Abstract
Purpose:
The aim of this study was to evaluate the relationship of cardiovascular disease (CVD) on middle cerebral blood flow velocity (MCAv) at rest and during exercise. A secondary aim was to explore the relationship between MCAv and 1) the presence of white matter lesions and 2) cognitive function.
Methods:
We recruited individuals who were cognitively normal older adults. CVD risk was assessed by the Pooled Cohort atherosclerotic cardiovascular disease (ASCVD) risk score. Transcranial Doppler ultrasound measured middle cerebral artery at rest and during a bout of moderate intensity exercise. We quantified white matter lesions from MRI and cognitive function outcomes included executive function, language, processing speed, and attention.
Results:
Seventy-two participants 70.1 ± 4.7 years of age completed the study protocol. ASCVD risk score was significantly associated with resting and exercise MCAv (p<0.01) but not associated with white matter lesions (p>0.468). We observed a significant association between resting and exercise MCAv and language processing (p=0.010) but not other cognitive domains.
Conclusion:
In cognitively normal older adults, higher ASCVD risk score was associated with blunted resting and exercise MCAv and with lower language processing performance. These results highlight the need for CVD risk management to maintain optimal brain health.
Keywords: ultrasound imaging, brain health, aging
Introduction and Purpose
Cardiovascular disease (CVD) risk factors play a major role in late-life cognitive function, as well as the occurrence of stroke, vascular dementia, and Alzheimer’s disease.1,2 Impaired cerebrovascular regulation may be the foundational link between CVD risk and poor brain health.3,4,5–8 Further, higher CVD risk in 142 middle aged and older adults was a significant predictor for lower brain blood flow velocity in both the carotid and middle cerebral arteries (MCA).4 CVD risk and aortic stiffness can negatively affect cerebral blood flow in healthy, cognitively normal adults.9 However, over time, impaired cerebral blood flow regulation may lead to repeated ischemic injury, such as white matter lesions (WML),10 stroke, and even Alzheimer’s disease.11 Therefore, healthcare professionals on the front lines of care should assess CVD risk to maximize optimal brain health.
Understanding MCAv at rest and during an acute exercise challenge may provide valuable information for individuals at risk for cerebral pathology or suboptimal brain aging. Our prior work was the first to show a blunted cerebrovascular response during exercise in individuals with elevated beta-amyloid, a known risk factor for Alzheimer’s disease12 when compared to those who were non-elevated. With further examination between groups, we reported that participants with elevated beta-amyloid also had greater CVD risk. The already present elevated beta-amyloid potentially obscured our ability to identify the unique contributions of CVD risk to brain health. Therefore, to better understand the relationship between CVD risk on MCAv, we designed the present study to focus on individuals characterized as non-elevated for the presence of beta-amyloid and without cognitive impairment. The current study evaluated the association of CVD risk (defined as the atherosclerotic cardiovascular disease (ASCVD) risk score)13 on MCAv at rest and during a single bout of moderate intensity exercise.
We hypothesized that individuals with higher ASCVD risk would have blunted MCAv at rest and during exercise. A second study objective was to explore the relationship between MCAv and 1) WML and 2) cognitive function. We hypothesized that blunted MCAv at rest and during exercise would be associated with higher WML and reduced cognitive function.
Methods
Participants
Participants were recruited from a registry of individuals at the University of XXX Alzheimer’s Disease Center. Inclusion criteria were: 1) 65–90 years of age; 2) cognitively normal/non-demented based on neuropsychological testing and a Clinical Dementia Rating = 0; and 3) completion of [18F] Florbetapir positron emission tomography (PET) scan within 6 months of our experimental procedures. Exclusion criteria included: 1) Diagnostic and Statistical Manual of Mental Disorders-IV defined drug or alcohol abuse within the previous 2 years; 2) clinically significant depression or anxiety; 3) insulin-dependent diabetes; 4) myocardial infarction or symptoms of coronary artery disease within the previous 2 years; 5) acute decompensated congestive heart failure or class IV heart failure; 6) major orthopedic disability; 7) inability to exercise due to pain or physician restrictions. For this study, we excluded individuals who were characterized as having elevated beta-amyloid status as previously reported.12
Written informed consent was obtained for all participants prior to any data collection. Approval for this study was granted by the Institutional Review Board at the University of XXX Medical Center.
Experimental procedure
All participants began study procedures between 7:30 and 9:00am. Participants abstained from caffeine for 12 hours, physical activity for 24 hours and a large meal for 2 hours.12,14 Participants were asked to refrain from taking their morning medications until after the procedure. After consent, health questionnaires including assessment of CVD risk factors were completed followed by the experimental protocol to assess cerebrovascular regulation.
Cardiovascular disease risk
We calculated ASCVD risk using the Pooled Cohort Equation provided by the American Heart Association and the American College of Cardiology Guideline on the Assessment of Cardiovascular Risk.13 The ASCVD risk score was calculated using the Pooled Cohort equation which incorporates gender, age, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure (SBP), as well as smoking, diabetes and hypertension treatment status.13 Supine SBP was assessed after 20 minutes of rest. Cholesterol values were obtained during the clinic visit to the XX Alzheimer’s Disease Center between 1–2 months prior to the TCD measures.
Middle Cerebral Artery Blood Flow Velocity
The laboratory room for the experimental session was dimly lit, the temperature was maintained between 22–24 degrees Celsius and external stimuli were kept to a minimum during testing.15–17 Unpublished data from an existing dataset (n = 70) in our laboratory demonstrated that the mean intra-trial coefficient of variation during the resting condition was 7.7% for MCA velocity (MCAvmean), 6.7% for mean arterial pressure (MAP) and 8.6% for end-tidal carbon dioxide (PETCO2) and during the exercise condition MCAvmean was 10.0%, 7.9% for MAP and 8.0% for PETCO2.
All participants sat quietly on the exercise device for 15 minutes during the experimental protocol set up. The study team member (YL, SP) performing the transcranial Doppler (TCD) ultrasound scan was blinded to any information related to medical history, cardiovascular risk status and any imaging data related to amyloid or white matter lesions. The MCA was measured using transcranial Doppler (TCD) ultrasound. The headset with a 2-MHz robotic probe (RobotoC2MD, Multigon Industries) was placed on the temporal window and fixed in place. The left MCA was the primary vessel of interest. If a signal was not obtainable, then the right MCA was used.12,15 Once the optimal signal was identified, the imaging process began for mean MCAv (MCAvmean) at rest and during exercise. MAP was measured using a finger plethysmograph (Finometer Pro, Finapres Medical Systems), which was placed on the middle finger of the left hand. A nasal cannula was placed in the participants’ nares and adjusted as needed to ensure optimal PETCO2 reading. (BCI Capnocheck 9004) We monitored PETCO2 during exercise to ensure participants were not hyperventilating, which is known to induce cerebral vasoconstriction and lower cerebral blood flow.18 Heart rate (HR) was measured using a 5-lead electrocardiogram. MAP, PETCO2 and MCAvmean were averaged across each condition (rest and exercise).
For the seated rest condition, participants sat quietly on a recumbent stepper (NuStep, T5XR). Baseline data for all variables was recorded for 8 minutes. After the rest condition, participants performed a single bout of exercise at moderate intensity using the recumbent stepper. Moderate intensity was defined as 40% - 60% of age-predicted heart rate (HR) reserve.19 Participants were instructed to maintain a step rate of approximately 90 steps per minute.20 All participants began the exercise at 40 watts. The resistance was then increased until the target HR range was reached. Once participants were in steady state for one continuous minute, the 8-minute exercise session began. Data were sampled at 500 Hz using an analog to digital data acquisition board (National Instruments) and custom script written for MATLAB (v2015, Mathworks).
White matter lesions
The NeuroImaging Core of the XX Alzheimer’s Disease Center performed data acquisition for the MRI according to the Alzheimer’s Disease Neuroimaging Initiative, which a multisite longitudinal study of aging and dementia. Our neuroimaging facility uses a Siemens 3.0 Tesla scanner high-resolution T1 weighted and T2 for anatomical assessment (MP-RAGE; 1*1*1.2mm voxels; TR = 2300ms, TE = 2.98ms, TI = 900ms, FOV 256mm, 9 degree flip angle). Lesions were segmented by the lesion growth algorithm21 as recommended and implemented in the LST toolbox version 2.0.15 (www.statisticalmodelling.de/lst.html) for SPM12. The algorithm first segments the MP-RAGE T1 images into the three main tissue classes (cerebrospinal fluid, gray matter, white matter). This information is then combined with the co-registered FLAIR (0.9*0.9*5mm voxels; TR: 9000ms, TE=91ms, TI=2500ms, FOV 240mm, 150-degree flip angle) intensities in order to calculate lesion belief maps. By thresholding these maps with a pre-chosen initial threshold (k=.13) an initial binary lesion map is obtained and subsequently grown according to hyperintensities in the FLAIR image to produce a lesion probability map. The optimal initial lesion threshold was identified as the consensus of 3 raters visually inspecting lesion probability maps in two independent data sets (n=5 age-matched older adults, n=5 individuals with multiple sclerosis). Total volume of WML was quantified.
Cognitive function evaluation
Participants were evaluated for dementia at the XX Alzheimer’s Disease Center using the National Alzheimer’s Coordinating Center’s Uniform Data Set (UDS) neuropsychological test battery scale employed by the United States Alzheimer’s Disease Center (ADC) network.22,23 This allows for collaboration, standardized data collection and longitudinal studies across the ADC network.24–26 We calculated cognitive domain scores for executive function, language, processing speed, and attention normalized to a cognitively normal sample of older adults as previously reported.27 During the study, the National Alzheimer’s Coordinating Center modified their battery of tests and this resulted in the inability to maintain uniform testing across all participants, particularly in the memory domain. Consequently, we used only the tests common to both batteries, and the summed free recall of the Free and Cued Selective Reminding Test28 for memory domain of all participants which our site had been additionally administering to all participants. We normalized this memory domain to a similar population.29
Statistical analysis
Data was assessed for normality using Shapiro-Wilk and appropriate statistical analyses were conducted. To examine differences between individuals having a low ASCVD risk (ASCVD score <7.5%) and those having an elevated ASCVD risk (ASCVD score ≥7.5%),13 Welch’s t-test, Wilcoxon Rank Sum test or Test of Proportions were performed. To evaluate the influence of ASCVD risk on resting and exercise MCAvmean, linear regressions were used.
Age is an important covariate of MCAvmean. However, age was not adjusted for in these initial linear regression models due to its large contribution to the ASCVD risk score.13 We did perform a sub-analysis to evaluate these linear regression models adjusting for age. Furthermore, race was not included in the analysis of the ASCVD risk score subcomponents secondary to the entire study sample identifying as white, non-Hispanic. To examine the relationship between MCAvmean and WML, both simple linear regressions and linear regression models adjusting for ASCVD risk were used. Additionally, linear regressions adjusted for age, gender and education were used to evaluate the relationship between MCAvmean and cognitive function. All data analysis were performed in Stata 15 (StataCorp; LLC,College Station, Texas, USA).
Results
Participant characteristics
Eighty-five participants enrolled in the present study and seventy-two participants had complete data sets for the primary analysis. Reasons for incomplete datasets and not being included in the analyses were: Missing cholesterol values (n=9) and either an unobtainable MCAv signal or artifact during exercise (n=4). Resting and exercise MCAv data for participants (n = 20) in the present study has also been previously published.11 Participants were educated, white non-Hispanic (100%) females (69%) with a mean age of 70.0 (SD= 4.7) (Table 1). All participants identified as being physically inactive30 and had varying levels of WML (M=2.72, SD=3.04). Nine participants were taking beta blockers and 8 reported taking calcium channel blockers. All participants reached the target HR range using the appropriate estimated HR equations. Participants had no history of cerebrovascular disease by clinical presentation and MRI.1
Table 1.
Participant Characteristics
| Demographics | Overall (n=72) |
Elevated ASCVD risk (n=54) | Low ASCVD risk (n=18) |
P-value |
|---|---|---|---|---|
| Age (years) | 70.0 (4.7) | 71.3 (4.7) | 66.3 (1.5) | <0.0001 |
| Female (%) | 69 | 59.3 | 100 | 0.001 |
| White non-Hispanic (%) | 100 | 100 | 100 | ----- |
| Education (years) | 16.8 (2.5) | 16.5 (2.4) | 17.7 (2.3) | 0.076 |
| BMI (kg/m2) | 26.7 (4.4) | 27.4 (4.6) | 24.8 (2.8) | 0.015 |
| Cardiovascular Disease Risk Characteristics | ||||
| ASCVD Score | 15.0 (9.0) | 18.2 (8.2) | 5.5 (1.2) | <0.0001 |
| Systolic Blood Pressure (mmHg) | 131.0 (15.0) | 134.4 (14.2) | 120.6 (13.1) | 0.001 |
| Total Cholesterol (mmol/L) | 190.2 (35.3) | 185.9 (35.2) | 203.3 (33.3) | 0.066 |
| HDL Cholesterol (mmol/L) | 60.0 (18.1) | 58.2 (17.7) | 65.4 (19.0) | 0.123 |
| Blood Pressure Treatment % | 31.0 | 40.7 | 0 | 0.001 |
| Diabetes % | 3.0 | 3.7 | 0 | 0.408 |
| Smoking Status % | 1.4 | 1.9 | 0 | 0.561 |
| Resting and Exercise Parameters | ||||
| Resting MCAvmean (cm/s) | 47.1 (11.1) | 44.8 (11.0) | 53.9 (8.3) | 0.001 |
| Exercise MCAvmean (cm/s) | 53.4 (12.6) | 50.4 (11.5) | 62.1 (11.9) | 0.001 |
| Resting Mean Arterial Pressure (mmHg) | 73.5 (12.6) | 74.1 (10.4) | 71.8 (17.8) | 0.129 |
| Exercise Mean Arterial Pressure (mmHg) | 106.3 (20.9) | 105.6 (18.8) | 108.1 (26.8) | 0.792 |
| Resting CO2 (mmHg) | 33.9 (5.2) | 33.4 (5.2) | 35.4 (5.1) | 0.071 |
| Exercise CO2 (mmHg) | 37.9 (4.5) | 37.5 (4.3) | 39.1 (4.8) | 0.183 |
| Cognitive Domains | ||||
| Processing Speed | 0.012 (0.397) | −0.015 (0.421) | 0.092 (0.312) | 0.257 |
| Executive Function | −0.172 (0.816) | −0.103 (0.886) | −0.377 (0.523) | 0.156 |
| Language Processing | 0.265 (0.705) | 0.243 (0.725) | 0.331 (0.653) | 0.634 |
| Attention | −0.214 (0.828) | −0.253 (0.795) | −0.097 (0.934) | 0.558 |
| Memory | 0.592 (0.924) | 0.546 (0.964) | 0.728 (0.802) | 0.435 |
Data are presented as mean (standard deviation). BMI indicates body mass index. ASCVD score indicates atherosclerotic cardiovascular disease. MCAv indicates mean middle cerebral artery blood flow velocity. Cognitive domain scores are normalized.
Relationship of CVD risk on MCAv
Evaluating differences between individuals having a low ASCVD risk (ASCVD score <7.5%) and those having an elevated ASCVD risk (ASCVD score ≥7.5%) revealed between group differences. Participants with an elevated CVD risk had significantly lower resting and exercise MCAvmean. We report a significant difference in resting MCAvmean between those with a low ASCVD risk (M=53.95cm/s, SD=8.27) and whose with an elevated ASCVD risk (M=44.79cm/s, SD=11.00); t(40.8)= 3.72, p=0.001. Similarly, exercise MCAvmean was significantly different between individuals characterized as low ASCVD risk (M=62.12cm/s, SD=11.93) and those with an elevated ASCVD risk (M=50.43cm/s, SD=11.53); t(29.6)= 3.63, p=0.001. Resting and exercise MAP and PETCO2 were not different (see Table 1)
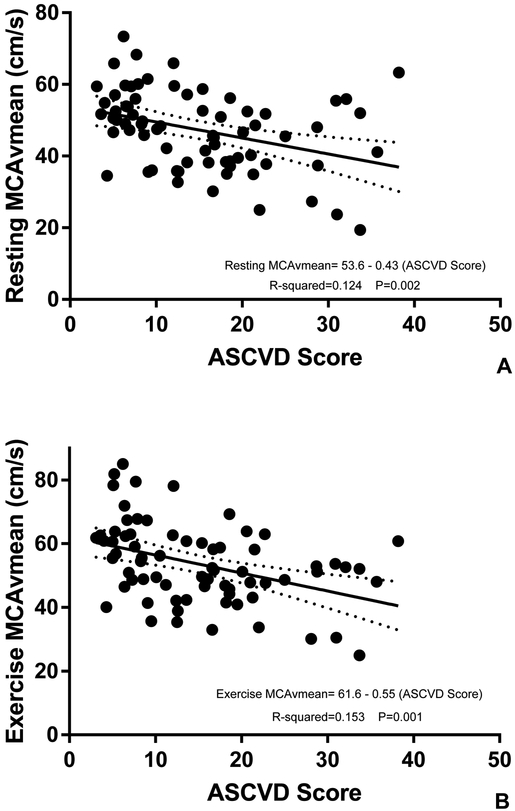
Resting MCAvmean significantly decreased on average by 0.43 cm/s (r2= 0.124, P=0.002) for every unit increase in ASCVD score (Figure 1). Exercise MCAvmean significantly decreased on average by 0.55 cm/s (r2=0.153, P=0.001) for every unit increase in ASCVD score (Figure 1). In the sub-analysis, adjusting these models for age revealed resting MCAvmean decreased on average by 0.47 cm/s (r2=0.124, P=0.046) for every unit increase in ASCVD score while exercise MCAvmean decreased by 0.58 cm/s (r2=0.153, P=0.027) for every unit increase in ASCVD score.
Figure 1. Associations between ASCVD Risk Score and MCAvmean;
(A) resting MCAvmean and (B) exercise MCAvmean. P values, R2 values and regression equations are from simple linear regression models evaluating the influence of CVD risk on cerebrovascular regulation.
Evaluating the subcomponents of the ASCVD score showed that age influenced resting MCAvmean. For every additional year, resting MCAvmean decreased by 0.64 cm/s (r2=0.072, P=0.022) and exercise MCAvmean decreased by 0.81 cm/s on average (r2=0.091, P=0.010). Males had a significantly lower resting MCAvmean by 5.70 cm/s compared to females (r2=0.571, P=0.043,). Individuals with diabetes exhibited a lower resting MCAvmean by 17.79 cm/s (r2=0.071, P=0.024,) and exercise MCAvmean was blunted by 17.46 cm/s (r2=0.052, P=0.053) on average compared to those without diabetes. No other ASCVD risk subcomponent was associated with MCAvmean (Table 2).
Table 2.
β Coefficients for Cardiovascular Disease Risk and MCAv Characteristics
| Resting MCAvmean | Exercise MCAvmean | |
|---|---|---|
| ASCVD Score | −0.43 (P=0.002)** | −0.55 (P=0.001)** |
| Age | −0.64 (P=0.022)* | −0.81 (P=0.010)* |
| Gender | −5.70 (P=0.043)* | −4.82 (P=0.136) |
| Total Cholesterol | 0.04 (P=0.284) | 0.05 (P=0.262) |
| HDL Cholesterol | 0.10 (P=0.159) | 0.12 (P=0.133) |
| Systolic Blood Pressure | −0.08 (P=0.370) | −0.16 (P=0.106) |
| Blood Pressure Treatment | −0.89 (P=0.756) | −2.52 (P=0.437) |
| Diabetes | −17.79 (P=0.024)* | −17.46 (P=0.053) |
| Smoking | −11.70 (P=0.297) | −12.17 (P=0.342) |
P<0.05,
P<0.01. ASCVD score indicates atherosclerotic cardiovascular disease. SBP indicates systolic blood pressure. BP indicates blood pressure. MCAvmean indicates mean middle cerebral artery blood flow velocity. β Coefficients are from individual simple linear regressions of MCAv values with ACSVD score and its subcomponents.
MCAv and ASCVD score with WML
Neither resting MCAvmean (β= −0.03 cm/s, r2=0.008, P=0.468) nor exercise MCAvmean (β= −0.02 cm/s, r2=0.004, P=0.595) were associated with WML (n= 66). Models adjusting for ASCVD risk score did not alter associations between resting or exercise MCAvmean and WML. These adjusted models revealed that ASCVD risk score was significantly associated with WML. Further investigation demonstrated for every unit increase in ASCVD risk score, WML increased by 0.10 mL (r2=0.082, P=0.020). This relationship was maintained after a sensitivity analysis removing outliers.
MCAv and cognitive function
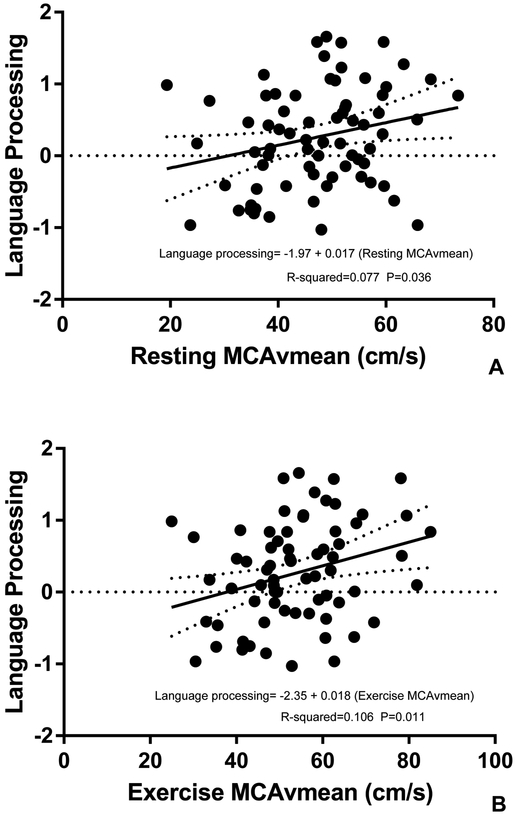
Adjusting models for age, education and gender revealed that language processing was significantly associated with both resting and exercise MCAvmean. Language processing significantly increased by 0.02 for every cm/s increase in resting MCAvmean (r2=0.077, P=0.036) (Figure 2) and by 0.02 for every cm/s increase in exercise MCAvmean (r2=0.106, P=0.011) (Figure 2).
Figure 2. Associations between MCAvmean and Language Processing (normed z-scores);
(A) resting MCAvmean and (B) exercise MCAvmean. P values, R2 values and regression equations are from linear regression models evaluating the relationship of cerebrovascular regulation and language processing adjusting for age, education and gender.
Adjusting models for age, education and gender revealed that executive function was not significantly associated with resting MCAvmean (β= −0.004 cm/s, r2=0.042, P=0.727) or exercise MCAvmean (β= −0.009 cm/s, r2=0.058, P=0.264). Adjusting models for age, education and gender revealed that memory was not associated resting MCAvmean (β= 0.01 cm/s, r2=0.098, P=0.243) or exercise MCAvmean (β= 0.02 cm/s, r2=0.116, P=0.100). Adjusting models for age, education and gender revealed that processing speed was not associated with resting MCAvmean (β= −0.004 cm/s, r2=0.069, P=0.351) or exercise MCAvmean (β= 0.0008 cm/s, r2=0.057, P=0.835). Similarly, attention was not associated with resting MCAvmean (β= 0.01 cm/s, r2=0.056, P=0.492) or exercise MCAvmean (β= 0.01 cm/s, r2=0.069, P=0.229).
Discussion
The present investigation resulted in several novel findings. First, older adults characterized as non-demented and “healthy brain aging” (no cerebrovascular disease such as stroke and non-elevated for beta amyloid) with higher ASCVD risk had blunted resting and exercise MCAvmean than those with lower ASCVD risk. Second, resting and exercise MCAvmean were not associated with WML. However, WML volume was associated with ASCVD risk, such that individuals with a higher ASCVD risk had a higher WML. Third, higher resting and exercise MCAvmean was associated with language processing skills but not with other measures of cognition. These results extend the current evidence and highlight those with higher CVD risk have reduced MCAv during exercise, which may be an indicator of cerebrovascular health.3,31–35
CVD risk and MCAv
MCAvmean during rest provides valuable information for establishing a baseline. However, the addition of exercise MCAvmean provides a much more comprehensive evaluation of MCAv response to a physical demand. Exercise is a physiological challenge to the cerebrovasculature due to increases in cardiac output, MAP, and sympathetic nervous system activity.36,37 Thus, evaluating the response of MCAvmean to exercise provides a unique assessment of cerebrovascular control12 and perhaps could be used to characterize the risk and progression of poor brain health.
Further, there is evidence to suggest that lower resting cerebral blood flow velocity is associated with similar measures of CV risk. The Framingham General Cardiovascular Risk Profile was related to lower resting MCAvmean in 160 healthy adults with a mean age of 59 (n=160).4 Similarly, another study reported a higher Framingham Risk Score was associated with lower resting cerebral blood flow using MRI techniques in large dataset (n = 576) of adults with a mean age of 46 years.3 The present study extends the current scientific knowledge by adding an exercise challenge to the MCAv assessment. Similar to rest, we report higher ASCVD score resulted in a lower exercise MCAvmean, further supporting the importance of maintaining cardiovascular health. Additionally, when comparing individuals having a low ASCVD risk (ASCVD score <7.5%) and those having an elevated ASCVD risk (ASCVD score ≥7.5%) we found that those with an elevated risk had significantly lower resting and exercise MCAvmean. Overall, the results of the present study provide support that CVD risk influences MCAvmean. We provide evidence that rest and exercise MCAvmean is blunted in those with elevated ASCVD risk and the importance of monitoring CVD risk for optimal brain health.
In addition to the ASCVD risk score, the present study assessed the ASCVD subcomponents to ascertain whether specific components were driving the relationship between the ASCVD score and resting and exercise MCAvmean. The findings of the present study revealed that age, gender, and diabetes were associated with either resting or exercise MCAvmean. These results are consistent with studies reporting that individuals who are older, male and diabetic have suboptimal cerebrovascular health.38–41 Given the current emphasis on cerebrovascular health and chronic disease, we provide support for the perspective that individuals with diabetes may be at risk for cerebrovascular dysfunction. Although only 2 participants had a diagnosis of diabetes, we found these individuals had a significantly lower resting (−17.8 cm/s) and exercise MCAvmean (−17.5 cm/s) compared to those without diabetes. Several mechanisms could explain our findings in these 2 participants. Diabetes is associated with increased blood viscosity which impairs blood flow, thereby reducing cerebral blood flow.42–44 Hyperglycemia results in a loss of vessel elasticity, which has been linked to reduced cerebral blood flow.45–47 Additionally, diabetes has a high prevalence of hypertension,48 which is known to result in both structural and functional cerebrovascular alterations, such as hypertrophy and remodeling of the cerebrovasculature, increases in resistance, impaired functional hyperemia (neurovascular decoupling) and reductions in cerebral blood flow.49 Targeting therapeutic and lifestyle interventions to reduce ASCVD risk, especially those at greatest risk for diabetes, is likely to be very important given the association between diabetes and dementia.50 Thus, further research in this area is warranted to elucidate the mechanisms contributing to reduced resting and exercise MCAvmean in people with diabetes.
ASCVD Risk, cerebrovascular regulation and WML
Current evidence suggests that lower cerebral blood flow and blunted cerebrovascular regulation are associated with WML.38,51,52 However, our results with MCAvmean did not support the hypothesis that these measures would be associated with higher WML. This discrepancy may be due to differences in methodology. In the present study, we assessed resting and exercise MCAvmean and not cerebrovascular regulation. Previous studies used MRI and acetazolamide administration to measure cerebral blood flow response. These different methods could lead to different results and thus further research is needed. We believe that exercise provides a unique physiologic challenge to the human system and provides insight into the interconnection between cerebrovascular health and dysfunction.12 Exercise has great ecological validity as it is clinically prescribed and is recommended that healthcare providers assess and promote physical activity.53 Although not a primary aim of the study, higher ASCVD score was associated with a higher WML. This is consistent with previous work demonstrating that CVD risk factors are positively associated with WML39,54 and underscore the importance to minimize CVD risk factors across the lifespan.
MCAv and cognitive function
Reduced cerebral blood flow is associated with cognitive decline and an increased risk for stroke and Alzheimer’s disease.55 Our results partly support the association between reduced MCAvmean with cognitive function. We found resting and exercise MCAvmean to be associated with reduced language processing. Prior work has reported cerebrovascular regulation to be associated with overall cognitive function and other cognitive domains.12,56,57 These differences may, in part, be due to cognitive assessments, participant demographics or our methodology as we didn’t directly assess cerebrovascular regulation. Our participants were well-characterized cognitively normal older adults and at lower risk of developing AD as evidenced by non-elevated beta-amyloid levels, which likely affected the relationship between resting and exercise MCAvmean and cognition. The cohort underwent standardized cognitive evaluation using the UDS neuropsychological test battery and the CDR, providing a rich, standardized cognitive profile and reducing the risk of contamination of our sample by prodromal dementia.22,23 This allowed us to explore the specific contribution of CVD risk factors to resting and exercise MCAvmean and cognitive function.
Study participants self-identified as white, non-Hispanic, which limits the interpretation of results to individuals from other racial and ethnic backgrounds who may be at higher risk for CVD and have differing cardiac risk factors. Additionally, study participants were mostly female, which could have affected our results, given the known sex differences in MCAv.58 As in our prior work, we have acknowledged TCD is an indirect measure of cerebral blood flow and does not account for changes in vessel diameter.59 Though it’s been reported that vessel diameter does not change,60 this has not been unequivocally established.61,62 Moreover, cholesterol values were collected during clinic visit and not drawn at the same visit as the MCAv experimental protocol, which could alter the ASCVD score. Finally, it would have been preferable to maintain a complete and consistent cognitive battery throughout the study. However, as noted in this manuscript, norming was performed either through a published UDS normative calculator or, in the case of the Free and Cued Selective Reminding Test, to a separate age, sex, education appropriate population of individuals from our prior work.29 Finally, we must acknowledge the r-square values are small which suggests that the variables are not accounting for much of the explained variability. Future research should explore other potential variables that may influence brain health.
Considerations for Clinical Practice
The findings presented here highlight the importance of maintaining heart health for brain health. This topic is timely as we observe increased life expectancy and the occurrence of non-communicable diseases is projected to rise.63 A timely publication by the American Heart Association, Defining Optimal Brain Health in Adults, strongly recommends humans minimize CVD risk factor across the lifespan to maintain both cardiovascular and brain health.64 As physical therapists, we should regularly evaluate our patients CVD risk using several metrics (blood pressure, diet, body weight and exercise) and educate patients on CVD risk management that are optimal for heart and brain health. These findings add to a growing body of evidence that the ASCVD score has potential clinical utility to assess CVD risk either through the downloadable applications or adapting the “medical record using patient data and published equations.”13 However, further characterization about the relationship between resting and exercise MCAvmean and ASCVD score, including longitudinal studies are necessary.
Conclusions
Individuals with elevated ASCVD risk present with blunted resting and exercise MCAvmean compared to those with lower ASCVD risk. We report that specific subcomponents of ASCVD risk was associated with blunted resting and exercise MCAvmean. Finally, resting and exercise MCAvmean was associated with reduced language processing. While these results suggest CVD risk management may be important for optimal brain health, more research is needed to examine other factors that may influence CVD health.
Sources of Funding:
This study was funded in part by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Dr. Billinger) and by the American Heart Association Grant 16GRNT30450008 (Dr. Billinger). Dr. Sisante was supported in part by T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. This project was supported by the University of Kansas Alzheimer’s Disease Center (P30 AG035982) and by an Institutional Clinical and Translational Science Award, NIH/NCATS Grant Number UL1TR000001. Avid Radiopharmaceutical, Eli Lilly and Co., and the NIA (R01 AG043962) provided funds that supported the florbetapir imaging procedure. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or other funding agencies. Dr. Billinger receives support from the Wohlgemuth Faculty Scholar Award. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund.
Footnotes
Institutional Review Board: This study was approved by the University of Kansas Medical Center, approval number STUDY00001444.
References
- 1.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings JR, Heim AF, Kuan DC-H, Gianaros PJ, Muldoon MF, Manuck SB. Use of total cerebral blood flow as an imaging biomarker of known cardiovascular risks. Stroke. 2013;44(9):2480–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43(10):2803–2805. [DOI] [PubMed] [Google Scholar]
- 5.White RP, Markus HS. Impaired dynamic cerebral autoregulation in carotid artery stenosis. Stroke. 1997;28(7):1340–1344. [DOI] [PubMed] [Google Scholar]
- 6.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiological reviews. 1998;78(1):53–97. [DOI] [PubMed] [Google Scholar]
- 7.Mayhan WG. Cerebral circulation during diabetes mellitus. Pharmacology & therapeutics. 1993;57(2–3):377–391. [DOI] [PubMed] [Google Scholar]
- 8.Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(5):H1890–H1899. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson AL, Cambronero FE, Liu D, et al. Higher Aortic Stiffness Is Related to Lower Cerebral Blood Flow and Preserved Cerebrovascular Reactivity in Older Adults. Circulation. 2018;138(18):1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarumi T, Zhang R. Cerebral hemodynamics of the aging brain: risk of Alzheimer disease and benefit of aerobic exercise. Frontiers in physiology. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pimentel‐Coelho PM, Rivest S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. European Journal of Neuroscience. 2012;35(12):1917–1937. [DOI] [PubMed] [Google Scholar]
- 12.Sisante J-FV, Vidoni ED, Kirkendoll K, et al. Blunted cerebrovascular response is associated with elevated beta-amyloid. Journal of Cerebral Blood Flow & Metabolism. 2017:0271678X17732449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 14.Tyndall AV, Davenport MH, Wilson BJ, et al. The brain-in-motion study: effect of a 6-month aerobic exercise intervention on cerebrovascular regulation and cognitive function in older adults. BMC geriatrics. 2013;13(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billinger SA, Craig JC, Kwapiszeski SJ, et al. Dynamics of middle cerebral artery blood flow velocity during moderate-intensity exercise. Journal of Applied Physiology. 2017;122(5):1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billinger SA, Sisante J-FV, Alqahtani AS, Pasnoor M, Kluding PM. Aerobic exercise improves measures of vascular health in diabetic peripheral neuropathy. International Journal of Neuroscience. 2017;127(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billinger SA, Sisante J-FV, Mattlage AE, et al. The relationship of pro-inflammatory markers to vascular endothelial function after acute stroke. International Journal of Neuroscience. 2017;127(6):486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willie C, Macleod D, Shaw A, et al. Regional brain blood flow in man during acute changes in arterial blood gases. The Journal of physiology. 2012;590(14):3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medicine ACoS. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 20.Billinger SA, Tseng BY, Kluding PM. Modified total-body recumbent stepper exercise test for assessing peak oxygen consumption in people with chronic stroke. Physical therapy. 2008;88(10):1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774–3783. [DOI] [PubMed] [Google Scholar]
- 22.Beekly DL, Ramos EM, Van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Disease & Associated Disorders. 2004;18(4):270–277. [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993. [DOI] [PubMed] [Google Scholar]
- 24.Besser L, Kukull W, Knopman DS, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ Neuropsychological Test Battery in the Uniform Data Set (UDS). Alzheimer Dis Assoc Disord. 2018;32(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monsell SE, Dodge HH, Zhou XH, et al. Results From the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord. 2016;30(2):134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirk SD, Mitchell MB, Shaughnessy LW, et al. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s research & therapy. 2011;3(6):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38(6):900–900. [DOI] [PubMed] [Google Scholar]
- 29.Vidoni ED, Johnson DK, Morris JK, et al. Dose-response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PloS one. 2015;10(7):e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ACSM. Guidelines for Exercise Testing and Prescription. 8th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 31.Jefferson AL, Liu D, Gupta DK, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. 2017: 10.1212/WNL.0000000000004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf PA, D’agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. [DOI] [PubMed] [Google Scholar]
- 33.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovascular psychiatry and neurology. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences. Stroke. 2008;39(10):2712–2719. [DOI] [PubMed] [Google Scholar]
- 35.de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC medicine. 2014;12(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Progress in neurobiology. 2000;61(4):397–414. [DOI] [PubMed] [Google Scholar]
- 37.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Medicine. 2007;37(9):765–782. [DOI] [PubMed] [Google Scholar]
- 38.Novak V, Last D, Alsop DC, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes care. 2006;29(7):1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fazekas F, Niederkorn K, Schmidt R, et al. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19(10):1285–1288. [DOI] [PubMed] [Google Scholar]
- 40.Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophrenia Bulletin. 1990;16(2):247. [DOI] [PubMed] [Google Scholar]
- 41.Melamed E, Lavy S, Bentin S, Cooper G, Rinot Y. Reduction in regional cerebral blood flow during normal aging in man. Stroke. 1980;11(1):31–35. [DOI] [PubMed] [Google Scholar]
- 42.Dandona P, James I, Newbury P, Woollard M, Beckett A. Cerebral blood flow in diabetes mellitus: evidence of abnormal cerebrovascular reactivity. Br Med J. 1978;2(6133):325–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes A, Locke P, Scudder P, Dormandy T, Dormandy J, Slack J. Is hyperviscosity a treatable component of diabetic microcirculatory disease? The Lancet. 1977;310(8042):789–791. [DOI] [PubMed] [Google Scholar]
- 44.Thomas D, Marshall J, Russell RR, et al. Effect of haematocrit on cerebral blood-flow in man. The Lancet. 1977;310(8045):941–943. [DOI] [PubMed] [Google Scholar]
- 45.Lee AT, Cerami A. Role of glycation in aging. Annals of the New York Academy of Sciences. 1992;663(1):63–70. [DOI] [PubMed] [Google Scholar]
- 46.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mechanisms of ageing and development. 2001;122(7):735–755. [DOI] [PubMed] [Google Scholar]
- 47.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. American journal of hypertension. 2011;24(10):1108–1113. [DOI] [PubMed] [Google Scholar]
- 48.Control CfD, Prevention: National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2014. In:2017. [Google Scholar]
- 49.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell metabolism. 2008;7(6):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid-and late-life diabetes in relation to the risk of dementia. Diabetes. 2009;58(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marstrand J, Garde E, Rostrup E, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33(4):972–976. [DOI] [PubMed] [Google Scholar]
- 52.Alosco ML, Brickman AM, Spitznagel MB, et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congestive heart failure. 2013;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobelo F, Rohm Young D, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137(18):e495–e522. [DOI] [PubMed] [Google Scholar]
- 54.Knopman DS, Penman A, Catellier D, et al. Vascular risk factors and longitudinal changes on brain MRI The ARIC study. Neurology. 2011;76(22):1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Reviews Neuroscience. 2011;12(12):723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown AD, McMorris CA, Longman RS, et al. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of aging. 2010;31(12):2047–2057. [DOI] [PubMed] [Google Scholar]
- 57.Guiney H, Lucas SJ, Cotter JD, Machado L. Evidence cerebral blood-flow regulation mediates exercise–cognition links in healthy young adults. Neuropsychology. 2015;29(1):1. [DOI] [PubMed] [Google Scholar]
- 58.Vriens E, Kraaier V, Musbach M, Wieneke G, Van Huffelen A. Transcranial pulsed Doppler measurements of blood velocity in the middle cerebral artery: reference values at rest and during hyperventilation in healthy volunteers in relation to age and sex. Ultrasound in medicine & biology. 1989;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 59.Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care. International journal of vascular medicine. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke. 1989;20(1):1–3. [DOI] [PubMed] [Google Scholar]
- 61.Brothers RM, Zhang R. CrossTalk opposing view: The middle cerebral artery diameter does not change during alterations in arterial blood gases and blood pressure. The Journal of physiology. 2016;594(15):4077–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoiland RL, Ainslie PN. CrossTalk proposal: the middle cerebral artery diameter does change during alterations in arterial blood gases and blood pressure. The Journal of physiology. 2016;594(15):4073–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorelick PB, Furie KL, Iadecola C, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]