Abstract
Cognitive neuroscience research relies, in part, on homologies between the brains of human and non-human primates. A quandary therefore arises when presumed anatomical homologues exhibit different functional properties. Such a situation has recently arisen in the case of the anterior cingulate cortex (ACC). In humans, numerous studies suggest a role for ACC in detecting conflicts in information processing. Studies of macaque monkey ACC, in contrast, have failed to find conflict-related responses. We consider several interpretations of this discrepancy, including differences in research methodology and cross-species differences in functional neuroanatomy. New directions for future research are outlined, emphasizing the importance of distinguishing illusory cross-species differences from the true evolutionary differences that make our species unique.
Introduction
Effective action often requires choices between competing alternatives. In many cases, such competition is highly asymmetric, and the decision is easy. However, in other cases, everyday behavior can give rise to conflict. An example of a task involving conflict monitoring is illustrated in Figure 1A. Extensive theoretical and computational modeling work has suggested that monitoring for conflicts—cases in which several mutually exclusive response options are simultaneously active—could signal the need for increased cognitive control [1, 2, 3]. According to this influential view, activity in a conflict monitoring system can trigger adjustments in cognitive control to resolve current conflicts and prevent future ones [4] (see Figure 1). Here we consider the neural basis of conflict monitoring, including several novel hypotheses that attempt to reconcile cross-species discrepancies from recent studies of conflict monitoring in monkeys and humans.
Figure 1 – Conflict monitoring in a simple decision task.
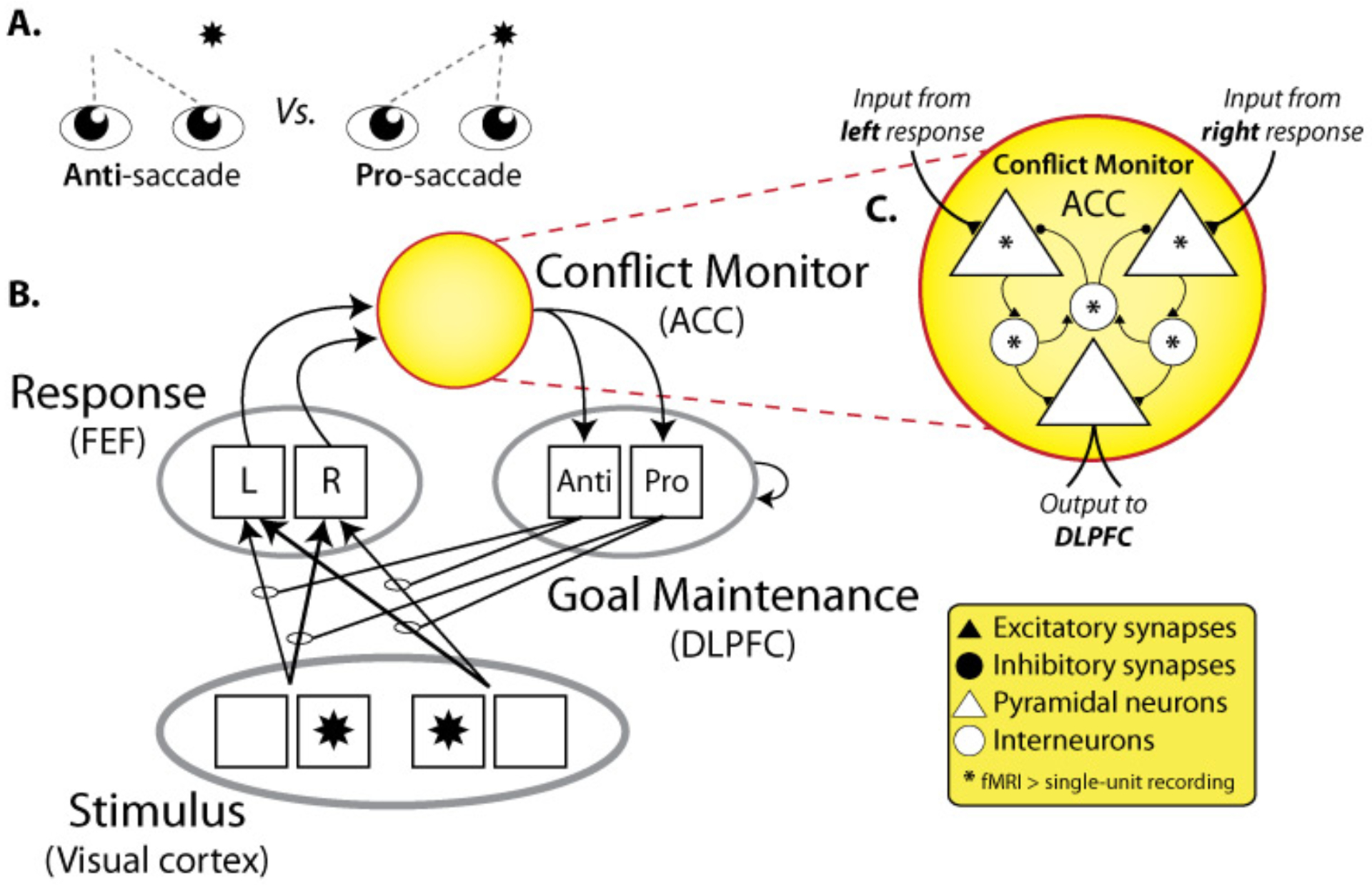
A) The anti-saccade task. The anti-saccade condition requires an eye-movement away from a presented target, while the pro-saccade condition requires an eye-movement toward the target. There is an innate tendency to look toward sudden-onset stimuli, creating more conflict for the anti-saccade condition. Note that a recent functional MRI (fMRI) study has reported possible conflict sensitivity in monkey ACC during an anti-saccade task [32]. B) A model illustration of the anti-saccade task based on previous models of conflict monitoring in other task contexts [9]. Anterior cingulate cortex (ACC) monitors for conflict between response units, and drives activity in dorso-lateral prefrontal cortex (DLPFC) according to the amount of conflict. DLPFC increases its bias on stimulus-response associations based on its maintained goal/task representation, causing either the left (L) or right (R) eye movement plan in the frontal eye fields (FEF) to win the competition, reducing conflict. This model has been applied to Stroop, Eriksen flanker, and other tasks [3]. Note that the ‘response’ module could be replaced by task-specific activity patterns to be monitored for conflict if variable binding was included in the conflict monitor, as may be the case for non-motor decision conflict monitoring in area 32′ (see text). C) A hypothetical model of ACC. Since fMRI is biased toward synaptic activity, it is likely to detect the inputs to ACC as well as the interactions between interneurons in the region (indicated by asterisks). In contrast, single unit activity (SUA) recording is biased toward pyramidal neuron output, and so may be at a disadvantage for detecting conflict-related activity in ACC. Note that most neural computation takes place in dendrites via synaptic activity [68], suggesting that fMRI may be generally more sensitive to a wider range of neural processing. This diagram incorporates established assumptions regarding pyramidal communication between brain regions, lateral inhibition between pyramidal neurons, and populations of excitatory (e.g., spiny stellate) and inhibitory (e.g., basket) interneurons mediating pyramidal activity.
It has been suggested that human dorsal-caudal anterior cingulate cortex (ACC; also referred to as anterior mid-cingulate cortex [5]; Figure 2A) acts as a conflict monitor [2, 3, 6] (for alternative views of the region’s function see [7, 8]). Converging support for this hypothesis comes from functional MRI (fMRI) [9, 10], event-related potentials (ERP) [6], local field potentials (LFP) [11], single-unit activity (SUA) [12, 13], and lesion studies [14, 15] in humans. However, several recent studies have tested for conflict sensitivity in macaque monkey ACC using SUA recordings [16, 17], LFP recordings [18], and lesions [19], but have revealed negative results. The conclusion often drawn from the animal research is that results from human studies have been misinterpreted [17, 20]. However, if one looks carefully at the accumulated data, one finds not disconfirmation but frank discrepancy: The data from monkeys appear to be simply incommensurable with the human data.
Figure 2 – Anatomy of Anterior Cingulate Cortex.
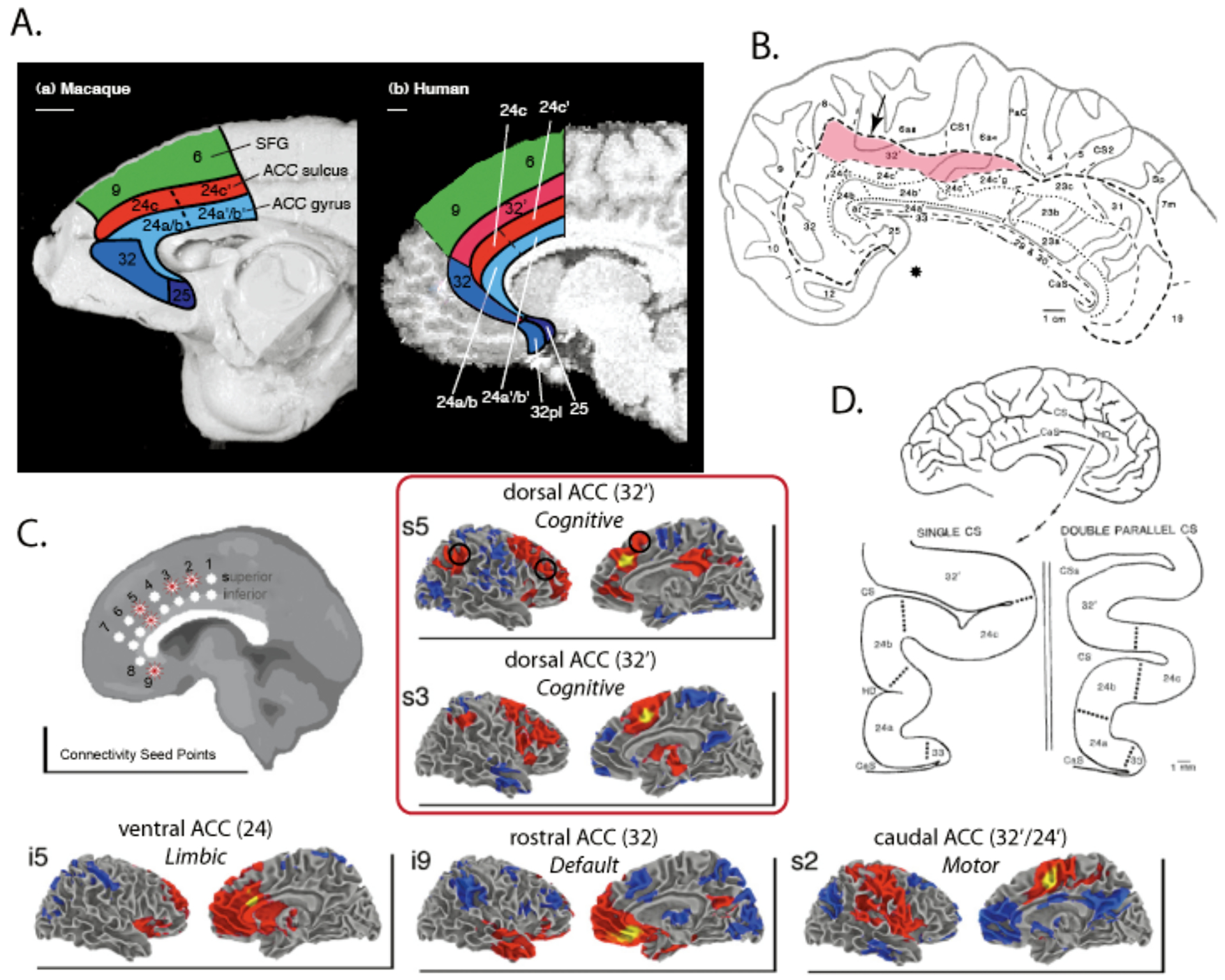
A) Macaque (left) and human (right) primate brains are illustrated with corresponding anatomical areas illustrated. Importantly, a dorsal-caudal extension of area 32, area 32′, is present only in the human brain. Figure adapted from [20]. B) A flatmap illustration of human anterior cingulate, illustrating the location and extent of area 32′. The arrow indicates that area 32′ often extends onto the dorsal medial wall surface. Figure adapted from [42]. C) Resting-state functional connectivity MRI maps of seed points along human ACC. Area 32′ extends from superior point 3 (s3) to s6, and shows connectivity with cognitive control regions posterior parietal cortex, DLPFC, and possibly nearby medial frontal regions. This region is separated connectively from caudal ACC in posterior area 32′/24′ (which appears to be a motor area), ventral ACC in area 24 (which appears to be a limbic area), and rostral ACC in area 32 (which is part of the “default state” network; [46]). Figure adapted from [45]. D) The anatomy of ACC is extremely variable between individuals. 30–50% of humans have a double cingulate in at least one hemisphere [24, 36]. Figure from [42].
A series of examples reflect this point. For instance, Ito et al. found no conflict-related activity within monkey ACC using a saccade countermanding task (in which eye movement plans must be withheld just before execution), while Curtis et al. [21] found conflict-related activity in single human subjects within ACC with the same task. Emeric et al. [18] saw a lack of conflict-related LFP in monkey ACC, while such activity has been found in human ACC with ERP [6] and LFP [11]. Mansouri et al. [19] found no effect of monkey ACC lesions on behavioral reactions to conflict, while human ACC lesions are associated with changes in such reactions [14, 15]. Ito et al. [16] and Nakamura et al. [17] found no conflict-related SUA in monkey ACC, while Davis et al. [12, 13] did find such SUA in human ACC.
What might explain these discrepancies? In what follows, we summarize what we see as the most plausible accounts available. For clarity, we organize these into two major categories. The first involves explanations relating to differences in the methods used to study monkeys and humans. The second looks to the perhaps neglected possibility that fundamental differences may exist between humans and monkeys at the level of functional neuroanatomy.
Differences in Methodology
The vast majority of research on human ACC has involved the use of fMRI or ERP, techniques with poor spatial resolution relative to SUA recordings, the dominant technique in monkey research. This has led some to suggest that human research has simply mislocalized conflict-related activity [20, 22]. More specifically, SUA studies in monkeys have detected apparent conflict-related activity in the pre-supplementary motor area (pre-SMA) and supplementary eye fields (SEF), raising the possibility that such activity actually occurs there, rather than ACC, in humans as well [17, 23].
Given the wide variability in localization apparent in fMRI studies of conflict monitoring [8], partially due to variability in the underlying neuroanatomy [24] (see Figure 2D), the case for a mislocalization to ACC initially seems plausible. However, other data appears to undermine that case. First, even if conflict sensitivity has been mislocalized to ACC in humans, other findings suggest that neurons in monkey pre-SMA and SEF do not actually monitor conflict, but may instead simply show modulations in movement-related representations in conditions of conflict [17, 23]. Second, in contrast to studies with monkeys, several human studies have found conflict-related activity concurrently in both pre-SMA/SEF and ACC [7, 10, 25]. Indeed, Curtis et al. [21] observed engagement of ACC (and SEF) during performance of a saccade countermanding task that had been specifically found not to engage ACC in monkeys. The larger-scale pattern of findings from human research is indicated in Figure 3A–C, which summarizes a large meta-analysis of studies involving the Stroop task [26]. The Stroop effect, the best characterized and most replicated conflict effect [27], involves naming colors of color words while withholding the automatic tendency to read those words (e.g., responding “red” to ‘BLUE’ in a red font). As the figure indicates, the most likely locus of activity across studies of the Stroop effect lies within ACC.
Figure 3 – Functional Activity During the Stroop Task.
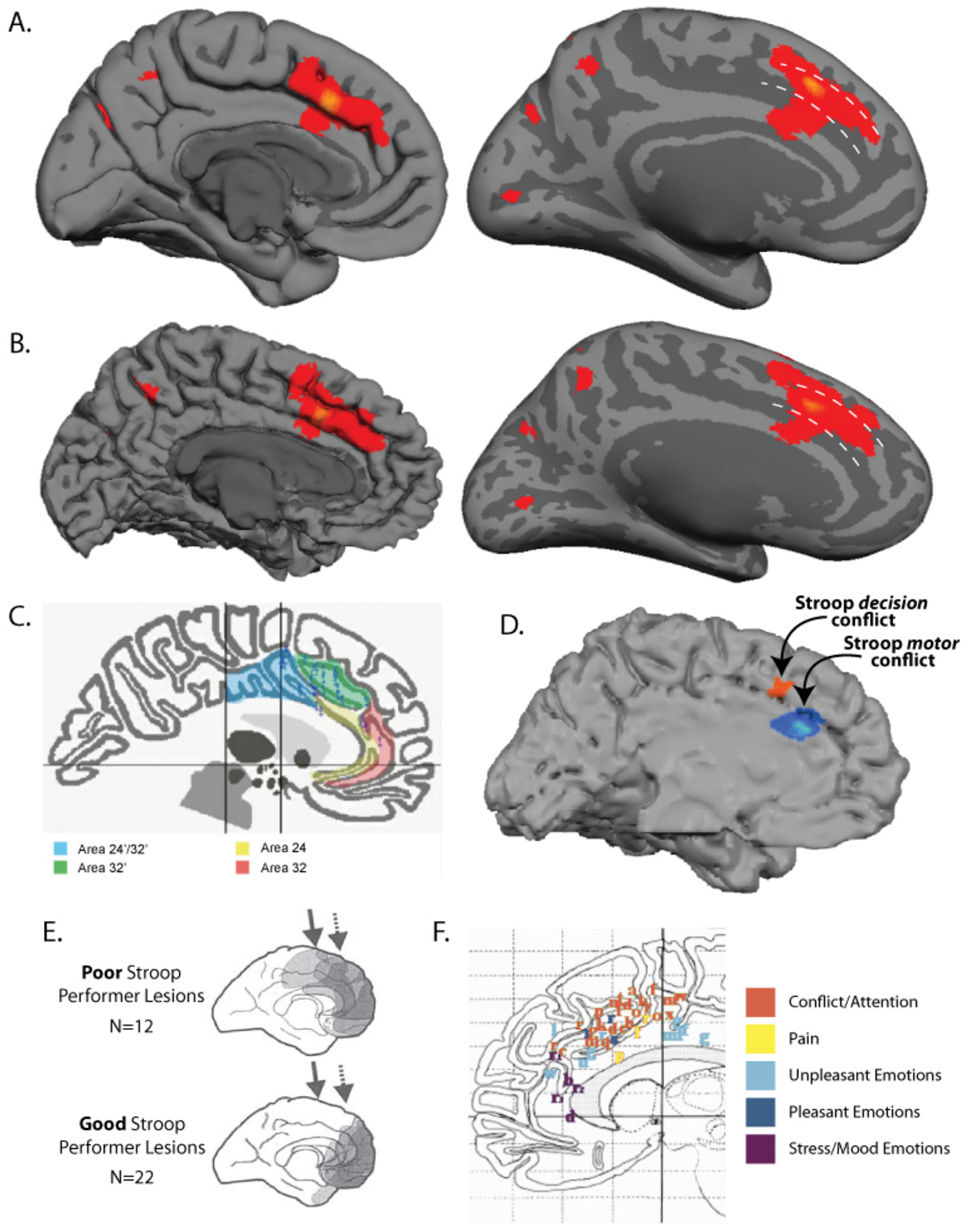
Activation likelihood estimation (ALE) meta-analytical results from Laird et al., 2005 [26] encompassing 19 fMRI and PET studies (27 experiments) involving approximately 250 subjects (A-C). A) The focus of the ALE probability map from Laird et al. 2005 in ACC is shown on the MNI152 template brain (average T1 brain image from 152 normal subjects at the Montreal Neurological Institute, Montreal, QC, Canada). Note that the spatial extent of the map is uncertain due to spatial smoothing (here 10mm FWHM). The MNI152 template brain is likely an averaged mix of mostly single and some double cingulate sulci. The pial surface is on the left and the inflated white-matter surface is on the right. The main focus of activity is on the dorsal bank of the cingulate sulcus and just above it (likely area 32′), extending down into the sulcus (area 32′ and 24/24′) and up onto the medial wall surface (area 32′ and 6). The dashed white lines indicate the approximate dorsal and ventral borders of area 32′, based on humans having a 60%/40% mix of single and double cingulates [36] and a systematic shifting of area 32′ in these cases (see Figure 2D). Note that some atlases fail to illustrate that area 32′ can extend onto the medial wall surface (see Figure 2B and 2D). Also note that further research is necessary to verify using histology and/or connectivity that the location of functional activity is in area 32′. B) Medial wall ALE statistical map on an individual with a double cingulate. Typical neuroimaging results spatially smooth data (by ~10mm) and average activations across subjects, making localization problematic. Vogt et al. 1995 [42] showed that area 32′ is centered on the gyrus between the two cingulate sulci in this case (see Figure 2D). 30–50% of humans have a double cingulate in at least one hemisphere [24, 36]. C) Foci of activation across experiments, used to create the above surface maps, are shown on a double-cingulate Talairach template image. Figure adapted from [26]. D) Stroop activation dissociating what is likely area 32′ from area 24/24′ by non-motor decision and motor conflict, respectively.Note that studies of motor conflict may show area 32′ activity, since many situations with motor conflict will have corresponding decision conflict as well. Figure adapted from [53]. E) Lesion locations associated with poor (top) and good (bottom) Stroop incongruent trial performance [14]. Only lesions including caudal ACC (indicated by the solid arrow; rostral ACC is indicated by the dashed arrow) were associated with poor Stroop performance. Figure adapted from [14]. F) Reported peak activations from a meta-analysis looking at ACC locations of PET and fMRI activations during emotional and cognitive tasks [38]. Peaks tend to be in area 24, rostral area 32, and area 24′ for emotional tasks, and area 32′ for cognitive tasks. The borders between ACC regions are known to vary tremendously between subjects, making any minor functional overlaps in these between-subject maps inconclusive. Figure adapted from [38].
Further evidence against mislocalization is provided by human SUA and LFP studies, which have revealed conflict-related responses within ACC [11, 12, 13], in addition to replicating findings from monkey ACC [28]. It is possible that these human SUA results are unreliable because time constraints during surgery limited the number of conflict-sensitive cells that could be found. Conversely, the fact that any conflict-related activity was discovered given these constraints might indicate the robustness of conflict activity in the human ACC. Furthermore, although not all neuropsychological findings are consistent [29], lesions of ACC have been shown in several studies to cause deficits in cognitive control, including disruptions of conflict-related behavioral adjustment [14, 15] (Figure 3E). Taken together, the evidence suggests that conflict-monitoring functions in humans can be reliably localized to ACC.
It appears, therefore, that the limited spatial resolution of human neuroimaging is unlikely to be the cause of discrepancies between the species. It remains possible, however, that methodological features typical of research with monkeys may be the cause. Studies with monkeys, as opposed to humans, have typically recorded SUA, used eye-movements as the response modality, and used highly over-trained subjects. We consider implications of each of these features in turn.
The inherent bias toward recording large pyramidal neurons, over other kinds of neurons, limits SUA recording’s sensitivity to neural processes that do not substantially alter spiking of pyramidal neurons [30]. In contrast, the blood oxygen level dependent (BOLD) response, as measured with fMRI, is likely sensitive to a larger variety of neural processing types, including inhibitory competition or local signal accumulation among networks of small interneurons, given that BOLD likely reflects metabolic demand associated with synaptic activity [31]. Importantly, conflict monitoring may rely on local competition among interneurons [3] (Figure 1C). If so, then methods such as fMRI that are sensitive to such activity should be especially effective for detecting conflict responses in ACC. In other words, ACC coding of conflict may be present in both humans and monkeys, but may be easier to detect in humans because of the sensitivity of fMRI to a wider variety of neural processes than other methods. Consistent with this explanation, a recent fMRI study in monkeys found activity in the ACC [32] that may be due to conflict monitoring (but could alternatively be due to error-processing [7]; see discussion below).
Another limitation of SUA recording is its limited field of view [30], which may have reduced the chance of discovering conflict-related neural responses that are present within monkey ACC but only in a circumscribed—and, to date, unsampled—area. The appeal of this explanation is limited by the fact that complementary methods (lesions, LFP) have been used in monkey ACC, which partially compensate for the limitations of recording SUA. For instance, Mansouri et al. [19] lesioned the entire monkey anterior cingulate sulcus yet saw no change in conflict-induced behavioral adjustment, while Emeric et al. [18] recorded LFPs from monkey ACC and found neural responses to errors and feedback, but not conflict. Therefore, although it remains possible that further investigation will reveal a portion of monkey ACC that responds to conflict, this outcome appears unlikely. Note, however, that possible conflict sensitivity was found with fMRI [32] primarily in the monkey cingulate gyrus, whereas the above-mentioned lesion and LFP studies focused on the cingulate sulcus (its primary location in humans), leaving open the possibility that even these studies missed the true location of conflict-sensitivity in monkey ACC.
Could the discrepancies reflect differences between the tasks typically used for the different species? Monkey studies often use saccadic eye movements, whereas human studies of conflict tend to use button presses. Motor control of the eyes and hands are radically different in several respects, including their degrees of freedom (effectively just two for the eyes, many more for arms and hands), their relative need to take account of gravity and physical obstacles and, neuroanatomically, whether or not the respective cortical systems project directly to motor neurons. It is therefore plausible that the oculomotor and skeletomotor systems might express conflict in very different ways. However, several human fMRI studies employing saccadic responses have reported clear conflict-related ACC activity [21, 33, 34], as has the recent monkey fMRI study mentioned above [32]. A meta-analysis comparing verbal and manual versions of the Stroop task reported some differences in the likelihood of activation, but also significant overlap in ACC [26]. These studies suggest that effector differences are unlikely to account for the cross-species discrepancies.
Still another methods-based explanation for the discrepancies between human and monkey research relates to training. Human studies typically investigate cognitive task performance after only minimal practice, while monkeys are usually studied after months of task-specific training. Such extended training may give rise to differences in task representation or performance monitoring, explaining differences in ACC activity (which in humans has been found to decrease following extended training [35]). One challenge for this explanation is that, despite differences in training duration, human and monkey ACC have been found to display similar responses along other dimensions, including responses to errors and action outcomes [28, 36]. Nevertheless, a role for training duration in driving the inter-species discrepancies cannot be ruled out on the basis of currently available data.
To conclude this review of methods-based explanations for cross-species discrepancies, we suggest that the discrepancies are unlikely to be due to the distinct spatial sampling limitations of fMRI and SUA recording, or to differences in motor responses often used across species. More plausible, and perhaps more intriguing, is the possibility that these discrepancies provide insight into the specific neural processes implementing conflict monitoring. Specifically, conflict monitoring may occur primarily in populations of ACC interneurons (Figure 1C), which may be more detectable with fMRI (typically used for human studies) than with SUA recording (typically used for monkey studies). Further research is necessary to decisively test this hypothesis.
Differences in Functional Neuroanatomy
The explanations considered so far implicitly accept the default assumption that monkey ACC is functionally equivalent to human ACC. However, this assumption may be incorrect. Indeed, a close look at current evidence lends credence to the hypothesis that the conflict-sensitive portion of human ACC has no direct homologue in monkeys.
It is tempting to reject this idea out of hand, given that several parallels have been found between human and monkey ACC, including responses to errors [37], pain [38], and changes in motivation [39]. However, conflict effects in humans are at least partially dissociable from these other effects. In particular, there are subregions of human anterior cingulate (pregenual areas 24 and 32) that—like monkey ACC—show error, pain and reward/punishment sensitivity, but not conflict responses [5, 37, 40, 41] (see Figure 3F).
The case for species differences becomes more compelling when one looks closely at the location of conflict-related responses in humans. As detailed in Figures 2 and 3, the focus of conflict-related activity across studies likely lies within the region labeled area 32′ [42]. This anatomical area has been delineated in carefully executed cytoarchitectonic studies of human cingulate cortex by Vogt et al. [42], who distinguish this area from neighboring areas 32, 6, 24, and 24′. These cytoarchitectonic distinctions are mirrored by corresponding regional differences in neurotransmitter receptor architecture and anatomical connectivity [5, 43]. Importantly, Vogt and colleagues describe area 32′ as a distinctive feature of human ACC, commenting that only the human cingulate contains a region of “cingulofrontal transition cortex” where area 32′ forms a dorsal border for areas 24 and 24′ [42] (Figure 2A and 2B). For clarity, note that Vogt and colleagues [5, 44] consider area 32′ to fall within ‘anterior midcingulate’, reserving the term ‘anterior cingulate’ for more rostral portions of the cingulate.
In addition to cytoarchitectonic differences, area 32′ also appears to differ from neighboring areas in terms of both connectivity (see Figure 2C) [45] and function (see Figure 3F) [38, 41]. In particular, area 32′ is connected to cortical regions implicated in executive function, including dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC) [45]. In contrast, area 24 interacts with limbic regions (e.g., insula, midbrain; see Figure 2C) and is implicated in emotional tasks [38, 45]. Meanwhile, area 32 is connected to ‘default mode’ network regions and is co-active with them during rest [46, 47] and is also involved in emotional tasks [38]. Lesions of area 32 do not affect Stroop task performance, whereas lesions in the vicinity of area 32′ do [14] (see Figure 2E). Finally, area 24′, unlike area 32′, is connected to M1 [45], is active during motor tasks [48], and is associated with processing of pain [5] and emotion [38].
Overall, area 32′ appears to share a closer functional relationship with pre-SMA (area 6) [8, 49] than with subregions of ACC proper. However, even here it is possible to find dissociations in function. In particular, unlike area 32′, pre-SMA is associated with response selection in the absence of conflict [50]. Meanwhile, unlike pre-SMA, area 32′ responds to shifts in motivation [28, 37, 39], although both regions show error-related responses [37] (possibly due to limbic or executive functions).
Localization of conflict activity to area 32′ (see Figure 3) remains somewhat tentative since it is based on surface anatomy that varies markedly across individuals (but see [51]). Nonetheless, as Figure 2D illustrates, there are systematic relationships between surface anatomy and the location of area 32′. Specifically, area 32′ tends to lie on the upper bank of the cingulate sulcus when a single cingulate sulcus occurs, but on the gyral surface when a paracingulate sulcus is present [42]. Figure 3A illustrates activity consistent across studies on anatomy averaged across 152 individuals, reflecting a mixture of mostly single and some double cingulates (only 30–50% of individuals have a double cingulate [36]). Figure 3B illustrates these statistics on a single individual’s anatomy with a double cingulate. In both cases the locations of activity across Stroop studies are consistent with area 32′.
The centering of conflict-related activity on human area 32′—an anatomically and functionally distinct region for which there is no known monkey homologue—gives considerable impetus to the idea that discrepancies between human and monkey research may reflect species differences rather than methodological differences. This idea suggests that the additional region in humans may provide additional behavioral functions, possibly increasing cognitive flexibility in humans relative to monkeys. If one function of this uniquely human region is conflict monitoring [52], then monkeys should lack behavior that reflects the impact of this monitoring function, such as conflict-induced adjustments in controlled behavior [3, 4].
Box 1 considers a challenge to this idea that emerges from a recent study by Mansouri and colleagues [19], which appears to show DLPFC-mediated behavioral adjustments to conflict in monkeys. We suggest that the data from this study are open to an alternative interpretation. However, it is also possible that ventral area 24′, not investigated by Mansouri et al., monitors for motor conflict in monkeys and humans, while area 32′ provides monitoring of more general decision conflict in humans. Motivation for this distinction comes from a recent human fMRI study using the Stroop task, in which response-level conflict engaged area 24′, while conflict at the level of color identification (putatively absent motor conflict) engaged area 32′ [53] (see Figure 3D). This suggests the possibility that both monkeys and humans monitor for motor conflict (area 24′), while only humans monitor for non-motor decision conflict (area 32′).
Box 1 -. Do monkeys perform conflict monitoring?
An important conclusion from human research is that detection of response conflict by ACC triggers compensatory adjustments in cognitive control [3, 9]. In line with this idea, Kerns et al. [4] found that ACC activation was higher in incongruent (conflict inducing) trials in the Stroop task, and that trials associated with particularly high ACC activation tended to be followed by shifts toward more focused or controlled Stroop performance.
In a recent study, Mansouri and colleagues [19] claim to have found a similar dynamic in monkeys, but without a role for ACC. Their animals performed a matching task in which an initial cue stimulus, defined by a particular shape and color, was soon flanked by three probe objects. The monkeys’ task was to identify a target object that matched the cue on the relevant dimension (either color or shape). On high conflict (H) trials, distractor objects matched the cue on the irrelevant dimension. On low conflict (L) trials, the distractors did not match the cue on either dimension. The key findings were: (1) faster responses on H trials following other H trials than on H trials following L trials, (2) disappearance of this effect following DLPFC but not ACC lesions, (3) differential responses in DLPFC neurons during H versus L trials.
Mansouri et al. [19] interpreted the last of these finding as indicating that DLPFC neurons were coding for the degree of conflict involved in each trial-type. However, there is another possible interpretation. Note that L trials permitted the animal to use a strategy unavailable on H trials. Specifically, on L trials, target selection could rely on visual grouping effects to draw attention toward the global match. It is therefore possible that the DLPFC neurons studied were coding not for conflict, but for the strategy used on H versus L trials (i.e., feature-based search versus grouping-based search). This interpretation might be more parsimonious than the one offered by Mansouri and colleagues, given that DLPFC neurons coding for task rules have been extensively reported [61].
The hypothesis that animals in this study used different strategies on H and L trials would also explain the trial-type sequence effects observed, since transitioning from an L to an H trial would effectively involve a task switch, with attendant performance costs and sensitivity to DLPFC lesions [62]. This interpretation involves no role for conflict monitoring, suggesting that the reported results do not provide unambiguous evidence for conflict monitoring in monkeys. Absent such evidence, the implications of a failure of ACC lesions to affect trial-type sequence effects are unclear. However, it should be noted that other studies beyond the scope of the present review have reported conflict adjustments in monkeys [17, 63] (but see [64]). In any case, we suggest that the question of whether macaque monkeys show conflict sensitivity in ACC, analogous to observations seen in humans, remains open.
Toward a resolution
The relationship between monkey and human ACC clearly needs elucidation. Monkey fMRI has proven useful in clarifying cross-species differences in functional neuroanatomy of other regions, such as the intraparietal sulcus [54]. Here this technique could contribute its large field-of-view and sensitivity to a large variety of neural processes to survey medial frontal cortex, potentially revealing conflict-related activity that was missed by previous neurophysiological studies.
The promise of this approach is suggested by a recent study reporting evidence of conflict-related activity in monkey ACC [32]. However, differences in the frequency of errors between conditions, as well as a possible selection bias toward high-effort anti-saccade trials due to removal of blocks with many errors, may have confounded the results. Further, no connection was made to previous monkey ACC findings using reward, punishment, pain, or explicit error manipulations. Such manipulations would test for dissociations between regions previously identified with SUA recording and the (hypothesized) new region found with fMRI, reconciling this new finding with previous findings in monkey medial frontal cortex.
Upon identifying a region showing conflict-related activity, an important next step would be to record from single neurons and local neural populations within the region. Such a coordinated use of fMRI and SUA recording has proven useful in identifying monkey homologues for human brain structures such as the fusiform face area [55]. SUA within a conflict-sensitive area identified with fMRI could indicate whether the involved neurons respond specifically to conflict, or in a response-specific way, as observed in monkey SEF [17]. It is also possible that little conflict sensitivity will be found in the spiking output of ACC, but that this sensitivity will be clearly evident in local interactive networks of interneurons (see Figure 1C), the activity of which is reflected in LFPs or recordings from multi-unit arrays.
If clear conflict-related activity were identified within monkey ACC, it would also be desirable to determine if monkey ACC is tied to subsequent shifts in behavioral performance, as is the case with human ACC [4, 9]. If so, lesions to the region should disrupt these sequential adjustment effects.
As discussed above, it remains plausible that there is no monkey equivalent of the human conflict monitoring system. Thus, it is foreseeable that the approach we propose will yield null results. However, even with null results, it would be difficult to claim that there is conflict sensitivity in monkey ACC if other functions (motivation, emotion, error, pain, motor processes, etc.) are mapped onto all parts of ACC, and no conflict sensitivity is found using a wide field-of-view method (e.g., fMRI) with extensive statistical power. Thus, we see future experiments using functional neuroimaging in primates as key to resolving the inter-species discrepancies discussed here, regardless of the outcome of those experiments.
Conclusion
The discrepancies between monkey and human ACC research present a riddle. As we have discussed, the answer to this riddle may turn out to be quite mundane. It may be that researchers studying monkeys using SUA recording have not yet hit upon the appropriate region of cingulate cortex, or that differences in training regimes explain the difference in findings. However, we have also considered more intriguing possibilities: that conflict monitoring involves neural processes that are likely to be detected by fMRI but missed by SUA recording in monkeys, or that there exist crucial differences in species-specific functional neuroanatomy.
The literature suggests that there might be both methodological and functional neuroanatomical differences. It may be that fMRI better detects conflict monitoring processes in both species’ ACC (see Figure 1C; also [32]) and that humans have an additional region in ACC for monitoring conflict (see Figure 2). These views are reconciled by the possibility that humans have two conflict monitoring regions in ACC, both of which are involved in tasks like the Stroop task in which both motor-level and decision-level conflict are present [53]. Figure 3D illustrates evidence that area 24′ (common to both monkeys and humans) is sensitive to motor conflict, while area 32′ (unique to humans) is sensitive to non-motor decision conflict. Such sensitivity to non-motor decision conflict in human ACC has been demonstrated by several recent studies [49, 52]. One might speculate that area 32′ evolved from nearby areas 32/24/24′, expanding from motor conflict monitoring to much more flexible and generalized decision conflict monitoring in humans. Non-motor decision conflict may allow for conflict monitoring of decisions not tied to specific motor outputs (such as conflict between conceptual or linguistically encoded decision outcomes), which would provide conflict-driven regulation of cognitive control during a wide variety of difficult decisions (see Figure 1B and [56, 57]). Of course, further research is necessary to fully confirm this hypothesis.
The primary impetus for studying ACC function in monkeys has been to use the species as a model for the human case, based on the assumption of functional and anatomical homology. Thus, if species differences turn out to explain the contradictions between monkey and human results, this would belie a fundamental presumption of monkey cingulate research. On the other hand, such species differences would never come to light without parallel comparative investigations of humans and monkeys, removing the opportunity to identify cerebral and cognitive functions that may be unique to each species. These differences, in turn, can provide insight into the nature of human brain evolution [58, 59, 60]. Thus, resolving the current discrepancies between human and monkey findings on the ACC presents an important challenge, and addressing this challenge may provide new insight into the cognitive abilities that make our species unique.
Box 2 – Questions for future research.
What is the functional role of human area 32′? Is this role, like the anatomy of the region, evolutionarily distinct?
What are the specific functions and inter-subject anatomical variability of nearby ACC and medial frontal regions in humans?
What neural and metabolic processes take place in ACC during conflict, and how might these processes lead to greater sensitivity to detection by fMRI?
To what extent does conflict-related activity in ACC reflect synaptic activity at pyramidal neurons versus interneurons?
Is monkey area 32 functionally equivalent to human area 32, human area 32′, or some other region? (Note that Brodmann himself did not consider the monkey area he labeled 32 as homologous to his human area 32 [65]).
How might new, more objective and in vivo methods for identifying anatomical areas [66, 67] shed light on the neuroanatomical differences that make our species unique?
To what extent should the macaque monkey model, which is known to differ from humans both behaviorally and by 25 million years of separate evolution [58], be relied on to make inferences about the human brain?
References:
- 1.vanVeen V and Carter C (2006).“Conflict and cognitive control in the brain.” Current Directions in Psychological Science 15(5): 237–240. [Google Scholar]
- 2.Botvinick M (2007).“Conflict monitoring and decision making: Reconciling two perspectives on anterior cingulate function.” Cognitive. [DOI] [PubMed] [Google Scholar]
- 3.Botvinick MM, Braver TS, Barch DM, Carter CS and Cohen JD (2001). “Conflict monitoring and cognitive control.” Psychological Review 108(3): 624–652. [DOI] [PubMed] [Google Scholar]
- 4.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA and Carter CS (2004). “Anterior cingulate conflict monitoring and adjustments in control. ” Science 303(5660): 1023–6. [DOI] [PubMed] [Google Scholar]
- 5.Vogt BA, Bergerand GR Derbyshire SW (2003). “Structural and functional dichotomy of human midcingulate cortex.” Eur J Neurosci 18(11): 3134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung N, Cohen JD and Botvinick M (2004). “The neural basis of error detection: conflict monitoring and the error-related negativity.” Psychol Rev 111(4): 931–59. [DOI] [PubMed] [Google Scholar]
- 7.Brown JS and Braver TS (2005). “Learned predictions of error likelihood in the anterior cingulate cortex.” Science 307(5712): 1118–1121. [DOI] [PubMed] [Google Scholar]
- 8.Ridderinkhof KR, Ullsperger M, Crone EA and Nieuwenhuis S (2004). “The role of the medial frontal cortex in cognitive control.” Science 306(5695): 443–7. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick M, Cohen JD and Carter CS (2004). “Conflict monitoring and anterior cingulate cortex: an update.” Trends Cogn Sci 8(12): 539–46. [DOI] [PubMed] [Google Scholar]
- 10.Cole MW and Schneider W (2007). “The cognitive control network: Integrated cortical regions with dissociable functions.” Neuroimage 37(1): 343–60. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Ulbert I, Schomer DL, Marinkovic K and Halgren E (2005).“Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting.” J Neurosci 25(3): 604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis KD, Hutchison WD, Lozano AM, Tasker RR and Dostrovsky JO (2000). “Human anterior cingulate cortex neurons modulated by attention-demanding tasks.” Journal of Neurophysiology 83(6): 3575–7. [DOI] [PubMed] [Google Scholar]
- 13.Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO and Lozano AM (2005). “Human anterior cingulate cortex neurons encode cognitive and emotional demands.” J Neurosci 25(37): 8402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuss DT, Floden D, Alexander MP, Levine B and Katz D (2001).“Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location.” Neuropsychologia 39(8): 771–86. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA and Rauch SL (2001). “Deficits in visual cognition and attention following bilateral anterior cingulotomy.” Neuropsychologia 39(3): 219–30. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Stuphorn V, Brown JW and Schall JD (2003). “Performance monitoring by the anterior cingulate cortex during saccade countermanding.” Science 302(5642): 120–2. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Roesch MR and Olson CR (2005). “Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict.” J Neurophysiol 93(2): 884–908. [DOI] [PubMed] [Google Scholar]
- 18.Emeric EE, Brown JW, Leslie M, Pouget P, Stuphorn V and Schall JD(2008). “Performance monitoring local field potentials in the medial frontal cortex of primates: anterior cingulate cortex.” Journal of Neurophysiology 99(2): 759–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansouri FA, Buckley MJ and Tanaka K (2007). “Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment.” Science 318(5852): 987–90. [DOI] [PubMed] [Google Scholar]
- 20.Rushworth MF, Walton ME, Kennerley SW and Bannerman DM (2004). “Action sets and decisions in the medial frontal cortex.” Trends Cogn Sci 8(9): 410–7. [DOI] [PubMed] [Google Scholar]
- 21.Curtis CE, Cole MW, Rao VY and D’Esposito M (2005). “Canceling planned action: an FMRI study of countermanding saccades.” Cereb Cortex 15(9): 1281–9. [DOI] [PubMed] [Google Scholar]
- 22.Schall JD and Boucher L (2007). “Executive control of gaze by the frontal lobes.” Cognitive, Affective & Behavioral Neuroscience 7(4): 396–412. [DOI] [PubMed] [Google Scholar]
- 23.Isoda M and Hikosaka O (2007). “Switching from automatic to controlled action by monkey medial frontal cortex.” Nature Neuroscience. [DOI] [PubMed] [Google Scholar]
- 24.Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R and Evans AC (1996). “Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map.” Cereb Cortex 6(2): 207–14. [DOI] [PubMed] [Google Scholar]
- 25.Petit L, Courtney SM, Ungerleider LG and Haxby JV(1998).“Sustained activity in the medial wall during working memory delays.” J Neurosci 18(22): 9429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laird A, Mcmillan K, Lancaster J, Kochunov P, Turkeltaub P, Pardo J and Fox P (2005). “A comparison of label-based review and ALE meta-analysis in the Stroop task.” Human brain mapping 25(1): 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLeod CM (1991). “Half a century of research on the Stroop effect: an integrative review.” Psychol Bull 109(2): 163–203. [DOI] [PubMed] [Google Scholar]
- 28.Williams Z, Bush G, Rauch S, Cosgrove G and Eskandar E (2004). “Human anterior cingulate neurons and the integration of monetary reward with motor responses.” Nature Neuroscience 7(12): 1370–1375. [DOI] [PubMed] [Google Scholar]
- 29.Fellows LK and Farah MJ (2005). “Is anterior cingulate cortex necessary for cognitive control?” Brain 128(Pt 4): 788–96. [DOI] [PubMed] [Google Scholar]
- 30.Olshausen BA and Field DJ (2004). “What is the other 85% of V1 doing.” Problems in Systems Neuroscience. [Google Scholar]
- 31.Logothetis NK and Wandell B (2004). “Interpreting the BOLD signal.” Annu. Rev. Physiol 66: 735–69. [DOI] [PubMed] [Google Scholar]
- 32.Ford KA, Gati JS, Menon RS and Everling S (2009). “BOLD fMRI activation for anti-saccades in nonhuman primates.” Neuroimage 45(2): 470–476. [DOI] [PubMed] [Google Scholar]
- 33.Brown MR, Vilis T and Everling S (2008). “Isolation of saccade inhibition processes: rapid event-related fMRI of saccades and nogo trials.” Neuroimage 39(2): 793–804. [DOI] [PubMed] [Google Scholar]
- 34.Curtis CE and D’Esposito M (2003). “Success and failure suppressing reflexive behavior.” Journal of Cognitive Neuroscience 15(3): 409–18. [DOI] [PubMed] [Google Scholar]
- 35.Chein JM and Schneider W (2005). “Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning.” Brain Res Cogn Brain Res 25(3): 607–23. [DOI] [PubMed] [Google Scholar]
- 36.Paus T (2001). “Primate anterior cingulate cortex: where motor control, drive and cognition interface.” Nat. Rev. Neurosci 2(6): 417–24. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA and Gehring WJ (2006). “Medial frontal cortex activity and loss-related responses to errors.” J Neurosci 26(15): 4063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyron R, Laurent B and García-Larrea L (2000). “Functional imaging of brain responses to pain. A review and meta-analysis (2000).” Neurophysiologie clinique = Clinical neurophysiology 30(5): 263–88. [DOI] [PubMed] [Google Scholar]
- 39.Knutson B, Taylor J, Kaufman M, Peterson R and Glover G (2005). “Distributed neural representation of expected value.” J Neurosci 25(19): 4806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garavan H, Ross TJ, Kaufman J and Stein EA (2003). “A midline dissociation between error-processing and response-conflict monitoring.” Neuroimage 20(2): 1132–9. [DOI] [PubMed] [Google Scholar]
- 41.Bush G, Luu P and Posner MI (2000). “Cognitive and emotional influences in anterior cingulate cortex.” Trends Cogn Sci 4(6): 215–222. [DOI] [PubMed] [Google Scholar]
- 42.Vogt BA, Nimchinsky EA, Vogt LJ and Hof PR (1995). “Human cingulate cortex: surface features, flat maps, and cytoarchitecture.” The Journal of Comparative Neurology 359(3): 490–506. [DOI] [PubMed] [Google Scholar]
- 43.Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS and Zilles K (2008). “Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model.” Hum. Brain Mapp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palomero-Gallagher N, Mohlberg H, Zilles K and Vogt B (2008). “Cytology and receptor architecture of human anterior cingulate cortex.” The Journal of Comparative Neurology 508(6): 906–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX and Milham MP (2007). “Mapping the functional connectivity of anterior cingulate cortex.” Neuroimage 37(2): 579–88. [DOI] [PubMed] [Google Scholar]
- 46.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA and Shulman GL (2001). “A default mode of brain function.” Proc Natl Acad Sci USA 98(2): 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent JL, Patel GH, Fox MD, Snyder A, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M and Raichle ME (2007). “Intrinsic functional architecture in the anaesthetized monkey brain.” Nature 447(7140): 83–6. [DOI] [PubMed] [Google Scholar]
- 48.Kollias SS, Alkadhi H, Jaermann T, Crelier G and Hepp-Reymond MC (2001). “Identification of multiple nonprimary motor cortical areas with simple movements.” Brain Res Brain Res Rev 36(2–3): 185–95. [DOI] [PubMed] [Google Scholar]
- 49.Pochon JB, Riis J, Sanfey AG, Nystrom LE and Cohen JD (2008). “Functional imaging of decision conflict.” J Neurosci 28(13): 3468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lau HC, Rogers RD and Passingham RE (2006). “Dissociating response selection and conflict in the medial frontal surface.” Neuroimage 29(2): 446–451. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K and Zilles K (2008). “Cortical folding patterns and predicting cytoarchitecture.” Cereb Cortex 18(8): 1973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter CS and van Veen V (2007). “Anterior cingulate cortex and conflict detection: an update of theory and data.” Cognitive, Affective & Behavioral Neuroscience 7(4): 367–79. [DOI] [PubMed] [Google Scholar]
- 53.van Veen V and Carter CS (2005). “Separating semantic conflict and response conflict in the Stroop task: A functional MRI study.” Neuroimage 27: 497–504. [DOI] [PubMed] [Google Scholar]
- 54.Orban G, Claeys K, Nelissen K, Smans R, Sunaert S, Todd J, Wardak C, Durand J and Vanduffel W (2006). “Mapping the parietal cortex of human and non-human primates.” Neuropsychologia 44(13): 2647–2667. [DOI] [PubMed] [Google Scholar]
- 55.Tsao DY, Freiwald WA, Tootell RBH and Livingstone MS (2006). “A Cortical Region Consisting Entirely of Face-Selective Cells.” Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J and Aston-Jones G (1999). “The role of locus coeruleus in the regulation of cognitive performance.” Science 283(5401): 549–54. [DOI] [PubMed] [Google Scholar]
- 57.Badre D and Wagner AD (2006). “Computational and neurobiological mechanisms underlying cognitive flexibility.” Proc Natl Acad Sci USA 103(18): 7186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passingham R(2009). “How good is the macaque monkey model of the human brain?” Curr Opin Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Passingham RE (2008). “What is special about the human brain?” Oxford University Press Oxford. [Google Scholar]
- 60.Gazzaniga M (2008). “Human: The Science Behind What Makes Us Unique.” HarperCollins: 352. [Google Scholar]
- 61.Wallis JD and Miller EK (2003). “From rule to response: neuronal processes in the premotor and prefrontal cortex.” J Neurophysiol 90(3): 1790–806. [DOI] [PubMed] [Google Scholar]
- 62.Monsell S (2003). “Task switching.”Trends in Cognitive Sciences. [DOI] [PubMed] [Google Scholar]
- 63.Emeric EE, Brown JW, Boucher L, Carpenter RH, Hanes DP, Harris R, Logan GD, Mashru RN, Paré M, Pouget P, Stuphorn V, Taylor TL and Schall JD (2007). “Influence of history on saccade countermanding performance in humans and macaque monkeys.” Vision Res 47(1): 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayr U, Awh E and Laurey P (2003). “Conflict adaptation effects in the absence of executive control.” Nature Neuroscience 6(5): 450–2. [DOI] [PubMed] [Google Scholar]
- 65.Öngür D, Ferry A and Price J (2003). “Architectonic subdivision of the human orbital and medial prefrontal cortex.” The Journal of Comparative Neurology 460(3): 425–449. [DOI] [PubMed] [Google Scholar]
- 66.Walters N, Eickhoff S, Schleicher A, Zilles K, Amunts K, Egan G and Watson JD (2007). “Observer-independent analysis of high-resolution MR images of the human cerebral cortex: in vivo delineation of cortical areas.” Hum. Brain Mapp 28(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schleicher A, Amunts K, Geyer S, Kowalski T, Schormann T, Palomero-Gallagher N and Zilles K (2000). “A stereological approach to human cortical architecture: identification and delineation of cortical areas.” J Chem Neuroanat 20(1): 31–47. [DOI] [PubMed] [Google Scholar]
- 68.London M and Häusser M (2005). “Dendritic computation.” Annual Review of Neuroscience 28: 503–32. [DOI] [PubMed] [Google Scholar]


