Abstract
Background:
Inflammatory bowel diseases (IBD), which include Crohn disease and ulcerative colitis, affect several million individuals worldwide. Curcumin as a complementary therapy has been used to cure the IBD, yet the efficacy and safety of curcumin remains to be assessed. In this study, we aim to draw up a protocol for systematic review to evaluate the efficacy and safety of curcumin for IBD.
Methods:
We will search the following electronic databases from inception to September 31, 2020: PubMed, Cochrane Library, EMBASE, Web of Science, Medline, the China National Knowledge Infrastructure Database, Wan Fang Database, the Chinese Scientific Journal Database, and Chinese Biomedical Literature Database. Clinical trial registrations, potential gray literatures, relevant conference abstracts and reference list of identified studies will also be searched. Relevant randomized controlled clinical trials were enrolled and analyzed. The literature selection, data extraction, and quality assessment will be completed by 2 independent authors. Either the fixed-effects or random-effects model will be used for data synthesis based on the heterogeneity test. Clinical remission will be evaluated as the primary outcome. Clinical response, endoscopic remission, inflammatory markers and adverse events will be assessed as the secondary outcomes. The RevManV.5.3.5 will be used for Meta-analysis. Subgroup analyses of doses, delivery way, frequency of treatment and the degree of IBD severity or different forms of IBD were also conducted.
Results:
This study will provide a synthesis of current evidence of curcumin for IBD from several aspects, such as clinical remission, clinical response, endoscopic remission, inflammatory markers, and adverse events.
Conclusion:
The conclusion of our study will provide updated evidence to judge whether curcumin is an effective solution to IBD patients.
INPLASY registration number:
INPLASY202090065.
Keywords: curcumin, inflammatory bowel diseases, protocol, systematic review
1. Introduction
Inflammatory bowel diseases (IBD), which include Crohn disease (CD)and ulcerative colitis (UC), affect more than 3.5 million people, and their incidence is increasing worldwide.[1] These diseases are characterized by debilitating and chronic relapsing and remitting inflammation of the gastrointestinal tract (for CD) or the colon (in UC).[2] The pathogenesis is multifactorial, involving genetic predisposition, epithelial barrier defects, dysregulated immune responses, and environmental factors.[3] IBD are attracting increased attention at present for a number of reasons.
The conventional approach to IBD aims to induce and maintain clinical remission free of corticosteroids, thus minimizing the impact on quality of life.[4] Currently, treatment options for both diseases, including drugs such as 5-aminosalicylates,[5] corticosteroids,[6] anti-tumor necrosis factor alpha drugs,[7] antibiotics,[8] probiotics,[9] and immunosuppressants,[10] are costly, often involve significant side effects, and are limited in effectiveness and specificity. Studies indicate that a substantial proportion of patients fail to respond to mesalamine for remission induction will often relay to corticosteroids and/or immunomodulators to control the disease.[11] Corticosteroid resistance/refractoriness rates range from 8.9% to 25% in individuals with IBD.[12] Despite an early and wide use of immunosuppressants and biologics, approximately one half of the patients with Crohn disease still needed bowel resection within 10 years after the diagnosis.[13] Patients with long-standing inflammatory bowel disease (IBD) involving at least 1/3 of the colon are at increased risk for colorectal cancer (CRC).[14] Therefore, the need of new compounds that can induce and maintain remission in IBD without serious side effects is around the corner.
Curcumin, a polyphenol derived from the turmeric plant (Curcuma longa),[15] has been demonstrated to hold many health-related benefits and pharmacological effects, including anti-inflammatory, anti-oxidant, immunomodulatory effects, as well as its potential to alter the intestinal microbiome.[16–18] Curcumin as a complementary therapy in ulcerative colitis has become quite common, two randomized, controlled trials investigated the efficacy and safety of curcumin in patients with crohn's disease recently.[19–27] The mechanism of its anti-inflammatory action deemed to be the most relevant is the inhibition of NF-κB, by blocking IκB kinase, thereby inhibiting the expression of pro-inflammatory cytokines (IL-1, IL-6, and TNF-α.[28] Therefore, it is necessary to conduct a meta-analysis of curcumin for IBD. In this study, we aim to analyze the studies published so far, to review the positive or negative effects of the use of curcumin, and to determine whether it is safe and effective as a complementary therapy in the management of IBD.
2. Methods
2.1. Study registration
The systematic review protocol has been registered on the International Platform of Registered Systematic Review and Meta-Analysis Protocols (INPLASY). The registration number was INPLASY202090065 (DOI number is 10.37766/inplasy2020.9.0065, https://inplasy.com/inplasy-2020-9-0065/). This meta-analysis is a secondary research which based on some previously published data. Therefore, the ethical approval or informed consent was not required in this study.
2.2. Inclusion criteria for study selection
2.2.1. Types of studies
All relevant randomized controlled trials (RCTs) in English and Chinese will be included. Duplicated publications, review articles, animal studies, editorial letters, in-vitro studies, observational, and descriptive studies, such as case reports and case series will be excluded.
2.2.2. Types of participants
Study participants in different age ranges with IBD can be included in the study without restricting nationality, sex, race, occupation, or education.
2.2.3. Types of interventions
The control group: basic treatment (including conventional medication and health education) and Placebo. The experimental group: basic treatment and curcumin.
2.2.4. Types of outcome measures
2.2.4.1. Primary outcomes
Clinical remission will be evaluated as the primary outcome.
2.2.4.2. Secondary outcomes
The secondary outcomes of this review will include:
-
1.
Clinical response
-
2.
Endoscopic remission
-
3.
Inflammatory markers (hs-CRP, ESR, inflammatory factor levels)
-
4.
Adverse events.
2.3. Search methods for identification of studies
2.3.1. Electronic searches
We will search the following electronic databases from inception to September 31, 2020: PubMed, Cochrane Library, EMBASE, Web of Science, Medline, the China National Knowledge Infrastructure Database, Wan Fang Database, the Chinese Scientific Journal Database, and Chinese Biomedical Literature Database. The search strategy for Medline (via PubMed) is shown in Table 1, other electronic databases will also be searched based on this strategy.
Table 1.
Search strategy for Medline (via PubMed).
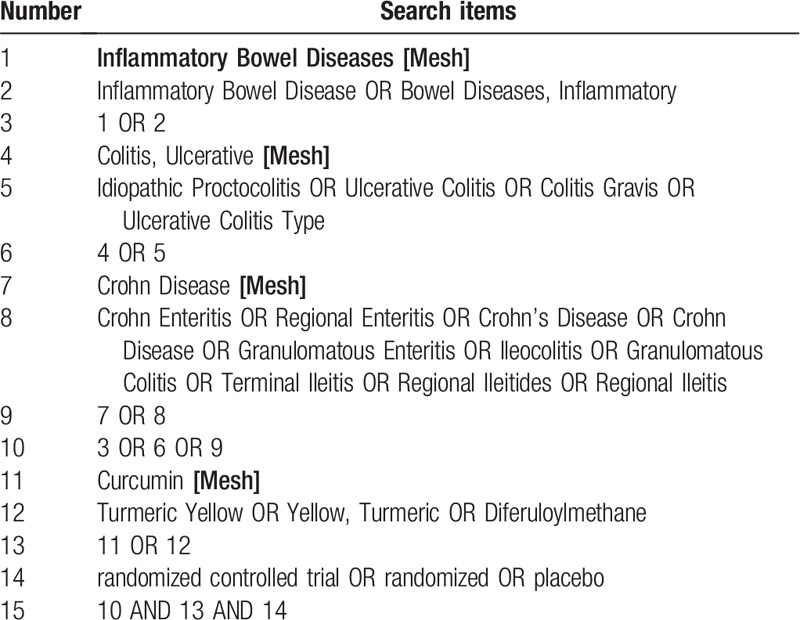
2.3.2. Searching other resources
The reference lists of studies and systematic reviews will be examined and retrieved for additional trials. Potential gray literatures will be searched in OpenGrey.eu. We will search relevant conference abstracts for eligible trials. In addition, we will also search the WHO International Clinical Trials Registry Platform and the ClinicalTrials.gov for all new reviews relevant to this topic.
2.4. Data collection and analysis
2.4.1. Selection of studies
We search the specified databases to obtain relevant literature, import them into a database created by Endnote X8 and screen out the duplicate documents. Two review authors will independently screen the titles, abstracts, and keywords of all retrieved records. If necessary, full texts will be examined according to the inclusion criteria for further assessment. We will resolve disagreements by discussing with the third author. The screening flow diagrams of this study will be shown in Figure 1.
Figure 1.
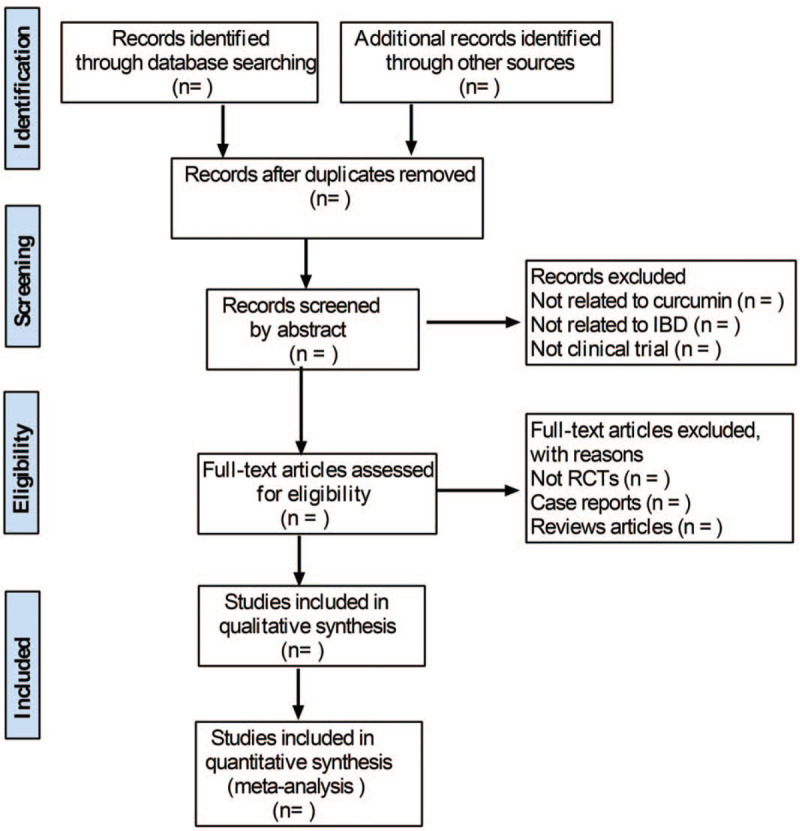
Study selection process for the meta-analysis.
2.4.2. Data extraction and management
We will make a standard data collection sheet before data extraction. Two reviewers will independently extract data from the selected studies and fill in the data collection sheet. Discrepancies and uncertainties will be resolved by consensus between the 2 review authors or by asking the third author to make a final decision. We will extract the following data:
-
1.
General information: the first author, title, the journal, publication type, publication year, country.
-
2.
Methods: study design, sample size, randomization, allocation concealment, blinding methods, inclusion criteria, and exclusion criteria.
-
3.
Participants: age, gender, severity of IBD, baseline disease activity.
-
4.
Interventions: type of control, duration of treatment, frequency of treatment.
-
5.
Outcomes: primary and secondary outcomes, adverse effects, and follow up.
2.4.3. Assessment of bias in the included studies
Two review authors will independently use the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias in the included studies. The following 6 domains in the Cochrane “Risk of bias tool” will be assessed: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias. We will grade each potential trial of bias as high, low, and unclear. Any disagreement will be resolved by discussing or by asking the 3rd author to make a final decision.
2.4.4. Measures of treatment effect
The continuous data will be expressed as mean difference (MD) or standard MD (SMD) with 95% confidence intervals (CIs), and the dichotomous outcomes will be estimated by the risk ratio (RR) with 95% CIs.
2.4.5. Unit of analysis issues
In order to avoid carryover effects, we will only extract the first experimental period data of cross-over trials. For trials with multiple intervention groups, we will combine all relevant control intervention groups and experimental intervention groups of the trial into a single group to avoid a unit-of-analysis error.
2.4.6. Management with missing data
For missing data, we will try to contact the original author to obtain the relevant data by email or phone. If we are unable to obtain missing data, the analysis will base on available data.
2.4.7. Assessment of heterogeneity
Heterogeneity will be assessed by visual inspection of the forest plots and detected by standard Chi-squared test and I2 statistic. I2 < 50% will be considered that the included studies are homogeneous, while I2 > 50% will be taken as evidence of representing substantial heterogeneity. Sensitivity analysis and subgroup analysis will be selected to detect the possible reason of substantial heterogeneity.
2.4.8. Assessment of reporting bias
When the number of included studies is more than 10, the funnel plot will be used to detect the reporting bias.
2.4.9. Data synthesis
RevManV.5.3.5 will be used for data analysis and synthesis. Continuous data will be expressed as MD/SMD with 95% CIs, while the dichotomous outcomes will be presented as RR with 95% CIs. When I2 < 50%, the fixed effect model will be adopted to analyze. Otherwise, the random effect model will be selected. Additionally, we will use the sensitivity analysis and subgroup analysis to explore the causes of heterogeneity.
2.4.10. Subgroup analysis
Subgroup analysis will be carried out based on the age of patients, different types of curcumin therapies, duration of treatment, frequency of treatment and the degree of IBD severity or different types of IBD.
2.4.11. Sensitivity analysis
If there are sufficient studies included, we will take sensitivity analyses to test the robustness and reliability of the results. The sensitivity analysis focuses on research characteristics or types such as methodological quality, and examines the effects of total effects by excluding certain low quality studies or unblinded studies.
3. Discussion
Curcumin, with the advantages of low-cost, reliable sources and low side effects, is holding considerable attention for IBD treatment. Curcumin, also known as diferuloylmethane, has been a popular supplement largely because of its affordability and safety, with no known toxic side effects in humans up to doses of 12 g/day.[29] Its significant NF-κB inhibition, expressed by regulating NF-κB/IκB pathway and down-regulating expression of pro-inflammatory cytokines, was thought to be the key mechanism for the anti-inflammatory.[30–32] In AP-1 signaling, curcumin can inhibit MAPK, ERK1/2, JNK, and p38, both directly and indirectly, thereby limiting transcription of inflammatory target genes.[33] We hope this systematic review will provide more reliable evidence in the management of IBD.
There are still some limitations in this review. Limited by our language capability, we just collect studies in English and Chinese. Meanwhile, the dose, delivery way, intervention time, and form of curcumin were different among them, which might add bias to our study outcomes.
Author contributions
Conceptualization: Zhenhuan Yang, Wenjing Liu, Shukun Yao
Data curation: Xiaoran Zhu, Feiya Suo
Formal analysis: Wenjing Liu
Project administration: Zhenhuan Yang, Shukun Yao
Supervision: Xuefeng Zhou, Xiaoran Zhu, Feiya Suo
Writing – original draft: Zhenhuan Yang, Wenjing Liu
Writing – review & editing: Zhenhuan Yang, Shukun Yao
Footnotes
Abbreviations: CD = Crohn's disease, IBD = inflammatory bowel disease, MD = mean difference, RCTs = randomized controlled trials, RR = risk ratio, SMD = standard mean difference, UC = ulcerative colitis.
How to cite this article: Yang Z, Liu W, Zhou X, Zhu X, Suo F, Yao S. The effectiveness and safety of curcumin as a complementary therapy in inflammatory bowel disease: a protocol of systematic review and meta-analysis. Medicine. 2020;99:43(e22916).
ZHY, WJL, and XFZ contributed equally to this work as the first author.
This study was financed by the Leap-forward Development Program for Beijing Biopharmaceutical Industry (G20) (Z171100001717008) and National Key Research and Development Program, No. 2017YFC0910000.
The authors declare no conflicts of interests.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England) 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- [2].Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019;569:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol 2020;145:16–27. [DOI] [PubMed] [Google Scholar]
- [4].D’Haens GR, Sartor RB, Silverberg MS, et al. Future directions in inflammatory bowel disease management. J Crohns Colitis 2014;8:726–34. [DOI] [PubMed] [Google Scholar]
- [5].Nikfar S, Rahimi R, Rezaie A, et al. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig Dis Sci 2009;54:1157–70. [DOI] [PubMed] [Google Scholar]
- [6].De Cassan C, Fiorino G, Danese S. Second-generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects? Dig Dis (Basel, Switzerland) 2012;30:368–75. [DOI] [PubMed] [Google Scholar]
- [7].Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ (Clinical research ed) 2017;357:j2505. [DOI] [PubMed] [Google Scholar]
- [8].Nitzan O, Elias M, Peretz A, et al. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol 2016;22:1078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Derwa Y, Gracie DJ, Hamlin PJ, et al. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:389–400. [DOI] [PubMed] [Google Scholar]
- [10].Zabana Y, Rodríguez L, Lobatón T, et al. Relevant infections in inflammatory bowel disease, and their relationship with immunosuppressive therapy and their effects on disease mortality. J Crohns Colitis 2019;13:828–37. [DOI] [PubMed] [Google Scholar]
- [11].Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet (London, England) 2007;369:1641–57. [DOI] [PubMed] [Google Scholar]
- [12].Li J, Wang F, Zhang HJ, et al. Corticosteroid therapy in ulcerative colitis: Clinical response and predictors. World J Gastroenterol 2015;21:3005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Peyrin-Biroulet L, Loftus EV, Jr, Columbel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- [14].Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol 2019;25:4148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors (Oxford, England) 2013;39:2–13. [DOI] [PubMed] [Google Scholar]
- [16].Alizadeh F, Javadi M, Karami AA, et al. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother Res 2018;32:514–21. [DOI] [PubMed] [Google Scholar]
- [17].Asteriou E, Gkoutzourelas A, Mavropoulos A, et al. Curcumin for the management of periodontitis and early ACPA-positive rheumatoid arthritis: killing two birds with one stone. Nutrients 2018;10:908–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shen L, Liu L, Ji HF. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr Res 2017;61:1361780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Banerjee R, Pal P, Penmetsa A, et al. Novel bioenhanced curcumin with mesalamine for induction of clinical and endoscopic remission in mild-to-moderate ulcerative colitis: a randomized double-blind placebo-controlled pilot study. J Clin Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- [20].Kedia S, Bhatia V, Thareja S, et al. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J Gastrointest Pharmacol Ther 2017;8:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol 2015;13:1444–9. e1441. [DOI] [PubMed] [Google Scholar]
- [22].Singla V, Pratap Mouli V, Garg SK, et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis 2014;8:208–14. [DOI] [PubMed] [Google Scholar]
- [23].Sadeghi N, Mansoori A, Shayesteh A, et al. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother Res 2020;34:1123–33. [DOI] [PubMed] [Google Scholar]
- [24].Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2006;4:1502–6. [DOI] [PubMed] [Google Scholar]
- [25].Masoodi M, Mahdiabadi MA, Mokhtare M, et al. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: a randomized double-blind controlled trial. J Cell Biochem 2018;119:9552–9. [DOI] [PubMed] [Google Scholar]
- [26].Sugimoto K, Ikeya K, Bamba S, et al. Highly bioavailable curcumin derivative ameliorates Crohn's disease symptoms: a randomized, double-blind, multicenter study. J Crohns Colitis 2020. [DOI] [PubMed] [Google Scholar]
- [27].Bommelaer G, Laharie D, Nancey S, et al. Oral curcumin no more effective than placebo in preventing recurrence of Crohn's disease after surgery in a randomized controlled trial. Clin Gastroenterol Hepatol 2020;18:1553–60. e1551. [DOI] [PubMed] [Google Scholar]
- [28].Sreedhar R, Arumugam S, Thandavarayan RA, et al. Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discov Today 2016;21:843–9. [DOI] [PubMed] [Google Scholar]
- [29].Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006;6:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Y, Tang Q, Duan P, et al. Curcumin as a therapeutic agent for blocking NF-κB activation in ulcerative colitis. Immunopharmacol Immunotoxicol 2018;40:476–82. [DOI] [PubMed] [Google Scholar]
- [31].Burge K, Gunasekaran A, Eckert J, et al. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. Int J Mol Sci 2019;20:1912–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baliga MS, Joseph N, Venkataranganna MV, et al. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct 2012;3:1109–17. [DOI] [PubMed] [Google Scholar]
- [33].Boozari M, Butler AE, Sahebkar A. Impact of curcumin on toll-like receptors. J Cell Physiol 2019;234:12471–82. [DOI] [PubMed] [Google Scholar]


