no significant differences in Eln expression between groups, I-Bgn−/− tendons trended toward increased Fmod expression compared to tendons from all other genotypes (Fig. 3). No differences in Mmp13, Col2a1, nor Acan expression were observed across genotypes.
Fig. 3.
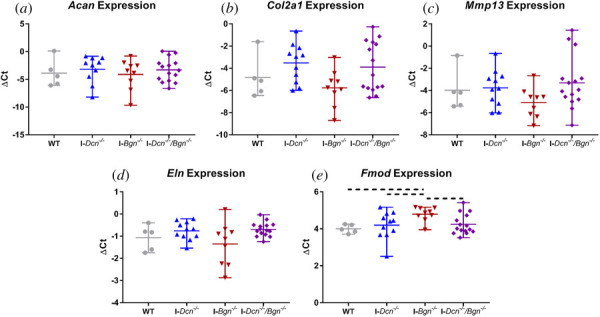
Deficiency of decorin or biglycan led to minimal changes in aged tendon gene expression. No differences in expression were observed across genotypes for Acan (a), Col2a1 (b), Mmp13 (c), or Eln (d). I-Bgn−/− tendons had trending increases in Fmod expression (e). Dotted lines denote p ≤ 0.1.
Quasi-Static Mechanical Properties Were Largely Unaffected by Small Leucine-Rich Proteoglycan Knockdown.
No differences in tendon CSA were observed across genotypes (Fig. 4(a)). No differences in stiffness or maximum load were observed across genotypes (Figs. 4(b) and 4(c)). No differences in insertion or midsubstance modulus were observed across genotypes (Figs. 4(d) and 4(e)). I-Dcn−/− tendons trended toward decreased maximum stress compared to WT tendons (Fig. 4(f)). I-Dcn−/−/Bgn−/− tendons had significantly increased maximum stress compared to I-Dcn−/− tendons and a trending increase in maximum stress compared to I-Bgn−/− tendons.
Fig. 4.
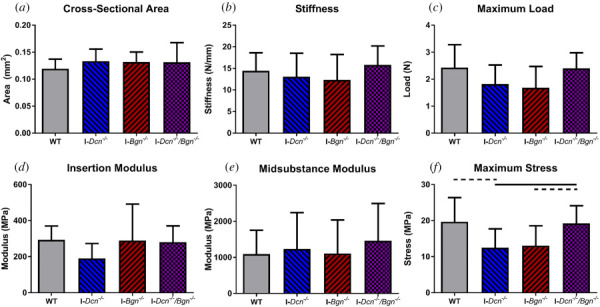
Decorin and biglycan knockdown led to minimal changes in aged tendon quasi-static properties. No differences inCSA (a), stiffness (b), maximum load (c), insertion modulus (d), or midsubstance (e) were observed across genotypes. I-Dcn−/− tendons had decreased maximum stress relative to WT and I-Dcn−/−/Bgn−/− tendons (f). I-Bgn−/− tendons had decreased maximum stress relative to I-Dcn−/−/Bgn−/− tendons. Solid lines denote p ≤ 0.05, and dotted lines denote p ≤ 0.1.
Decorin-Deficient Tendons Continued Fiber Realignment Further Into Ramp to Failure.
In the tendon midsubstance, WT and I-Bgn−/− tendons experienced significant realignment between 1% and 3% strain of the ramp-to-failure, while I-Dcn−/− and I-Dcn−/−/Bgn−/− tendons significantly realigned between both 1% and 3% strain and 3% and 5% strain (Fig. S2, in the Supplemental Materials on the ASME Digital Collection). No differences in realignment magnitude were observed at any strain level across genotypes, including 0% strain. In the tendon insertion, WT, I-Dcn−/−, and I-Bgn−/− tendons significantly realigned between 1% and 3% strain (data not shown). I-Dcn−/−/Bgn−/− significantly realigned between 1% and 3% strain and 3% and 5% strain within the insertion, as they did in the midsubstance. No differences in realignment magnitude were observed at any strain level across genotypes, including 0% strain.
Decorin Deficiency, Alone and in Tandem With Biglycan Deficiency, Increased Tendon Viscoelastic Properties.
I-Dcn−/− tendons exhibited a trending increase in stress relaxation at 4% strain relative to WT tendons (Fig. 5). I-Dcn−/−/Bgn−/− tendons exhibited significantly increased stress relaxation at 3% and 4% strain, and trended toward an increase at 5% strain, relative to WT and I-Bgn−/− tendons. No differences in dynamic moduli were observed across genotypes at any strain level or frequency (Fig. S3, in the Supplemental Materials on the ASME Digital Collection).
Fig. 5.
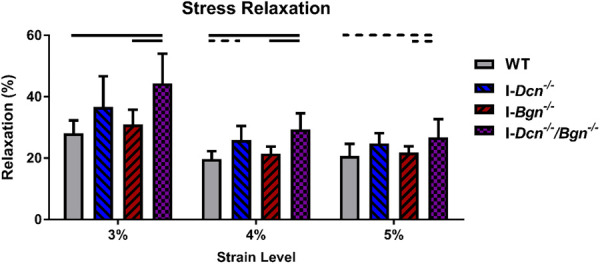
Decorin deficiency, alone and in tandem with biglycan deficiency, increased aged tendon stress relaxation. I-Dcn−/− tendons had a trending increase in stress relaxation at 4% strain. I-Dcn−/−/Bgn−/− tendons exhibited larger changes in stress relaxation, with increased relaxation relative to WT and I-Bgn−/− tendons at all strain levels. Solid lines denote p ≤ 0.05, and dotted lines denote p ≤ 0.1.
At 3% strain, I-Dcn−/− tendons had trending increases in tan(δ) values relative to WT tendons at 5 Hz and 10 Hz (Fig. 6(a)). I-Dcn−/−/Bgn−/− tendons had significantly increased tan(δ) values relative to WT tendons at all frequencies. I-Dcn−/−/Bgn−/− tendons had significantly increased tan(δ) values relative to I-Bgn−/− tendons at 0.1 Hz and 1 Hz, with trending increases at 5 Hz and 10 Hz.
Fig. 6.
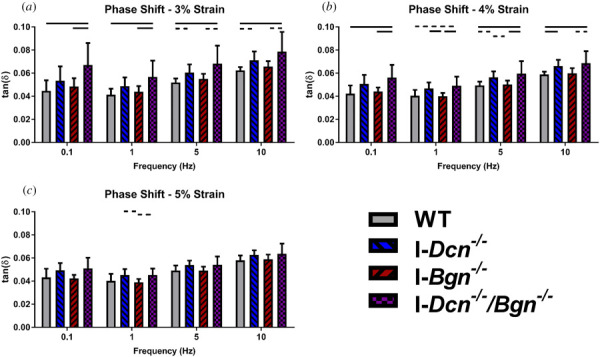
Decorin deficiency, alone and in tandem with biglycan deficiency, increased aged tendon phase shift. I-Dcn−/− tendons exhibited increased phase shift relative to WT and I-Bgn−/− tendons through multiple frequencies at 3% (a) and 4% (b) strain. These increases were more pronounced in I-Dcn−/−/Bgn−/− tendons. At 5% strain (c), these changes effectively disappeared. Solid lines denote p ≤ 0.05, and dotted lines denote p ≤ 0.1.
At 4% strain, I-Dcn−/− tendons had significantly increased tan(δ) values relative to WT tendons at 10 Hz, with a trending increase at 5 Hz (Fig. 6(b)). I-Dcn−/− tendons had significantly increased tan(δ) values relative to I-Bgn−/− tendons at 1 Hz, with a trending increase at 5 Hz. I-Dcn−/−/Bgn−/− tendons had significantly increased tan(δ) values relative to WT tendons at 0.1 Hz, 5 Hz, and 10 Hz, with a trending increase at 1 Hz. I-Dcn−/−/Bgn−/− tendons had significantly increased tan(δ) values relative to I-Bgn−/− tendons at 0.1 Hz, 1 Hz, and 5 Hz, with a trending increase at 10 Hz.
At 5% strain, I-Dcn−/− tendons trended toward increased tan(δ) values relative to I-Bgn−/− tendons at 1 Hz (Fig. 6(c)). I-Dcn−/−/Bgn−/− tendons trended toward increased tan(δ) values relative to I-Bgn−/− tendons at 1 Hz.
Viscoelastic Properties Correlated With Decorin Expression.
For each mouse, Dcn and Bgn expression of the right patellar tendon was plotted against stress relaxation and tan(δ) values at 4% strain of the left patellar tendon. Dcn expression significantly correlated with stress relaxation and phase shift at every frequency (Table S1, in the Supplemental Materials on the ASME Digital Collection). Bgn expression did not significantly correlate with any of these viscoelastic parameters.
Discussion
Small Leucine-Rich Proteoglycan Knockdown Led to Minimal Changes in Tendon Gene Expression.
Our mouse models demonstrated effective knockdown of targeted genes, with more than 5.5-fold and 4.5-fold reduction in expression of Dcn and Bgn, respectively. PCA revealed that the second principal component (PC2) separated decorin-deficient tendons from WT and I-Bgn−/− tendons. PC2 scores correlated with standard deviation of ΔΔCt values across all genes. This relationship suggests that decorin knockdown led to perturbations in overall expression profiles across the genes analyzed. However, these array-level changes did not translate to statistical differences in expression of individual genes. Mmp13 and Col2a1 had high PC2 loading scores and appeared to be upregulated in decorin-deficient tendons, but no statistical differences were found in expression of these genes.
Fmod expression had a trending increase with knockdown of biglycan alone. Prior data suggest that biglycan and fibromodulin function in tandem to regulate tendon properties [16,30]. Here, upregulation of Fmod in I-Bgn−/− tendons supports a compensatory function of fibromodulin in the absence of biglycan.
Overall, the lack of robust genetic changes seen in response to decorin and/or biglycan knockdown implies that the inducible knockdown models used here allow for direct analysis of the role of these SLRPs in regulating aged tendon mechanical properties. Any mechanical changes seen are likely due to the direct function of decorin or biglycan protein rather than the result of cellular response to SLRP knockdown.
Small Leucine-Rich Proteoglycan Knockdown Lead to Minimal Changes in Quasi-Static Properties and Fiber Realignment Dynamics.
Aside from trending decreases in maximum stress in I-Dcn−/− tendons, no quasi-static differences were observed between genotypes. This result corroborates previous findings that decorin and biglycan do not regulate quasi-static mechanical properties in the patellar tendon [19,20]. Decorin-deficient tendons experienced significant fiber realignment later in the ramp-to-failure compared to WT and I-Bgn−/− tendons. However, no differences in realignment were observed between genotypes at any strain level. This suggests that decorin deficiency led to subtle changes in fiber realignment dynamics that do not translate to significant decreases in realignment at any given strain.
Decorin Deficiency, Alone and in Tandem With Biglycan Deficiency, Increased Tendon Viscoelastic Properties.
This study found that induced knockdown of decorin, alone and in tandem with biglycan knockdown, increased the viscoelastic properties of aged tendons. Compared to WT tendons, I-Dcn−/− tendons had a trending increase in stress relaxation at 4% strain and trending or significant increases in phase shift at 3% and 4% strain across multiple frequencies (Fig. 6). Increased phase shift indicates a lag in tendon stress in response to strain, and increased tan(δ) values represent a higher ratio of loss modulus to storage modulus. Both changes are indicative of increased tissue viscosity. When biglycan was knocked down in tandem with decorin, increases in stress relaxation and phase shift seen with decorin deficiency alone became more pronounced. Compared to WT tendons, I-Dcn−/−/Bgn−/− tendons had significant increases in stress relaxation at 3% and 4% strain, and a trending increase in stress relaxation at 5% strain. I-Dcn−/−/Bgn−/− tendons had significant increases in phase shift at 3% and 4% strain across all frequencies (except at 4% strain and 1 Hz, which was a trending increase). This result suggests that biglycan can mitigate the effects of decorin deficiency. These findings support our hypothesis, as decorin deficiency led to larger changes in viscoelastic properties than changes seen with biglycan deficiency. Knockdown of both SLRPs led to larger changes in viscoelastic properties than those seen with knockdown of either SLRP alone.
Decorin Directly Modulated Aged Tendon Viscoelastic Properties.
The structure–function role of decorin remains controversial. Decorin knockout studies demonstrate a role of decorin in regulating tendon viscoelastic properties [17,19,24,31]. It is hypothesized that decorin directly regulates tendon mechanical properties via matrix interactions with its GAG chain [32,33]. When GAGs are digested in ex vivo tendons, however, no changes in tendon viscoelastic properties are observed [34–36]. These findings suggest that decorin does not regulate viscoelastic properties through its functional GAG chain. Here, Dcn expression correlated with viscoelastic parameters while Bgn expression did not. This relationship strengthens the conclusion that decorin modulates tendon viscoelastic properties. Given that no changes in expression of analyzed genes were observed with decorin knockdown at the time of mechanical testing, this regulation of viscoelastic properties likely originates from the direct function of decorin rather than compensatory expression of other genes. While this study corroborates that decorin modulates tendon viscoelastic properties, this regulation may not occur directly through the GAG chain structure. Decorin may regulate tendon viscoelastic properties by maintaining proper collagen fibril size.
As mentioned, decorin deficiency through tendon development and aging led to less pronounced age-associated changes in mechanical properties [7]. Here, when decorin is knocked down in aged tendons, the opposite effect is seen. Decorin deficiency increased viscoelastic properties—a change seen in WT aging. This result suggests that the role of decorin in aged tendon mechanics is predicated on the duration of decorin deficiency. Tendons that are decorin-deficient from birth may adapt to this deficiency through aging, while aged tendons do not have the capacity to adapt to decorin deficiency.
In this study, biglycan deficiency alone did not lead to meaningful changes in aged tendon mechanics. It is hypothesized that biglycan comprises a stem cell “niche” within the tendon, leading to its large regulatory role within the tissue [37]. Due to the old age of the mice studied here, there is likely a decreased role of resident stem cells in regulating tendon functional properties. This may explain the lack of changes seen in biglycan-deficient tendons, as stem cell regulation is less impactful in aged tendons. Better understanding of the relationship between biglycan and tendon cells is needed to fully explain these results.
Limitations and Future Directions.
This study is not without limitations. Gene expression was measured 30 days post-knockdown, and changes in gene expression that lead to functional matrix changes may have been missed at this later timepoint. While expression data at this timepoint suggests effective knockdown of decorin and/or biglycan, this knockdown was not further validated with matrix protein measurements. However, previous work using these models demonstrated significant reduction in matrix presence of decorin and biglycan 30 days post-TM injections [24]. While our results suggest that decorin regulates tendon viscoelastic properties without a genetic response from other matrix molecules, this study lacks a true mechanistic understanding of this viscoelastic regulation. Future work will analyze matrix content and fibril size distributions to better explain the mechanisms by which decorin alters tendon viscoelasticity.
This study found that Col2a1 and Mmp13 had high PC2 loading scores, and PC2 score was significantly increased in decorin-deficient tendons. A subset of decorin-deficient tendons also demonstrated elevated Acan expression. These results would suggest a relationship between Dcn, Col2a1, Acan, and Mmp13 expression. While no statistical differences in expression of these genes was observed, such a change may be apparent in other musculoskeletal tissues. Tissues that experience compression, such as cartilage, have higher levels of collagen II, aggrecan, and MMP-13. Increased presence of these molecules may lead to more robust expression changes in response to decorin knockdown. Therefore, future work will define the role of decorin in regulating expression and presence of these molecules in compressive tissues.
Conclusions
Overall, this work demonstrated that decorin regulates viscoelastic properties in homeostatic-aged tendons. This regulation is in part coordinated with biglycan, as biglycan moderately compensates for decorin deficiency. These changes in viscoelastic properties were not accompanied by altered expression of other matrix proteins, however, suggesting a more direct role of SLRPs in regulating viscoelastic properties of aged tendons. This work provides a better understanding of the roles of decorin and biglycan in aged tendon homeostasis. Understanding the age-dependent function of SLRPs can better inform therapeutic targets to mitigate the age-associated increases in tendon injury risk.
Supplementary Material
Supplementary Material PDF
Acknowledgment
The authors thank Dr. David Birk and Mei Sun (University of South Florida) for providing the mice for this study. The authors also thank Hetty Rodriguez of the Molecular Profiling Facility (University of Pennsylvania) for running the Fluidigm dynamic array. RJL, SSS, SNW, and LJS designed and implemented the study. RJL collected data. RJL, NAD, and LJS interpreted results. RJL wrote the paper in consultation of all authors.
Funding Data
NIH (Grant Nos. P30AR069619 and R01AR068057; Funder ID: 10.13039/100000002).
NSF GRFP (Fellow ID: 2017242213; Funder ID: 10.13039/100000001).
References
- [1]. Clayton, R. A. E. , and Court-Brown, C. M. , 2008, “ The Epidemiology of Musculoskeletal Tendinous and Ligamentous Injuries,” Injury, 39(12), pp. 1338–1344. 10.1016/j.injury.2008.06.021 [DOI] [PubMed] [Google Scholar]
- [2]. Yamaguchi, K. , Ditsios, K. , Middleton, W. D. , Hildebolt, C. F. , Galatz, L. M. , and Teefey, S. A. , 2006, “ The Demographic and Morphological Features of Rotator Cuff Disease: A Comparison of Asymptomatic and Symptomatic Shoulders,” J. Bone Jt. Surg. Ser. A, 88(8), pp. 1699–1704. 10.2106/00004623-200608000-00002 [DOI] [PubMed] [Google Scholar]
- [3]. Lemme, N. J. , Li, N. Y. , DeFroda, S. F. , Kleiner, J. , and Owens, B. D. , 2018, “ Epidemiology of Achilles Tendon Ruptures in the United States: Athletic and Nonathletic Injuries From 2012 to 2016,” Orthop. J. Sport. Med., 6(11), pp. 1–7. 10.1177/2325967118808238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Pardes, A. M. , Beach, Z. M. , Raja, H. , Rodriguez, A. B. , Freedman, B. R. , and Soslowsky, L. J. , 2017, “ Aging Leads to Inferior Achilles Tendon Mechanics and Altered Ankle Function in Rodents,” J. Biomech., 60, pp. 30–38. 10.1016/j.jbiomech.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Dressler, M. R. , Butler, D. L. , Wenstrup, R. , Awad, H. A. , Smith, F. , and Boivin, G. P. , 2002, “ A Potential Mechanism for Age-Related Declines in Patellar Tendon Biomechanics,” J. Orthop. Res., 20(6), pp. 1315–1322. 10.1016/S0736-0266(02)00052-9 [DOI] [PubMed] [Google Scholar]
- [6]. Zuskov, A. , Freedman, B. R. , Gordon, J. A. , Sarver, J. J. , Buckley, M. R. , and Soslowsky, L. J. , 2020, “ Tendon Biomechanics and Crimp Properties Following Fatigue Loading Are Influenced by Tendon Type and Age in Mice,” J. Orthop. Res., 38(1), pp. 36–42. 10.1002/jor.24407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Dunkman, A. A. , Buckley, M. R. , Mienaltowski, M. J. , Adams, S. M. , Thomas, S. J. , Satchell, L. , Kumar, A. , Pathmanathan, L. , Beason, D. P. , Iozzo, R. V. , Birk, D. E. , and Soslowsky, L. J. , 2013, “ Decorin Expression is Important for Age-Related Changes in Tendon Structure and Mechanical Properties,” Matrix Biol., 32(1), pp. 3–13. 10.1016/j.matbio.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ackerman, J. E. , Bah, I. , Jonason, J. H. , Buckley, M. R. , and Loiselle, A. E. , 2017, “ Aging Does Not Alter Tendon Mechanical Properties During Homeostasis, but Does Impair Flexor Tendon Healing,” J. Orthop. Res., 35(12), pp. 2716–2724. 10.1002/jor.23580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Haut, R. C. , Lancaster, R. L. , and DeCamp, C. E. , 1992, “ Mechanical Properties of the Canine Patellar Tendon: Some Correlations With Age and the Content of Collagen,” J. Biomech., 25(2), pp. 163–173. 10.1016/0021-9290(92)90273-4 [DOI] [PubMed] [Google Scholar]
- [10]. Thorpe, C. T. , Udeze, C. P. , Birch, H. L. , Clegg, P. D. , and Screen, H. R. C. , 2013, “ Capacity for Sliding Between Tendon Fascicles Decreases With Ageing in Injury Prone Equine Tendons: A Possible Mechanism for Age-Related Tendinopathy?,” Eur. Cells Mater., 25, pp. 48–60. 10.22203/eCM.v025a04 [DOI] [PubMed] [Google Scholar]
- [11]. Johnson, G. A. , Tramaglini, D. M. , Levine, R. E. , Ohno, K. , Choi, N.-Y. , and L-Y. Woo, S. , 1994, “ Tensile and Viscoelastic Properties of Human Patellar Tendon,” J. Orthop. Res., 12(6), pp. 796–803. 10.1002/jor.1100120607 [DOI] [PubMed] [Google Scholar]
- [12]. Blevins, F. T. , Hecker, A. T. , Bigler, G. T. , Boland, A. L. , and Hayes, W. C. , 1994, “ The Effects of Donor Age and Strain Rate on the Biomechanical Properties of Bone-Patellar Tendon-Bone Allografts,” Am. J. Sports. Med., 22(3), pp. 328–333. 10.1177/036354659402200306 [DOI] [PubMed] [Google Scholar]
- [13]. Wong, A. K. , Calvo M, R. , Schaffler, B. C. , Nixon, R. A. , Carrero, L. C. , Neufeld, E. V. , Grande, D. A. , and Calvo R, R. , 2019, “ Biomechanical and Geometric Characterization of Peroneus Longus Allografts With Respect to Age,” Clin. Biomech., 67, pp. 90–95. 10.1016/j.clinbiomech.2019.04.017 [DOI] [PubMed] [Google Scholar]
- [14]. Chen, S. , and Birk, D. E. , 2013, “ The Regulatory Roles of Small Leucine-Rich Proteoglycans in Extracellular Matrix Assembly,” Febs J., 280(10), pp. 2120–2137. 10.1111/febs.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Zhang, G. , Ezura, Y. , Chervoneva, I. , Robinson, P. S. , Beason, D. P. , Carine, E. T. , Soslowsky, L. J. , Iozzo, R. V. , and Birk, D. E. , 2006, “ Decorin Regulates Assembly of Collagen Fibrils and Acquisition of Biomechanical Properties During Tendon Development,” J. Cell. Biochem., 98(6), pp. 1436–1449. 10.1002/jcb.20776 [DOI] [PubMed] [Google Scholar]
- [16]. Ameye, L. , Aria, D. , Jepsen, K. , Oldberg, A. , Xu, T. , and Young, M. F. , 2002, “ Abnormal Collagen Fibrils in Tendons of Biglycan/Fibromodulin-Deficient Mice Lead to Gait Impairment, Ectopic Ossification, and Osteoarthritis,” Faseb J., 16(7), pp. 673–680. 10.1096/fj.01-0848com [DOI] [PubMed] [Google Scholar]
- [17]. Elliott, D. M. , Robinson, P. S. , Gimbel, J. A. , Sarver, J. J. , Abboud, J. A. , Iozzo, R. V. , and Soslowsky, L. J. , 2003, “ Effect of Altered Matrix Proteins on Quasilinear Viscoelastic Properties in Transgenic Mouse Tail Tendons,” Ann. Biomed. Eng., 31(5), pp. 599–605. 10.1114/1.1567282 [DOI] [PubMed] [Google Scholar]
- [18]. Robinson, P. S. , Lin, T. W. , Jawad, A. F. , Iozzo, R. V. , and Soslowsky, L. J. , 2004, “ Investigating Tendon Fascicle Structure-Function Relationships in a Transgenic-Age Mouse Model Using Multiple Regression Models,” Ann. Biomed. Eng., 32(7), pp. 924–931. 10.1023/B:ABME.0000032455.78459.56 [DOI] [PubMed] [Google Scholar]
- [19]. Dourte, L. M. , Pathmanathan, L. , Jawad, A. F. , Iozzo, R. V. , Mienaltowski, M. J. , Birk, D. E. , and Soslowsky, L. J. , 2012, “ Influence of Decorin on the Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon,” ASME J. Biomech. Eng., 134(3), p. 31005. 10.1115/1.4006200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Dourte, L. M. , Pathmanathan, L. , Mienaltowski, M. J. , Jawad, A. F. , Birk, D. E. , and Soslowsky, L. J. , 2013, “ Mechanical, Compositional, and Structural Properties of the Mouse Patellar Tendon With Changes in Biglycan Gene Expression,” J. Orthop. Res., 31(9), pp. 1430–1437. 10.1002/jor.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Gordon, J. A. , Freedman, B. R. , Zuskov, A. , Iozzo, R. V. , Birk, D. E. , and Soslowsky, L. J. , 2015, “ Achilles Tendons From Decorin- and Biglycan-Null Mouse Models Have Inferior Mechanical and Structural Properties Predicted by an Image-Based Empirical Damage Model,” J. Biomech., 48(10), pp. 2110–2115. 10.1016/j.jbiomech.2015.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Zhang, G. , Chen, S. , Goldoni, S. , Calder, B. W. , Simpson, H. C. , Owens, R. T. , McQuillan, D. J. , Young, M. F. , Iozzo, R. V. , and Birk, D. E. , 2009, “ Genetic Evidence for the Coordinated Regulation of Collagen Fibrillogenesis in the Cornea by Decorin and Biglycan,” J. Biol. Chem., 284(13), pp. 8888–8897. 10.1074/jbc.M806590200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Corsi, A. , Xu, T. , Chen, X.-D. , Boyde, A. , Liang, J. , Mankani, M. , Sommer, B. , Iozzo, R. V. , Eichstetter, I. , Robey, P. G. , Bianco, P. , and Young, M. F. , 2002, “ Phenotypic Effects of Biglycan Deficiency Are Linked to Collagen Fibril Abnormalities, Are Synergized by Decorin Deficiency, and Mimic Ehlers-Danlos-Like Changes in Bone and Other Connective Tissues,” J. Bone Miner. Res., 17(7), pp. 1180–1189. 10.1359/jbmr.2002.17.7.1180 [DOI] [PubMed] [Google Scholar]
- [24]. Robinson, K. A. , Sun, M. , Barnum, C. E. , Weiss, S. N. , Huegel, J. , Shetye, S. S. , Lin, L. , Saez, D. , Adams, S. M. , Iozzo, R. V. , Soslowsky, L. J. , and Birk, D. E. , 2017, “ Decorin and Biglycan Are Necessary for Maintaining Collagen Fibril Structure, Fiber Realignment, and Mechanical Properties of Mature Tendons,” Matrix Biol., 64, pp. 81–93. 10.1016/j.matbio.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Connizzo, B. K. , Sarver, J. J. , Birk, D. E. , Soslowsky, L. J. , and Iozzo, R. V. , 2013, “ Effect of Age and Proteoglycan Deficiency on Collagen Fiber Re-Alignment and Mechanical Properties in Mouse Supraspinatus Tendon,” ASME J. Biomech. Eng., 135(2), p. 021019. 10.1115/1.4023234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Favata, M. , 2006, “ Scarless Healing in the Fetus: Implications and Strategies for Postnatal Tendon Repair,” Ph.D. dissertation, University of Pennsylvania, Philadelphia, PA.https://repository.upenn.edu/dissertations/AAI3246156/
- [27]. Miller, K. S. , Connizzo, B. K. , Feeney, E. , and Soslowsky, L. J. , 2012, “ Characterizing Local Collagen Fiber Re-Alignment and Crimp Behavior Throughout Mechanical Testing in a Mature Mouse Supraspinatus Tendon Model,” J. Biomech., 45(12), pp. 2061–2065. 10.1016/j.jbiomech.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Lake, S. P. , Miller, K. S. , Elliott, D. M. , and Soslowsky, L. J. , 2009, “ Effect of Fiber Distribution and Realignment on the Nonlinear and Inhomogeneous Mechanical Properties of Human Supraspinatus Tendon Under Longitudinal Tensile Loading,” J. Orthop. Res., 27(12), pp. 1596–1602. 10.1002/jor.20938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Metsalu, T. , and Vilo, J. , 2015, “ ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap,” Nucl. Acids Res., 43(W1), pp. W566–W570. 10.1093/nar/gkv468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Kilts, T. , Ameye, L. , Syed-Picard, F. , Ono, M. , Berendsen, A. D. , Oldberg, A. , Heegaard, A.-M. , Bi, Y. , and Young, M. F. , 2009, “ Potential Roles for the Small Leucine-Rich Proteoglycans Biglycan and Fibromodulin in Ectopic Ossification of Tendon Induced by Exercise and in Modulating Rotarod Performance,” Scand. J. Med. Sci. Sports, 19(4), pp. 536–546. 10.1111/j.1600-0838.2009.00909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Robinson, P. S. , Huang, T.-F. , Kazam, E. , Iozzo, R. V. , Birk, D. E. , and Soslowsky, L. J. , 2005, “ Influence of Decorin and Biglycan on Mechanical Properties of Multiple Tendons in Knockout Mice,” ASME J. Biomech. Eng., 127(1), pp. 181–185. 10.1115/1.1835363 [DOI] [PubMed] [Google Scholar]
- [32]. Cribb, A. M. , and Scott, J. E. , 1995, “ Tendon Response to Tensile Stress: An Ultrastructural Investigation of Collagen:Proteoglycan Interactions in Stressed Tendon,” J. Anat., 187(Pt. 2), pp. 423–428.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1167437/ [PMC free article] [PubMed] [Google Scholar]
- [33]. Liao, J. , and Vesely, I. , 2007, “ Skewness Angle of Interfibrillar Proteoglycans Increases With Applied Load on Mitral Valve Chordae Tendineae,” J. Biomech., 40(2), pp. 390–398. 10.1016/j.jbiomech.2005.12.011 [DOI] [PubMed] [Google Scholar]
- [34]. Lujan, T. J. , Underwood, C. J. , Henninger, H. B. , Thompson, B. M. , and Weiss, J. A. , 2007, “ Effect of Dermatan Sulfate Glycosaminoglycans on the Quasi-Static Material Properties of the Human Medial Collateral Ligament,” J. Orthop. Res., 25(7), pp. 894–903. 10.1002/jor.20351 [DOI] [PubMed] [Google Scholar]
- [35]. Lujan, T. J. , Underwood, C. J. , Jacobs, N. T. , and Weiss, J. A. , 2009, “ Contribution of Glycosaminoglycans to Viscoelastic Tensile Behavior of Human Ligament,” J. Appl. Physiol., 106(2), pp. 423–31. 10.1152/japplphysiol.90748.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Fessel, G. , and Snedeker, J. G. , 2009, “ Evidence Against Proteoglycan Mediated Collagen Fibril Load Transmission and Dynamic Viscoelasticity in Tendon,” Matrix Biol., 28(8), pp. 503–510. 10.1016/j.matbio.2009.08.002 [DOI] [PubMed] [Google Scholar]
- [37]. Bi, Y. , Ehirchiou, D. , Kilts, T. M. , Inkson, C. A. , Embree, M. C. , Sonoyama, W. , Li, L. , Leet, A. I. , Seo, B. M. , Zhang, L. , Shi, S. , and Young, M. F. , 2007, “ Identification of Tendon Stem/Progenitor Cells and the Role of the Extracellular Matrix in Their Niche,” Nat. Med., 13(10), pp. 1219–1227. 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material PDF


