Abstract
Purpose:
Exploitation of altered glycosylation in cancer is a major goal for the design of new cancer therapy. Here designed a novel chimeric signal peptide-Galectin-3 conjugate (sGal-3) and investigated its ability to induce cancer-specific cell death by targeting aberrantly N-glycosylated cell surface receptors in cancer cells.
Experimental Design:
sGal-3 was genetically engineered from Gal-3 by extending its N-terminus with a non-cleavable signal peptide from tissue plasminogen activator (tPA). sGal-3 killing ability was tested on normal and tumor cells in vitro and its anti-tumor activity evaluated in subcutaneous lung cancer and orthotopic malignant glioma models. The mechanism of killing was investigated through assays detecting sGal-3 interaction with specific glycans at the surface of tumor cells and the elicited downstream pro-apoptotic signaling.
Results:
We found sGal-3 preferentially binds to β1 integrin on the surface of tumor cells due to aberrant N-glycosylation resulting from cancer-associated upregulation of several glycosyltransferases. This interaction induces potent cancer-specific death by triggering an oncoglycan-β1/calpain/caspase-9 pro-apoptotic signaling cascade. sGal-3 could reduce the growth of subcutaneous lung cancers and malignant gliomas in brain, leading to increased animal survival.
Conclusions:
We demonstrate that sGal-3 kills aberrantly glycosylated tumor cells and antagonizes tumor growth through a novel integrin β1-dependent cell-extrinsic apoptotic pathway. These findings provide proof of concept that aberrant N-oncoglycans represent valid cancer targets and support further translation of the chimeric sGal-3 peptide conjugate for cancer therapy.
Keywords: cancer, N-glycan, Galectin-3, β1 integrin, apoptosis
Introduction
Altered glycosylation of cell surface proteins is a characteristic of cancer and leads to abnormal glycoproteins with N- and O-glycans on human tumors (1). Tumor-associated glycosylation occurs through two mechanisms: ‘incomplete synthesis’, which leads to truncated forms of normal glycan structures (2), and ‘neo-synthesis’, where abnormal cancer-specific glycans are generated through cancer-associated changes in expression of glycosyltransferases (3). Aberrantly-enhanced glycosylation contributes to tumorigenesis and tumor immune evasion (4). Carcinomas show increased expression of N-acetylglucosaminyltransferase V (MGAT5), an enzyme that catalyzes the synthesis of GlcNAcβ1,6-Mannose glycan branches, leading to the formation of tetra-antennary N-glycans (5), which carry poly-N-acetyllactosamine, the preferred ligand for Galectin-3 (Gal-3). Consequently, there is a strong interest in exploiting aberrantly glycosylated tumor cell surface proteins as targets for cancer therapy (3), but this objective has not been realized to date (6).
Integrins are cell surface adhesion molecules composed of dimers of α and β subunits and their signaling is important for cell proliferation, survival, cytoskeletal re-organization and migration. Upon activation, integrins undergo rapid, reversible conformational changes, which promotes recruitment of intracellular signaling molecules (7). Modification of integrin extracellular N-termini through glycosylation is required for dimer formation, ligand binding and functional activation (8–10). Alteration in N-linked glycans on cell surface integrins increases the migration and metastasis potential of several cancer types (11).
Lectins are carbohydrate-binding proteins that each recognizes distinct glycan moieties on glycoproteins or glycolipids through carbohydrate recognition domains (CRD). Gal-3 belongs to the β-galactoside binding galectin family, is secreted through a non-classical secretion pathway, and is unique among galectins in that it carries a long collagen-like N-terminal domain that permits oligomerization, and for its dual role in apoptosis (12). Intracellular Gal-3 has a well-documented role as anti-apoptotic factor in cytoplasm and at the mitochondrial membrane through carbohydrate-independent mechanisms (13,14). In contrast, carbohydrate-dependent pro-apoptotic responses of extracellular Gal-3 have been observed at supra-physiological concentrations (1–10 μM) in lymphocytic cells (15), but not in other adherent cell types where Gal-3 regulates migration through the modulation of cell adhesion molecules and extracellular matrix (16).
Here we sought to determine whether the weak pro-apoptotic activity of extracellular Gal-3 could be enhanced through a peptide conjugate approach in its N-terminus, and harnessed for targeting aberrantly glycosylated cancer cells. Peptide conjugates have been developed to selectively enhance cell surface receptor binding (17). Our study demonstrates that aberrant β1-integrin glycosylation can be exploited for tumor-specific targeting with a signal peptide conjugated Gal-3, supporting its further development for therapeutic applications.
Materials and Methods
Chemicals, Cell lines, and primary cells.
Recombinant human galectin-3, sugars (lactose, sucrose, and melibiose), apoptosis inhibitors (caspase-3 (Ac-DEVD-CHO), Caspase-8 (Z-IETD-FMK), caspase-9 (Z-LEHD-FMK)), glycosylation inhibitors (Kifunensin for N-linked glycosylation and Benzyl 2-acetamido-2-deoxy-α-D-galactopyranoside for O-linked glycosylation), signaling pathway inhibitors (LY294002 for PI3K/Akt, Cpd22 for ILK, MDL28170 for Calpain, and Verapamil hydrochloride for calcium channels), Transfection reagents (GenePorter and GeneSilencer), glycan purification material (unconjugated Phaseolus Vulgaris Leucoagglutinin (PHA-L), agarose-bound L-PHA, and agarose bound Ricinus Communis Agglutinin (RCA)) were purchased from commercial sources (see Supplemental data). Human glioblastoma cell lines LN-Z308 (p53 null) parental (18), LN229 parental, clone LN229-L16 (tet-on) (19), U87MG and SF767 (20), and embryonic kidney (293HEK), breast (MCF7 and MD468), lung (A549), colon (HCT116) and prostate (LnCaP) cancer cell lines, and human foreskin fibroblasts (HFF1) were grown in DMEM supplemented with 5–10% tetracycline-free FCS (Gibco, Grand island, NY). Primary cultures of human dermal fibroblasts (HDF), human dermal microvascular endothelial cells (HDMEC), human normal breast epithelial cells (MCF10), human astrocytes and glioma neurospheres (N08–74) were grown as described in supplemental data. Cell lines were tested for mycoplasma and their identity verified by SNP analysis.
Generation of sGal-3 expression vectors.
The generation of sGal-3 transient (pUMVC7-Gal3) and stable vectors (pTRE2-sGal3) is described in supplemental data.
Transient cDNA or siRNA transfection studies.
For β1 integrin knockdown, cells were transfected with β1 integrin (sc-35674, sc-44310) or control (RNA-A; sc-37007; Santa Cruz BioTechnology) siRNAs (100 nM) for 72 hrs with siRNA-specific transfectant (Gene Silencer, Genlantis) after which the cells were seeded at 5,000 cells per well in 96-well plates. For sGal3 treatment, 24 hrs after cell splitting, 200 μl of 1x control or sGal-3 conditioned media (CM) were added to each well. For MGAT5 overexpression or knockdown studies, the pCXN2-MGAT5 and pSUPER-MGAT5 expression vectors were used (11,21).
Generation of stably transfected cells.
Doxycycline-inducible sGal-3 transfected clones were generated in LN229-L16 glioma cells (Tet-on clone derived from LN229)(22) by transfecting them with xxx and yyy plasmids (1:10 ratio). 293 cells stably expressing MGAT5 were generated as described (23).
Production of sGal-3.
293 cells were transiently transfected using GenePORTER reagent (Genlantis) with the pUMVC7-sGal-3 expression vector or pCMV-LacZ as control, and switched to serum free media 16 h later. The CM was collected after 48 hrs, purified, and was either stored in frozen aliquots at –20°C or used undiluted (1x) on target cells in cell viability assays. Purification of sGal-3 from CM was performed using a lactosyl-sepharose column as described (24). Preparation and purification of His-tag sGal3 was performed as described (25), see details in supplemental methods.
RT/PCR and Western Blot Analyses.
Detailed information for immunoblot preparation is provided in supplemental methods.
Antibody-mediated activity blocking assays.
Neutralization of β1 integrin on cells was achieved by addition of 5 μg/ml anti-human β1 integrin inhibitory antibodies (clones P5D2 and AIIB2; The Developmental Studies Hybridoma Bank, Iowa city, IA) or control immunoglobulins (normal mouse IgG, Santa Cruz) 24h. before sGal-3 CM treatment.
GST-Gal-3 pulldown assays.
Recombinant GST-Gal-3 constructs were used for the production of rGal-3 in bacteria and used for pulldown assays as described (26) with some modification (see supplemental methods).
Co-immunoprecipitation assays.
To examine the interaction of sGal-3 with tumor cell surface integrins, sGal-3 CM was added to live cultured cells. Briefly, 3 ml of 2x sGal-3 CM was added to 5x106 cells in 10 cm diameter culture dishes (serum-starved for 24 hrs) grown in 10 cm petri dishes and rocked for 4 hrs at room temperature. After sGal-3 CM removal, cells were washed three times with PBS and lysed with CHAPS buffer (30 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1mM EDTA, 0.25% CHAPS (A.G. Scientific, San Diego, CA)) supplemented with a protease inhibitor cocktail (Roche). Aliquots of 750 μg of whole cell extracts at 1μg/μl were pre-cleared with protein G agarose beads at 4°C for 20 min. Subsequently, anti-human galectin-3, β1 integrin, α5 integrin antibodies or non-specific immunoglobulins (1.5 μg/ml; Santa Cruz Biotechnology) were used for immunoprecipitation overnight at 4°C. Protein G agarose beads (20 μl; 50% (W/V), Roche) were added and incubated for another 4 hrs at 4°C. Beads were recovered by centrifugation, washed 3 times with lysis buffer, boiled in Laemmli sample buffer and the immunoprecipitated proteins analyzed by western blot.
For the detection of MGAT5 synthesized glycans on cell surface integrin β1, L-PHA-cell surface integrin β1 co-immunoprecipitation assays were performed as above except the cultured cells were pre-treated with a pure L-PHA solution (2.5µg/ml)(Vector Labs, Burlingame, CA) for 2 hrs at room temperature. After washing 3x with PBS, a cell extract was prepared by lysis in CHAPS buffer and anti-L-PHA antibodies (1.5 µ/ml Vector Labs) used to immunoprecipitate the L-PHA-bound glycan residues, followed by Western blot for β1 integrin.
Plant lectin pull down assays.
For the detection of glycans synthesized by MGAT5 on β1 integrin, L-PHA agarose-integrin β1 pulldown assays were performed. For the detection of β4GalT-transferred glycan branches, Ricinus communis agglutinin (RCA)-agarose-β1 integrin pulldown assays were used with RCA agarose beads (Vector Lab). Detailed procedures are described in supplemental data.
Apoptosis assays.
For apoptosis analysis, cells were treated with either CM containing secreted Gal-3 or with purified sGal-3, with or without 100 nM AC-DEVD-CHO caspase-3/7 specific inhibitor (BD Bioscience), 20 μM of Z-IETD-FMK caspase-8 inhibitor, or 20 μM of Z-LEHD-FMK caspase-9 inhibitor (MBL international). Caspase-3/7 or −9 Glo assays (Promega, Madison, WI) were performed on sGal-3-treated cells as per the manufacturer’s instructions. The luminescence value (RLU, blank subtracted) was converted to fold induction and the values from control vector-transfected CM-treated cells were considered as 1.
Calpain GLO protease assays.
For calpain protease activity analysis, cells were treated with either CM containing sGal-3 alone or supplemented with 500 nM of calpain inhibitor III (MDL28170, Cayman Chemical, CA). As controls, cells were treated with rGal-3 or sGal-3 CM pretreated with 25 mM lactose or 25 mM melibiose for 30 min. Calpain GLO protease assays (Promega) was performed on sGal-3-treated cells as per the manufacturer’s instructions. The luminescence value (RLU, blank subtracted) was converted to fold induction and the value from 0 h sample was considered as 1. All assays were repeated 3 times independently (n=3) in triplicate.
Calcium colorimetric assays.
For calcium influx accumulation analysis, cells were treated with sGal-3 CM for indicated times. As controls, cells were pre-treated with 50 μM of verapamil (calcium channel blocker, Sigma Aldrich) for 24 hrs or with sGal-3 CM pretreated with 25 mM lactose for 30 min. Calcium colorimetric assay was performed as per the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI). For further details see supplementary data.
Crystal Violet cytotoxicity assays.
Cells were plated at 5,000 cells/well in 96-well plates and treated with 1x control of Dox-induced sGal-3 CM (~500 ng/ml sGal-3) for 24 to 120 hrs. Thereafter, the cells were fixed in a crystal violet (0.2%) /ethanol (2%) solution for 10 min., washed in water and solubilized in 1% SDS. Relative cell number was quantified by acquiring absorbance at 575 nm using a spectrophotometer.
Soft-agar Colony Formation assays.
Six-well plates were layered with 2 ml of 1% agar in DMEM medium supplemented with 10% Tet-free serum. This bottom layer was overlaid with 5,000 cells mixed in 0.33% agar with DMEM and 10% Tet-free serum. One ml of 10% Tet-tested serum containing media +/− 5 μg/ml of Doxycycline (dox) was added on top of the agar and replaced every 72 hrs. After 21 days the colonies were fixed using 100% methanol and visualized using Giemsa stain according to the manufacturer’s protocol (Sigma). The plates were air-dried to flatten the agar discs, the colonies counted and photographed at 20x. The experiment was repeated three times in triplicate (n=3).
In vivo tumorigenicity experiments.
All animal experiments were performed under Institutional Animal Care and Use Committee (IACUC) guidelines. For the subcutaneous tumor growth experiments 6-week old female athymic nude mice (NCI) (8–10/ group) were injected subcutaneously with 5x106 cells of the indicated cell lines. Mice with LN229-sGal3 tet-on gliomas received oral doxycycline (dox; 2 mg/ml) in drinking water containing 4% sucrose to induce expression of sGal-3 one week post injection of tumor cells until termination of the experiment. Lung cancer cells were preincubated with His-tag sGal3 (500 ng/ml) for 20 minutes at room temperature, then mixed with an equal volume of matrigel (Corning Life Sciences, Tewksbury, MA; cat. No 356234) and injected subcutaneously. Tumor volume was calculated in mm3 = (length x width2)/2.
For the orthotopic brain tumor experiments, 6-week old female athymic nude (NCI) mice were injected intracranially with 5 x 105 LN229-L16 sGal-3 Tet-on cells (clone #11) and divided into two groups (+/− Dox) of 11 mice each. Sixty-three days after the intracranial tumor injection, 10 nM of IR-labeled 2-deoxyglucose (2-DG) (LI-COR, Lincoln, NE) was tail-vein injected and the intensity of dye-stained brain tumor was analyzed 24 hrs later with Olympus FV-1000 microscopy (IR wavelength = 750 nm). Mice were terminated as per IACUC criteria. The Kaplan-Meier survival curve was established using SPSS and MedCalc statistical software.
Statistics.
Statistical analysis was performed using GraphPad Prism v6.01 software (GraphPad Software Inc.). Results are presented as mean ± SEM. For comparison of sample versus control, unpaired t-test was used. For Kaplan-Meir survival study, p-value was calculated by Logrank test. A p-value less than or equal to 0.05 was considered significant. For results p-values are presented as follows: *<0.05, **<0.01, ***<0.001, and ****<0.0001.
Study approval.
All animal work was performed according to the guidelines for animal experimentation and welfare and approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Results
N-terminus-modified Gal-3 reduces cancer cell viability in vitro:
To study the importance of the N-terminus in the pro-apoptotic function of Gal-3 (15), we previously made a series of expression constructs that either shortened or extended the N-terminus of Gal-3 and tested them for in vitro cytotoxicity against cancer cells (27). One construct produced a form of Gal-3 with dramatically increased cytotoxicity as compared to wild type Gal-3. This construct generates a ~33-kDa Gal-3 protein (sGal-3) due to the N-terminus conjugation of the signal peptide from tissue-plasminogen activator protein (tPA) (Suppl. Fig. 1A). The engineered signal peptide of sGal-3 functions as a classical secretion signal, but is incompletely processed by signal peptidases, and only the uncleaved form is efficiently secreted. Upon transient expression in mammalian cells (HEK293), sGal-3 is produced abundantly in the conditioned medium (CM) and can easily be distinguished from the endogenous ~27kDa Gal-3 (Fig. 1A). While Gal-3 is now secreted through the Golgi apparatus, there was no evidence for glycosylation (Suppl. Fig. 1B)
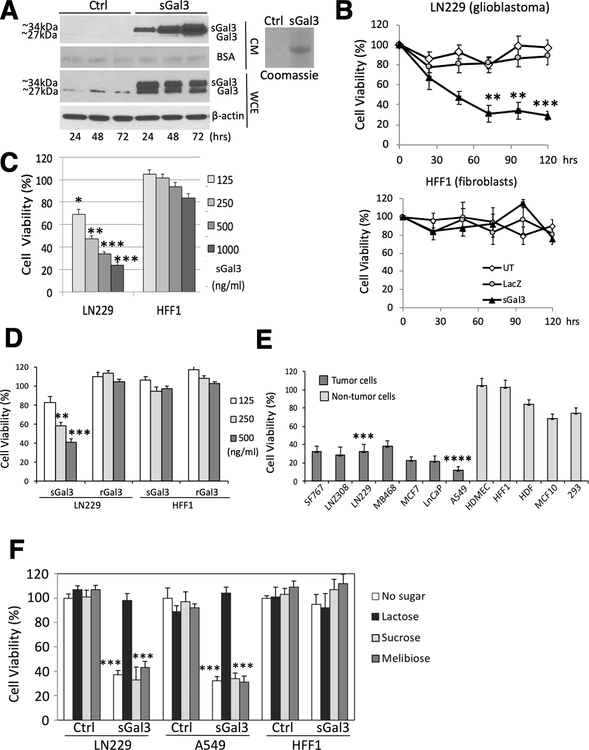
Figure 1: Secreted Gal-3 inhibits tumor cell viability in vitro.
A) Western blot showing levels of endogenous (Gal-3) and secreted (sGal-3) forms of Galectin-3. Left panel: cell extracts (WCE) and supernatants (CM) from 293 cells 48 hrs after transient transfection with an expression vector encoding LGALS3 cDNA fused to a classical secretion signal (pUMVC7) or control vector pCMV-LacZ (Ctrl.) were analyzed. BSA, Ponceau staining of bovine serum albumin (BSA). Right panel: Coomassie blue staining shows lactosyl-sepharose-purified sGal3 from CM.
B) Crystal violet cell viability assay showing tumor cell-specific toxicity of sGal-3. Human glioma cells (LN229, upper panel), and human fibroblasts (HFF-1, lower panel) were treated in triplicate for 30–120 hrs. with CM from 293 cells either untransfected (UT), or transfected with control (LacZ) or sGal3-expressing vectors. **p <0.01, ***p <0.001 (unpaired t-test).
C) Comparison between sGal-3 CM and rGal-3-mediated cytotoxicity in LN229 and HFF-1 cells after 48 hrs. treatment (upper panel). Quantification is presented as percent of sGal-3/vector control CM crystal violet staining from triplicates. *p <0.05, **p <0.01, ***p < 0.001 compared to control CM (unpaired t-test).
D) Dose-response of purified sGal-3 on LN229 and HFF-1 cell viability by crystal violet assay after 48 hrs. sGal-3 CM was purified through lactosyl-Sepharose column and quantified by ELISA. Quantification from triplicates as above. **p < 0.01 ***p < 0.001 (unpaired t-test). sGal-3 treatment of LN229 cells induced caspase-3 and PARP cleavage (lower panel).
E) Crystal violet assay demonstrating sGal-3 CM induced death at 48 hrs in genetically and biologically heterogeneous malignant human cancer cell lines (black) derived from brain (SF767, LN-Z308 and LN229), breast (MD468 and MCF7), lung (A549 and H1289) and prostate (LnCaP and PC3) tumors. In contrast, primary cultures of human endothelial cells (HDMEC) or fibroblasts (HDF and HFF-1) or normal breast epithelial cells (MCF10) or embryonic neuroepithelial 293 cells (grey) did not show significant decreases in cell viability. Cell viability is expressed as percentage sGal-3 over control CM. Three independent experiments were performed in triplicate (n=3). ***p < 0.001, ****p < 0.0001 compared to control CM (unpaired t-test).
F) Crystal violet assay showing neutralization of sGal-3 CM with lactose. sGal-3 CM was pre-treated for 1 hr with 20 mM (final concentration) of lactose, sucrose, and melibiose, then used to treat tumor (LN229, A549) and normal (HFF-1) cells for 48 hrs. Quantified as percent of sGal-3/control CM crystal violet staining from triplicates. ***p < 0.001 compared to control CM (unpaired t-test).
Treatment of malignant human glioma cells (LN229) with sGal-3-containing CM mediated tumor cell killing in a time- and dose-dependent fashion, while human foreskin fibroblasts (HFF-1) remained unaffected (Fig. 1B and Suppl. Fig. 1C). Purification of recombinant sGal-3 from the CM using a lactosyl-Sepharose column showed that sGal-3 per se induced tumor cell killing with an IC50 of ~250 ng/ml (Fig. 1C). In contrast, bacterially-produced recombinant Gal-3 (full length, CRD only or artificially elongated to 33 kDa by duplicating collagen repeats 4,5,6) had no tumor killing effect at the same concentration (Fig. 1D and Suppl. 1D), even when mixed with control CM (Suppl. Fig. 1E). Only 100–200-fold higher concentrations of rGal-3 (30–60 μg/ml) were able to induce apoptosis in Jurkat cells, consistent with the literature (15)(Suppl. Fig. 1F). sGal-3 showed tumor-specific killing in a panel of genetically and biologically heterogeneous cancer cell lines, while a variety of non-tumoral cells were resistant to sGal-3 cytotoxicity (Fig. 1E).
The carbohydrate-recognition domain (CRD) of Gal-3 is known to interact with multiple cell surface receptors carrying glycosylated branches containing β-galactoside modifications and this binding can be competed with high concentrations (>mM) of lactose (β-D-galactopyranosyl-(1→4)-D-glucose). Addition of lactose neutralized sGal-3-mediated tumor killing, while the control sugars sucrose (α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside) and melibiose (α-D-galactopyranosyl-(1→6)-D-glucose), which do not bind Gal-3, had no effect (Fig. 1F). The neutralizing effect was not due to a direct survival effect mediated by lactose cell binding per se, as pre-incubation of the tumor cells with lactose did not have a protective effect (Suppl. Fig. 1G). Altogether, these data showed that sGal-3-mediates tumor cell-specific killing, and this is dependent upon its β-galactoside binding function.
Secreted Gal-3 reduces in vitro and in vivo tumorigenicity
To examine whether the presence of sGal-3 in the extracellular milieu would reduce tumor growth, we generated human glioblastoma cells with inducible sGal-3-expression (clones LN229-sGal-3 tet-on Med and High). Both clones showed tight doxycycline-mediated induction with abundant secretion of sGal-3 in the conditioned medium under induced conditions (Fig. 2A). To examine sGal-3 effect on anchorage-independent growth, we performed soft-agar colony formation assays. sGal-3 induction resulted in a ~4–6- fold decrease in colony number, proportional to the amount of sGal-3 produced in the CM (Fig. 2B). Reduced growth upon sGal3 treatment was not related to a reduction in cell proliferation as there were no changes in cell cycle distribution (Suppl Fig. 2A). Induction of sGal-3 strongly reduced subcutaneous and intracranial in vivo tumor formation and increased animal survival while being well tolerated (Fig. 2C, D, and Suppl. Fig. 2B, C). Furthermore, sGal-3-mediated in vivo antitumor effects were not limited to brain tumors as a short exposure of aggressive lung cancer cells to recombinant His-tagged-sGal3 completely prevented subcutaneous tumor development (Suppl. Fig 2E and F). These data show that sGal-3 has potent anti-tumor activity in vitro and in vivo.
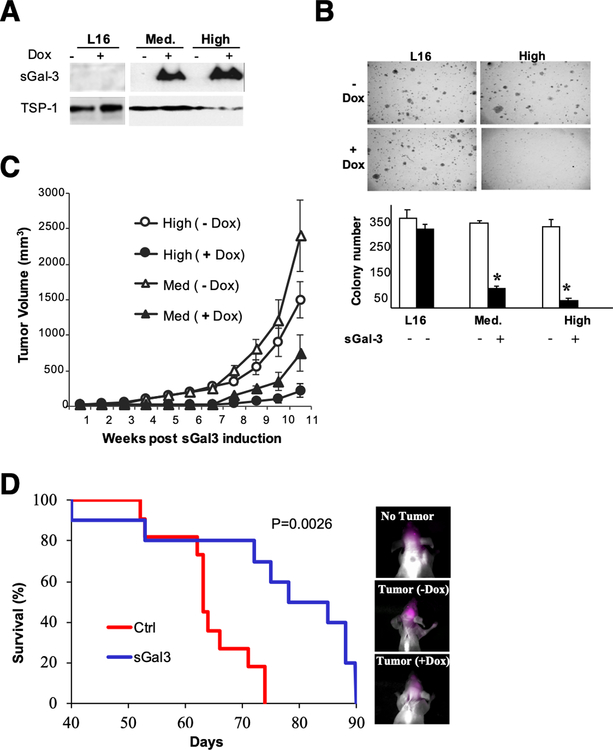
Figure 2: Gal-3 secretion reduces the tumorigenicity of malignant glioma cells in vitro and in vivo.
A) Western blot analysis showing doxycycline (dox)-dependent sGal-3 expression in CM of two sGal-3 inducible clones (Med and High) derived from LN229-L16-tet-on cells after 48 hrs of induction. Thrombospondin-1 (TSP-1) was used as loading control.
B) Soft-agar colony formation assays show dose-dependent inhibition of colony formation upon dox-inducible sGal-3 expression in both clones.
C) sGal-3 inhibits subcutaneous tumor growth. Athymic nude mice were injected s.c. with 5 x 106 cells of each clone (Med and High) and divided into two groups (9 mice/group; 2 tumors/mouse). One group/cell line was left untreated while the second group was given 2 mg/ ml Dox in drinking water containing 5% sucrose to induce expression of sGal-3 one week after tumor cell implantation until termination of the experiment. Average tumor volumes at termination were 4 to 6-fold smaller than controls (p<0.02; unpaired t-test). Dox had no effect on LN229-L16 control tumor growth (data not shown).
D) Kaplan-Meier survival curve for nude mice intracranially injected with 5 x 105 High clone #11 cells (n=12/group). Mean survival time: 63 days for control, and 78 days for sGal-3 group (p = 0.0026; Logrank test). Near-infrared imaging 24 h after injection of fluorescent 2-deoxy-glucose (10 nM) showed increased tumor burden in un-induced mice at day 63, which preceded their rapid decline (right panel, and Suppl. Fig. 2A). No systemic or brain toxicity were observed with production of sGal-3 by the tumor cells did (not shown). Dox treatment per se had no effect on survival of mice implanted with parental LN229-L16 cells (Suppl. Fig. 2B).
Secreted Gal-3 selectively induces apoptosis in cancer cells
Next we investigated the mechanism of sGal-3 induced tumor cell death. sGal-3-treated glioma cells displayed activation of caspases-3 and −9 and PARP cleavage, and these effects were inhibited by inhibitors of caspase-3 and −9, but not caspase-8 (Fig. 3A–D and SFig. 3A). In contrast, sGal-3 elicited no caspase activation in normal fibroblasts (Fig. 3B–D and SFig. 3B). sGal-3 treatment also induced caspase-3/7 activation in glioma stem-like cells (GSCs) and blocked neurosphere formation, while normal neural progenitor cells were unaffected (Fig. 3E,F). sGal-3 also efficiently induced caspase-7 activation in caspase-3-deficient MCF-7 cells (Suppl. Fig. 3C). To identify apoptosis-related proteins altered by sGal-3 in tumor cells, we performed a protein array and found upregulation of cleaved caspase-3 and pro-apoptotic Bax in the presence of sGal-3, while survivin, a member of the inhibitor of apoptosis (IAP) family was downregulated (Suppl. Fig. 3D). Western blot confirmed these results (Suppl. Fig. 3E,F). These data suggest that sGal-3 induces cancer cell apoptosis through a caspase-9 dependent mitochondrial pathway by disrupting the balance between pro-and anti-apoptotic proteins.
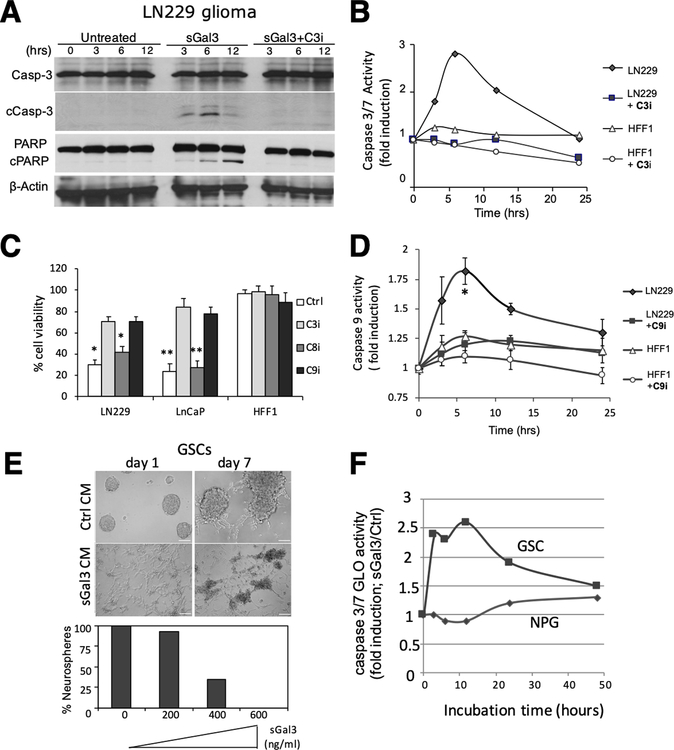
Figure 3: sGal-3 induces apoptosis specifically in tumor cells:
A) Western blot showing cleaved caspase-3 (17 and 19 kDa) and PARP (89 kDa) in sGal-3 treated LN229 cells at indicated times. Cleavage was inhibited by a caspase-3 inhibitor (C3I; Ac-DEVD-CHO, 100 nM).
B) Caspase-3/7 GLO assay shows kinetics of induction of caspase-3/7 cleavage following treatment with sGal-3 in LN229 but not HFF-1 cells.
C) Crystal violet cell survival assay showing that caspase-3 and −9 inhibitors prevent sGal-3-mediated cancer cell (LN229 and LnCaP prostate) death at 72 hrs. Inhibitors: caspase-3 (C3I; Ac-DEVD-CHO; 100 nM), caspase-8 (C8I; Z-IETD-FMK; 20 μM) and caspase-9 (C9I; Z-LEHD-FMK; 20 μM). Quantification is percent of sGal-3/control CM crystal violet staining. N=2 (in triplicates). *p<0.05, **p<0.01 compared to control CM (unpaired t-test)
D) Caspase-9 GLO assay shows induction kinetics of caspase-9 cleavage following treatment with 2x sGal-3 CM in LN229, but not HFF-1 cells. (N = 2)
E) sGal3 CM reduces neurosphere formation by CD133+ glioblastoma patient derived cancer stem-like cells (GSCs) in a dose-dependent fashion. Number of neurospheres formed is expressed as percent in sGal-3/control CM groups. Scale bar: 100 μm.
F) Caspase-3/7 GLO assay shows induction kinetics of caspase-3/7 cleavage after GSC treatment with sGal-3 (600 ng/ml). Normal human neural progenitor cells (NPGs) were not affected.
β1 integrin is required for sGal3-mediated cancer cell death.
Extracellular Gal-3 interacts with a variety of cell surface proteins mediating its multiple functions (12). In search of candidate cell surface ligands as mediators of the pro-apoptotic signal of sGal-3, we tested death receptors (Fas, DR4, DR5) and members of the integrin family. We did not find involvement of the death receptors (data not shown), consistent with the lack of caspase-8 activation upon sGal-3-induced apoptosis (Fig. 3C). In contrast, siRNA and neutralizing antibodies against integrin β1 largely prevented sGal-3 mediated activation of caspase-3/7, PARP cleavage, and tumor cell killing (Fig. 4A and Suppl. Fig. 4A,B). Immunofluorescence staining confirmed co-localization of sGal-3 and integrin β1 at the tumor cell surface (Suppl. Fig. 4D). Conversely, overexpression of either α5 or β1 or both integrins in 293 cells induced β1-Gal3 interaction in GST pull down experiments (Suppl. Fig. 4E), and sensitized the cells to sGal-3 induced apoptosis (Suppl. Fig. 4F). (Initially reviewer 3 wants to remove this!!) This data demonstrates that sGal-3-mediated tumor cell death requires β1 integrin expression; however, β1 expression alone is not sufficient to explain cancer-specific cytotoxicity. Indeed, HFF-1 and LN229 cells express similar levels of β1 integrin, but only LN229 cells die in response to sGal-3 (Suppl. Fig. 4A,B). Of note, β1 integrin is of higher molecular weight in cancer cells, suggesting a potential role for post-translational modifications.
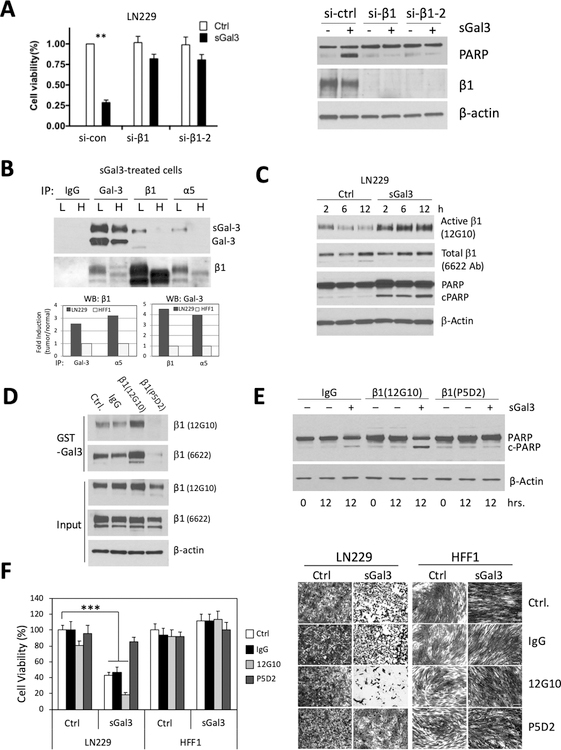
Figure 4: β1 integrin is required for sGal-3-mediated cell death and shows preferential activation and interaction with sGal3 in cancer cells due to altered glycosylation.
A) (Left panel) Integrin β1 knockdown protects cancer cells from sGal-3-activated cell death. LN229 cells were pre-treated with either control (si-con) or two independent β1 siRNAs for 72hrs, before being seeded at 5,000 cells/well in 96-well plates. 24 hrs later, cells were treated with 200 μl of 1x control or sGal-3 CM. Cell viability was examined 72 hrs later by SRB assay in triplicates. **p < 0.01; unpaired t-test. (Right panel) Western blot showing efficient siRNA knockdown of β1 protects cells from SGal3 induction of PARP cleavage, a marker of late-stage apoptosis.
B) Co-immunoprecipitation experiments show increased binding of sGal-3 to cell surface β1 integrin on LN229 cells (L) versus HFF-1 cells (H). Cells were cultured for 3 hrs. at RT with sGal-3 CM, then lysed and immunoprecipitated with anti-Gal-3 and β1 and α5 integrin antibodies. Immunoprecipitated proteins were subjected to Western blotting for Gal-3 and β1 integrin. Note that co-IPs between sGal-3 and β1 and α5 integrins are only found in LN229 cells. Intensity of Gal-3-β1 integrin and Gal-3-α5 integrin binding was compared between tumor and normal cells by densitometry (lower panel). Data are graphed as fold binding induction based on co-IP band intensity between tumor and normal cells (normal cell binding was set at 1). The β1/β1 and Gal-3/Gal-3 co-IPs show input levels for each protein in both cell lines.
C) Western blot showing that sGal-3 treatment switches β1 integrin into its active conformation and activates PARP cleavage.
D) GST-Gal-3 pull down experiment shows that 24 hrs pretreatment with antibodies that respectively switch β1 integrin into its active (12G10) or inactive (P5D2) conformation respectively augment or inhibit interaction with Gal-3.
E) Western blot showing that β1 integrin activation antibody (12G10) augments PARP cleavage in response to sGal-3 in LN229 cells.
F) Crystal violet cell viability assay showing that 24 hrs pretreatment with antibodies that mediate β1 integrin switch towards active or inactive conformations induces or prevents sGal3-mediated killing in LN229 cells, but has no effect in HFF-1 cells. Left panel, quantification from triplicates (*** p<0.001 unpaired t-test). Right panel, representative images.
sGal3-β1 integrin interaction is increased in tumor cells.
To further assess the interaction of sGal-3 with β1 integrin and examine whether integrin β1 exhibits higher affinity for sGal-3 in tumor versus normal cells, we performed co-immunoprecipitation (co-IP) assays. We treated cultured cells with sGal-3, washed off unbound lectin, lysed cells and performed co-IPs to assess sGal-3−β1 interactions at the cell surface. We focused on α5β1 integrin, due to its elevated expression in glioblastoma compared to adjacent normal brain tissue (28). Cell surface interactions between sGal-3 and α5β1 integrin were evidenced in LN229 glioma, but not HFF-1 fibroblasts (Fig. 4B), even though both cell types express comparable levels of α5β1 and bind similar amounts of exogenous sGal-3 at the cell surface (Suppl. Fig. 4G,H). Combined, these experiments show that sGal-3’s affinity for cell surface α5/β1 integrins is increased in tumor cells, and upon binding induces pro-apoptotic signaling.
sGal-3 switches β1 integrin into its active conformation, and β1 activation status regulates sGal-3-mediated tumor cell killing.
The activation status of integrins is dependent upon conformational changes in their extracellular domains, which regulates their ligand affinity (29). We first examined whether sGal-3 treatment could alter β1 integrin conformation, and found that sGal-3 treatment led to a rapid β1 integrin conformation switch into its active form, which was accompanied by induction of PARP cleavage (Fig. 4C). To determine whether conformation of β1 integrin complexes per se influences its binding to Gal-3, we used neutralizing (P5D2) and activating (12G10) anti-β1 integrin antibodies (Fig. 4D). Activating antibody increased β1 integrin affinity for Gal-3 as demonstrated by a GST-Gal-3 pull down experiment, while blocking antibody abrogated β1 integrin-Gal-3 interaction. Congruently, antibody-mediated activation of β1 integrin complex further sensitized tumor cells to sGal-3-mediated PARP cleavage and concomitant death, while a blocking antibody reduced killing (Fig. 4E,F). These results show that sGal-3 interaction and death-inducing signaling is dependent upon the activation state of the β1 integrin complex and suggest that sGal3 can actively switch β1 towards its active state.
sGal-3 mediates tumor cell killing through a calpain/GSK3β-dependent signaling cascade.
Next, we investigated which signaling cascade was involved in sGal-3 triggered apoptosis. By examining downstream mediators of integrin signaling, we found that sGal-3 rapidly (within 30 min.) reduced the level of the inactive form of GSK3β (phosphorylated at Ser 9) in an integrin-linked kinase (ILK)-independent fashion (Fig. 5A, lanes 4,5 and 9,10). To verify ILK inhibitor (Cpd22) activity, we examined phosphorylation of Akt (Ser 473), one of ILK’s known substrates (30), and observed inhibition (Fig. 5A, lane 2 vs. 7). These data show that sGal-3 induces both ILK-dependent and -independent integrin signaling. The ILK/Akt axis was not involved in sGal-3-mediated killing as ILK and Akt inhibitors failed to prevent cell death (Suppl. Fig. 5A, B).
Figure 5: sGal-3 induces calcium influx and triggers a calpain-dependent pro-apoptotic signaling cascade.
A) Western blot showing that sGal-3 CM (500 ng/ml, 80 min) reduces GSK3β serine 9 phosphorylation and increases Akt serine 473 phosphorylation in LN229 cells. ILK inhibitor cpd22 (0.5 μM, 12 hr pre-treatment/co-treatment) blocked Akt phosphorylation, but had no effect on GSK3β.
B) Western blot showing that sGal-3 (500 ng/ml, 12 hrs) binding to LN229 cells induces a pro-apoptotic response by calpain-dependent cleavage of GSK3β, Bax and Bad. Caspase-8-dependent Bid was not affected. Calpain inhibitor III (10μM cotreatment, 12hrs) blocked the cleavage.
C) Time course of calpain activation in LN229 cells by sGal-3 (500 ng/ml) using a Calpain GLO assay. Calpain activation was absent in Calpain inhibitor III (10 μM co-treatment, 12 hrs) treated cells and in non-tumor cells (HFF-1). N=3 (triplicates). *p <0.05, **p <0.01, ***p < 0.001 (unpaired t-test)
D) sGal-3-mediated activation of calpain (GLO assay) in LN229 cells is blocked by pre-treatment (30 min.) with lactose (β-D-Galp-β(1–4)-D-Glc; 25 mM), but not melibiose (D-Gal-α(1–6)-D-Glc; 25 mM). N=3 (triplicates). ***p <0.001 (unpaired t-test)
E) Calpain GLO assay showing that sGal-3 CM (500 ng/ml), but not control CM supplemented with rGal-3 (500 ng/ml) activates calpain in LN229 cells. N=3 (triplicates). ***p <0.001 (unpaired t-test)
F) Calcium colorimetric assay showing that sGal-3 treatment induces cytosolic calcium accumulation in LN229 cells. Verapamil (50 μM, 24 hr pretreatment), a calcium channel blocker, and lactose-pretreated sGal-3 neutralized this effect. N=3 (triplicates). **p <0.01 (unpaired t-test)
Interestingly, phospho-GSK3β downregulation was accompanied by ILK-independent cleavage of total GSK3α/β (Fig. 5A, lanes 4 and 9), an effect which dramatically increased over time (Fig. 5B). Such cleavage is reminiscent of calpain-mediated cleavage on the N-terminus of GSK3β (31), therefore we tested calpain involvement in sGal-3-mediated cell killing. Calpain inhibitor (MDL28170) strongly reduced GSK3α/β cleavage and restored phospho-GSK3β levels (Fig. 5B). A calpain assay confirmed that sGal-3 treatment rapidly increased calpain activity in tumor cells but not in control fibroblasts (Fig. 5C), while a pre-treatment with lactose (competitive Gal-3 ligand) prevented calpain activation (Fig. 5D). The observed calpain activation was specific to sGal-3, as rGal-3 failed to achieve it (Fig. 5E). Importantly, calpain activation was necessary for sGal-3-mediated apoptosis, as calpain inhibition decreased levels of both cleaved Bax and PARP (Fig. 5B) and prevented tumor cell death (Suppl. Fig. 5).
Elevated intracellular calcium level is responsible for calpain activation (32), and integrin ligation can increase calcium channel influx (33). To analyze whether sGal-3 could trigger Ca2+ transport, we performed a calcium colorimetric assay in LN229 cells and found sGal-3 rapidly increased cytosolic calcium accumulation (Fig. 5F). Pretreatment with lactose or a calcium channel blocker (verapamil) neutralized this effect. Furthermore, verapamil treatment abrogated sGal3-mediated cell death (Suppl. Fig. 5).
In aggregate, these data show that the mechanism underlying sGal-3-mediated tumor cell death sequentially involves integrin activation, calcium channel facilitated Ca2+ influx, Ca2+-mediated calpain activation, and induction of the pro-apoptotic GSK3β/Bax signaling cascade following calpain-mediated cleavage.
N-linked glycosylation is required for sGal-3-mediated killing.
Considering that aberrant N-glycosylation is associated with malignant transformation (10), and integrins are major carriers of N-glycans (34), we examined whether aberrant N-glycosylation of β1 integrin in tumor cells contributes to their susceptibility to sGal-3. Tumor cell treatment with kifunensine, an inhibitor of α-mannosidases important in N-glycan biosynthesis and formation of complex N-glycans, reduced the molecular weight of β1 integrin, and abrogated GST-Gal-3/β1 interaction (Fig. 6A), while treatment with Benzyl-2-α-GalNAc, an O-glycosylation inhibitor (OGI) at 2 mM concentrations, affected neither Gal-3/β1 integrin interaction nor β1 integrin molecular weight. Furthermore, kifunensine protected tumor cells from sGal-3-mediated killing, whereas Benzyl-2-α-GalNAc did not (Fig. 6B). These results show that complex N-glycans, recognized by galectins, are necessary for sGal-3/β1 integrin interaction and sGal-3-mediated apoptosis.
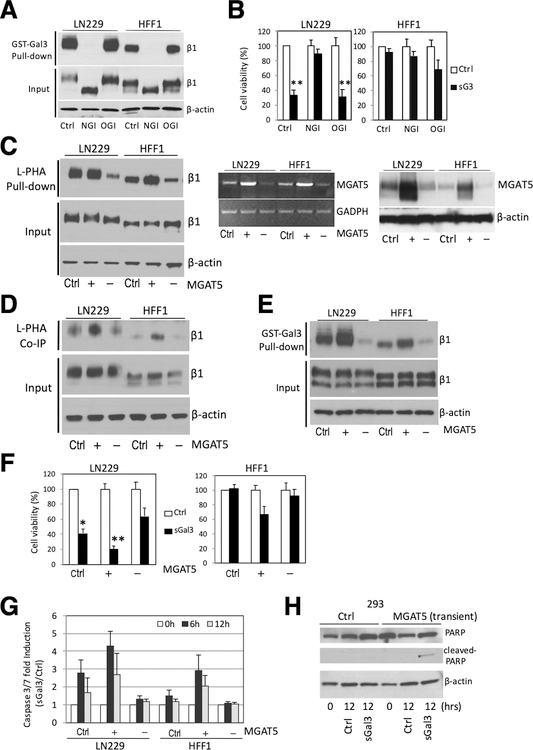
Figure 6. Alteration in MGAT5 expression effects complex N-glycan formation on β1 integrin, sGal-3-β1 integrin interaction and sGal-3-mediated cell killing.
A) GST-Gal-3 pull-down assay showing that kifunensine (100 μM), an α-mannosidase inhibitor that blocks N-glycan processing (NGI), abrogated GST-Gal-3-β1 integrin interaction. Loss of N-glycans reduces size of β1 integrin (see input). Treatment with benzyl-N-acetyl-α-D-galactosamide (α-benzyl-GalNAc) (2 mM), an O-glycosylation inhibitor (OGI) had no effect.
B) Crystal violet cell viability assay showing kifunensine pre-treatment (24 hrs) prevented sGal-3-mediated cell death in LN229 cells. Cells were pre-treated with NGI or OGI for 24 hrs, then 1x control or sGal-3 CM was added and cells incubated for another 72 hrs. N=3 (in triplicates). **p<0.01 (unpaired t-test)
C) L-PHA pulldown assay shows MGAT5 overexpression (+) increases, while MGAT5 knockdown (–) decreases L-PHA binding to β1 integrin. Cells were transiently transfected with control plasmid (Ctrl.) or expression vectors for MGAT5 (+) or shRNA for MGAT5 (–) and cell extracts analyzed after 48 hrs. L-PHA-agarose beads were used to specifically pull-down proteins carrying N-glycan branches synthesized by MGAT5, followed by Western blot for β1 integrin (left panel). RT-PCR analysis (middle panel) and Western blot (right panel) show increase/decrease in MGAT5 mRNA/protein levels.
D) Co-immunoprecipitation experiments showing increased/decreased binding of L-PHA to cell surface β1 integrins with overexpression (+) or knockdown (–) of MGAT5. Cells were transiently transfected as in C), then incubated with purified L-PHA (2.5 μg/ml) for 2 hrs at RT and cell extracts prepared. Cell surface proteins bound to L-PHA were then immunoprecipitated with anti-L-PHA antibodies (2.5 μg/ml) and protein G agarose beads followed by β1 integrin western blot. (Ctrl., +, -) as above.
E) GST-Gal-3 pulldown assay shows that alteration in MGAT5 expression modulates Gal-3 interaction with β1 integrin. Cells were transiently transfected as in C), then cell extracts were incubated with GST-Gal-3 beads and bound β1 integrin detected by Western blot. (Ctrl., +, -) as above.
F) Crystal violet cell viability assay showing modulation of MGAT5 expression alters cell sensitivity to sGal-3-mediated killing. Note that increased MGAT5 expression sensitizes HFF-1 cells to sGal-3 killing. Cells were transiently transfected as in C), then treated with sGal-3 CM for 72 hrs. N=triplicates; *p < 0.05, **p < 0.01 (unpaired t-test).
G) GLO assay showing modulation of MGAT5 expression alters the cell sensitivity to sGal3-induced apoptosis. Cells were transiently transfected as in C), then treated with sGal-3 for 6 to 12 hrs and caspase-3/7 activation measured. Note that increased MGAT5 expression sensitizes HFF-1 cells to sGal3-induced caspase 3/7 activation, while MGAT5 knockdown reduces cell death in LN229 cells. Fold induction is based on ratio of sGal-3/control luciferase units. Fold ratio at 0 hr is 1. (n = 2).
H) Western blot showing that transient transfection of MGAT5 renders 293 cells susceptible to sGal-3-mediated PARP cleavage.
MGAT5 regulates Gal-3/ β1 integrin interaction.
MGAT5 is the gatekeeper enzyme that controls the synthesis of complex tetra-antennary N-glycans on glycoproteins. It is one of the most important enzymes involved in the regulation of the biosynthesis of glycoprotein oligosaccharides and is believed to underlie aberrant N-glycosylation in cancer (5). MGAT-5 is an N-acetylglucosaminyltransferase that catalyzes the addition of N-acetylglucosamine in β1–6 linkage to the α-linked mannose of biantennary N-linked oligosaccharides present on newly synthesized glycoproteins in the Golgi apparatus (35). Addition of N-acetylglucosamine to α-mannose residues is the limiting step for further branch elongation into poly-N-acetyllactosamines (oligosaccharides comprised of repeating galactose and N-acetylglucosamine residues), which are preferential Gal-3 ligands (36). To determine whether MGAT5 modulation affects Gal-3/β1 integrin interactions, we used overexpression and shRNA neutralization studies. Increased MGAT5 expression augmented the presence of complex N-glycans on β1 integrin of both LN229 and HFF-1 cells as shown in L-PHA pull-down studies of cell extracts (Fig. 6C). L-PHA, is a phytohemagglutinin that has a carbohydrate-binding specificity for the complex N-glycans synthesized by MGAT5. Conversely, MGAT5 knockdown reduced L-PHA binding.
These results were confirmed in a more physiological setting, where cultured cells were treated with pure L-PHA, washed and lysed, and L-PHA bound to cell surface β1 integrin immunoprecipitated with anti-L-PHA antibodies followed by Western blot for β1 integrin (Fig. 6D). Importantly, alteration in MGAT5 expression only affected the composition of cell surface N-glycans, without altering expression of β1 integrin. We then determined whether this post-translational modification altered sGal-3 binding and mediated killing (Fig. 6E,F). Increased MGAT5 augmented β1 integrin binding and sGal-3 killing potency in LN229 cells, and remarkably, somewhat sensitized HFF-1 cells to Gal3-induced cytotoxicity (Fig. 6F). Conversely, reduction in MGAT5 expression reduced LN229 susceptibility to sGal-3-mediated killing. These changes in cytotoxicity were accompanied with alterations in activation of caspase 3/7 (Fig. 6G). Overexpression of MGAT5 rendered HEK293 cells sensitive to sGal3 killing in an N-glycan-dependent fashion (Fig. 6H and Suppl. Fig. 6A).
Overexpression of MGAT5 in glioma cells led to robust activation of β1 integrin as evidenced by talin binding to the cytoplasmic tail of β1 integrin (Suppl. Fig. 6B); a similar response was observed in fibroblasts despite lower transfection efficiency. Co-transfection of MGAT5 and β1 integrin in 293 cells also augmented β1 integrin activation (Suppl. Fig. 6C). Altogether, these results show that MGAT5 promotes β1 integrin conformational activation and the resultant binding to sGal3.
Expression of N-glycosyltransferases is increased in tumor cells.
Three enzymes are critical in the sequential synthesis of high-affinity glycan ligands for Gal-3, which are comprised of tetra-antennary branches containing poly-N-acetyl-lactosamines. Briefly, MGAT5 creates the GlcNAcβ(1–6) Man branched structure, which is further elaborated by β1,4-galactosyltransferases (B4GALT1–8). Subsequent and repetitive reactions by β1,3-acetylglucosaminyltransferases (B3GNT1–4) and β1,4-galactosyltransferases create the tandem repeats of Gal and GlcNAc that constitute poly-N-acetyl-lactosamines. To determine whether tumor cells express aberrant levels of these glycosyltransferases, we performed RT-PCR to assess their mRNA expression. Indeed, tumor cells expressed significantly higher levels of β3GnT2 and β4GalT1, 2, 5 and MGAT5 (Suppl. Fig. 7A,B,D). The expression levels of MGAT5, β3GnT2 and β4GalT5 were also elevated in CD133+ glioma stem cells (GSCs) compared to adult brain neural progenitor cells (NPCs) (Suppl. Fig. 7C).
To quantify the glycosyltransferase activities of these enzymes, we probed for the specific glycan structures they synthesize on β1 integrin using branch-specific pull-down assays followed by western blot (Suppl. Fig. 7D). MGAT5-specific glycans were pulled down with L-PHA-agarose, β4GalTs-specific glycans with Ricinus communis agglutinin (RCA)-agarose (37), and for the detection of N-acetyl-lactosamine, the joined product of β3GnT2 and β4GalTs action, we used the Gal-3-GST pull-down system. Tumor cells showed significantly higher amounts of glycan branches than normal cells (Suppl. Fig. 7E). In sum, these results show that tumor cells overexpress multiple glycosyltransferases (Suppl. Fig. 7B), which leads to the coordinated formation of tetra-antennary branches with poly-N-acetyl-lactosamine on β1 integrin (Suppl. Fig. 7D), and explaining why tumor cells display enhanced susceptibility to sGal-3-mediated killing.
Discussion
We have engineered a chimeric form of secreted Galectin-3 (sGal-3), a major glycan binding protein, by extending its N-terminus through the conjugation of a non-cleaved signal peptide. sGal-3 exhibits ~100-fold greater pro-apoptotic activity towards cancer cells than recombinant wild type Gal-3. sGal-3 preferentially binds to cancer cells, which harbor aberrant complex N-glycans on their cell surface due to oncogenesis-related upregulation of glycosyltransferases (38). sGal-3 displays selective and potent cytotoxicity towards cancer cells, and induces a novel oncoglycanated- β1 integrin/calpain/caspase 9 pro-apoptotic signaling axis that is independent of anoikis, which is caspase 8-dependent. sGal-3 is cytotoxic to a variety of genetically heterogeneous cancer cell lines from different organs, even death-resistant cells that have hyperactivation of survival signaling or defective apoptosis pathways (39). Thus, we unveil a new neoglycan-based cancer cell susceptibility to cell death, which can be further exploited for cancer treatment.
Our data evidences that β1 integrin expression is necessary but not sufficient for sGal-3-mediated killing. While both normal and tumor cells express variable levels of β1 integrin, cancer cells display aberrantly-enhanced glycosylation of β1 integrin and we show this underlies the selective pro-apoptotic effect of sGal-3 on cancer cells. Alteration in cell surface β1 integrin glycosylation can promote tumor dissemination (11), and we now show that such tumor-specific epitopes can be targeted for cytotoxic therapy. Because β1 integrin has 11 N-glycosylated Asn residues on its extracellular domain, further work is necessary to determine which of these carry the critical N-glycans responsible for killing selectivity and what novel types of glycan chains are formed. Determining which of the 12 possible αxβ1 integrin dimers can initiate apoptosis in response to sGal3 also needs further investigation.
Additional work is also warranted to determine why sGal-3 is ~60–100-fold more potent at tumor cell killing than rGal-3. One possibility is that preparation of rGal-3 from bacteria compromises its binding activity. To address this caveat, we demonstrated efficient Gal-3-β1 integrin interaction in a pull-down experiment, using recombinant GST-Gal-3. While endogenous Gal-3 is secreted through a non-classical Golgi apparatus-independent secretion mechanism, the N-terminal signal peptide of sGal-3 will direct it towards classical secretion. The involvement of the Golgi apparatus in sGal-3 maturation may lead to post-translational modifications not present on endogenous Gal-3. To address this possibility, we produced sGal-3 in cells treated with either N- or O-glycosylation inhibitors, but did not see a change in sGal-3 molecular weight. A more plausible explanation for the novel death-inducing activity of sGal-3 is that the conjugated chimeric N-terminal signal peptide alters protein conformation and behavior. Indeed, we showed that it can induce a shift in β1 integrin conformation to the active state. The tissue plasminogen activator (tPA) signal peptide possesses 15 hydrophobic amino acids in α-helical structure, which may facilitate α-helix-mediated protein-protein interaction. Since extracellular Gal-3 can oligomerize through its N-terminus (40,41), the chimeric signal peptide of sGal-3 could potentially facilitate this process and augment Gal-3-β1 integrin binding, and potentiate downstream signaling via cross-linked integrin complexes. Further work is necessary to address this hypothesis, but it is supported by prior work with Galectin-1, where addition of rigid α-helix or flexible random coil linkers between CRD domains promoted multimerization, lattice formation and augmented glycan binding (42).
Conceptually, sGal-3 binding to β1 integrin could either activate a pro-apoptotic signaling cascade downstream of the activated integrin, or alternatively antagonize pre-existing ligand activated β1 integrin-mediated survival signals. The fact that anoikis, a form of apoptosis induced by cell detachment from the extracellular matrix (ECM), is caspase-8-dependent, rather than caspase-9 in our study; and our finding that knockdown of β1-integrin prevents sGal-3-induced apoptosis, argue against integrin survival signaling blockage as a major mechanism. While growing our cells on standard negatively charged polystyrene (no ECM coating), we considered that integrin-mediated survival signaling might be activated by the cells’ own secreted ECM components. To test this, we examined the activation status of focal adhesion kinase (FAK), a hallmark of ECM-mediated integrin survival signaling, but found no FAK phosphorylation at Y397 (as a positive control we showed fibronectin induced phospho-FAK; data not shown).
Further investigation into the sGal-3 killing mechanism allowed us to define its critical mediators. sGal-3 binds activated β1 integrin, and thereby rapidly triggers intracellular Ca2+ influx and subsequent calpain activation. Calpain is a member of a family of calcium-dependent cysteine proteases, and can activate GSK3β through N-terminal cleavage (43). Calpain can coordinate apoptosis induction via proteolytic activation of pro-apoptotic factors at the mitochondria (44). Concordantly, we found that sGal-3 treatment triggered a calpain-dependent pro-apoptotic cascade, starting from cleavage-mediated activation of GSK3α/β, to downstream Bax, Bad and PARP cleavage. Calpain activation is calcium-dependent, which is regulated by calcium channels (33), and we found that inhibition of calcium channel function successfully interfered with sGal-3/integrin-mediated calpain activation and ensuing apoptotic cell death signals.
Abnormal glycan structures are present in cancer due to altered glycosyltransferase activities (3). Aberrant glycosylation was once thought to be a passenger event in cellular transformation, but is now increasingly recognized as a driver in tumor formation (1). Elevated MGAT5 expression has been reported in various tumor cells and is believed to facilitate tumor growth, angiogenesis and metastasis; making it a therapeutic target for cancer (45). Our study evidenced overexpression of MGAT5 in a panel of genetically heterogeneous cancer cells and showed this was, at least in part, the basis for their susceptibility to sGal-3-mediated killing. Neutralization of MGAT5 expression by RNA interference induced resistance to sGal-3 in tumor cells, whereas sensitivity to sGal3-mediated killing could be transferred to normal cells by forced expression of MGAT5. In addition, overexpression of MGAT5 switched β1 integrin into its active confirmation and elevated β1-talin interaction in tumor cells.
Our study further showed that the expression levels of glycosyltransferases (β4GalTs and β3GnT2) that act downstream of MGAT5 in mediating the elongation of tetra-antennary N-glycans into poly-N-acetyl-lactosamine chains were also overexpressed in cancer cells in a coordinated fashion. This led to enhanced N-glycan formation on β1 integrin as detected by glycan-specific plant lectin binding assays. Consistently, inhibition of either β1 integrin expression or N-glycosylation abrogated sGal-3-induced apoptosis. While cancer cell overexpression of individual glycosyltransferases was in the range of 2–2.5 fold, we anticipate that their combined upregulation has a synergistic effect in generating more complex N-glycans on β1 integrin in tumor cells. This likely explains tumor cell hypersensitivity to sGal-3 and suggests a promising therapeutic index for cancer treatment.
Our results shed further light on the glycosylation-dependent pro-apoptotic role of sGal-3 and its possible exploitation for cancer therapy. We showed cancer cells are more sensitive to sGal-3-induced apoptosis compared to normal cells and sGal-3 strongly inhibited tumor cell growth and prolonged mouse survival in subcutaneous and intracranial mouse models. Local delivery of sGal-3 to the tumor microenvironment had no obvious toxicity and was well tolerated by the animals. Therefore, our results support further investigations into using tPA signal peptide-Gal-3 conjugate as a wide-spectrum anti-cancer therapeutic. Novel therapeutic agents have been developed by conjugating natural peptides, peptide analogues, and chemical agents for improved selectivity, efficiency and safety (17,46). The normal function of N-terminal signal peptides is to guide protein sorting and secretion, but it has been shown that uncleaved signal peptide can modify its function (47,48).
In conclusion, our study provides proof of principle for the targeting of neo-synthesized glycoantigens in cancer and shows this can be achieved through treatment with a novel signal peptide-galectins-3 conjugate, which potently and specifically induces cancer cell death. It further demonstrates the potential of chimeric signal peptide conjugation as a novel approach to dramatically enhance protein therapeutic activity.
Supplementary Material
Clinical Significance.
Tumor-selective targeting is a critical requirement for successful cancer therapeutics. A major difference between normal and cancer cells is the presence of aberrantly-enhanced glycans on tumor cells. Yet, successful exploitation of augmented glycosylation in cancer for the design of new cancer therapy has remained elusive. Here we show that an engineered chimeric form of Galectin-3 can potently induce cancer-specific cell death by targeting aberrantly-enhanced N-glycans on tumor cells. These findings provide proof-of-principle that aberrant N-oncoglycans represent valid cancer targets that can be successfully targeted for therapeutic gain.
Acknowledgments
We thank Dr. W. Stallcup for GST-Gal-3 expression vectors, Dr. E. Miyoshi, for the pCXN2-MGAT5 plasmid, and Dr. M. Pierce for the pSUPER-MGAT5 plasmid. This work was supported by NIH grants R01-CA086335, R01-CA163722, R01-NS096236 (to EGVM), U01-CA168930 (to RDC), the PBTFUS (to EGVM), the Goldhirsh Foundation (to EGVM), the Genetics and Molecular Biology program of the Laney Graduate School of Emory University (to FWK), and the NSF (PRISM; DGE0231900 to FWK).
Footnotes
Conflict of interest:
The authors declare no potential conflicts of interest.
References
- 1.Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Frontiers in bioscience : a journal and virtual library 2012;17:670–99. [DOI] [PubMed] [Google Scholar]
- 2.Stowell SR, Ju T, Cummings RD Protein Glycosylation in Cancer. Annual Review of Pathology: Mechanisms of Disease 2015;10:473–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinho SS, Reis CA Glycosylation in cancer: mechanisms and clinical implications. Nature Reviews Cancer 2015;15:540–55. [DOI] [PubMed] [Google Scholar]
- 4.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, et al. Essentials of Glycobiology, 2nd Edition. Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 5.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 2004;306(5693):120–4. [DOI] [PubMed] [Google Scholar]
- 6.Yu LG. Glycosylation Change in Cancer as Therapeutic Target: Opportunities and Challenges. Journal of Glycobiology 2013;S1:e001. [Google Scholar]
- 7.Luo BH, Carman CV, Springer TA Structural basis of integrin regulation and signaling. Annu Rev Immunol 2007;25:619–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng M, Fang H, Hakomori S Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 beta 1 subunits and concomitant loss of fibronectin binding activity. Journal of Biological Chemistry 1994;269(16):12325–31. [PubMed] [Google Scholar]
- 9.Isaji T, Sato Y, Fukuda T, Gu J N-glycosylation of the I-like domain of beta 1 integrin is essential for beta 1 integrin expression and biological function: identification of the minimal N-glycosylation requirement for alpha 5beta1. Journal of Biological Chemistry 2009;248(18):12207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J, Taniguchi N. Regulation of integrin functions by N-glycans. Glycoconj J 2004;21(1–2):9–15 doi 5277444 [pii] 10.1023/B:GLYC.0000043741.47559.30. [DOI] [PubMed] [Google Scholar]
- 11.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res 2002;62(23):6837–45. [PubMed] [Google Scholar]
- 12.Dumic J, Dabelic S, Flogel M. Galectin-3: An open-ended story. Biochim Biophys Acta 2006;1760((4)):616–35. [DOI] [PubMed] [Google Scholar]
- 13.Nangia-Makker P, Nakahara S, Hogan V and Raz A Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr 2007;39((1)):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyer KK, Pang M, Gui D, Shintaku IP, Kuwabara I, Liu FT, Said JW, Baum LG, Teitell MA An Anti-Apoptotic Role for Galectin-3 in Diffuse Large B-Cell Lymphomas. American Journal of Pathology 2004;164(3):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res 2003;63(23):8302–11. [PubMed] [Google Scholar]
- 16.Ochieng J, Furtak V, Lukyanov P Extracellular function of galectin-3. Glycoconjugate Journal 2002;19(7–9):527–35. [DOI] [PubMed] [Google Scholar]
- 17.Fosgerau K, Hoffmann T Peptide therapeutics: current status and future directions. Drug Discovery Today 2015;20(1):122–8. [DOI] [PubMed] [Google Scholar]
- 18.Albertoni M, Daub DM, Arden KC, Viars CS, Powell C, Van Meir EG. Genetic instability leads to loss of both p53 alleles in a human glioblastoma. Oncogene 1998;16(3):321–6. [DOI] [PubMed] [Google Scholar]
- 19.Kaur B, Brat DJ, Devi NS, Van Meir EG. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene 2005;24(22):3632–42. [DOI] [PubMed] [Google Scholar]
- 20.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol 1999;9(3):469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis : an international journal on programmed cell death 2005;10(2):267–75 doi 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 22.Kaur B, Cork SM, Sandberg EM, Devi NS, Zhang Z, Klenotic PA, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res 2009;69(3):1212–20 doi 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niwa H, Yamamura K, Miyazaki J Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991;108:193–200. [DOI] [PubMed] [Google Scholar]
- 24.Massa SM, Cooper DN, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993;32(1):260–7. [DOI] [PubMed] [Google Scholar]
- 25.Bornhorst JA, Falke JJ [16] Purification of Protein Using Polyhistidine Affinity Tags. Methods in enzymology 2000;326:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Y, Makagiansar IT, Fukushi J, Liu FT, Fukuda MN, Stallcup WB. Molecular basis of interaction between NG2 proteoglycan and galectin-3. Journal of cellular biochemistry 2006;98(1):115–27 doi 10.1002/jcb.20768. [DOI] [PubMed] [Google Scholar]
- 27.Tyler K Determining the role of the galectin-3 N-terminus in tumor-specific apoptosis of human glioblastoma cells Master Thesis, Emory University, Atlanta, GA: 2016. [Google Scholar]
- 28.Gingras MC, Roussel E, Brunner JM, Branch CD, Moser RP Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. Journal of Neuroimmunology 1995;57:143–53. [DOI] [PubMed] [Google Scholar]
- 29.Shattil SJ, Kim C, Ginsberg MH The final steps of integrin activation: the end game. Nature Reviews Molecular Cell Biology 2010;11:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persad S, Dedhar S The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev 2003;22(4):375–84. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y, Xia Y, Shu X, Ge H, Zeng K, Wang J, Wang X Cleavage of GSK-3β by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3β activity induced by H2O2. Journal of Neurochemistry 2013;126(2):234–42. [DOI] [PubMed] [Google Scholar]
- 32.Pasquet JM, Dachary-Prigent J, Nurden AT Calcium influx is a determing factor of calpain activation and microparticle formation in platelets. European Journal of Biochemistry 1996;239(3):647–54. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ Regulation of the L-type Calcium Channel by a5b1 Integrin Requires Sigaling between Focal Adhesion Proteins. Journal of Biological Chemistry 2001;276(32):30285–92. [DOI] [PubMed] [Google Scholar]
- 34.Hou S HQ, Isaji T, Lu J, Fukuda T, Gu J Importance of membrane-proximal N-glycosylation on integrin B1 in its activation and complex formation. FASEB J 2016;30(12):4120–31. [DOI] [PubMed] [Google Scholar]
- 35.Shoreibah M, Perng GS, Adler B, Weinstein J, Basu R, Cupples R, Wen D, Browne JK, Buckhaults P, Fregien N, Pierce M Isolation, characterization, and expression of a cDNA encoding N-acetylglucosaminyltransferase V. Journal of Biological Chemistry 1993;268(21):15381–5. [PubMed] [Google Scholar]
- 36.Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chemistry & biology 2009;16(1):36–47 doi 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oubihi M, Kitajima K, Kobayashi K, Adachi T, Aoki N, Matsuda T. Development of an enzyme-linked immunosorbent assay-based method for measuring galactosyltransferase activity using a synthetic glycopolymer acceptor substrate. Analytical biochemistry 1998;257(2):169–75 doi 10.1006/abio.1997.2551. [DOI] [PubMed] [Google Scholar]
- 38.Hakomori S Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci U S A 2002;99(16):10231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YC, Tzeng CC, Song JH, Tsia FJ, Hsieh LJ, Liao SJ, et al. Genomic alterations in human malignant glioma cells associate with the cell resistance to the combination treatment with tumor necrosis factor-related apoptosis-inducing ligand and chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research 2006;12(9):2716–29 doi 10.1158/1078-0432.CCR-05-1980. [DOI] [PubMed] [Google Scholar]
- 40.Nabi IR, Shankar J, Dennis JW The galectin lattice at a glance. Journal of Cell Science 2015;128(13):2213–9. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogenous Cross-linked Complexes. Journal of Biological Chemistry 2004;279(12):10841–7. [DOI] [PubMed] [Google Scholar]
- 42.Earl LA, Bi S., Baum L Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology 2011;21(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goni-Oliver P, Lucas JJ, Avila J, Hernandez F N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. Journal of Biological Chemistry 2007;282(31):22406–13. [DOI] [PubMed] [Google Scholar]
- 44.Gao G, Dou QP N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes Bcl-2-independent cytochrome C release and apoptotic cell death. Journal of Cellular Biochemistry 2000;80(1):53–72. [DOI] [PubMed] [Google Scholar]
- 45.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology 2008;18(10):750–60 doi 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 46.Majumdar S, Siabaan TJ Peptide-mediated Targeted Drug Delivery. Medicinal Research Reviews 2012;32(3):637–58. [DOI] [PubMed] [Google Scholar]
- 47.Liu M, Allegood J, Zhu X, Seo J, Gebre AK, Boudyguina E, Cheng D, Chuang CC, Shelness GS, Spiegel S, Parks JS Uncleaved apoM signal peptide is required for formation of large apoM/S1P enriched HDL particles. Journal of Biological Chemistry 2015;290:7861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye RD, Wun TC, Sadler JE Mammalian Protein Secretion without Signal Peptide Removal. Journal of Biological Chemistry 1988;263(10):4869–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.