Abstract
The treatment of severe traumatic brain injury (TBI) with brain herniation is challenging because outcomes are often associated with high mortality and morbidity. Our aim was to identity factors contributing to decompressive craniectomy (DC) and evaluate treatment outcomes in patients with severe TBI with brain herniation.
In this retrospective study, we analyzed medical records of severe TBI with brain herniation from May 2009 to December 2013. We reviewed their demographic data, mechanism of injury, Glasgow Coma Scale (GCS) score, pupil status, computed tomography findings, surgical treatment methods, time interval between brain herniation and surgery, as well as outcomes. GCS and pupil status are clinical parameters for detecting increase intracranial pressure while brain parenchyma bulged above the inner plate of the skull during operation indicated brain swelling as well as increased intracranial pressure on which basis the decision to perform DC or craniotomy was determined intraoperatively.
One hundred ninety-four patients were included in the study. We performed DC in 143 of the patients while 51 of them we performed craniotomy. There were no statistically significant differences in the age, gender, or injury mechanism between the 2 groups. GCS, pupillary dilation, midline shift, hematoma type and timing of surgery were associated with DC. Nevertheless, logistic regression analysis revealed that hematoma type and timing of surgery were significantly associated with favorable DC outcomes (P < .001 and P = .023). Subdural hematoma and timing of surgery >1 hour were both identified as risk factors for DC. Six months after TBI, 34.0% of patients exhibited favorable outcomes. Overall mortality rate was 30.4%. Age, GCS, pupil dilation, hematoma type, and timing of surgery were all associated with patient outcomes. Further logistic regression analysis revealed that, lower GCS, bilateral pupil dilation, timing of surgery >1 hour, and advanced age were independent risk factors for poor outcomes (P = .001, P = .037, P = .028, and P = .001, respectively).
Our study revealed that, DC is not mandatory for all TBI patients with brain herniation. Nevertheless, DC decreases mortality rate in severe TBI patients with brain herniation. Subdural hematoma and timing of surgery >1 hour are key indicators for DC. Lower GCS, bilateral pupil dilation, delayed timing of surgery and advance age are indicators of poor outcomes.
Keywords: craniectomy, decompressive, herniation, intracranial pressure, outcome, traumatic brain injury
1. Introduction
Treatment of severe traumatic brain injury (TBI) is challenging and often associated with high mortality and morbidity.[1,2] The mortality rate can be as high as 60% to 84.6% in TBI cases with brain herniation.[3] Although decompressive craniectomy (DC) has been used to treat severe TBI for decades, it is still controversial because of its inherent complications and treatment outcomes.[4–6] Whereas DC can reduce mortality, it can also increase the incidence of unfavorable outcomes; consequently, the decision to perform DC is always challenging.[7,8] Furthermore, there is significant uncertainty with regards to selecting the appropriate candidates for this procedure, and choosing the optimal time for surgery.[9,10]
Brain herniation is a critical side effect of TBI and DC maybe an appropriate choice of treatment for these patients. However, the outcome of DC for these patients can be affected by a range of factors including the Glasgow Coma Scale score (GCS), pupil enlargement, midline shift (MLS), and the type of intracranial hematoma.[11–13] To the best of our knowledge, the existing literature is very limited in terms of the treatment of patients with severe TBI with brain herniation.
At present, there is significant uncertainty as to whether it is necessary to perform DC for all patients with herniation. Therefore, we retrospectively collated and analyzed clinical data of 194 patients with severe TBI accompanied by brain herniation. Our aim was to identity factors which might need to be considered for DC and evaluate the outcomes of patients who underwent DC treatment due to TBI with brain herniations.
2. Method and materials
Clinical data from patients with severe TBI who were treated in West China Hospital, Sichuan University between May 2009 and December 2013 were retrospectively collected and analyzed. The ethical committee waived the informed consent requirement because this was a retrospective analysis. A clinical profile which included GCS score after resuscitation, pupillary status, patient's demographic data, injury mechanism, CT scans, surgical procedure, intra-operative findings, treatment outcome, and the time interval between brain herniation as well as craniectomy/craniotomy was created for each patient. Pupil status was the key clinical parameter for detecting increase intracranial pressure (ICP).
2.1. Patients inclusion criteria
Our specific inclusion criteria were as follows: closed TBI (Closed TBI results when an outside force impacts the head, but the skull is not broken, fractured, or penetrated); GCS ≤8 after resuscitation; signs of brain herniation; massive hematoma (volume >30 mL) confirmed by computed tomography (CT) scans; and surgery. Brain herniation in this study specifically refers to uncal herniation which was diagnosed by a series of criteria. The criteria were coma, pupillary enlargement, and signs of brain stem compression on CT scans. All these criteria were met before the patient was included in the study.
2.2. Patients exclusion criteria
Exclusion criteria were as follows:
-
(1)
history of craniotomy or any serious chronic illness;
-
(2)
penetrating injury;
-
(3)
respiratory or circulatory failure, or dead on arrival;
-
(4)
any extracranial injury that could affect the outcome; and
-
(5)
diffuse axonal injury without the presence of intracranial hematoma.
2.3. Radiological evaluation
Head CT was ranked in accordance with the Marshall CT classification.[14] MLS and basal cistern status were evaluated according to Bullock et al criteria.[15] MLS was recorded on a 3-point scale as one of the following: MLS < 5 mm, 5 mm ≤ MLS < 10 mm or MLS ≥ 10 mm. Hematomas were categorized into 3 sub-types: epidural hematoma (EDH); subdural hematoma (SDH) and intracerebral hematoma (ICH, including brain contusion). If multiple intracranial hematomas were present, they were classified according to the predominant hematoma.[16]
2.4. Surgical procedures
Emergency surgery was performed immediately after admission. Timing of surgery was defined as the time period between herniation detection and skin incision in the operating room, because the brain herniation may not come into formation at the same time of TBI events (as seen in delayed intracranial hematoma). Ultra-early surgery was defined as the commencement of surgery within 1 hour after the detection of herniation. A large fronto-temporo-parietal craniotomy was often used. After evacuating hematoma, the bone flap was removed or replaced, based on surgical findings. If the brain parenchyma bulged above the inner plate of the skull, the bone flap was removed with expansion duraplasty and DC was achieved. However, if the brain parenchyma did not bulge above the inner plate of the skull, and was pulsing normally, craniotomy was indicated and the bone flap replaced. Thus, brain parenchyma bulged above the inner plate of the skull during operation indicated brain swelling as well as increased ICP on which basis the decision to perform DC or craniotomy was determined intraoperatively. Furthermore, an ICP monitor (Codman MicroSensor, Johnson &Johnson Professional, Inc., Raynham, MA) was used. Basic treatments such as sedation, analgesia, hyperosmolar therapy as well as hypothermia therapy were administered in accordance to guidelines for the management of severe head injury.[17]
2.5. Patients outcomes
Outcomes were determined by the Glasgow Outcome Scale (GOS) score 6 months after injury. The 5 GOS categories (dead, vegetative state, severe disability, moderate disability, and mild disability) were reduced to 3 groups for data analysis (favorable, unfavorable, and dead). A favorable outcome was defined as a GOS score of 4 to 5. Patients were divided into 2 groups:
-
(1)
DC group, in which the bone flaps were removed without replacement and
-
(2)
craniotomy group, in which bone flaps were replaced during surgery.
2.6. Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS 25, Chicago, IL). Analysis included the Kruskal–Wallis test, Mann–Whitney U test, Chi square test, linear-by-linear association test and logistic regression analysis. P < .05 was considered to be statistically significant.
3. Results
Clinical data of severe TBI patients with brain herniation are described in Table 1. The patient consisted of 150 (77.3%) males out of the 194 patients included in the study while females were 44 (22.7%). The ages of the patients ranged from 3 to 83 years with a mean age of 40.9 years. Six pediatric patients who aged from 3 to 13 years old were also included due to the fact that most children over 3 years old are usually past the preverbal stage which normal refers to the infants under 2 years old. Hence, their language skills did not hinder the accurate judgment of GCS score and posed little bias to the study. Road traffic accidents involving 114 (58.8%) patients out of the 194 patients was the predominant cause of TBI with brain herniation. The basal cisterns were compressed in all 194 patients. Also, hematomas were consistent with Marshall CT classification V, in 88 patients (45.4%) with SDH, 56 patients (28.9%) with ICH and 50 patients (25.7%) with EDH.
Table 1.
Patient characteristics on admission (N = 194).

A total of 143 patients underwent DC and 32 patients (22.4%) experienced cerebral infarction. On the other hand, 51 patients underwent craniotomy and 1 patient (1.9%) suffered infarction. The rate of cerebral infarction in the DC group was significantly higher than that of the craniotomy group (P = .002). Furthermore, 62 patients (31.9%) underwent ultra-early surgery after brain herniation. The rate of DC in the ultra-early surgery patients was significantly lower than for other patients (33.8% vs 92.4%, P < .001) as in Table 2.
Table 2.
Relationship between different factors and DC.

Our analysis did not find any significant differences between the DC and craniotomy groups in terms of age, gender or the mechanism of injury. Compared to the craniotomy group, the DC group had lower GCS and a greater number of patients with bilateral pupil dilation, SDH, and ICP ≥30 mm Hg. GCS, pupillary dilation, MLS, hematoma type and time to surgery were all associated with DC (Table 2). However, logistic regression analysis revealed that only hematoma type and timing of surgery were significantly associated with DC. EDH was an independent factor associated with reduced DC. Patients with EDH had more chances of bone flap replacement after evacuation of hematoma (odds ration = 0.15). SDH and timing of surgery >1 h were risk factors for DC (Table 3). In other words, patients with SDH had higher chance of undergoing DC. More specifically, SDH patients are 3.6 times more likely to receive DC than ICH patients (odds ratio = 3.603).
Table 3.
Logistic regression analysis of factors relating to DC.

Patients’ GOS scores 6 months after TBI are shown in Table 4. In total, 34.0% of patients achieved a favorable outcome. Overall, the mortality rate was 30.4%. There was a significantly lower numbers of patients with favorable outcomes in the DC group than in the craniotomy group (34/143 = 23.7% vs 32/51 = 62.7%, P < .001). Mortality in the DC group was significantly higher than in the craniotomy group (54/143 = 3 7.8% vs 5/51 = 9.8%, P < .001). Only 106 of our patients underwent ICP monitoring according to the data retrieved for this retrospective study. However, analysis showed that patient outcomes were significantly different at various ICP levels (P < .001). The mortality rates for patients with an ICP ≥25 mm Hg, ICP ≥30 mm Hg and ICP ≥35 mm Hg was 62.7% (32/51), 84.8% (28/33) and 95.4% (21/22), respectively. GOS scores declined as ICP increased and a higher ICP was significantly associated with worse outcomes (linear-by-linear association test, P < .001). Nevertheless, ICP was not included in our logistic regression analysis because it could have caused statistical bias since all patients did not received ICP monitoring according to our retrieved data.
Table 4.
Postoperative clinical outcomes according to various predisposing factors.

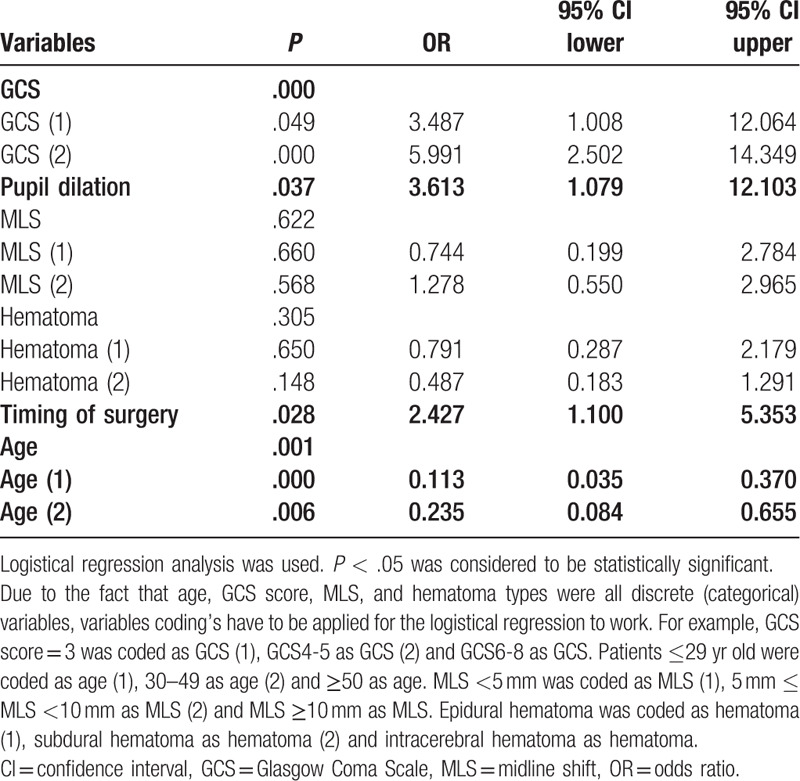
Age, GCS, pupil dilation, type of hematoma, and timing of surgery were all associated with patients’ outcomes (Table 5). Furthermore, logistic regression analysis showed that lower GCS, bilateral pupil dilation, timing of surgery >1 hour, and advanced age were independent risk factors of poor outcomes. Advanced age with a decreased GCS resulted in worse patients’ outcomes (P = .001 and P = .000, respectively). Patients with bilateral pupil dilation and timing of surgery >1 hour tend to have worse outcomes (odds ratio = 3.613 and 2.427, respectively). Pupil dilation signified brain swelling as well as ICP.
Table 5.
Logistic regression analysis for clinical outcome.
4. Discussion
The effectiveness of DC has been proven by many authors[18–20] but long-term research has shown that DC is associated with unfavorable outcomes.[21] Some studies have indicated that, DC does not necessarily lead to better outcomes as compared to maximal medical management for patients with severe TBI.[22,23] In areas with limited medical resources, DC has proven to be beneficial when performed <5 hours after injury in younger patients with a GCS >5.[22] Grandhi et al indicated, that DC was associated with worse postoperative outcome and places a considerable burden on society.[9] Nevertheless, brain herniation is a risk factor for poor outcomes in TBI patients. Therefore, our study emphases on the efficiency of DC in TBI patients with brain herniation as well as associated risk factors. We observed that, hematoma type and timing of surgery were significantly associated with favorable DC outcomes (P < .001 and P = .023). Initial studies did not indicate that, these parameters are factors contributing to outcomes in DC patients.
Severe TBI patients with brain herniation has a high chance of developing intracranial hypertension or cerebral infarction after craniotomy. Therefore, DC could be performed to prevent a secondary operation. All patients presented with mass effect with MLS of the brain that necessitated DC. DC was performed only when the brain matter bulged above the inner plate of the skull after evacuation of the hematomas. Most importantly, brain parenchyma bulged above the inner plate of the skull during operation indicated brain swelling as well as increased ICP on which basis the decision to perform DC or craniotomy was determined intraoperatively. Furthermore, although all patients did not receive ICP monitoring, the level of ICP in the craniotomy group seemed lower than that in the DC group. Only 11.1% of patients (3/27) presented with an ICP ≥30 mm Hg in the craniotomy group. The rate of infarction in the craniotomy group was lower than that in the DC group probably due less brain swelling as well as unelevated ICP in this patient category. We believe that, post-surgical infarction was as a result of vasospasms associated with the pathophysiological process of the TBI with brain herniation and not a consequence of DC or bone-flap replacement.
Nirula et al indicated that, early DC does not improve outcomes in patients with refractory intracranial hypertension as compared to medical treatment.[24] Park et al also indicated that, ultra-early DC for intracranial hypertension does not improve patient outcome. In their study, they compared early and late DC in the management of severe TBI patients.[25] Nevertheless, Bor-Seng-Shu et al observed that, not all patients who undergo DC developed brain edema or intracranial hypertension.[26] They therefore concluded that, DC was not necessary for some patient's category after evacuation of the hematoma. We noted that, SDH and timing of surgery were significantly associated with favorable DC outcomes (P < .001 and P = .023).
Our study revealed that, GCS, pupillary dilation, MLS, hematoma type and timing of surgery influence the decision to perform DC. Furthermore, GCS, pupil enlargement, MLS, as well as types of injury were clear indicators of severity TBI. The more severe the injury, the higher likelihood that a patient would develop intracranial hypertension as well as intraoperative bulging of brain matter above the inner plate of the skull as a result of brain swelling as well as ICP. The rate of DC in patients with a GCS ≤6 was significantly higher than that of patients with a GCS of 7 to 8 (P = .008). Phan et al reported that, a lower GCS correlated with a higher proportion of patients requiring DC.[27] In our study, the rate of DC in patients with bilateral pupil dilation was significantly higher than that in patients with unilateral pupil dilation (88.5% vs 68.3%, P = .005). We also found that, patients with SDH had the highest chances of receiving DC, while patients with EDH had the lowest chances of receiving DC. Studies have demonstrated that, in cases with acute SDH, the shear force at the time of trauma caused greater damage to the cerebral vasculature resulting into immediate ischemic brain damage, which subsequently leads to acute cerebral swelling.[18,25] Therefore, patients who suffered from SDH or ICH were more likely to develop excessive brain swelling intra-operatively, even after evacuation of the mass lesion, making them more likely candidates for DC. Also, patients with excessive brain swelling are more likely to develop ICP than patients with less brain swelling after TBI.
Currently, the timing of early surgical decompression is still controversial. Nevertheless, several authors are of the view that, early DC is capable of reversing expectant or initially secondary ischemia, or axonal injury due to refractory intracranial hypertension in TBI patients.[23,28,29] We advocate that, DC should be performed before irreversible neurological deficit sets in, especially when brain herniation has occurred. The longer the duration of herniation, the more likely it is that patient will develop intracranial hypertension as well as ischemia. Our logistic regression analysis revealed that, patients with EDH has lesser chances of undergoing DC while patients with SDH as well as timing of surgery >1 hour were also identified DC indicators. We advocate that, DC should be perform in SDH or ICH patient with GCS ≤6 and timing to surgery >1 hour. On other hand, during surgery, we advocate that, the bone flap should be replaced after evacuation of the mass lesion if the brain matter did not herniate above the skull intraoperatively.
Our study revealed that, age, GCS, pupil dilation, type of hematoma, post-operation ICP, and timing of surgery were associated with patient outcome. Studies have proven that, GCS, pupillary reactivity, and types of mass lesion are indicators of outcome in patients with TBI.[30,31] Also, MLS was an indicator of hematoma volume and thus an indicator of patients’ outcomes.[32] However, in the current study, we found that MLS was not associated with outcomes. Also, Sauvigny et al did not find any significant differences between pre-surgical and post-surgical MLS TBI patients.[33] Lee et al demonstrated that, the prognostic value of MLS should be limited to patients with unilateral mass lesions.[28]
Several authors have reported the relationship between old age and high mortality. Howard et al reported a mortality rate of 74% in those >65 years of age, in contrast to an 18% mortality rate in those aged between 18 and 40 years.[18] Wilberger et al also found a significantly higher mortality rate in >65-years group than <65-years group (82% vs 54%).[34,35] Our present study yielded results that were similar to these earlier studies. This was probably due to the high rate of extracranial complications associated with the older age group after severe TBI.[23,28,29]
Bor-Seng-Shu et al indicated that, DC can effectively reduce ICP and increase cerebral perfusion pressure in patients with TBI and refractory elevated ICP.[36] However, majority of deaths following decompression occurred as a result of uncontrolled brain swelling and extensive brain infarction.[37] Using multivariate analysis, Grille et al. also found that only the presence of post-DC intracranial hypertension was significantly associated with poor outcomes.[38] We also found that higher postoperative ICP was associated with worse outcomes. However, ICP was not used in our logistic regression analysis because it could have caused statistical bias. Our retrieved data indicated that, only 106 patients received ICP monitoring. ICP monitoring results correlated well with our criteria that, brain parenchyma bulged above the inner plate of the skull during operation indicated brain swelling as well as increased ICP on which basis the decision to perform DC or craniotomy was determined intraoperatively. Further logistic regression analysis showed that lower GCS, bilateral pupil dilation, a timing of surgery >1 hour, and advanced age were independent risk factors of poor outcomes.
Cooper et al DC trial for patients with severe TBI (DECRA) indicated that, DC could increase mortality and was associated with more unfavorable outcomes.[39] In our study, logistic regression analysis revealed that only hematoma type (SDH and ICH) and timing of surgery were significantly associated with favorable DC outcomes (P < .001 and P = .023). Also, our study revealed 23.7% favorable outcomes following DC as compare to craniotomy. Furthermore, the randomized evaluation of surgery with craniectomy for uncontrollable elevation of intracranial pressure trial indicated that DC is capable of reducing mortality.[19,40] In our current study, the proportion of mortality and unfavorable outcomes in the DC group were higher than those in the craniotomy group because of associated brain herniations.
Our present study could be limited because it was a single center, retrospective study. A multi-center study will be more conclusive. Also, post-operative ICP status were not available for all the patients. Furthermore, factors such as cerebral perfusion pressure and cerebral blood flow could not be quantified due to limited data on ICP monitoring.
5. Conclusions
Our study demonstrated that, hematoma type (SDH and ICH) and timing of DC were key influential outcome factors in TBI patients with brain herniation. SDH and a timing of surgery >1 hour are indicators for DC. Lower GCS, bilateral pupil dilation, and advance age are indicators of poor outcomes. Also, DC does not necessarily increase mortality in severe TBI patients with brain herniation. Also, brain parenchyma bulged above the inner plate of the skull during operation indicated brain swelling as well as increased ICP which could be adopted as a possible criterion for DC or craniotomy in severe TBI patients with brain herniation.
Author contributions
Conceptualization: Zhigang Lan, Seidu A Richard, Qiang Li, Cong Wu, Qiao Zhang, Ruiqi Chen, Chaohua Yang.
Data curation: Zhigang Lan, Seidu A Richard, Qiang Li, Cong Wu, Qiao Zhang, Ruiqi Chen, Chaohua Yang.
Formal analysis: Zhigang Lan, Seidu A Richard, Qiang Li, Cong Wu, Qiao Zhang, Ruiqi Chen, Chaohua Yang.
Funding acquisition: Chaohua Yang.
Methodology: Zhigang Lan, Seidu A Richard, Qiang Li, Cong Wu, Qiao Zhang, Ruiqi Chen, Chaohua Yang.
Resources: Chaohua Yang.
Supervision: Chaohua Yang.
Writing – original draft: Zhigang Lan, Seidu A Richard, Chaohua Yang.
Writing – review & editing: Seidu A Richard, Qiang Li, Cong Wu, Qiao Zhang, Ruiqi Chen, Chaohua Yang.
Footnotes
Abbreviations: CT = computed tomography, DC = decompressive craniectomy, EDH = epidural hematoma, GCS = Glasgow Coma Scale score, GOS = Glasgow Outcome Scale, ICH = intracerebral hematoma, ICP = intracranial pressure, MLS = midline shift, SDH = subdural hematoma, TBI = traumatic brain injury.
How to cite this article: Lan Z, Richard SA, Li Q, Wu C, Zhang Q, Chen R, Yang C. Outcomes of patients undergoing craniotomy and decompressive craniectomy for severe traumatic brain injury with brain herniation: A retrospective study. Medicine. 2020;99:43(e22742).
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
This work was supported by the Natural Science Foundation of Sichuan Province of China (Grant Number: 0040205302272).
The authors have no conflicts of interest to disclose.
References
- [1].Hutchinson PJ, Kolias AG, Tajsic T, et al. Consensus statement from the international consensus meeting on the role of decompressive craniectomy in the management of traumatic brain injury. Acta Neurochirurgica 2019;161:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Manfiotto M, Beccaria K, Rolland A, et al. Decompressive craniectomy in children with severe traumatic brain injury: a multicenter retrospective study and literature review. World Neurosurg 2019;129:e56–62. [DOI] [PubMed] [Google Scholar]
- [3].2001;Clusmann H, Schaller C, Schramm J. Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: management and outcome, Journal of neurology, neurosurgery, and psychiatry. 71:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database System Rev 2006;CD003983. [DOI] [PubMed] [Google Scholar]
- [5].Liang Q, Chen X, Liu D, et al. Analysis of the Curative Effect of a New Decompressive Craniectomy on the Treatment of Severe Craniocerebral Injury. Journal of Brain and Neurology, 2018;1 [Google Scholar]
- [6].Goker B, Guclu DG, Dolas I, et al. Clinical study of decompressive craniectomy in children. Turkish Neurosurg 2020;30:225–30. [DOI] [PubMed] [Google Scholar]
- [7].Honeybul S, Ho KM, Lind CRP, et al. The current role of decompressive craniectomy for severe traumatic brain injury. J Clin Neurosci 2017;43:11–5. [DOI] [PubMed] [Google Scholar]
- [8].Shim HK, Yu SH, Kim BC, et al. Relationship between clinical outcomes and superior sagittal sinus to bone flap distance during unilateral decompressive craniectomy in patients with traumatic brain injury: experience at a single trauma center. Korean J Neurotrauma 2018;14:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grandhi R, Bonfield CM, Newman WC, et al. Surgical management of traumatic brain injury: a review of guidelines, pathophysiology, neurophysiology, outcomes, and controversies. J Neurosurg Sci 2014;58:249–59. [PubMed] [Google Scholar]
- [10].Shah A, Almenawer S, Hawryluk GW. Timing of decompressive hemicraniectomy for ischemic stroke and traumatic brain injury: a review. Front Neurol 2019;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang JY, Gao GY, Li WP, et al. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma 2002;19:869–74. [DOI] [PubMed] [Google Scholar]
- [12].Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury: update of the brain trauma foundation guidelines, executive summary. Neurosurgery 2019;84:1169–78. [DOI] [PubMed] [Google Scholar]
- [14].Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma 1992;9: Suppl 1: S287–92. [PubMed] [Google Scholar]
- [15].Bullock R, Chesnut R, Ghajar J, et al. Guidelines for the surgical management of traumatic brain injury 2006;58:S2–62. [Google Scholar]
- [16].Caroli M, Locatelli M, Campanella R, et al. Multiple intracranial lesions in head injury: clinical considerations, prognostic factors, management, and results in 95 patients. Surg Neurol 2001;56:82–8. [DOI] [PubMed] [Google Scholar]
- [17].Carney N, Totten AM, Oʼreilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017;80:6–15. [DOI] [PubMed] [Google Scholar]
- [18].Howard JL, Cipolle MD, Anderson M, et al. Outcome after decompressive craniectomy for the treatment of severe traumatic brain injury. J Trauma 2008;65:380–5. [DOI] [PubMed] [Google Scholar]
- [19].Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 2016;375:1119–30. [DOI] [PubMed] [Google Scholar]
- [20].Jiang JY, Xu W, Li WP, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma 2005;22:623–8. [DOI] [PubMed] [Google Scholar]
- [21].Quintard H, Lebourdon X, Staccini P, et al. Decompression surgery for severe traumatic brain injury (TBI): A long-term, single-centre experience. Anaesth Crit Care Pain Med 2015;34:79–82. [DOI] [PubMed] [Google Scholar]
- [22].Barthelemy EJ, Melis M, Gordon E, et al. Decompressive craniectomy for severe traumatic brain injury: a systematic review. World Neurosurg 2016;88:411–20. [DOI] [PubMed] [Google Scholar]
- [23].Woertgen C, Rothoerl RD, Schebesch KM, et al. Comparison of craniotomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci 2006;13:718–21. [DOI] [PubMed] [Google Scholar]
- [24].Nirula R, Millar D, Greene T, et al. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. J Trauma Acute Care Surg 2014;76:944–52. [DOI] [PubMed] [Google Scholar]
- [25].Park JH, Park JE, Kim SH, et al. Outcomes of ultra-early decompressive craniectomy after severe traumatic brain injury-treatment outcomes after severe TBI. Korean J Neurotrauma 2014;10:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bor-Seng-Shu E, Figueiredo EG, Fonoff ET, et al. Decompressive craniectomy and head injury: brain morphometry, ICP, cerebral hemodynamics, cerebral microvascular reactivity, and neurochemistry. Neurosurg Rev 2013;36:361–70. [DOI] [PubMed] [Google Scholar]
- [27].Phan K, Moore JM, Griessenauer C, et al. Craniotomy versus decompressive craniectomy for acute subdural hematoma: systematic review and meta-analysis. World Neurosurg 2017;101:677–85.e2. [DOI] [PubMed] [Google Scholar]
- [28].Hu Y, Sun H, Yuan Y, et al. Acute bilateral mass-occupying lesions in non-penetrating traumatic brain injury: a retrospective study. BMC Surg 2015;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lee JJ, Segar DJ, Morrison JF, et al. Subdural hematoma as a major determinant of short-term outcomes in traumatic brain injury. J Neurosurg 2018;128:236–49. [DOI] [PubMed] [Google Scholar]
- [30].Arbabi S, Jurkovich GJ, Wahl WL, et al. A comparison of prehospital and hospital data in trauma patients. J Trauma 2004;56:1029–32. [DOI] [PubMed] [Google Scholar]
- [31].Majdan M, Steyerberg EW, Nieboer D, et al. Glasgow coma scale motor score and pupillary reaction to predict six-month mortality in patients with traumatic brain injury: comparison of field and admission assessment. J Neurotrauma 2015;32:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nelson DW, Nystrom H, MacCallum RM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma 2010;27:51–64. [DOI] [PubMed] [Google Scholar]
- [33].Sauvigny T, Gottsche J, Vettorazzi E, et al. New radiologic parameters predict clinical outcome after decompressive craniectomy. World Neurosurg 2016;88:519–25.e1. [DOI] [PubMed] [Google Scholar]
- [34].Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 1991;74:212–8. [DOI] [PubMed] [Google Scholar]
- [35].Wilberger JE, Jr, Harris M, Diamond DL. Acute subdural hematoma: morbidity and mortality related to timing of operative intervention. J Trauma 1990;30:733–6. [PubMed] [Google Scholar]
- [36].Brain Trauma F. American Association of Neurological S, Congress of Neurological S, Joint Section on N, Critical Care AC, Bratton SL, et al. Guidelines for the management of severe traumatic brain injury. XIV. Hyperventilation. J Neurotrauma 2007;24: Suppl 1: S87–90. [DOI] [PubMed] [Google Scholar]
- [37].Huang YH, Lee TC, Lee TH, et al. Thirty-day mortality in traumatically brain-injured patients undergoing decompressive craniectomy. J Neurosurg 2013;118:1329–35. [DOI] [PubMed] [Google Scholar]
- [38].Grille P, Tommasino N. Decompressive craniectomy in severe traumatic brain injury: prognostic factors and complications. Rev Bras Ter Intensiva 2015;27:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011;364:1493–502. [DOI] [PubMed] [Google Scholar]
- [40].Sharma R, Garg K. Role of decompressive craniectomy in traumatic brain injury—how much wiser are we after randomized evaluation of surgery with craniectomy for uncontrollable elevation of intracranial pressure trial? Neurosurgery 2017;81:E58–60. [DOI] [PubMed] [Google Scholar]


