Abstract
Extranodal natural killer (NK)/T-cell lymphoma (ENKTCL) is a rare type of Non-Hodgkin’s lymphoma which rarely metastasizes to the central nervous system (CNS). Ten of 60 patients (16.7%) with ENKTCL followed at Memorial Sloan Kettering Cancer Center (MSKCC) were diagnosed with CNS involvement between 1995 and 2016. Eight patients had systemic disease at the time of CNS diagnosis; one patient never developed systemic disease and another was in remission at the time of CNS relapse. Median overall survival was 3.8 months; at time of this report 9 patients have died and one who underwent autologous stem cell transplant (ASCT) is alive 27 months after CNS diagnosis. Five patients achieved a complete response in the CNS; one is still alive, one died of systemic disease, and three died of infection. CNS ENKTCL portends a grim prognosis, with no standard treatment. Prospective study on ASCT and immunotherapy in CNS ENKTCL is warranted.
Keywords: Natural Killer/T-cell lymphoma, extranodal natural killer/T-cell lymphoma, central nervous system lymphoma, central nervous system metastasis, leptomeningeal metastasis
Introduction
Extra-nodal natural killer cell T-cell lymphoma (ENKTCL) is a rare and aggressive type of non-Hodgkin’s lymphoma (NHL). In the World Health Organization (WHO) 2016 guidelines, ENKTCL is grouped with 25 other lymphoma or leukemia types as ‘mature T and NK neoplasms’[1]. There are three natural killer (NK)/T-cell-specific tumor types in the WHO’s mature T and NK neoplasm category: aggressive NK leukemia, chronic lymphoproliferative disorder of NK cells, and ENKTCL (nasal type) [1]. Historically referred to as lethal midline granulomas, histopathologic characterization of these locally invasive midline lesions led to their reclassification as ENKTCL [2]. Generally considered to have both NK cell and T-cell origins, ENKTCL is typically CD2+, surface CD3−, cytoplasmic CD3+, and CD56+. Though the neoplastic cells are almost always infected with Epstein-Barr virus (EBV), this association may not be as concrete as once believed [1,3].
Globally, ENKTCL is more common in Asian than Western countries, comprising 22% vs 5% of lymphoma cases, respectively [4]. In the United States, the incidence of ENKTCL is much higher in Asian/Pacific Islanders, American Indian/Alaskan Natives, and Hispanic Whites than Non-Hispanic Whites and Blacks [5]. The upper aerodigestive tract is the primary site of ENKTCL involvement in 70–80% of patients. In the remaining 20–30% of patients, lymphoma is present in only extra-nasal sites including the gastrointestinal tract and skin [4,6,7]. Central nervous system (CNS) involvement is rare, cited to occur in 0–11% of patients with ENKTCL [8–12]. Due to the rarity of CNS involvement of ENKTCL, the natural history and treatment response are unknown. The purpose of this study was to assess patient characteristics, treatment, and outcomes of patients with CNS ENKTCL.
Methods
Patients
We reviewed the records of patients with ENKTCL treated at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1995 and September 2016. Patients were identified retrospectively through chart review; this study was approved by the MSKCC Institutional Review Board. Patients included in this manuscript had to meet the following criteria: confirmed histopathological diagnosis of ENKTCL on MSKCC pathology review, age 18 or older, and at least 2 months follow-up or death from any cause. NK leukemia, chronic lymphoproliferative disorder of NK cells, peripheral T-cell lymphoma, and other leukemias and lymphomas were not included in the analysis. Some exceptional cases included in this study lacked one immunophenotypic marker typical of ENKTCL or Epstein-Barr Virus (EBV) presence; in each exceptional case the patient was diagnosed with ENKTCL by both expert pathologists at MSKCC and the clinical treatment team, and harbored other typical characteristics of ENKTCL.
Diagnosis of CNS disease
CNS involvement of ENKTCL was defined by one of the following criteria: positive CSF cytology, suspicious CSF cytology with the appropriate neurological clinical signs or symptoms of leptomeningeal disease, or unequivocal evidence of metastasis on contrast-enhanced MRI or CT of the brain or spine. Suspicious cytology was defined in the pathology reports as lymphocytes with atypical morphology that stained positive for cytoplasmic CD3 and negative for CD20. All CNS imaging was interpreted by a neuroradiologist. Diagnosis of CNS involvement during first line or salvage chemotherapy for relapsed systemic disease, or while in remission from systemic disease was considered CNS relapsed disease. Diagnosis of CNS involvement at time of initial ENKTCL diagnosis or shortly after was considered concurrent disease. Patients with relapsed or concurrent CNS ENKTCL were considered to have secondary CNS ENKTCL. Diagnosis of CNS ENKTCL without prior history or evidence of systemic involvement at CNS diagnosis was considered primary CNS ENKTCL.
Patient characteristics, treatment, and outcome
Symptoms, patient demographics, and pathology reports documented or performed at time of CNS disease were reviewed. Initial stage of disease was based on the Ann Arbor Staging system (I-IV, based on location and degree of tumor involvement), and the NK Prognostic Index (NKPI, based on the presence of ‘B’ symptoms, Ann Arbor Stage, lactate dehydrogenase level, and regional lymph nodes) [13,14]. Treatments received both prior to (if applicable) and after CNS diagnosis was documented. Time to CNS disease was defined as the time from systemic disease diagnosis to diagnosis of CNS involvement. Survival was determined both from time of initial diagnosis to death of any cause, and time from CNS diagnosis to death of any cause. Interpretation of characteristics, treatments, and outcomes is descriptive due to the small sample size
Results
Patient characteristics and diagnosis
Sixty patients with ENKTCL met inclusion criteria for in-depth chart review, and 10 (16.7%) had CNS involvement (Tables 1 and 2). Median age at CNS diagnosis was 57 years; 6 patients were women and 4 were men. Most common symptoms at presentation of CNS involvement were altered mental status, cranial nerve deficit(s), and headache. Patients also presented with seizure, gait instability, and back pain. One patient was asymptomatic and incidentally diagnosed with leptomeningeal metastasis by identification of neoplastic NK/T-cells on CSF cytology from a lumbar puncture performed during the first administration of prophylactic intrathecal (IT) methotrexate.
Table 1.
Patient Characteristics with CNS ENKTCL.
| Patients with CNS involvement | N = 10 (10/60, 16.7%) |
|---|---|
| Age, y | |
| Median (range) | 57 (44–83) |
| Sex | N (%) |
| Male | 4 (40) |
| Female | 6 (60) |
| Symptom(s) at presentation of CNS disease, Na | |
| Confusion | 5 |
| Cranial neuropathy | 3 |
| Headache | 3 |
| Gait Instability | 1 |
| Seizure | 1 |
| Back pain | 1 |
| Noneb | 1 |
| CNS diagnosis, CSF/Imaging/Both | N (%) |
| Positive MRI or CT only | 2 (20) |
| Positive CSF cytology only | 4 (40) |
| Positive imaging and cytology | 4 (40) |
| Pattern of CNS involvement | N (%) |
| Relapsed disease | 6 (60%) |
| Concurrent disease | 3 (30%) |
| Primary CNS ENKTCL | 1 (10) |
Four patients had more than one symptom at presentation;
Diagnosis of leptomeningeal metastasis was made incidentally by cytology during prophylactic IT chemotherapy treatment.
Table 2.
Tumor characteristics of 10 patients with CNS ENKTCL.
| Patient | Disease Pattern | Primary Tumor Location | NKPI score at ENKL diagnosis | Ann Arbor Stage | Lymph node involvement | Tumor cytotoxic markers | Tumor EBV status |
|---|---|---|---|---|---|---|---|
| 1 | Relapse | Upper aerodigestive tract | I | I | No | +cytoplasmic CD3, −surface CD3, +CD 56 | Unknown |
| 2 | Relapse | Small intestine | III | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 3 | Relapse | Liver | IV | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 4 | Relapse | Upper aerodigestive tract | IV | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 5 | Relapse | Upper aerodigestive tract | III | IV | No | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 6 | Relapse | Skin | IV | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 7 | Concurrent | Upper aerodigestive tract | IV | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 8 | Concurrent | Adrenal glands | III | IV | Yes | +cytoplasmic CD3, −surface CD3, −CD 56, −CD20 | Positive |
| 9 | Concurrent | Upper aerodigestive tract | IV | IV | Yes | +cytoplasmic CD3, −surface CD3, +CD 56 | Positive |
| 10 | Primary CNS | Cerebrospinal fluid | N/A | N/A | No/ N/A | +cytoplasmic CD3, −surface CD3, +CD 56, +CD2, −CD4, −CD5 | Negative, though limited by paucity of cells in CSF |
At diagnosis of CNS involvement, 6 patients met criteria for relapsed CNS ENKTCL, 3 patients had concurrent CNS ENKTCL, and 1 had primary CNS ENKTCL (Table 2). Primary tumor location at time of ENKTCL diagnosis for the 6 patients with relapsed CNS involvement included the nasopharynx, small intestine, liver, and skin; for the 3 patients with concurrent disease, primary disease sites included the adrenal glands, skin, and nasopharynx. NKPI score at the time of ENKTCL diagnosis ranged from 1–4, Ann Arbor stage also ranged from 1–4. All but three patients had positive cytoplasmic CD3, negative surface CD3, positive CD56, and positive EBV by in situ hybridization staining of encoded small RNA. One patient had unknown EBV status (not tested), and pathology tissue is no longer available as patient had original biopsy at an outside institution two decades ago; however, that patient had otherwise classical clinical presentation of ENKTCL. The patient with primary CNS ENKTCL had negative EBV PCR and negative EBV in situ hybridization (both in the CSF), though in situ hybridization was limited by paucity of lymphoma cells. This patient is now deceased, and EBV serum PCR was not checked prior to death. However, based on + CD56, +cytoplasmic CD3, −surface CD3, −CD4, −CD5, +CD2, −CD20, and negative other B-cell cytotoxic markers, the patient was determined to have ENKTCL by both MSKCC pathologists and clinical experts.
Time from diagnosis of ENKTCL to CNS involvement was generally short with a median interval of 2.4 months, ranging from 0–12.5 months. One patient had CNS relapse after a complete response (CR) systemically and was planning for autologous stem cell transplant, the remaining patients with secondary CNS ENKTCL had active systemic disease at the time of CNS metastasis.
Patterns of CNS involvement were mostly leptomeningeal: 8 patients had leptomeningeal ENKTCL, 1 patient had parenchymal brain involvement, and 1 patient had both leptomeningeal and CNS parenchymal disease. Of the 8 patients with leptomeningeal metastasis as the only site of CNS disease, 4 patients had NK/T-cells identified on CSF flow cytometry and/or cytology with a normal MRI of the brain and/or spine, 3 patients had evidence of CNS ENKTCL identified both on imaging and in the CSF, and 1 had a normal CSF cytology/flow cytometry with leptomeningeal metastasis only identified radiographically. The one patient with parenchymal involvement only had classic radiographic findings of lymphoma brain metastasis, the one patient with both leptomeningeal disease and parenchymal disease was diagnosed with positive CSF cytology and MRI findings. Figures 1 and 2 show the radiographic patterns of involvement in two patients from this series.
Figure 1.
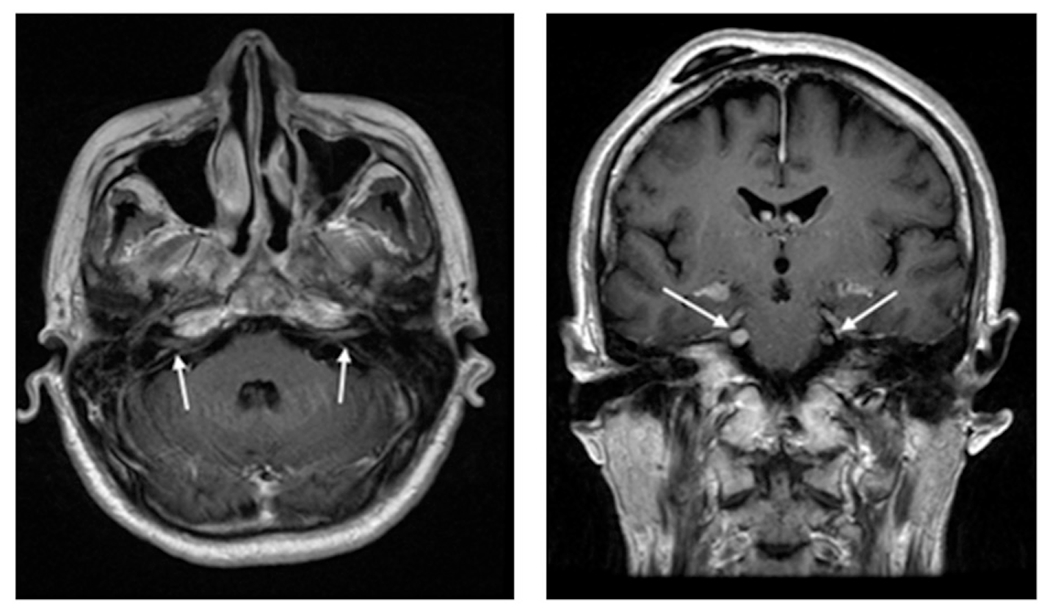
Gadolinium-enhanced MRI of a patient with leptomeningeal CNS ENKTCL disease. This patient had CNS relapsed disease, with concurrent systemic progression while on salvage chemotherapy that included high-dose methotrexate regimen. Patient developed bilateral hearing loss, facial numbness, and difficulty chewing, leading to this MRI that shows enhancement of bilateral vestibulocochlear and trigeminal nerves. CSF cytology was also positive.
Figure 2.
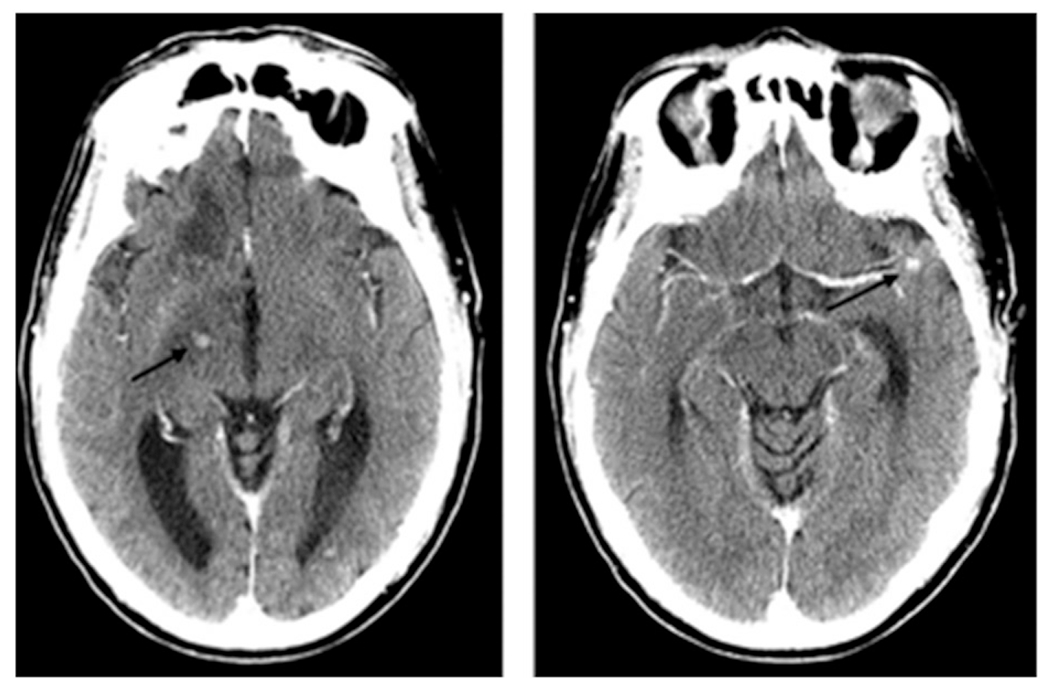
Contrast-enhanced CT of a patient with parenchymal CNS ENKTCL. This patient presented with confusion and gait imbalance, found to have concurrent CNS and systemic ENKTCL. He could not undergo MRI due to incompatible pacemaker. CSF profile was unremarkable. Symptoms improved and imaging abnormalities resolved with treatment, which included high-dose methotrexate. He died from complications related to recurrent pulmonary infections and adrenal insufficiency.
Treatments
All 6 patients with relapsed disease received at least one line of treatment for systemic ENKTCL prior to discovery of CNS metastases (Table 3). Treatment prior to CNS metastasis varied and included: dexamethasone, methotrexate (2g/m2), ifosfamide, L-asparaginase, and etoposide (SMILE regimen, 4 patients); ifosfamide, carboplatin, etoposide (ICE regimen, 2 patients); etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (VACOP-B regimen, 1 patient); etoposide, ifosfamide, cytarabine, and IT methotrexate (IVAC regimen, 1 patient); cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP regimen, 1 patient); nasopharyngeal radiation (1 patient); cisplatin (1 patient); IT methotrexate (1 patient); and allogenic bone marrow transplant (1 patient). Five patients received more than one treatment regimen prior to CNS involvement due to incomplete response or relapsed systemic disease after front-line therapy. Five of the 6 patients who received chemotherapy prior to CNS metastasis had at least one chemotherapy regimen that addressed the CNS, either high dose methotrexate (individual doses ≥2g/m2), N = 4 or IT methotrexate, N = 1.
Table 3.
Treatments and outcomes in 10 patients with CNS ENKTCL.
| Patient | Disease Pattern | Treatment prior to CNS ENKTCL | CNS prophylaxis | CNS treatment | Best CNS response | Survival from CNS diagnosis | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | Relapse | CHOPa x6, ICEb x2 | None | IT methotrexate, focal radiation to the spine, then ASCT | CR prior to ASCT | 4.3 months | Septic shock |
| 2 | Relapse | Cisplatin, focal radiation, SMILEc x4 | High dose methotrexate x4 as part of SMILE | High dose methotrexate x2, IT Methotrexate x1, IT cytarabine x3 | PR (MRI improved, CSF remained positive for malignant cells) | 3.4 months | Septic and Hemorrhagic shock |
| 3 | Relapse | Modified SMILEc (no methotrexate in cycle one, dose reduced thereafter) x2, ICEb x3 | High dose methotrexate x1as part of SMILE | High dose methotrexate x4, IT methotrexate x6 | PR (MRI improved, CSF cleared) | 3.6 months | CNS progression of disease |
| 4 | Relapse | VACOP-Bd plus IT methotrexate, focal radiation | IT methotrexate (unknown doses) | IT methotrexate, IT cytarabine | PR (MRI improved) | 8.8 months | Pulmonary infection and progression of systemic disease |
| 5 | Relapse | SMILEc x3 | High dose methotrexate x3 as part of SMILE | MVPe IT cytarabine x2, high dose myeloablative chemotherapy (with plan for ASCT, but died prior to engraftment) | CR prior to myeloablative chemotherapy | 4.3 months | Septic shock |
| 6 | Relapse | SMILEc x3, ASCT | High dose methotrexate x3 as part of SMILE | IT methotrexate x8 | CR | 2.1 months | Progression of systemic disease |
| 7 | Concurrent | None | N/A | IT methotrexate x1 | Unevaluable | 0.5 months | Unclear |
| 8 | Concurrent | None | N/A | High dose methotrexate x5 (one dose as part of SMILE) | CR | 4.0 months | Multiple infections, adrenal insufficiency |
| 9 | Concurrent | None | N/A | High dose methotrexate x4 as part of SMILE, IT methotrexate x1, IT cytarabine x1, then ASCT | CR | 27 months | Still alive |
| 10 | Primary CNS | None | N/A | IT methotrexate x1, then whole brain radiation | Progressive disease | 3.3 months | HSV encephalitis, progression of disease |
Cyclophosphamide, doxorubicin, vincristine, prednisone
Isofosfamide, carboplatin, etoposide
Dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide
Etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, bleomycin
High dose methotrexate, vincristine, procarbazine
All patients received at least one cancer-directed treatment targeting the CNS after diagnosis of CNS ENKTCL (Table 3). While all patients received either IT methotrexate, high-dose methotrexate, or both as part of CNS-directed therapy, the remainder of each treatment regimen varied. Other treatments included non-methotrexate IT chemotherapy, various CNS-penetrant systemic chemotherapies, radiation, and high-dose chemotherapy with autologous stem cell transplant (ASCT). One patient underwent whole-brain radiation therapy in an attempt to salvage progressive CNS disease after a single dose of IT MTX.
Outcome
Outcomes were poor in patients with CNS involvement, with a median overall survival (mOS) of 3.8 months from CNS diagnosis and 8.5 months from ENKTCL diagnosis (Table 2). Nine patients have died by the time of this report, 5 from infection-related complications (3 of whom had CR in the CNS at time of death), 2 from progressive CNS disease, 1 of unclear causes, and 1 after disease-related pancytopenia prohibited further cancer-directed treatment. One patient remains alive 27+ months after diagnosis of concurrent disease who received a high dose methotrexate-based regimen followed by ASCT. Five patients achieved a CR in the CNS after methotrexate-based regimens. Two patients with CR underwent ASCT: 1 is still alive, and the other died of infection 2 months after transplant. The three patients who achieved a partial response (PR) in the CNS also received methotrexate-based regimens. One patient had progressive disease after one dose of IT methotrexate and underwent whole brain radiation, another patient died within 15 days of first dose of IT methotrexate of unknown cause. Though limited by small sample size, there was no survival difference between patients with relapsed and concurrent CNS disease (mOS 3.5 months vs 4.3 months, respectively, p = .174), those who received high-dose methotrexate after CNS diagnosis and those who did not (mOS 3.6 vs 4.3 months, respectively, p = .768), and those who received IT chemotherapy after CNS diagnosis and those who did not (mOS 4.3 vs 3.3 months, respectively, p = .084).
In the 50 patients with ENKTCL without CNS involvement, mOS was 46.4 months, a significant difference compared to those with CNS involvement (46.4 vs 8.5 months, respectively, p = .0001). There was also a significant difference in survival between patients without CNS disease based on the stage of disease at diagnosis: those who had Ann Arbor stage I/II disease survived longer than those with stage III/IV disease (mOS 50.73 vs 13.75 months, respectively, p = .043). This difference was also maintained in NKPI stage I/II disease versus stage III/IV disease at diagnosis (mOS 79.27 vs 13.75 months respectively, p = .008). Among all patients with ENKTCL who had Ann Arbor stage IV disease at diagnosis (n = 24), there was no difference in survival between those who had secondary CNS involvement at any point in their disease course (n = 8) versus those who did not (n = 16), (mOS 8.47 vs 13.75 months, respectively, p = .302); this also held true for all patients with NKPI stage III-IV disease at diagnosis (mOS 8.47 vs 13.75 months respectively, p = .220).
Discussion
ENKTCL is a rare and aggressive form of NHL, with the cumulative probability of survival at 5 years ranging from 37.9% to 49.5% [14–16]. Spread of aggressive NHLs to the CNS portends a particularly poor prognosis, and ENKTCL is no exception. As ENKTCL is so rare, and CNS involvement unusual, not much is known about the patterns of disease, treatment or outcome.
We found a slightly higher rate of CNS involvement of ENKTCL (16.7%) than previously reported in other studies (0–11%), perhaps partly due to the high rate of stage III/IV disease at diagnosis in our series [8–11]. Just under half of the patients had stage III/IV disease at diagnosis in our series (40%) compared to that reported by Kim et al. (30.3%). Additionally, we included patients who were diagnosed with concurrent and primary CNS disease, unlike most reports on CNS ENKTCL, such as Kim et al., that only included patients with relapsed disease. In their study, they demonstrated Ann Arbor stage III/IV disease is a significant risk factor for CNS metastasis in patients with ENKTCL [8]. They also identified advanced NKPI (score of III or IV), location of ENKTCL involvement in the extra-upper aerodigestive tract as the primary site of involvement (vs upper aerodigestive tract), and lymph node involvement as other risk factors for CNS involvement [8]. Among patients with concurrent or relapsed disease in our study (N = 9), all but one had Ann Arbor stage IV disease, an NKPI score of III or IV, and many had lymph node involvement and extra-upper aerodigestive tract site as the primary site of disease.
Eighty percent of patients in this study were diagnosed with CNS involvement via positive or suspicious CSF cytology or flow cytometry. Most patients were diagnosed early in their ENTKCL disease course, consistent with other reports on ENKTCL or T-cell lymphomas [10,17,18]. The rapid onset of CNS involvement and frequency of leptomeningeal disease may justify early screening for the presence of leptomeningeal involvement among newly diagnosed patients at highest risk (i.e. stage III/IV, high NKPI stage, lymph node involvement, extra-upper aerodigestive tract involvement). The short interval to CNS diagnosis (and the one asymptomatic patient in this series diagnosed from CSF obtained during a lumbar puncture for CNS prophylactic therapy) supports the theory that some patients with advanced-stage disease may have low, but detectable levels of CSF lymphoma involvement prior to developing clinical manifestations. Although CNS prophylaxis in the highest-risk ENKTCL population theoretically should treat undiagnosed asymptomatic CNS metastatic disease, the effectiveness of CNS prophylaxis to prevent symptomatic CNS metastatic disease is unclear. Early identification of CNS metastasis prior to symptom manifestation may lead to more rigorous treatment and improved outcomes.
Given the rarity of CNS ENKTCL, optimal prevention strategies of peripheral T-cell lymphoma in the CNS are not well defined and only reported in small retrospective studies. In one study of 13 patients with CNS relapse of peripheral T-cell lymphomas, the specific treatment prior to CNS involvement (including regimens with systemic methotrexate dosed at 1 g/m2) was not associated with lower relapse rates in the CNS [12]. Extrapolating from data using IT and systemic CNS chemotherapy prophylaxis in other aggressive NHLs, the results are mixed in the post-rituximab era, but most studies report no benefit to CNS prophylaxis [19–23]. In the current study, 5 of 6 patients with relapsed CNS ENKTCL had received CNS prophylaxis as part of their initial systemic therapy. Among patients at highest risk for CNS relapse (Ann Arbor stage III/IV and/or NKPI stage III/IV patients) who did not have CNS involvement (n = 19), 14/19 received CNS prophylaxis (IT Methotrexate =3, high dose methotrexate ≥2g/m2=11). The 5 of 19 high-risk, advanced stage ENKTCL patients who did not receive CNS prophylaxis and did not go on to develop CNS metastases had a median overall survival of 19.4 months. This data suggests that traditional CNS prophylactic chemotherapy may not be highly effective in preventing ENKTCL CNS disease, though interpretation is limited by small sample size and prospective evaluation is required. The National Comprehensive Cancer Network does not recommend routine CNS prophylaxis in patients with NK or T-cell lymphomas.
There is no standard treatment after discovery of CNS disease, but typically includes IT or high-dose methotrexate based regimens, mimicking regimens used in aggressive B-cell lymphomas [24–26]. Despite lack of standardization, high dose methotrexate is often regarded as the backbone of treatment in parenchymal or leptomeningeal metastasis from lymphomas, though sometimes systemic therapy is deferred initially in favor of IT chemotherapy or radiation in the case of leptomeningeal disease only [27]. In our study, four patients with leptomeningeal metastasis only received IT chemotherapy alone or in combination with radiation for treatment of CNS disease, but without additional systemic CNS-directed treatment. These four patients did not receive systemic CNS-directed therapy due to one or more of the following: poor functional status, rapid neurological decline, and/or other co-morbidities (such as severe pancytopenia).
In more recent years, consolidative treatment with high-dose chemotherapy followed by autologous stem cell transplant for secondary B-cell CNS lymphoma has demonstrated prolonged overall and progression-free survival, surpassing historical controls [28,29]. Among patients with advanced systemic ENKTCL, retrospective studies show that consolidative stem cell transplant is effective in prolonging overall and progression-free survival [30,31]. Best outcomes in studies on transplant for lymphomas are seen in patients who achieve CR prior to transplant, though there is the possibility of benefit in patients who only achieve PR at the time of transplant. In our study, the long-term survivor underwent consolidative autologous stem cell transplant after achieving systemic and CNS CR. Of note, infections accounted for 5 patient deaths: 3 were treatment-related (all 3 with CR in the CNS, 2 of which also with CR systemically), and 2 were likely due to treatment and systemic disease. Of the 3 patients with CR in the CNS at time of infection-related death, one patient died a few months after ASCT, one died after receiving high-dose myeloablative chemotherapy but before the planned stem cell transplant could be performed, and one patient with improving systemic disease died of septic shock after experiencing recurrent multi-drug resistant infections throughout the treatment course. Of the 2 patients with active CNS/systemic disease who died of infection, 1 died of fungal pneumonia (though also had progression of liver metastasis at time of death), and the other of septic shock from bacteremia which led to disseminated intravascular coagulation, hemorrhage, and death. This points to the high toxicity associated with treatments directed at CNS disease, particularly ASCT; even when CR is achieved, the risk of mortality was high in this series.
Over the past year, pembrolizumab has been used to treat multiple refractory, relapsed, or advanced ENKTCL successfully, even among patients who had aggressive relapse after ASCT [32,33]. While none of the 14 patients treated between these two studies had CNS involvement of ENKTCL, the promising results in the systemic ENKTCL population and CNS B-cell lymphoma population gives hope that similar success with pembrolizumab may be seen in CNS ENKTCL [34].
In conclusion, CNS involvement of ENKTCL is relatively uncommon. Almost all patients with relapsed CNS disease had prior exposure to CNS prophylaxis, questioning the utility of CNS prophylaxis in ENKTCL. Survival among patients with CNS ENKTCL was poor, but ASCT may yield long-term survival in some. ASCT and immunotherapy warrant future investigation as treatments for CNS ENKTCL.
Acknowledgments
Funding
This research was funded in part through the NIH/NCI Cancer Center Support. Grant P30 CA008748.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http:\\<10.1080/10428194.2018.1551541>.
References
- [1].Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chim CS, Ooi GC, Shek TWH, et al. Lethal midline granuloma revisited: nasal T/Natural-killer cell lymphoma. Jco. 1999;17:1322–1325. [DOI] [PubMed] [Google Scholar]
- [3].Tsuyama N, Asaka R, Dobashi A, et al. Epstein-Barr virus-negative extranodal “true” natural killer-cell lymphoma harbouring a KDM6A mutation. Hematol Oncol. 2018;36:328–335. [DOI] [PubMed] [Google Scholar]
- [4].Au W-y, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. [DOI] [PubMed] [Google Scholar]
- [5].Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral t-cell lymphoma incidence and survival in the United States. Jco. 2016;34:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Suzuki R, Suzumiya J, Yamaguchi M, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010;21: 1032–1040. [DOI] [PubMed] [Google Scholar]
- [7].Jo J-C, Yoon DH, Kim S, et al. Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol. 2012;89:103–110. [DOI] [PubMed] [Google Scholar]
- [8].Kim SJ, Oh SY, Hong JY, et al. When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol. 2010;21:1058–1063. [DOI] [PubMed] [Google Scholar]
- [9].Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin’s lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. Jco. 1998;16:70–77. [DOI] [PubMed] [Google Scholar]
- [10].Gurion R, Mehta N, Migliacci JC, et al. Central nervous system involvement in T-cell lymphoma: A single center experience. Acta Oncol. 2016;55:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ellin F, Landstrom J, Jerkeman M, et al. Central nervous system relapse in peripheral T-cell lymphomas: a Swedish Lymphoma Registry study. Blood. 2015;126:36–41. [DOI] [PubMed] [Google Scholar]
- [12].Chihara D, et al. The risk of central nervous system (CNS) relapses in patients with peripheral T-cell lymphoma. PLoS One. 2018;14:e0191461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. [DOI] [PubMed] [Google Scholar]
- [14].Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. Jco. 2006;24:612–618. [DOI] [PubMed] [Google Scholar]
- [15].Lee J, Park YH, Kim WS, et al. Extranodal nasal type NK/T-cell lymphoma: elucidating clinical prognostic factors for risk-based stratification of therapy. Eur J Cancer. 2005;41:1402–1408. [DOI] [PubMed] [Google Scholar]
- [16].Cheung MMC, Chan JKC, Lau W-h, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002;54:182–190. [DOI] [PubMed] [Google Scholar]
- [17].Yi JH, Kim JH, Baek KK, et al. Elevated LDH and paranasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Ann Oncol. 2011;22:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pro B, Perini G. Central nervous system prophylaxis in peripheral T-cell lymphoma. Blood. 2010;115:5427. [DOI] [PubMed] [Google Scholar]
- [19].Kumar A, Vanderplas A, LaCasce AS, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118: 2944–2951. [DOI] [PubMed] [Google Scholar]
- [20].Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 - the Southwest Oncology Group. J Clin Oncol. 2009;27:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tomita N, Kodama F, Kanamori H, et al. Prophylactic intrathecal methotrexate and hydrocortisone reduces central nervous system recurrence and improves survival in aggressive non-hodgkin lymphoma. Cancer. 2002;95:576–580. [DOI] [PubMed] [Google Scholar]
- [22].Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol. 2012;159:39–49. [DOI] [PubMed] [Google Scholar]
- [23].Cheah CY, Herbert KE, O’Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer. 2014;111: 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim SJ, Oh SY, Kim JS, et al. Secondary central nervous system (CNS) involvement in patients with diffuse large B-cell lymphoma: a therapeutic dilemma. Ann Hematol. 2011;90:539–546. [DOI] [PubMed] [Google Scholar]
- [25].Bokstein F, Lossos A, Lossos IS, et al. Central nervous system relapse of systemic non-Hodgkin’s lymphoma: results of treatment based on high-dose methotrexate combination chemotherapy. Leuk Lymphoma. 2002; 43:587–593. [DOI] [PubMed] [Google Scholar]
- [26].El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer. 2018;93:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Taylor JW, Flanagan EP, O’Neill BP, et al. Primary leptomeningeal lymphoma: International Primary CNS Lymphoma Collaborative Group report. Neurology. 2013;81:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Welch MR, Sauter CS, Matasar MJ, et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56:361–367. [DOI] [PubMed] [Google Scholar]
- [29].Ferreri AJM, Donadoni G, Cabras MG, et al. High doses of antimetabolites followed by high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation in patients with systemic B-cell lymphoma and secondary CNS involvement: final results of a multicenter phase II trial. Jco. 2015;33:3903–3910. [DOI] [PubMed] [Google Scholar]
- [30].Brammer JE, Chihara D, Poon LM, et al. Management of advanced and relapsed/refractory extranodal natural killer T-cell lymphoma: an analysis of stem cell transplantation and chemotherapy outcomes. Clin Lymphoma Myeloma Leuk. 2018;18:e41–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee J, Au W-Y, Park MJ, et al. Autologous hematopoietic stem cell transplantation in extranodal natural killer/T cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant. 2008;141356–1364. [DOI] [PubMed] [Google Scholar]
- [32].Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kwong Y-L, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;1292437–2442. [DOI] [PubMed] [Google Scholar]
- [34].Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]


