Abstract
N-glycans are important players in a variety of pathologies including different types of cancer, (auto)immune diseases, and also viral infections. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) is an important tool for high-throughput N-glycan profiling and, upon use of tandem MS, for structure determination. By use of MALDI-MS imaging (MSI) in combination with PNGase F treatment, also spatially correlated N-glycan profiling from tissue sections becomes possible. Here we coupled laser-induced postionization, or MALDI-2, to a trapped ion mobility quadrupole time-of-flight mass spectrometer (timsTOF fleX MALDI-2, Bruker Daltonics). We demonstrate that with MALDI-2 the sensitivity for the detection of molecular [M – H]− species of N-glycans increased by about 3 orders of magnitude. Compared to the current gold standard, the positive ion mode analysis of [M + Na]+ adducts, a sensitivity increase by about a factor of 10 is achieved. By exploiting the advantageous fragmentation behavior of [M – H]− ions, exceedingly rich structural information on the composition of complex N-glycans was moreover obtained directly from thin tissue sections of human cerebellum and upon use of low-energy collision-induced dissociation tandem MS. In another set of experiments, in this case by use of a modified Synapt G2-S QTOF mass spectrometer (Waters), we investigated the influence of relevant input parameters, in particular pressure of the N2 cooling gas in the ion source, delay between the two laser pulses, and that of their pulse energies. In this way, analytical conditions were identified at which molecular ion abundances were maximized and fragmentation reactions minimized. The use of negative ion mode MALDI-2-MSI could constitute a valuable tool in glycobiology research.
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) is a popular tool for high-throughput analysis of oligosaccharides including N- and O-linked glycans. Typically, the oligosaccharides are derived from glycoproteins by enzymatic, or in the case of O-linked glycans, chemical cleavage (e.g., PNGase F or β-elimination). MALDI-MS has been used to profile glycans from a variety of biological matrices, including biofluids such as serum, blood, and urine, as well as other matrices (e.g., food sources and plant material).1−6 To also reveal important spatial information on glycans in tissue, MALDI-MS imaging (MALDI-MSI) can be used.7,8 Application examples of MALDI-MSI comprise, for example, the role specific glycosylation patterns play in the development and pathogenesis of ovarian and breast cancer and myxoid liposarcoma.9−13
Because of the better detection sensitivity, the majority of these applications relied on the detection of singly charged alkali-metal adduct ions, typically [M + Na]+. For structure elucidation, low-energy collision-induced dissociation (CID) is commonly used and in positive ion mode typically results in fragmentation of the glycosidic bond, with little formation of cross-ring fragments. In contrast, CID in negative ion mode predominantly yields diagnostic cross-ring fragments required for the unambiguous identification of intersaccharide linkages. Therefore, negative ion mode MS finds its application mostly in tandem MS analysis of oligosaccharides.14−16 A hindrance for more extensively exploiting this potential is given by the rather low ion yields of MALDI for oligosaccharides in the negative ion mode. Therefore, a plethora of derivatization strategies has been developed to stabilize and neutralize the notoriously labile terminal sialic acid residues,17−19 as well as functionalize the reducing end of the oligosaccharide with a stabilizing, often fluorescent, and permanently charged group.19−21 Although functional, these strategies often result in complex sample preparation protocols, which are prone to the inclusion of experimentally induced measurement variations. Moreover, chemical derivatization strategies are not always transferable to tissue sections, and therefore they may not be applicable to MALDI-MSI.
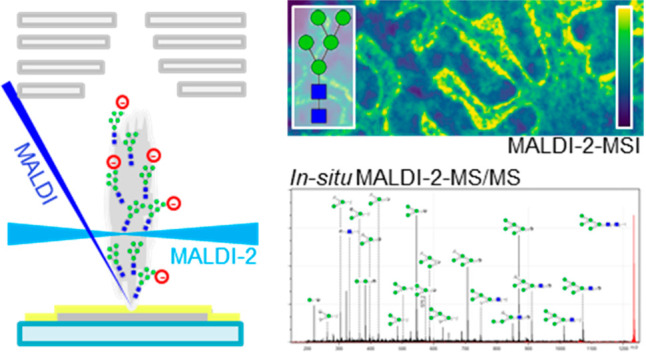
A powerful alternative to increase the ion yields of deprotonated oligosaccharides is MALDI-MS coupled with laser-induced postionization (PI), or MALDI-2-MSI.22 With this recently introduced technique, the initial MALDI-particle plume, which is formed in a fine vacuum environment of a few mbar of N2, is intercepted by the beam of a pulsed PI-laser (e.g., a q-switched Nd:YAG laser emitting at 266 nm23) a suitable distance and interlaser pulse delay. Very recently, also the first successful adaptation of MALDI-2 in combination with an atmospheric pressure (AP) ion source has been reported.24 Somewhat depending on matrix, analyte, spot size, and pulse energy of the ablation laser, optimal MALDI-2 conditions are typically found if a central distance of the PI laser beam to the primary laser spot of about 500 μm and an interlaser pulse delay of ∼10 μs are established (see the Results and Discussion section below). The interaction of the PI laser beam with the particle plume gives rise to a significant boost in ion yields for numerous classes of small molecules but also for more complex lipids like phospho- and glycosphingolipids. The current hypothesis on the working mechanism of MALDI-2, based predominantly on studies that were performed with glycerophospholipids, consists of two coexisting mechanisms.25 The first is based on resonance-enhanced two-photon ionization (REMPI) of the MALDI matrix. For aromatic MALDI matrices, this condition is generally met for wavelengths shorter than 310 nm. Because of energetically favorable reactions, the newly formed charges are transferred to neutral analytes in close proximity, presumably via gas phase collisions. Additionally, a mechanism similar to aerosol MALDI26 could result in a more conventional MALDI process upon involvement of larger clusters. Further work is needed to elucidate these pathways for different combinations of MALDI matrices and laser parameters.22,25,27,28
The application of MALDI-2 to the analysis of oligosaccharides in negative ion mode could potentially simplify sample preparation workflows and enhance ion yields and would therefore improve reproducibility and sensitivity of the analysis. While already indicated by an increase in signal intensity for small disaccharides (Hex2) by Soltwisch et al., beneficial effects for the analysis of larger, more complex oligosaccharides can, however, currently only be suspected.22 Therefore, we have studied these potential benefits of applying MALDI-2 for mass spectrometry-based glycomics approaches and N-glycan MSI at the examples of a set of standards and by the analysis of human brain sections at 50 μm pixel sizes. We also tested the potential of low-energy CID tandem MS for generating structurally informative fragments from [M – H]− precursors of complex N-glycans directly from human brain tissue.
Materials and Methods
Chemicals and Reagents
Acetonitrile (ACN) and LC-MS-grade deionized water (dH2O) were acquired from Carl ROTH (Karlsruhe, Germany). Nor-harmane (NOR), histological grade xylene, and Millipore Amicon Ultra-0.5 centrifugal filter units (10 kDa molecular-weight cutoff (MWCO) filters) were obtained from Sigma-Aldrich (Steinheim, Germany). Ethanol (EtOH) and 2,5-dihydroxyacetophenone (2,5-DHAP) were acquired from Merck (Darmstadt, Germany). Oligosaccharide standards (Maltoheptaose DP7 (DP7); N-glycan H5N4) were obtained from Biosynth Carbosynth (Compton, U.K.). Endoglycosidase PNGase F, recombinant (lyophilized) (Serva Electrophoresis, Heidelberg, Germany), and ESI-L low concentration tuning mix (Agilent Technologies, Santa Clara, CA, U.S.A.) were acquired at the indicated vendors. Superfrost microscope glass slides were acquired from Thermo Scientific (Schwerte, Germany).
Tissues and Tissue Treatment
Formalin-fixed and paraffin-embedded (FFPE) human brain (cerebellum region) tissue obtained from a 79-year-old male were obtained from the Department of Neuropathology of the University Hospital Münster (UKM). The individual died from cardiovascular disease without known neurological disorders. Tissues were sectioned at 5 μm thickness and were mounted on Superfrost glass slides (Thermo Scientific). Sections were stored at 4 °C in the dark until further processing. Tissues were used following in accordance with the research ethics board approval (1IIIPau; UKM, Münster, Germany).
Maltoheptaose Sensitivity Measurements
Sensitivity measurements for the MALDI-2-MS analysis of oligosaccharides were assessed using a timsTOF fleX MALDI-2 QTOF instrument (Bruker Daltonics; details on the performed modifications are provided in the Supporting Information) and in ref (29). Spectra (n = 5), recorded over an m/z-range of 500–1500, were acquired in positive ion mode using MALDI, and in negative ion mode both with and without MALDI-2; MALDI-2 in the positive ion mode was also tried but did not result in sizable differences in the ion abundances of the [M + Na]+ ions, and was therefore not used anymore in further experiments. Spectra (400 laser shots summed) were acquired at optimized settings: 3.0 mbar cooling gas pressure (N2), 50 × 50 μm2 pixel size (“M5 small” setting, ablation surface 1280 μm2), approximately 22 μJ or 52 μJ ablation laser pulse energies in positive and negative ion mode, respectively. All spectra were recorded using 1 kHz repetition rates for both ablation and PI lasers. For the MALDI-2 measurements in negative ion mode, approximately 350 μJ PI-laser pulse energy, and an interlaser pulse delay of 30 μs were used.
N-Glycan MALDI-2-MSI Sample Preparation and Analysis
FFPE sections were dewaxed using two 10 min-long washes in xylene. Following dewaxing, tissues were rehydrated in a series of EtOH washes (100% EtOH (3×), 70% EtOH (1×), 50% EtOH (1×), dH2O (1×), 1× equals 20 2 s dips) and washed in dH2O for an additional 5 min. Samples were dried under a stream of N2. Endoglycosidase PNGase F was buffer exchanged using 10 kDa MWCO filters, dissolved in dH2O (0.1 μg μL–1), and homogeneously applied on the tissue sections using the SimCoat spray-robot (SonoTek; 40 passes; flow rate, 30 μL min–1; XY-stage-speed, 30 mm s–1; line-to-line distance, 1.8 mm; ultrasound power (at 48 kHz), 1.5 W). Tissues were incubated overnight (18 h) at 37 °C in a saturated humid environment. Following incubation 41 mM NOR, and 5 μM maltoheptaose (in 50:50 (% v/v) ACN:dH2O) were cosprayed on the tissue using the following parameters: 40 passes; flow rate, 50 μL min–1; XY-stage-speed, 30 mm s–1; line-to-line distance, 1.8 mm; ultrasound power, 6.0 W (at 48 kHz). Unless otherwise noted, MALDI-2-MSI was performed on the timsTOF fleX MALDI-2 (Bruker Daltonics) in negative ion mode at optimized conditions: N2 cooling gas pressure, 3.0 mbar; ablation laser pulse energy, 34 μJ; interlaser pulse delay, 30 μs; PI laser pulse energy, 350 μJ; 50 laser shots per pixel. For the analyses assessing the influence of cooling gas pressure and interlaser pulse delay, the respective parameters were adjusted accordingly, while all other parameters were kept identical. Positive ion mode MALDI-MSI measurements were performed after applying 46 mM 2,5-DHAP and 5 μM maltoheptaose (in 50:50 (% v/v) ACN:dH2O) using the same spraying method used for applying NOR. Spectra were recorded at p = 3.0 mbar, using an ablation laser pulse energy of 34 μJ, and 500 shots per pixel. All spectra were recorded using 1 kHz repetition rates for both ablation and PI lasers, and over an m/z range between 900 and 3000 using a 50 × 50 μm2 pixel size (“M5 small”). After acquisition, data was imported and analyzed in SCiLS Lab MVS (2020a Pro, build 8.00.11593, Bruker Daltonics). Figures were prepared in Prism (8.1.1, build 330, GraphPad), mMass (5.5.0, www.mmass.org), and the Office 2016 software suite (Microsoft).
Results and Discussion
MALDI-2 of Oligosaccharides in Positive and Negative Ion Modes
First, we assessed whether the MALDI-MS-based detection of oligosaccharides can be improved by applying MALDI-2 with the analyses of oligosaccharide standards and then with endogenous N-glycans from tissue sections. Our goal was to study key effects of the MALDI-2 parameters, and not least to optimize experimental protocols for MALDI-2-MS(I) of N-glycans.
The intensities of the ablation and PI laser were systematically varied to study their effects on the (post)ionization of the oligosaccharide. In positive ion mode, the sodium adducts of maltoheptaose did not benefit from MALDI-2, and even decreased somewhat (by up to a factor of 3) with increasing PI-laser energy (Figure 1A). This observation is in line with previously published observations on lipids, where decreased MALDI-2 signal intensities of sodium adducts have been hypothesized to be an effect of either the elevated pressure in the ion source, leading to suboptimal ion transmission conditions, or increased de novo formation of negative ion species during the MALDI-2 procedure, having a neutralizing effect on positive ion species.25,30 The results displayed here led us to believe that these observations are not analyte class specific. Similar to the reports on MALDI-2 of lipids, the protonated maltoheptaose ion received a boost in signal intensity. However, to achieve this effect high ablation laser energies were required, which resulted in partial dissociation of maltoheptaose via the loss of single or more hexose units (Figure S1A,B, Supporting Information). The option to boost protonated molecular ions of oligosaccharides should perhaps be studied further in the future (e.g., by using different matrix systems).
Figure 1.
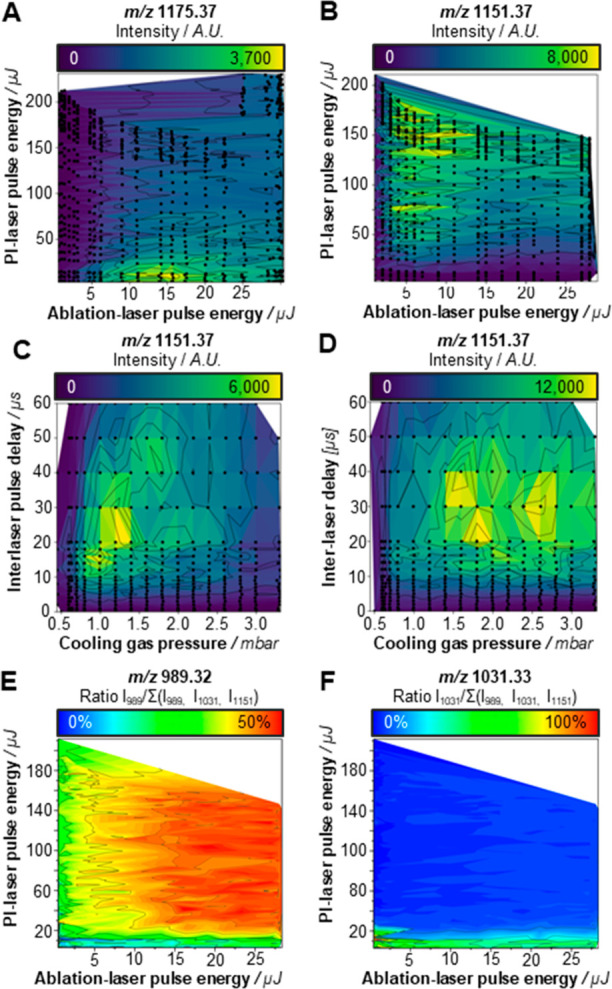
Optimization of PI parameters in negative ion mode MALDI-2 measurements. The influence of the ablation and PI-laser pulse energies (at a fixed cooling gas pressure of 2.0 mbar, and an interlaser pulse delay of 10 μs) on the signal intensity of (A) the [M + Na]+ signal of maltoheptaose (m/z 1175.37) and (B) the [M – H]− signal of the oligosaccharide (m/z 1151.37 [maltoheptaose – H]−). Additionally, the effects of cooling gas pressure, and the interlaser pulse delay on the signal intensity of deprotonated maltoheptaose molecules were assessed. Spectra were acquired using an ablation-laser pulse energy of (C) 5 and (D) 25 μJ, respectively. PI-laser pulse energy was at the maximum energy of approximately 200 μJ. Common for the MALDI-based analysis of neutral oligosaccharides, in-source fragmentation occurred. Different types of fragments were produced under different conditions; in panels A–D, black dots represent individual measurements (average of n = 10 pixels). (E) Loss of a hexose (m/z 989.32 [maltoheptaose–C6H10O5–H]−) occurred predominantly under MALDI-2 conditions (using both high ablation laser, and PI energies). (F) Higher-energy cross-ring saccharide fragmentation (m/z 1031.33 [maltoheptaose–C4H8O4– H]−) occurred exclusively under normal MALDI-conditions, and even at low ablation laser energies. Relative fragmentation (E and F) was calculated as the fragment-intensity over the sum of intact maltoheptaose and all detected fragments.
In the negative ion mode, a clear boost in signal intensity as a function of increasing PI-laser energy was observed for the deprotonated maltoheptaose molecule. However, it is well-known that [M–H]− ions of neutral oligosaccharides are prone to in-source dissociation (ISD) - these fragment peaks were indeed detected in both MALDI and MALDI-2 analyses (Figure S1C,D, Supporting Information). However, under identical conditions (besides applying PI) the intensity of the intact maltoheptaose ion was enhanced much stronger by MALDI-2 compared to its ISD fragments (Figure S2, Supporting Information). Previously, several groups successfully stabilized deprotonated oligosaccharide ions by adding sodium iodide to the MALDI matrix and therefore promote the formation of iodide adducts.31,32 This strategy did not result in increased MALDI-2 signals in negative (data not shown), which is in line with the findings on detection of adducts in positive ion mode described earlier.
Optimization of MALDI-2 Parameters
To address this and further mechanistic questions, next we studied the effects of the experimental parameters of MALDI-2 with a focus on the negative ion mode analysis. By varying τ and p in combination with a relatively “low” ablation laser pulse energy, not far from the ion detection threshold, we observed two sets of conditions, or regimes, that resulted in increased maltoheptaose ion yields (Figure 1C): regime (i) 0.5 mbar < p < 1 mbar and 10 μs < τ < 20 μs and regime (ii) 1.0 mbar < p < 1.5 mbar and 20 μs < τ < 30 μs.
At high ablation laser pulse energy conditions (Figure 1D), the highest maltoheptaose intensities were found at elevated pressure (1.5 mbar < p < 3.0 mbar) and 20 μs < τ < 40 μs. The maximum absolute intensity of the deprotonated maltoheptaose ion in the high ablation laser pulse energy experiment was doubled compared to the intensity recorded at low ablation laser pulse energy. At both low and high ablation laser pulse energy settings, fragmentation occurred predominantly at low pressure (p < 1 mbar) and short interlaser pulse delay (10 μs < τ < 20 μs) conditions (Figure S3A,B, Supporting Information). These results indicate the presence of (at least) two ion populations following the MALDI event. One being a population of molecular ions exhibiting a high mean velocity, generated (most likely) through a “thermal” desorption process,33 and one slower population with larger clusters, ejected from the tissue surface through an ablation mechanism. Very similar findings were obtained through modeling MALDI-plume development by Knochenmuss34 and have been experimentally described by Niehaus and Soltwisch for glycerophospholipids and glycolipids,27 as well as by other authors for various analyte/matrix systems and summarized in a review by Dreisewerd.35
Next, we asked if the negative ion mode MALDI-2-MSI mechanisms, as revealed with the maltoheptaose standards, may also apply for more complex glycans, namely enzymatically released N-glycans. To account for possible tissue-type (and area)-dependent variations in ion suppression effects,28,36−39 we homogeneously sprayed maltoheptaose (5 μM) onto human cerebellum tissues and recorded MALDI-2-MSI data at a set of different p (between 2.0–3.0 mbar, Figure S4A, Supporting Information) and τ (between 5–60 μs, Figure S4D, Supporting Information) values. The [M – H]− maltoheptaose signals were used for normalization.
As for the pure maltoheptaose samples, the MALDI-2-MSI data again show a strong pressure dependence on the oligosaccharide/glycan signal intensity. Higher pressures, as well as an optimal interlaser pulse delay around 30 μs were found favorable for the analysis of N-glycans from tissue. However, the effect of the interlaser pulse delay on the signal intensity was much less pronounced than for the sample system above.
In-Source Dissociation in the Negative Ion Mode
Next, we recommenced to ask if fragmentation of oligosaccharides was increased by the MALDI-2 process or whether it rather results from the MALDI process, and fragment signals were merely enhanced by MALDI-2. We found that the relative majority of cross-ring fragmentation occurred under MALDI conditions (no PI-laser energy applied; Figure 1E). Similar to the observations in the positive ion mode, dissociation of the glycosidic bond resulting in the loss of a complete hexose residue, occurred predominantly as an effect of increased ablation-laser energy (Figure 1F). Also, in the MALDI-2-MSI analyses, we detected fragment ions (cross-ring as well as loss of hexose) for both the homogeneously sprayed maltoheptaose internal standard, and endogenous N-glycans. When varying the cooling gas pressure, the absolute intensities of the fragments followed the same trends as the intact endogenous glycan (Figure S4B, SI), but the relative abundances of fragments and intact oligosaccharide remained the same (Figure S4C, SI). Similar observations were made when changing the interlaser pulse delay (Figure S4E,F, SI). However, while the relative abundance of the intact glycan did not change much under the influence of τ, there were relative changes between the type of fragments that were favored between short and longer interlaser pulse delays (Figure S4F, SI). Short interlaser pulse delays resulted predominantly in the formation of cross-ring fragments, while longer interlaser pulse delays favored glycosidic bond fragmentation. As cross-ring fragmentation generally requires more fragmentation energy, and short interlaser pulse delays result in PI of faster, high-energy single ions formed through “thermal” desorption, these observations fit very well with the presence of multiple ion populations in the MALDI plume described above. Moreover, from these data we were able to finally conclude that the observed in-source fragmentation is mainly generated in the ablation process. The MALDI-derived fragment ions were enhanced by MALDI-2, but under the tested conditions no additional fragmentation induced by the second laser pulse seemed to occur.
Improved MALDI-2 Measurement Sensitivity
Although [M–H]− signal increases were observed for negative ion mode MALDI-2 measurements, the ultimate question was whether the signal increase would translate to an increase in measurement sensitivity for MALDI-2. Therefore, a maltoheptaose dilution series was prepared and analyzed in positive ion mode without PI, and in negative ion mode both with and without PI. Due to the homogeneous preparation, and the high pixel fidelity of the timsTOF fleX, the amount of maltoheptaose per 50 × 50 μm2 pixel could be calculated (Supplementary Table S-1, Supporting Information). In negative ion mode, MALDI-2 analysis (Figure 2A) resulted in the smallest lower limit of detection (LLOD) of 2 amol per pixel. This LLOD improved by 3 orders of magnitude in the equivalent negative ion mode measurement without MALDI-2 (LLOD: 2 fmol per pixel; Figure 2B) and 1 order of magnitude in the positive ion mode MALDI measurements of the maltoheptaose sodium adduct (LLOD: 22 amol per pixel; Figure 2C). Further studies are required to assess whether similar performance benefits are achieved for various glycan classes, and for in situ analysis from tissue.
Figure 2.
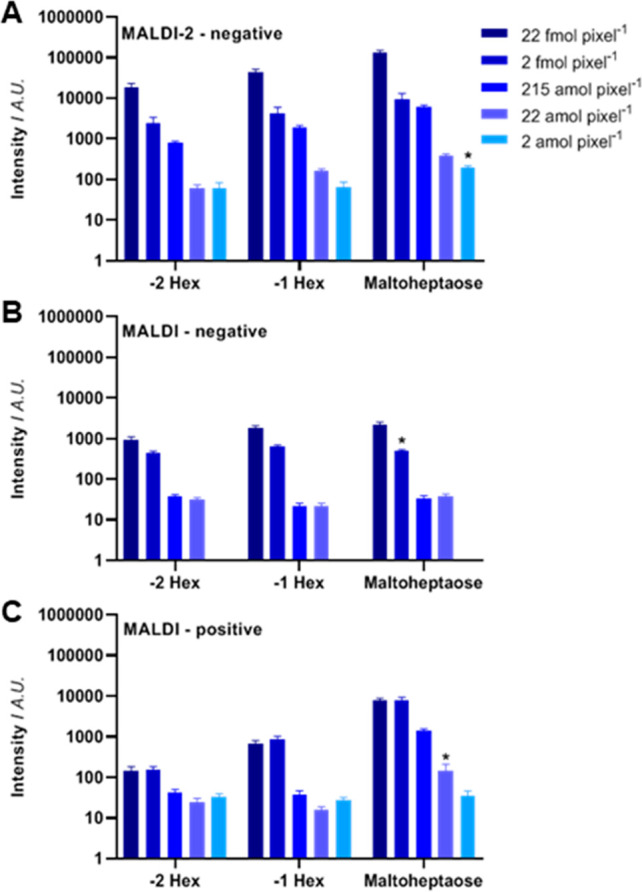
Oligosaccharide sensitivity. A dilution series of spraycoated maltoheptaose on glass slide was analyzed by (A) MALDI- 2-MS in negative ion mode, (B) MALDI-MS in negative ion mode, and (C) MALDI-MS in positive ion mode. The bars show the mean intensity of the performed experiments (n = 5). Asterisks (*) highlight the lower limit of detection (signal-to-noise ratio (SNR) ≥ 3), and error bars represent the SEM.
N-Glycan MALDI-2-MSI
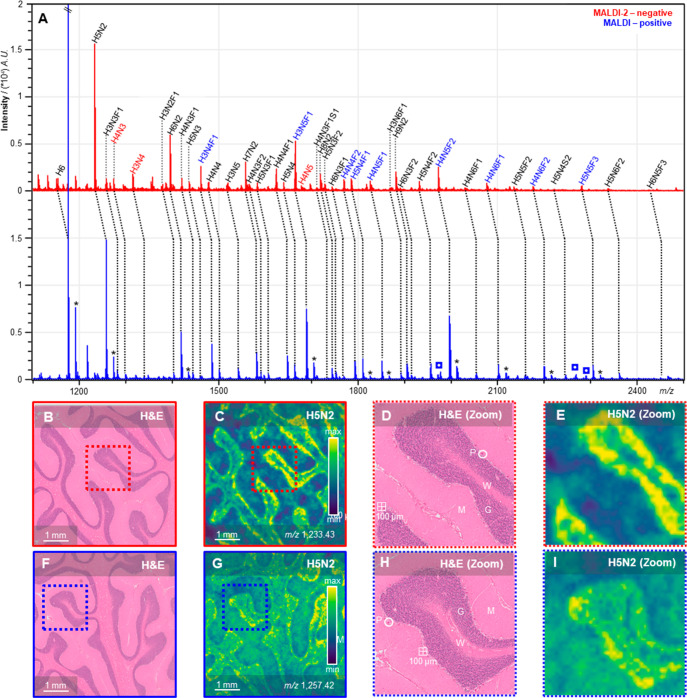
To evaluate whether ion species generated from the same N-glycans in both positive ion mode MALDI and negative ion mode MALDI-2 would show the same profiles and spatial distributions in tissue, a comparison experiment was performed on human cerebellum tissues. Figure 3A shows the represetative average tissue spectra obtained from the two analyses. Tissue analysis using negative ion mode MALDI was also attempted, but the resulting spectra were of such poor quality that the MSI data would only comprise of noise. Due to the ISD in the negative ion mode, there seems to be a slight bias toward the smaller N-glycans in the MALDI-2-MSI analysis. The affected glycans with an intensity variation more than > ± 10% have been highlighted. However, as can be deduced from Figure 3B–I, the recorded distributions showed the same glycans to be present in similar morphological areas upon measurement with the two ion polarities.
Figure 3.
Spectra and images of positive-ion mode MALDI-MSI and negative ion-mode MALDI-2-MSI. (A) Average spectra for negative ion-mode MALDI-2-MSI (red) and positive ion-mode MALDI-MSI (blue) with assigned N-glycan species. Colored N-glycan compositions represent a > ± 10% variation of intensity between positive and negative ion-mode analyses. Peaks with an asterisk (*) in the positive ion mode spectrum are potassium adducts. (B,F) H&E stained consecutive section displaying cerebellar brain morphology. (C,G) Example images for N-glycan H5N2 in human cerebellum. (D,H) Zoom in on histology with different morphological structures annotated (P, Purkinje cell, G, granular layer, M, molecular layer, W, white matter). (E,I) Zoom in on example H5N2 images. In red, negative ion-mode MALDI-2-MSI images, and in blue, positive ion-mode MALDI-MSI images.
One striking observation is that, although varying notably in their structural composition, the majority of the registered N-glycans expess very similar expression profiles across the investigated brain area (Figure S5, Supporting Information). Presumably due to the general difficulty to access human brain samples for these analytical purposes, we could not find any previous MALDI-MSI-based data for N-glycans in the human cerebellum in the literature for comparison. However, both the Human Protein Atlas (http://www.proteinatlas.org) and the Allen Mouse Brain Atlas (http://www.brain-map.org) contain data on the expression of key-enzymes of the N-glycan biosynthesis pathway. Mannosidase 1, alpha (man1a1) is the enzyme responsible for trimming H9N2 to H8N2 in the endoplasmatic reticulum (ER). As it is one of the “early-stage” enzymes of the biosynthesis pathway, all N-glycans that are expressed have been processed by this enzyme, and therefore it is a good marker for the presence of N-glycosylated proteins. We found man1a1 to be predominantly expressed in the Purkinje cell layer in both human and mouse cerebellum (Figure S6, Supporting Information). Additional expression was found in the granular layer, although at lower levels compared to the Purkinje cells. These findings correspond well to the distributions found for the N-glycans detected by MSI. Especially the negative ion mode MALDI-2 analysis showed a very clear delineation of the Purkinje cells and granular layer in all of the recorded glycan distributions (Figures 3 and S5, Supporting Information).
An additional benefit of the analysis of oligosaccharides in the negative ion mode is the absence of peak-splitting that occurs in positive ion mode through the presence of multiple alkali-metal adducts for the same glycan (e.g., sodium and potassium adducts; Figure 3A). In MALDI-MSI experiments, local differences in salt concentrations can affect adduct formation, which will ulimately result in biased (semi)quantitative data.40,41 Although additional research is needed to assess potential differences in (post)ionization efficiencies of various glycan classes (e.g., high-mannose vs complex-type, sialylated and/or fucosylated species), in our view, these results show clearly that MALDI-2-MSI can be an exceedingly useful tool for the visualization of N-glycans in FFPE tissue sections.
In Situ MALDI-2-MS/MS
In mass spectrometry-based glycomics, the negative ion mode is commonly used for obtaining tandem MS spectra, as they contain unique isomer-specific cross-ring fragment ion signals not readily available in positive ion mode.31,42,43 In previous MSI-based studies on N-glycans, oligosaccharide compositions were determined based on mass-matching, often in combination with the off-tissue analysis of extracts by MALDI-MS/MS.17,43 This indirect, and laborious approach is often required as the sensitivity of MALDI-MS/MS is generally too low for structure elucidation directly from tissue.
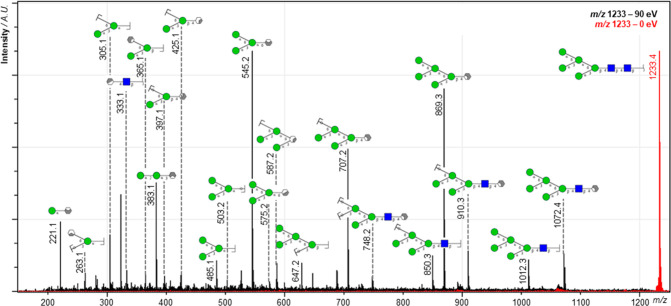
In our initial study, highlighting the general capabilities of the timsTOF fleX MALDI-2 instrument, we already included examples for the in situ MS/MS analysis of (glyco-)lipids recorded from murine tissue.29 Here we show that with this instrument similar advancement are achieved for on-tissue MS/MS of N-glycans. In total the identity of 14 out of the 38 tentatively assigned N-glycan species as observed in the MALDI-2-MSI analysis (Figure 3) was confirmed by MALDI-2-MS/MS directly from the previously analyzed tissue section (Table S2, Supporting Information). Figure 4 shows an example of an on-tissue MS/MS spectrum from H5N2, obtained using MALDI-2 in the negative ion mode. Consistent with work by Harvey, where MS/MS analyses of electrosprayed [M–NO3]− and [M–(NO3)2]2– ions from neutral high-mannose N-linked glycans and their 2-aminobenzamide derivatives was performed, the most abundant fragment peaks were present in the analyzed spectra.44
Figure 4.
MALDI-2 tandem MS spectrum of deprotonated H5N2 from human cerebellum. The red trace shows a pseudo-MS1 spectrum, where quadrupole filtering was applied but no collision energy (0 eV). The black trace shows the low-energy CID MS/MS spectrum that resulted by applying a collision energy of 100 eV (laboratory frame). Both spectra were acquired from a total of 20 50 × 50 μm2 wide pixels from a human cerebellum tissue previously analyzed by MALDI-2-MSI. Based on mass matching (mass error tolerance, ±10 ppm), the majority of high intensity peaks could be assigned with fragment identities. Note that the applied method, which is not incorporating the use of ion mobility spectrometry, does not allow for separation of isomeric fragments. For visualization, only one hypothetical isomer is therefore denoted. Blue squares represent N-acetylhexoses (HexNAc), green circles represent hexoses (Hex), and grayish hexagons represent various additional cross-ring fragments.
Conclusions
MALDI-2 performed in the negative ion mode induces a boost in [M–H]− ion yields for the analysis of oligosaccharides. As a result, this provides an order of magnitude higher sensitivity compared to the current “gold standard”, the positive ion mode MALDI analysis of sodium-adducts of oligosaccharides. In the mechanistic part of our study, we moreover optimized several key parameters for MALDI-2-MSI of N-glycans in the negative ion mode. Using the so optimized conditions, we showed how the increased ion yields could be highly beneficial for the acquisition of high-quality MS/MS spectra and structural analysis of N-glycans from minute sample amounts. Although having a focus on N-glycans and neutral standards (maltoheptaose), we foresee that the method will also reach out beyond these systems. It could, for instance, also comprise valuable for O-glycan analysis. And, although not studied in the context of this manuscript, making use of the trapped ion-mobility separation (TIMS) capabilities of the timsTOF fleX MALDI-2 instrument, valuable information on the presence and distribution of N-glycan isomers in tissue is expected to benefit strongly from MALDI-2. In summary, MALDI-2 could comprise a valuable tool for many applications in mass spectrometry-based glycomics research.
Acknowledgments
The authors would like to thank Annika Koch, Simeon Vens-Cappell, Arne Fütterer, Andreas Haase, Henning Peise, and Jens Höhndorf of Bruker Daltonics for their invaluable support. We are thankful to Astrid Jeibmann (Institute for Neuropathology, Münster University Hospital) for providing and sectioning the human cerebellum tissues. Financial support by the German Research Foundation (DFG; grants DR 416/9-1, Project No. 208319078 (to K.D.); DR 416/12-1 and SO 976/3-1, Project No. 290343045 (to K.D. and J.S.), and the Interdisciplinary Center for Clinical Research (IZKF) Münster (Grant Drei2/019/17; to K.D. and J.S.) are gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.0c02732.
Supplementary methods; supplementary references; supplementary figures showing: the effect of laser pulse energies on the fragmentation of oligosaccharides; the effect of MALDI-2 and negative ion mode on oligosaccharide fragmentation; the effect of cooling gas pressure and interlaser pulse delay on fragmentation; the effect of cooling gas pressure and interlaser pulse delay on MSI of N-glycans in human cerebellum tissue; comparison of example images of various N-glycans between negative ion mode MALDI-2-MSI and positive ion mode MALDI-MSI; mannosidase 1, alpha expression in human and mouse brain (PDF)
Supplementary table with a fragment database from the in situ tandem MS analyses of release endogenous N-glycans (XLSX)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): B.H. was supported by Bruker Daltonics during the course of this project. All other authors declare no conflict of interest.
Supplementary Material
References
- Hall P. L.; Lam C.; Alexander J. J.; Asif G.; Berry G. T.; Ferreira C.; Freeze H. H.; Gahl W. A.; Nickander K. K.; Sharer J. D.; Watson C. M.; Wolfe L.; Raymond K. M. Urine oligosaccharide screening by MALDI-TOF for the identification of NGLY1 deficiency. Mol. Genet. Metab. 2018, 124 (1), 82–86. 10.1016/j.ymgme.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding K. R.; Bondt A.; Hennig R.; Gardner R. A.; O’Flaherty R.; Trbojevic-Akmacic I.; Shubhakar A.; Hazes J. M. W.; Reichl U.; Fernandes D. L.; Pucic-Bakovic M.; Rapp E.; Spencer D. I. R.; Dolhain R. J. E. M.; Rudd P. M.; Lauc G.; Wuhrer M. High-throughput serum N-glycomics: method comparison and application to study rheumatoid arthritis and pregnancy-associated changes. Mol. Cell. Proteomics 2019, 18 (1), 3–15. 10.1074/mcp.RA117.000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsias M.; Kozak R. P.; Gardner R. A.; Wuhrer M.; Spencer D. I. R. Improved and semi-automated reductive β-elimination workflow for higher throughput protein O-glycosylation analysis. PLoS One 2019, 14 (1), e0210759 10.1371/journal.pone.0210759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeker G. C. M.; Bladergroen M. R.; Nicolardi S.; Mesker W. E.; Tollenaar R. A. E. M.; van der Burgt Y. E. M.; Wuhrer M. Dried blood spot N-glycome analysis by MALDI mass spectrometry. Talanta 2019, 205, 120104. 10.1016/j.talanta.2019.06.104. [DOI] [PubMed] [Google Scholar]
- Qu L.; Jiang Y.; Huang X.; Cui M.; Ning F.; Liu T.; Gao Y.; Wu D.; Nie Z.; Luo L. High-throughput monitoring of multiclass syrup adulterants in honey based on the oligosaccharide and polysaccharide profiles by MALDI mass spectrometry. J. Agric. Food Chem. 2019, 67 (40), 11256–11261. 10.1021/acs.jafc.9b05317. [DOI] [PubMed] [Google Scholar]
- Wang H.; Zhao X.; Huang Y.; Liao J.; Liu Y.; Pan Y. Rapid quality control of medicine and food dual purpose plant polysaccharides by matrix assisted laser desorption/ionization mass spectrometry. Analyst 2020, 145 (6), 2168–2175. 10.1039/C9AN02440A. [DOI] [PubMed] [Google Scholar]
- Powers T. W.; Neely B. A.; Shao Y.; Tang H.; Troyer D. A.; Mehta A. S.; Haab B. B.; Drake R. R. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS One 2014, 9 (9), e106255 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T. W.; Holst S.; Wuhrer M.; Mehta A. S.; Drake R. R. Two-dimensional N-glycan distribution mapping of hepatocellular carcinoma tissues by MALDI-imaging mass spectrometry. Biomolecules 2015, 5 (4), 2554–2572. 10.3390/biom5042554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everest-Dass A. V.; Briggs M. T.; Kaur G.; Oehler M. K.; Hoffmann P.; Packer N. H. N-glycan MALDI imaging mass spectrometry on formalin-fixed paraffin-embedded tissues enables the delineation of ovarian cancer tissues. Mol. Cell. Proteomics 2016, 15 (9), 3003–3016. 10.1074/mcp.M116.059816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. A.; Casadonte R.; Cardinali B.; Spruill L.; Mehta A. S.; Carli F.; Simone N.; Kriegsmann M.; Del Mastro L.; Kriegsmann J.; Drake R. R. Increases in tumor N-glycan polylactosamines associated with advanced HER2-positive and triple-negative breast cancer tissues. Proteomics: Clin. Appl. 2019, 13, 1800014. 10.1002/prca.201800014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. T.; Condina M. R.; Ho Y. Y.; Everest-Dass A. V.; Mittal P.; Kaur G.; Oehler M. K.; Packer N. H.; Hoffmann P. MALDI mass spectrometry imaging of early- and late-stage serous ovarian cancer tissue reveals stage specific N-glycans. Proteomics 2019, 19, 1800482. 10.1002/pmic.201800482. [DOI] [PubMed] [Google Scholar]
- Drake R. R.; McDowell C.; West C.; David F.; Powers T. W.; Nowling T.; Bruner E.; Mehta A. S.; Angel P. M.; Marlow L. A.; Tun H. W.; Copland J. A. Defining the human kidney N-glycome in normal and cancer tissues using MALDI imaging mass spectrometry. J. Mass Spectrom. 2020, 55, e4490 10.1002/jms.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijs B.; Holst-Bernal S.; de Graaff M. A.; Briaire-De Bruijn I. H.; Rodriguez-Girondo M.; van de Sande M. A. J.; Wuhrer M.; McDonnell L. A.; Bovee J. V. M. G. Molecular signatures of tumor progression in myxoid liposarcoma identified by N-glycan mass spectrometry imaging. Lab. Invest. 2020, 100, 1252. 10.1038/s41374-020-0435-2. [DOI] [PubMed] [Google Scholar]
- Jovanovic M.; Peter-Katalinić J. Negative ion MALDI-TOF MS, ISD, and PSD of neutral underivatized oligosaccharides without anionic dopant strategies, using 2,5-DHAP as a matrix. J. Mass Spectrom. 2016, 51 (2), 111–122. 10.1002/jms.3727. [DOI] [PubMed] [Google Scholar]
- Huang C.; Yan J.; Zhan L.; Zhao M.; Zhou J.; Gao H.; Xie W.; Li Y.; Chai W. Linkage and sequence analysis of neutral oligosaccharides by negative-ion MALDI tandem mass spectrometry with laser-induced dissociation. Anal. Chim. Acta 2019, 1071, 25–25. 10.1016/j.aca.2019.04.067. [DOI] [PubMed] [Google Scholar]
- Harvey D. J. Negative ion mass spectrometry for the analysis of N-linked glycans. Mass Spectrom. Rev. 2020, 39, 586–679. 10.1002/mas.21622. [DOI] [PubMed] [Google Scholar]
- Holst S.; Heijs B.; de Haan N.; van Zeijl R. J. M.; Briaire-De Bruijn I. H.; van Pelt G. W.; Mehta A. S.; Angel P. M.; Mesker W. E.; Tollenaar R. A.; Drake R. R.; Bovée J. V. M. G.; McDonnell L. A.; Wuhrer M. Linkage-specific in situ sialic acid derivatization for N-glycan mass spectrometry imaging from formalin-fixed paraffin-embedded tissues. Anal. Chem. 2016, 88 (11), 5904–5913. 10.1021/acs.analchem.6b00819. [DOI] [PubMed] [Google Scholar]
- Pongracz T.; Wuhrer M.; de Haan N. Expanding the reaction space of linkage-specific sialic acid derivatization. Molecules 2019, 24 (19), 3617. 10.3390/molecules24193617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Huang Y.; Ma G.; Liu Y.; Guo C.; He Q.; Wang H.; Liao J.; Pan Y. Parallel on-target derivatization for mass calibration and rapid profiling of N-glycans by MALDI-TOF MS. Anal. Chem. 2020, 92 (1), 991–998. 10.1021/acs.analchem.9b03932. [DOI] [PubMed] [Google Scholar]
- Ruhaak L. R.; Zauner G.; Huhn C.; Bruggink C.; Deelder A. M.; Wuhrer M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. 10.1007/s00216-010-3532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J.; Yang L.; Zhang Y.; Lu H. Hydrazinonicotinic acid derivatization for selective ionization and improved glycan structure characterization by MALDI-MS. Analyst 2015, 140, 5475–5480. 10.1039/C5AN00572H. [DOI] [PubMed] [Google Scholar]
- Soltwisch J.; Kettling H.; Vens-Cappell S.; Wiegelmann M.; Muthing J.; Dreisewerd K. Mass spectrometry imaging with laser-induced postionization. Science 2015, 348, 211–215. 10.1126/science.aaa1051. [DOI] [PubMed] [Google Scholar]
- Bednařík A.; Bölsker S.; Soltwisch J.; Dreisewerd K. An on-tissue Paternò-Büchi reaction for localization of carbon-carbon double bonds in phospholipids and lycolipids by matrix-assisted laser desorption/ionization mass spectrometry imaging. Angew. Chem., Int. Ed. 2018, 57, 12092. 10.1002/anie.201806635. [DOI] [PubMed] [Google Scholar]
- Niehaus M.; Robinson K. N.; Murta T.; Elia E. A.; Race A. M.; Yan B.; Steven R. T.; Bunch J. MALDI-2 at Atmospheric Pressure – Parameter Optimization and First Imaging Experiments. J. Am. Soc. Mass Spectrom. 2020, accepted manuscript 10.1021/jasms.0c00237. [DOI] [PubMed] [Google Scholar]
- Potthoff A.; Dreisewerd K.; Soltwisch J. Detailed characterization of the post-ionization efficiencies in MALDI-2 as a function of relevant input parameters. J. Am. Soc. Mass Spectrom. 2020, 31, 1844. 10.1021/jasms.0c00072. [DOI] [PubMed] [Google Scholar]
- Murray K. K.; Russell D. H. Aerosol Matrix-Assisted Laser Desorption Ionization Mass Spectrome-try. J. Am. Soc. Mass Spectrom. 1994, 5, 1–9. 10.1016/1044-0305(94)85077-1. [DOI] [PubMed] [Google Scholar]
- Niehaus M.; Soltwisch J. New insights into mechanisms of material ejection in MALDI mass spectrometry for a wide range of spot sizes. Sci. Rep. 2018, 8, 7755. 10.1038/s41598-018-25946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskamp M. S.; Soltwisch J. Charge distribution between different classes of glycerophospholipids in MALDI-MS imaging. Anal. Chem. 2020, 92 (7), 5222–5230. 10.1021/acs.analchem.9b05761. [DOI] [PubMed] [Google Scholar]
- Soltwisch J.; Heijs B.; Koch A.; Vens-Cappell S.; Höhndorf J.; Dreisewerd K. MALDI-2 on a trapped ion mobility quadrupole time-of-flight instrument for rapid mass spectrometry imaging and ion mobility separation of complex lipid profiles. Anal. Chem. 2020, 92, 8697–8703. 10.1021/acs.analchem.0c01747. [DOI] [PubMed] [Google Scholar]
- Ellis S. R.; Soltwisch J.; Paine M. R. L.; Dreisewerd K.; Heeren R. M. A. Laser post-ionisation combined with a high resolving power orbitrap mass spectrometer for enhanced MALDI-MS imaging of lipids. Chem. Commun. 2017, 53, 7246–7249. 10.1039/C7CC02325A. [DOI] [PubMed] [Google Scholar]
- Domann P.; Spencer D. I. R.; Harvey D. J. Production and fragmentation of negative ions from neutral N-linked carbohydrates ionized by matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 2012, 26 (4), 469–479. 10.1002/rcm.5322. [DOI] [PubMed] [Google Scholar]
- Calvano C. D.; Cataldi T. R. I.; Kogel J. F.; Monopoli A.; Palmisano F.; Sundermeyer J. Structural characterization of neutral saccharides by negative ion MALDI mass spectrometry using a superbasic proton sponge as deprotonating matrix. J. Am. Soc. Mass Spectrom. 2017, 28, 1666–1675. 10.1007/s13361-017-1679-y. [DOI] [PubMed] [Google Scholar]
- Dreisewerd K.; Schürenberg M.; Karas M.; Hillenkamp F. Influence of the laser intensity and spot size on the desorption of molecules and ions in matrix-assisted laser desorption/ionization with a uniform beam profile. Int. J. Mass Spectrom. Ion Processes 1995, 141 (2), 127–148. 10.1016/0168-1176(94)04108-J. [DOI] [Google Scholar]
- Knochenmuss R. Ion formation mechanisms in UV-MALDI. Analyst 2006, 131, 966–986. 10.1039/b605646f. [DOI] [PubMed] [Google Scholar]
- Dreisewerd K. The desorption process in MALDI. Chem. Rev. 2003, 103, 395–425. 10.1021/cr010375i. [DOI] [PubMed] [Google Scholar]
- Hamm G.; Bonnel D.; Legouffe R.; Pamelard F.; Delbos J.-M.; Bouzom F.; Stauber J. Quantitative mass spectrometry imaging of popranolol and olanzapine using tissue extinction calculation as normalization factor. J. Proteomics 2012, 75 (16), 4952–4961. 10.1016/j.jprot.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Heijs B.; Tolner E. A.; Bovée J. V. M. G.; van den Maagdenberg A. M. J. M.; McDonnell L. A. Brain region-specific dynamics of on-tissue protein digestions using MALDI mass spectrometry imaging. J. Proteome Res. 2015, 14 (12), 5348–5354. 10.1021/acs.jproteome.5b00849. [DOI] [PubMed] [Google Scholar]
- Taylor A. J.; Dexter A.; Bunch J. Exploring ion suppression in mass spectrometry imaging of a heterogeneous tissue. Anal. Chem. 2018, 90 (9), 5637–5645. 10.1021/acs.analchem.7b05005. [DOI] [PubMed] [Google Scholar]
- Shariatgorji M.; Nilsson A.; Fridjonsdottir E.; Vallianatou T.; Källback P.; Katan L.; Sävmarker J.; Mantas I.; Zhang X.; Bezard E.; Svenningsson P.; Odell L. R.; Andrén P. E. Comprehensive mapping of neurotransmitter networks by MALDI-MS imaging. Nat. Methods 2019, 16, 1021–1028. 10.1038/s41592-019-0551-3. [DOI] [PubMed] [Google Scholar]
- Leite J. F.; Hajivandi M. R.; Diller T.; Pope R. M. Remove of sodium and potassium adducts using a matrix additive during matrix-associated laser desorption/ionization time-of-flight mass spectrometeric analysis of peptides. Rapid Commun. Mass Spectrom. 2004, 18, 2953–2959. 10.1002/rcm.1711. [DOI] [PubMed] [Google Scholar]
- Lee C.; Lu I.-C.; Hsu H. C.; Lin H.-Y.; Liang S.-P.; Lee Y.-T.; Ni C.-K. Formation of metal-related ions in matrix-assisted laser desorption ionization. J. Am. Soc. Mass Spectrom. 2016, 27, 1491–1498. 10.1007/s13361-016-1424-y. [DOI] [PubMed] [Google Scholar]
- Harvey D. J.; Scarff C. A.; Crispin M.; Scanlan C. N.; Bonomelli C.; Scrivens J. H. MALDI-MS/MS with traveling wave ion mobility for the structural analysis of N-linked glycans. J. Am. Soc. Mass Spectrom. 2012, 23 (11), 1955–1966. 10.1007/s13361-012-0425-8. [DOI] [PubMed] [Google Scholar]
- Heijs B.; Holst S.; Briaire-De Bruijn I. H.; van Pelt G. W.; de Ru A. H.; van Veelen P. A.; Drake R. R.; Mehta A. S.; Mesker W. E.; Tollenaar R. A.; Bovée J. V. M. G.; Wuhrer M.; McDonnell L. A. Multimodal mass spectrometry imaging of N-glycans and proteons from the same tissue section. Anal. Chem. 2016, 88 (15), 7745–7753. 10.1021/acs.analchem.6b01739. [DOI] [PubMed] [Google Scholar]
- Harvey D. J. Fragmentation of negative ions from carbohydrates: part 2. Fragmentation of high-mannose N-linked glycans. J. Am. Soc. Mass Spectrom. 2005, 16 (5), 631–646. 10.1016/j.jasms.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.