Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, epoprostenol, nitric oxide, pulmonary vasodilator, refractory hypoxemia
Abstract
Objectives:
The objectives of this study were to evaluate the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide in patients with refractory hypoxemia secondary to coronavirus disease 2019.
Design:
Retrospective single-center study.
Setting:
ICUs at a large academic medical center in the United States.
Patients:
Thirty-eight adult critically ill patients with coronavirus disease 2019 and refractory hypoxemia treated with either inhaled epoprostenol or inhaled nitric oxide for at least 1 hour between March 1, 2020, and June 30, 2020.
Interventions:
Electronic chart review.
Measurements and Main Results:
Of 93 patients screened, 38 were included in the analysis, with mild (4, 10.5%), moderate (24, 63.2%), or severe (10, 26.3%), with acute respiratory distress syndrome. All patients were initiated on inhaled epoprostenol as the initial pulmonary vasodilator and the median time from intubation to initiation was 137 hours (68–228 h). The median change in Pao2/Fio2 was 0 (–12.8 to 31.6) immediately following administration of inhaled epoprostenol. Sixteen patients were classified as responders (increase Pao2/Fio2 > 10%) to inhaled epoprostenol, with a median increase in Pao2/Fio2 of 34.1 (24.3–53.9). The mean change in Pao2 and Spo2 was –0.55 ± 41.8 and –0.6 ± 4.7, respectively. Eleven patients transitioned to inhaled nitric oxide with a median change of 11 (3.6–24.8) in Pao2/Fio2. A logistic regression analysis did not identify any differences in outcomes or characteristics between the responders and the nonresponders. Minimal adverse events were seen in patients who received either inhaled epoprostenol or inhaled nitric oxide.
Conclusions:
We found that the initiation of inhaled epoprostenol and inhaled nitric oxide in patients with refractory hypoxemia secondary to coronavirus disease 2019, on average, did not produce significant increases in oxygenation metrics. However, a group of patients had significant improvement with inhaled epoprostenol and inhaled nitric oxide. Administration of inhaled epoprostenol or inhaled nitric oxide may be considered in patients with severe respiratory failure secondary to coronavirus disease 2019.
The acute respiratory distress syndrome (ARDS) is an inflammatory process that is associated with decreased lung compliance, severe hypoxemia, and increased pulmonary shunt (1). ARDS typically develops within 7 days of initial symptom onset or clinical insult and is associated with mortality rates of 40–45% (1–3). The pathophysiology of ARDS includes diffuse alveolar damage occurring early in the exudative phase, leaky alveolar capillaries, and pulmonary edema (1). ARDS can lead to a reduction in lung compliance and severe hypoxemia, along with insults to other organs. The Berlin criteria further define ARDS into three stages based on a patient’s Pao2/Fio2 on positive end-expiratory pressure (PEEP) greater than or equal to 5 cm H2O: mild (200 mm Hg < Pao2/Fio2 ≤ 300 mm Hg), moderate (100 mm Hg < Pao2/Fio2 ≤ 200 mm Hg), or severe (Pao2/Fio2 ≤ 100 mm Hg) (3).
Optimal management of ARDS is complex and centers around strategies that improve the efficacy and safety of mechanical ventilation, such utilization of lower tidal volumes and higher PEEP, prone-positioning, and use of neuromuscular blocking agents (1, 4–7). Inhaled pulmonary vasodilators, such as inhaled epoprostenol (iEPO) and inhaled nitric oxide (iNO), have been shown to improve hypoxemia by increasing blood flow to well-ventilated portions of the lung, leading to improvements in ventilation and perfusion matching (8–10). In addition to refractory hypoxemia secondary to ARDS, iEPO and iNO may provide benefits in patients with pulmonary arterial hypertension or right heart dysfunction (11). Despite a lack of data demonstrating improvements in end points such as mortality with pulmonary vasodilator use, iNO and iEPO are considered as adjunctive therapies in patients with ARDS or right ventricular dysfunction (12–15).
Patients who are diagnosed with coronavirus disease 2019 (COVID-19) from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may develop respiratory distress that requires mechanical ventilation (16–22). A recent analysis found 29% of patients COVID-19 had respiratory failure that progressed to ARDS (23). The differences, if any, between ARDS related to COVID-19 versus another etiology are still unknown, but inhaled vasodilators have been recommended as potential options for refractory hypoxemia (2, 16). The purpose of this analysis was to evaluate the efficacy and safety of pulmonary vasodilators (iEPO or iNO) in patients with COVID-19 and refractory hypoxemia.
MATERIALS AND METHODS
This was a retrospective, observational study at Brigham and Women’s Hospital that received Partners institutional review board approval. We identified consecutive adult patients with COVID-19 and ARDS that were admitted to any ICU and received either iEPO or iNO while mechanically ventilated. Patients had to receive at least 1 hour of either iEPO or iNO and have results from an arterial blood gas (ABG) within the first 6 hours of therapy. Patients were excluded if they received extracorporeal membrane oxygenation support prior to initiation of the pulmonary vasodilator, concomitant iEPO and iNO, or parenteral prostacyclin. Patients were also excluded if they did not have a baseline ABG, if their baseline ABG was greater than 6 hours prior to initiation, if were transferred from an outside institution on iNO or iEPO, or if neuromuscular blockade or prone-positioning was initiated between ABGs checked at baseline and after initiation of inhaled vasodilator therapy.
Brigham and Women’s Hospital’s full guideline related to caring for patients with COVID-19 can be found at covidprotocols.org (24). The definition of ARDS is consistent with Berlin criteria (acute onset, bilateral opacities, and Pao2/Fio2 < 300 mm Hg with a minimum PEEP of 5 cm H2O, not explained by cardiac failure or fluid overload). Our guideline recommends consideration of pulmonary vasodilator therapy in mechanically ventilated patients with ARDS after or in conjunction with other strategies, such as use of low tidal-volume ventilation, best PEEP protocol, prone-positioning, and neuromuscular blockade (24). Patients with alveolar hemorrhage or left ventricular dysfunction are excluded from receiving pulmonary vasodilators. Our recommended dose range for iNO and iEPO is 1–80 ppm and 0.01–0.05 mcg/kg/min, respectively. iEPO is our first-line pulmonary vasodilator and is initiated at 0.05 mcg/kg/min based on ideal body weight and recommended to continue if the Pao2 increases greater than 10% from baseline. If patients do not respond to iEPO (less than 10% improvement in Pao2 or Pao2/Fio2 ratio), iNO is considered after iEPO discontinuation and is initiated at 20 ppm, with a recommendation to titrate up to 80 ppm if the Pao2 does not increase greater than 10%. ABGs are recommended at baseline and 2 hours after pulmonary vasodilator therapies are initiated. Weaning of both agents is done slowly over several hours with recommendation to restart or uptitrate if Pao2 decreases.
Pertinent patient data were collected, such as Acute Physiology and Chronic Health Evaluation (APACHE) II score on ICU admission, Sequential Organ Failure Assessment score prior to iEPO initiation, baseline demographics, and relevant past medical history. The main end point was the change in Pao2/Fio2 ratio after initiation of pulmonary vasodilator therapy. Other oxygenation metrics, such as the change in Pao2, pulse oxygenation saturation (Spo2), and the percentage of patients with an increase of at least 10% in Pao2 and Pao2/Fio2 ratio, were collected. Patients were classified as “responders” to iEPO or iNO if they had an increase in Pao2/Fio2 ratio greater than 10% on the next ABG. Characteristics of responders and nonresponders were compared. Ventilator settings and oxygenation metrics were collected at baseline and during the first 24 hours of therapy. The average dose and duration for iEPO and iNO were assessed in all patients. Adverse drug events were collected using the following criteria: bleeding (International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee bleeding assessment tool), methemoglobinemia (> 2%), tachycardia (new onset heart rate > 100 beats/min or 20% increase within 2 hr), hypotension (new onset mean arterial pressure < 65 mm Hg or 20% increase in vasopressors in 2 hr), and thrombocytopenia (platelet count decreased to < 50,000/µL during therapy) were collected. Hemodynamic effects were assessed within 2 hours of initiation, whereas other adverse drug events were assessed throughout entire course of inhaled pulmonary vasodilator.
We summarized categorical data using frequencies and percentages. Continuous data were summarized using mean and sds or medians and interquartile ranges where appropriate. Chi-square test or Fisher exact test was used when appropriate for categorical data. Continuous data were analyzed using paired t test or unpaired t test, where appropriate. An alpha of less than or equal to 0.05 was deemed statistically significant for all tests. We used a multivariable logistic regression model to investigate possible predictors of responsiveness to iEPO therapy. We included variables with p value of less than 0.2 from the univariate analysis as well as ideal body weight and APACHE II score based on previous literature. Statistical tests were performed using Stata Statistical Software, Version 15.1 (StataCorp, College Station, TX).
RESULTS
Overall, 93 patients who received inhaled pulmonary vasodilators were screened for study inclusion, of which 47 were excluded for the following reasons: negative for SARS-CoV-2 (n = 41), ABG not drawn within 6 hours prior or after initiating iEPO (n = 3), no baseline ABG (n = 2), baseline Pao2/Fio2 greater than 300 (n = 1), and received iEPO prior to admission (n = 1). Of the 46 patients that met inclusion criteria, eight were subsequently excluded due to initiation of neuromuscular blockade and/or prone-positioning between the baseline and postinhaled vasodilator therapy ABGs. Baseline characteristics of the 38 remaining patients that were included are summarized in Table 1. Overall, most patients had moderate or severe ARDS at the time of inhaled pulmonary vasodilator initiation. Prone-positioning and neuromuscular blockade were initiated at some point during the ICU course in almost all patients. The median time from intubation to initiation of iEPO was 158 hours. Other therapies related to COVID-19 or critical illness, which were trialed prior to the start of inhaled pulmonary vasodilators, are listed in Table 1. Adjunctive therapies administered are displayed according to the date patients tested positive for SARS-CoV-2 in the Supplementary Table (http://links.lww.com/CCX/A385).
TABLE 1.
Baseline Demographics of Patients Receiving Inhaled Pulmonary Vasodilators
| Variable | n = 38 |
|---|---|
| Age, yr (mean ± sd) | 61 ± 12 |
| Female (n [%]) | 14 (36.8) |
| Height, in. (mean ± sd) | 66.9 ± 4 |
| Weight (actual), kg (mean ± sd) | 89.5 ± 19 |
| Weight (ideal), kg (mean ± sd) | 64.9 ± 11.5 |
| Body mass index, kg/m2 | 30.8 ± 5.5 |
| Acute Physiology and Chronic Health Evaluation II on ICU admission (mean ± sd) | 26.9 ± 8.5 |
| Sequential Organ Failure Assessment on iEPO initiation (mean ± sd) | 12.1 ± 2.8 |
| Past medical history (n [%]) | |
| Systolic heart failure | 2 (5.3) |
| Chronic obstructive pulmonary disease | 2 (5.3) |
| Asthma | 5 (13.2) |
| Hypertension | 23 (60.5) |
| Diabetes mellitus | 15 (39.5) |
| Coronary artery disease | 4 (10.5) |
| Pulmonary arterial hypertension | 0 |
| Malignancy | 4 (10.5) |
| Transplant | 1 (2.6) |
| Chronic kidney disease | 4 (10.5) |
| Liver disease | 1 (2.6) |
| Echo during ICU admission (n [%]) | 21 (55.3) |
| RV mild dilatation | 1 (2.6) |
| RV strain | 2 (5.3) |
| RV reduced function | 4 (10.5) |
| Initial inhaled pulmonary vasodilator (n [%]) | |
| iEPO | 38 (100) |
| Inhaled nitric oxide | 0 (0) |
| Time from intubation to iEPO, hr (mean ± sd) | 157.8 ± 114.8 |
| Transfer from another institution (n [%]) | 27 (71.1) |
| Intubated at another institution (n [%]) | 25 (65.8) |
| Acute respiratory distress syndrome severity at inhaled pulmonary vasodilator initiation (n [%]) | |
| Mild | 4 (10.5) |
| Moderate | 24 (63.2) |
| Severe | 10 (26.3) |
| Invasive mechanical ventilation (n [%]) | 38 (100) |
| Therapeutic anticoagulation (n [%]) | 12 (31.6) |
| Vasopressors (n [%]) | 25 (65.8) |
| Other therapies trialed prior to iEPO (n [%]) | |
| Neuromuscular blockade | 34 (89.5) |
| Prone-positioning | 33 (86.8) |
| Hydroxychloroquine | 22 (57.9) |
| Tocilizumab | 10 (26.3) |
| Steroids | 4 (10.5) |
| Dornase | 3 (7.9) |
iEPO = inhaled epoprostenol; iNO = inhaled nitric oxide; RV = right ventricle.
All 38 patients received iEPO as the initial inhaled pulmonary vasodilator and 11 transitioned to iNO during their admission. A complete list of ventilator settings, mechanics, and oxygenation metrics can be found in Table 2. The median starting dose of iEPO was 0.05 mcg/kg/min. The median change in the Pao2/Fio2 ratio and Pao2/Fio2 percentage was 0 (–12.8–31.6) and 0% (–9.5 to 30.4%), respectively (Fig. 1). Sixteen of 38 patients (42.1%) were classified as responders and 11 patients (28.9%) had an increase of at least 10% in Pao2. In the 16 responders to iEPO, the median change in Pao2/Fio2 and Pao2/Fio2 percentage was 34.1 (24.3–53.9) and 31.7% (25.7–40.5%), respectively (Fig. 2). The median change in Pao2 and Pao2 percentage was 22.5 mm Hg (7.5–38.3 mm Hg) and 29.4% (10.1–44.4%), respectively, among responders.
TABLE 2.
Effectiveness of Inhaled Pulmonary Vasodilators
| Outcome | Before iEPO (n = 38) | After iEPO (n = 38) | Before iNO (n = 11) | After iNO (n = 11) | p |
|---|---|---|---|---|---|
| Time of baseline ABG, hr (mean ± sd) | 2.6 ± 2.2 | 1.6 ± 1.4 | |||
| Time of first post-iPVD ABG, hr (mean ± sd) | 2.7 ± 2.3 | 2 ± 0.9 | |||
| Proned at time of iPVD start (n [%]) | 19 (50) | 19 (50) | 6 (54.5) | 6 (54.5) | |
| Paralyzed at time of iPVD start (n [%]) | 26 (68.4) | 26 (68.4) | 11 (100) | 11 (100) | |
| Baseline ABG while on iPVD (n [%]) | 0 (0) | 5 (45.5) | |||
| Pao2 (mean ± sd) | 90 ± 36 | 89 ± 38 | 84 ± 26 | 98 ± 27 | |
| Fio2 (mean ± sd) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | |
| Spo2 (mean ± sd) | 94 ± 4.1 | 93 ± 6 | 93 ± 5 | 96 ± 2 | |
| Pao2:Fio2 (mean ± sd) | 130 ± 49 | 138 ± 56 | 119 ± 51 | 133 ± 48 | |
| PEEP (mean ± sd) | 15 ± 3 | 15 ± 3 | 16 ± 3 | 16 ± 3 | |
| Tidal volume (mean ± sd) | 389 ± 90 | 392 ± 90 | 372 ± 74 | 384 ± 74 | |
| Respiratory rate | 25 ± 6 | 26 ± 5 | |||
| Compliance | 32 ± 10 (n = 26) | 35 ± 15 (n = 21) | |||
| Resistance | 13 ± 5 (n = 26) | 12 ± 3 (n = 22) | |||
| Plateau pressure | 27 ± 4 (n = 30) | 28 ± 4 (n = 24) | |||
| Change Pao2:Fio2 (mean ± sd) | 7.8 ± 40.8 | 13.6 ± 15.8 | 0.56 | ||
| Percent change Pao2:Fio2 (mean ± sd) | 9.6 ± 30.2 | 15.2 ± 16.4 | 0.58 | ||
| Increase > 10% Pao2:Fio2α | 16 (42.1) | 7 (63.6) | 0.21 | ||
| Change Pao2 (mean ± sd) | –0.55 ± 41.8 | 14 ± 14.3 | 0.23 | ||
| Pao2 increase > 10 mm Hg (mean ± sd) | 11 (28.9) | 6 (54.5) | 0.12 | ||
| Pao2 increase > 10%α | 12 (31.6) | 7 (63.6) | 0.06 | ||
| Change Spo2 (mean ± sd) | –0.6 ± 4.7 | 2.9 ± 4.3 | 0.14 | ||
| Increased PEEP (n [%]) | 2 (5.3) | 0 (0) | 1 | ||
| Change in PEEP (mean ± sd) | –0.14 ± 1.1 | 0 ± 0 | n/a | ||
| Increased Fio2 (n [%]) | 3 (7.9) | 2 (18.2) | 0.1 | ||
| Change in Fio2 (mean ± sd) | –0.05 ± 0.13 | 0.1 ± 0 | 0.37 |
ABG = arterial blood gas; iEPO = inhaled epoprostenol; iNO = inhaled nitric oxide; iPVD = inhaled pulmonary vasodilator; PEEP = positive end-expiratory pressure.
Figure 1.
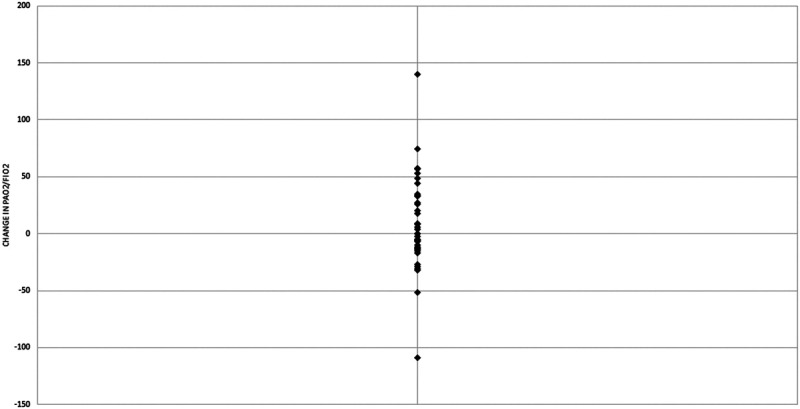
Change in Pao2/Fio2 following initiation of inhaled epoprostenol.
Figure 2.
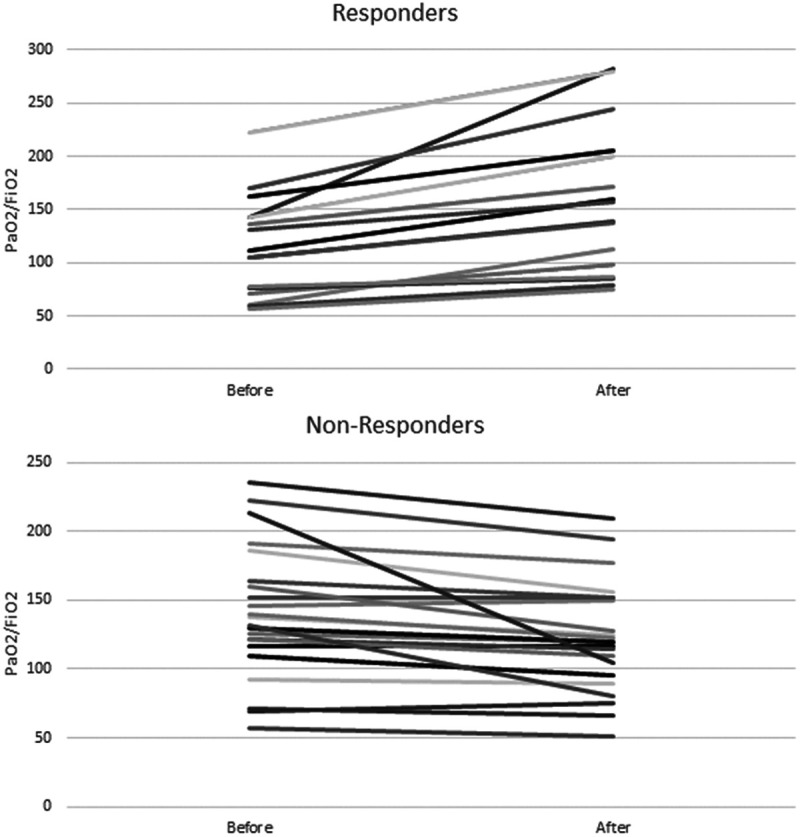
Pao2/Fio2 before and after inhaled epoprostenol in responders (n = 16) and nonresponders (n = 22).
The median starting dose of iNO in the 11 patients transitioned was 20 ppm (20–30 ppm). The iNO dose was eventually increased to 80 ppm in seven patients (64%). The median change in the Pao2/Fio2 ratio and Pao2/Fio2 percentage was 11 (3.6–24.8) and 16.7% (1.6–25.8%), respectively. There was an increase by at least 10% in seven of the 11 patients (63.4%) in both Pao2/Fio2 and Pao2. In the seven patients who were classified as responders to iNO, the median increase in Pao2/Fio2 and Pao2/Fio2 percentage was 23.2 (16.5–28.2) and 25.2% (18.3–30.7%), respectively. The median increase in Pao2 and Pao2 percentage was 26 mm Hg (16.5–29.5 mm Hg) and 34.9% (21–41.3%), respectively. There was a trend toward more significant improvements in Pao2 and Spo2 while receiving iNO compared with iEPO, but it was not statistically significant (Table 2).
The mean durations of infusion of iEPO and iNO are shown in Table 3. iEPO was continued for 77.7 hours in responders and 26.2 hours in nonresponders, whereas iNO was administered for 58.3 and 35.9 hours, respectively. Overall outcomes, such as adverse events, duration of mechanical ventilation, and length of stay, are found in Table 3. Few side effects, such as new onset hypotension or tachycardia, bleeding, or thrombocytopenia, occurred. Six patients (50%) that received iNO developed methemoglobin levels greater than 2%.
TABLE 3.
Outcomes of Patients on Inhaled Vasodilator Therapy
| Variable | iEPO (n = 38) | iNO (n = 11) | Overall (n = 38) |
|---|---|---|---|
| iEPO duration, hr (mean ± sd) | 47.9 ± 58.6 | ||
| iNO duration, hr (mean ± sd) | 50.2 ± 31.3 | ||
| Initial dose (mean ± sd) | 0.05 ± 0 mcg/kg/min | 29.1 ± 18.7 ppm | |
| New hypotension (n [%]) | 1 (2.6) | 0 (0) | |
| New tachycardia (n [%]) | 1 (2.6) | 0 (0) | |
| Bleeding (n [%]) | 4 (10.5) | 2 (18.2) | |
| Patients administered packed red blood cells (n [%]) | 2 (5.3) | 2 (18.2) | |
| Thrombocytopenia (n [%]) | 1 (2.6) | 0 (0) | |
| Methemoglobinemia (n [%]) | 0 (0) | 6 (55) | |
| Duration mechanical ventilation, hr (mean ± sd) | 443.9 ± 239.1 | ||
| ICU length of stay, hr (mean ± sd) | 524.5 ± 318.1 | ||
| ICU mortality (n [%]) | 19 (50) | ||
| Reintubations (n [%]) | 6 (15.8) | ||
| Tracheostomy (n [%]) | 6 (15.8) |
iEPO = inhaled epoprostenol, iNO = inhaled nitric oxide.
Responders to iEPO were compared with nonresponders in their baseline characteristics, concomitant therapeutic interventions, and time from intubation to initiation of iEPO (Table 4). No differences were observed to be statistically significant between the two groups. There was a trend toward higher tidal volumes and higher lung compliance in the responders when compared with nonresponders. We estimated the associations of APACHE II, ideal body weight, baseline tidal volume, baseline lung compliance, and classification of severe ARDS at the time of iEPO initiation regarding iEPO responsiveness using a multivariable logistic regression model. None of the variables tested were significantly different between the responders and nonresponders (Table 4).
TABLE 4.
Comparison of Responders to Nonresponders to Inhaled Epoprostenol
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Responders (n = 16) | Nonresponders (n = 22) | p | OR (95% CI) | P | |
| Age, yr (mean ± sd) | 63 ± 10 | 59 ± 15 | 0.36 | — | — |
| Height, in. (mean ± sd) | 67 ± 4.2 | 66.1 ± 3.5 | 0.48 | — | — |
| Ideal body weight, kg (mean ± sd) | 64.7 ± 10.9 | 63.4 ± 11.3 | 0.72 | 0.87 (0.63-1.22) | 0.42 |
| Acute Physiology and Chronic Health Evaluation II (mean ± sd) | 28.8 ± 10 | 25 ± 7 | 0.18 | 1.23 (0.96-1.59) | 0.09 |
| Time from intubation to iEPO, hr (mean ± sd) | 178.7 ± 129 | 142.7 ± 103.8 | 0.35 | -- | -- |
| Tidal volume at iEPO initiation, mL (mean ± sd) | 420 ± 80 | 365 ± 93 | 0.06 | 1.02 (0.97-1.07) | 0.42 |
| (n=20)a | |||||
| Compliance at iEPO initiation | 35.7 ± 12.3 | 29.5 ± 7.4 | 0.12 | 1.09 (0.95-1.25) | 0.22 |
| (n=11) | (n=15) | ||||
| Mild ARDS (n [%]) | 1 (6.3) | 3 (13.6) | 0.45 | -- | -- |
| Moderate ARDS (n [%]) | 9 (56.3) | 15 (68.2) | 0.45 | -- | -- |
| Severe ARDS (n [%]) | 6 (37.5) | 4 (18.2) | 0.18 | 10.6 (0.22-516.4) | 0.23 |
| Right ventricle strain or dysfunction (n [%]) | 2 (12.5) | 4 (18.2) | 0.63 | -- | -- |
| Baseline therapeutic anticoagulation (n [%]) | 5 (31.3) | 7 (31.8) | 0.86 | -- | -- |
| Duration mechanical ventilation, hr (mean ± sd) | 422.5 ± 244.1 | 459.5 ± 239.9 | 0.64 | -- | -- |
| ICU LOS, hr (mean ± sd) | 515.2 ± 352.1 | 527.6 ± 300.7 | 0.91 | -- | -- |
| ICU mortality (n [%]) | 9 (56.3) | 10 (45.5) | 0.52 | -- | -- |
ARDS = acute respiratory distress syndrome, iEPO = inhaled epoprostenol, LOS =length of stay.
aTwo patients in nonresponder group were receiving airway pressure release ventilation.
DISCUSSION
This study evaluated the use of iEPO and iNO in patients with refractory hypoxemia secondary to COVID-19. Overall, 41% of the patients were classified as responders to iEPO. However, we observed no change on average in the Pao2 or Spo2, and less than a 10% increase in median Pao2/Fio2. Although there were only 11 patients that transitioned to iNO, there was a similar observed change in Pao2/Fio2. These results are different from what previous studies have shown when inhaled vasodilators have been used for refractory hypoxemia in ARDS unrelated to COVID-19 (8, 9).
This study differs from previous data examining the use of iEPO and iNO in ARDS in that we exclusively evaluated patients with respiratory failure secondary to COVID-19. Both iEPO and iNO have had mixed results in patients with ARDS, demonstrating improvement in oxygenation but failing to improve mortality (12–14). Several analyses comparing both agents have shown iEPO to be noninferior to iNO in patients with ARDS regarding safety, changes in oxygenation, and improvement in clinical outcomes, such as ventilator-free days (8, 10, 25). It is unclear if and how ARDS in patients with COVID-19 differs, but we saw less of an improvement in oxygenation metrics compared with previous studies (8, 9, 26). Our patients had significant differences at baseline, specifically decreased use of corticosteroids and increased use of neuromuscular blockade and prone-positioning, compared with previous studies evaluating ARDS unrelated to COVID-19 (9, 26). Early data described risk factors of developing ARDS in patients with COVID-19 that included elevated inflammation-related indices such as C-reactive protein and ferritin (27). Additionally, SARS-CoV-2 may be linked with the induction of cytokine storm, leading to the worsening of disease severity and patient outcomes (28). However, although inflammatory markers may be elevated in patients with COVID-19-related ARDS, they may not be significantly different from patients who develop ARDS unrelated to COVID-19 (29). Overall, it is unclear if we observed less of an improvement with iEPO or iNO in our study due to the population being comprised completely of patients with COVID-19 or for some other reason, such as smaller sample size.
We did not detect a clear signal to help predict which patients with refractory hypoxemia secondary to COVID-19 may benefit from iEPO or iNO therapy. We hypothesized that earlier initiation of inhaled pulmonary vasodilators in relation to development of ARDS may be beneficial, but that was not observed. We did observe a trend toward higher tidal volumes in patients classified as responders when compared with nonresponders, possibly indicating that nonresponders may have had inadequate distribution of the medication to produce vasodilation and clinical effect. Although the baseline lung compliance in our cohort overall was low, we observed a trend toward higher compliance in responders to iEPO therapy when compared with nonresponders. Gattinoni et al (30) have described the different phenotypes of COVID-19-related ARDS: type L, characterized by normal or high compliance, low ventilation-to-perfusion ratio, small increase in lung weight, and low recruitability, and type H, characterized by low compliance, right-to-left shunt, significant increases in lung weight, and high recruitability. Although ARDS may be classified as type L early on, Camporota et al (31) describe the potential to shift to type H around 5–7 days, which may necessitate a change in ventilatory strategies. Roesthuis et al (32) evaluated 14 patients with COVID-19-related ARDS and demonstrated decreased PEEP was associated with an increase in lung compliance and a decrease in dead space ventilation. Our cohort received iEPO approximately 6.5 days after intubation on average. Since iEPO is more likely to be efficacious in patients with functional alveoli, it is possible that earlier administration of iEPO or iNO may have produced greater clinical effects. Larger studies should be done to see if trends that were seen in our data, such as increased tidal volumes, APACHE II scores, and percentage of severe ARDS in responders, are confirmed. Additionally, the efficacy and safety of iEPO should be looked at further in patients with different ARDS phenotypes.
Although the overall average changes in Pao2/Fio2, Pao2, and Spo2 were not significant, 41% of patients met the definition of responders based on the change in Pao2/Fio2 ratio. However, we observed that patients who did not initially respond were continued on both agents for over 24 hours, on average. It is likely that improvement in oxygenation due to inhaled pulmonary vasodilators should be realized quickly, if a response occurs at all. Therefore, these agents should be able to be trialed and assessed for an impact shortly, allowing for discontinuation if no initial benefit is seen since both are associated with high costs (33). Additionally, although we did not see a large amount of adverse reactions with either agent, possibly serious side effects such as hemodynamic instability and methemoglobinemia can occur.
While both iEPO and iNO were evaluated in this study, we cannot compare their efficacy, since the 11 patients who received iNO had previously trialed iEPO. iNO may have theoretical benefits based on in vitro studies demonstrating activity against SARS-CoV (34). A case of outpatient iNO administration in a patient with pulmonary arterial hypertension and COVID-19 was recently published and described significant improvement in respiratory symptoms (35). Additionally, a recent case series described oxygenation improvements with the use of iNO in pregnant patients admitted with severe COVID-19 (36). We observed a trend toward a more significant improvement in Spo2 after receiving iNO as well as a trend toward increasing the Pao2 and Pao2 percentage changes when compared with iEPO. However, it should be emphasized that only 11 patients were transitioned from iEPO to iNO.
This study has several limitations. First, this is a retrospective study at a single academic medical center. Although all patients in this study were admitted with respiratory failure secondary to COVID-19, it is possible that other populations of patients with COVID-19 may see a benefit with these agents. Another limitation of this study is that we did not look at the effects of iEPO and iNO in different groups of patients, and therefore cannot draw much from the efficacy compared with each other. Additionally, due to the retrospective nature of the study, we were unable to control for other management strategies and therapeutic changes that occurred in trying to optimize oxygenation. We attempted to minimize the effect of other interventions such as neuromuscular blockade or prone-positioning by excluding patients who were paralyzed or proned during the initiation of inhaled pulmonary vasodilators. Minimal changes on patients’ ventilator settings were made as iEPO or iNO was started; however, it is possible that other interventions interfered with the interpretation of drug effects. Additionally, we did not account for interventions and changes that occurred from the time of ordering iEPO or iNO and the time of initiation, which may have resulted in improvements in Pao2/Fio2, leading to the administration of these agents in some patients who transitioned from moderate to mild ARDS. Another limitation, despite having guidelines and recommendations related to initiation and weaning of inhaled pulmonary vasodilators, it is likely that providers used these agents differently. Finally, although this study is the largest analysis of inhaled pulmonary vasodilators in patients with COVID-19, it contains a relatively small number of patients.
CONCLUSIONS
We found that the initiation of iEPO and iNO in patients with refractory hypoxemia secondary to COVID-19, on average, did not produce significant increases in oxygenation metrics such as Pao2/Fio2, Pao2, or Spo2 despite minimal other confounding interventions. However, a group of patients had significant improvement in measured clinical parameters with iEPO and iNO. Administration of iEPO or iNO may be considered in patients with severe respiratory failure secondary to COVID-19.
ACKNOWLEDGMENT
We thank Leo F. Buckley, PharmD, and Gretchen Stern, PharmD, BCPS, for help with statistical analysis.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Papazian L, Aubron C, Brochard L, et al. Formal guideline: Management of acute respiratory distress syndrome. Ann Intensive Care. 2019; 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Ma X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit Care. 2020; 24:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 4.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 5.Forel JM, Roch A, Marin V, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006; 34:2749–2757 [DOI] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 7.Cornejo RA, Díaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013; 188:440–448 [DOI] [PubMed] [Google Scholar]
- 8.Torbic H, Szumita PM, Anger KE, et al. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care. 2013; 28:844–848 [DOI] [PubMed] [Google Scholar]
- 9.Hawn JM, Bauer SR, Wanek MR, et al. Effectiveness, safety, and economic comparison of inhaled epoprostenol brands, flolan and veletri, in acute respiratory distress syndrome. Ann Pharmacother. 2020; 54:434–441 [DOI] [PubMed] [Google Scholar]
- 10.Buckley M, Agarwal SK, Garcia-Orr R, et al. Comparison of fixed-dose inhaled epoprostenol and inhaled nitric oxide for acute respiratory distress syndrome in critically ill adults. J Intensive Care Med. 2020Mar 5. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.De Wet CJ, Affleck DG, Jacobsohn E, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004; 127:1058–1067 [DOI] [PubMed] [Google Scholar]
- 12.Fuller BM, Mohr NM, Skrupky L, et al. The use of inhaled prostaglandins in patients with ARDS: A systematic review and meta-analysis. Chest. 2015; 147:1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults (review). Cochrane Database Syst Rev. 2016; 2016:CD002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afshari A, Bastholm Bille A, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS) (review). Cochrane Database Syst Rev. 2017; 7:CD007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzierba AL, Abel EE, Buckley MS, et al. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014; 34:279–290 [DOI] [PubMed] [Google Scholar]
- 16.Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020; 48:e440–e469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed T, Shah RJ, Rahim SEG, et al. Coronavirus disease 2019 (COVID-19) complicated by acute respiratory distress syndrome: An internist’s perspective. Cureus. 2020; 12:e7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geier MR, Geier DA. Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses. 2020; 140:109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes, and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy Crit Care Resusc. 2020Apr 23. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauri T, Spinelli E, Scotti E, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020; 48:1129–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brigham and Women’s Hospital COVID-19 Task Force: Brigham and Women’s Hospital COVID-19 Clinical Guidelines. 2020 Available at: covidprotocols.org. Accessed June 23, 2020.
- 25.Ammar MA, Bauer SR, Bass SN, et al. Noninferiority of inhaled epoprostenol to inhaled nitric oxide for the treatment of ARDS. Ann Pharmacother. 2015; 49:1105–1112 [DOI] [PubMed] [Google Scholar]
- 26.Dunkley KA, Louzon PR, Lee J, et al. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: A pilot study. Ann Pharmacother. 2013; 47:790–796 [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen SF, Ho YC. SARS-CoV-2: A storm is raging. J Clin Invest. 2020; 130:2202–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020Jun 30. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020; 46:1099–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camporota L, Vasques F, Sanderson B, et al. Identification of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Respir Med. 2020; 8:752–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roesthuis L, Van den Berg M, Van der Hoeven H. Advanced respiratory monitoring in COVID-19: Use less PEEP! Crit Care. 2020; 24:230–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis SL, Crow JR, Fan JR, et al. Use and costs of inhaled nitric oxide and inhaled epoprostenol in adult critically ill patients: A quality improvement project. Am J Health Syst Pharm. 2019; 76:1413–1419 [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Liu P, Gao H, et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: A rescue trial in Beijing. Clin Infect Dis. 2004; 39:1531–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamanian RT, Pollack CV, Jr, Gentile MA, et al. Outpatient inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 infection. Am J Respir Crit Care Med. 2020; 202:130–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fakhr BS, Weigand SB, Pinciroli R, et al. High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus 2019 (COVID-19). Obstet Gynecol. 2020Aug 26. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


