Abstract
Frailty is a common geriatric condition due to aging and defined as a decline in strength and a decrease in the physiologic ability to maintain the homeostasis. Vitamin B12 (B12), water-soluble vitamins, are a cofactor in DNA synthesis and involved in the metabolism of every cell in the human body, including the central nervous system. Demyelination neuromuscular symptoms observed in the peripheral nervous system, along with signs of significant damage to nerve fibers, often cause weakness, numbness in distal limbs, impaired balance, gait ataxia, and even physical frailty. In this cross-sectional study, we aimed to investigate the relationship between frailty and B12 level in community-dwelling Korean older adults.
Using the data from the Korean Frailty and Aging Cohort Study, 2938 participants (1400 men and 1538 women) were recruited in this study. To evaluate frailty, we compared the frail group and not-frail group based on the modified Korean version of the cardiovascular health study frailty index developed by Fried. SARC-F is used to screen for sarcopenia. The short physical performance battery (SPPB) timed up and go (TUG) test and activities-specific balance confidence scale used to evaluate the physical function and fall risk of participants. B12 concentrations were classified into clinically relevant categories: insufficient (<350 pg/mL) and sufficient (≥350 pg/mL). Linear and logistic regression analyses were used to evaluate the relationship between frailty and B12 levels.
The mean age of the frail group was 77.8 (standard deviation = 3.7) years, while that of the not-frail group was 76.7 (SD = 4.0); of which the frail group's mean age was significantly high. In the unadjusted model, frailty was highly prevalent in the B12 insufficient group (odds ratio = 1.298). In the model fully adjusted for demographic data and comorbidities, these associations were attenuated. The B12 sufficiency group showed better total SPPB and TUG test scores. However, they were not statistically significant in the fully adjusted model.
In this cross-sectional study, low B12 increased the incidence of frailty and affected physical performance, but it does not increase the incidence of frailty when considering the confounding factors. Frailty is caused by several factors rather than 1 factor, and B12 is one of these factors.
Keywords: frailty, geriatrics, Korean Frailty and Aging Cohort study, vitamin B12
1. Introduction
Frailty is a common geriatric condition due to aging and defined as a decline in strength and a decrease in the physiologic ability to maintain the homeostasis.[1,2] It increases an individual's vulnerability to developing adverse health outcomes.[3] Although there is no gold standard for diagnosing frailty, cardiovascular health study (CHS) frail index developed by Fried is commonly used for frailty risk assessment and in epidemiologic studies.[4] Frailty can result in functional decline and prolonged hospitalization as well as increased mortality rate.[5] Frailty is not only a result of aging but is also associated with lower educational level, smoking, not married, ethnicity, depression, lack of exercise, poor nutrition, various chronic diseases, medications, cognitive impairment, and social isolation.[6–8] The prevalence of frailty among the geriatric population is 13.9%, and it increases with age. According to the American Medical Association, 40% of adults aged 80 years and older are frail.[9,10] A systematic review, based on 21 cohort studies, including 61,500 participants, reported that the prevalence of frailty in the community varies (from 4.0% to 59.1%). On average, 10.7% of community-dwelling adults aged 65 years and older are frail, and 41.6% have prefrail status.[11]
Vitamin B12 (B12), also known as cobalamin, is a cofactor in the DNA synthesis and involved in the metabolism of every cell in the human body.[12] In particular, it has an important role in the synthesis of myelin in the nervous system.[13] B12 deficiency is characterized by peripheral neuropathy, cognitive impairment, and blood cell disorder such as pernicious anemia.[14] Demyelinating disorders observed in the peripheral nervous system, along with significant nerve fiber damage, often cause weakness, numbness, and pain in the distal limbs, thus resulting in impaired balance, gait ataxia, and even physical frailty.[15–17] The leading cause of B12 deficiency is impaired absorption due to loss of gastric intrinsic factor and long-term antacid therapy, including proton pump inhibitors or H2 blockers, which are highly prevalent with advancing age.[18]
As both frailty and B12 insufficiency are highly prevalent in older adults, previous studies reported the association between frailty and B12 insufficiency. However, the results were controversial.[19,20] In this cross-sectional study, we aimed to investigate the relationship between frailty and B12 insufficiency in community-dwelling Korean older adults using the baseline data from the Korean Frailty and Aging Cohort Study (KFACS).
2. Methods
2.1. Data and study population
We used the data from KFACS to investigate the relationship between B12 level and physical frailty including frailty defined based on Fried's criteria, sarcopenia, activities-specific balance confidence[21] scale, and physical performance tests in community-dwelling individuals aged between 70 and 84 years. KFACS is a nationwide, multicenter study performed in 8 medical and 2 public health centers across Korea, and the goal is to identify and prevent frailty in older community dwellers (Won). Among the 3014 participants, 76 participants with incomplete data on B12 blood analysis, hemiplegia due to cerebrovascular accident, deformity or defect in the extremities, paraplegia, and blindness in 1 or both eyes, and who could not complete the physical performance test were excluded (Fig. 1). A total of 2938 participants (1400 men and 1538 women) were recruited in this study. Participants were individually interviewed and underwent blood and physical performance tests. Baseline demographic data and medical history were also collected, including age, sex, level of education, location of residence, smoking, alcohol consumption, depression, body mass index (BMI), marital status, annual income, and comorbidities. Current smokers and drinkers referred to participants who smoke more than 1 cigarette per week and drink alcohol at least once a week, respectively. Chronic comorbidities affecting physical function, such as cardio-pulmonary and musculoskeletal disease (hypertension, myocardial infarction, heart failure, peripheral vascular disease, chronic obstructive lung disease, osteoarthritis, rheumatoid arthritis, and osteoporosis) were evaluated in this study. The KFACS protocol was approved by the Institutional Review Board [22] of the Clinical Research Ethics Committee of the Kyung Hee University Medical Center, and all participants provided a written informed consent (IRB number: 2015-12-103).
Figure 1.
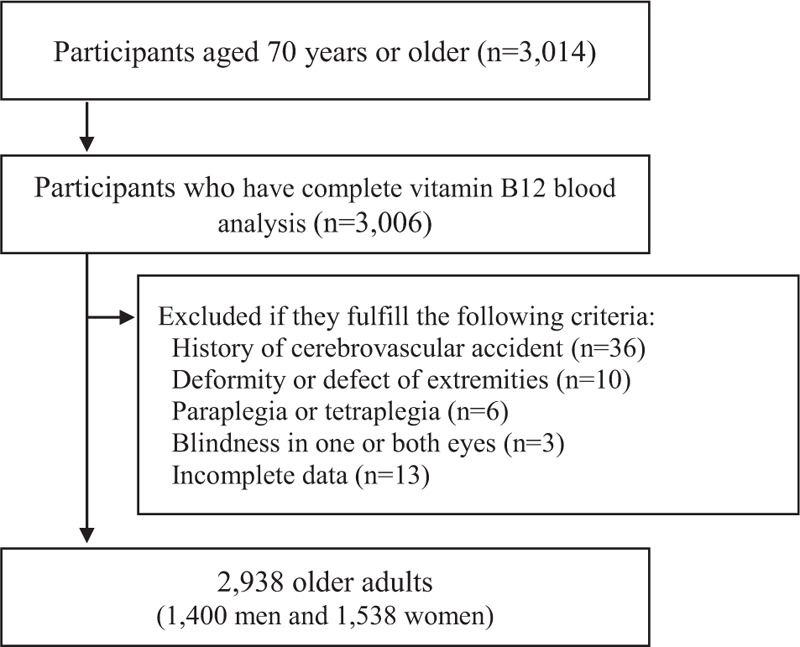
Flow chart of the process of participant recruitment.
2.1.1. Frailty
2.1.1.1. Cardiovascular health study (CHS) frailty index
To evaluate frailty, we used the CHS frailty index developed by Fried et al. [4,23] CHS frailty index has 5 components such as unintentional weight loss, exhaustion, reduced physical activity, reduced walking speed, and weakness. Participants who fulfilled 3 or more components were diagnosed with frailty:
Unintentional weight loss: Participants weighing more than 4.5 kg or having more than 5% of body weight compared with that in the previous year were considered frail.
Exhaustion: For those who positively responded to the questions “Did you experience difficulties in doing about everything?” or “Did you feel that you can’t do anything?,” a score of 1 point was provided if the participant experienced exhaustion 3 or more days per week, while 0 point if it was less than that.
-
1.
Reduced physical activity: Using the short-term International Physical Activity Questionnaire, which has verified for validity and reliability, calorie consumption for a week was calculated and divided into male and female groups (<494.65 kcal for men and <283.50 kcal for women) (1 point, lowest 20%).
-
2.
Reduced gait speed: Gait speed at 4 m was measured twice, and a Gait speed of less than 1.0 m/s was score as 1 point. The participants were instructed to walk at a usual pace through a 1-m zone for acceleration, a central 4-m test zone while timed with a digital stopwatch with a laser sensor, and a 1-m zone for deceleration.
-
3.
Weakness: Using a hand dynamometer (JAMAR, Bolingbrook, IL), hand grip strength (HGS) in both sides was measured twice, and the highest value was obtained (1 point: <26 kg for men and <18 kg for women). Based on the CHS frailty scale score, with the sum of each component, those who scored 1 point, 3 points, or more were classified as frail.
2.1.2. Sarcopenia
2.1.2.1. SARC-F
The Korean version of SARC-F is used to screen for sarcopenia.[24,25] The SARC-F is a screening tool for sarcopenia with high specificity and is composed of 5 items:
Strength: How difficult is it to lift and transport 4.5-kg box?
Assistance in walking aid: How difficult Can I walk from 1 corner of the room to the other?
Rise from chair: Is it difficult to get up from a chair or transfer to bed?
Climbing stairs: How difficult is it to climb 10 stairs without rest?
Fall history: How many times did you fall last year? Each item was scored (0 = not difficult at all, 1 = some difficult, and 2 = unable or need lots of aids), and the scores were summed up.
A total score of 4 points or more indicates sarcopenia.
2.2. Physical performance tests
2.2.1. Short physical performance battery
The short physical performance battery (SPPB) was developed to establish an epidemiological study in older adults and has been used to evaluate their physical function.[26] The SPPB is a well-established performance test that evaluates the physical function of the lower extremity. It is composed of 3 items: a standing balance test, gait speed, and 5 repeated chair stands. Each test was scored from 0 to 4 points, with a total of 12 points. A higher score indicates a better lower extremity physical function.
Standing balance: Standing balance consists of 3 subitems of side-by-side stance, semitandem stance, and tandem stance. Each posture is demonstrated to the patient by a skilled examiner. After that, the examiner evaluates the patient's ability to perform the different postures. The participants obtain a score if they are able to maintain each posture, and the total score is 4.
Gait speed: Evaluated as the time it takes to walk a distance of 4 m. Each speed is rated from 0 to 4, with 0 indicating inability to perform the test and 4 representing the highest category of performance: 1 point for over 8.7 seconds, 2 points for 6.21 to 8.7 seconds, 3 points for 4.82 to 6.20 seconds, and 4 points for less than 4.82 seconds. Gait speed is measured twice and evaluated based on the fastest time. In the same way, if walking is possible using a walking aid such as a walking stick, the same method is performed.
Repeated chair stands: Evaluated as the time it takes to stand up and repeat the task 5 times on a chair with arms folded to the chest. It is rated as follows: 0 point, if the participant failed to complete the test within 60 seconds; 1 point, if the test was completed more than 16.7 seconds; 2 points, 13.7 to 16.7 seconds; 3 points, 11.2 to 13.7 seconds; and 4 points, 11.2 seconds or less. If the participants are concerned about the safety, such as standing using hands or fall during the test, the tester may stop the test and a score of 0 point is provided.
2.2.2. Timed up and go test
Timed up and go (TUG) test is used to measure functional motor ability and determine fall risk.[27] During the test, the participant is asked to sit on a chair, stand up, and walk 3 m at a comfortable pace. Then, the participant is asked to turn around the return point and sit on the chair. The return point is installed on the floor in front. The examiner measures the total time to complete the task. The TUG test has good validity and reliability for community-dwelling older adults.
2.2.3. Activities-specific balance confidence scale
This 16-item tool measures the individual's ability to perform functional tasks.[28] The test assesses balance confidence in performing various activities. Each question is rated on a scale of 0% (not confident at all) to 100% (I’m completely confident). A higher percentage indicates a higher level of activity-specific balance confidence. The ABC scale is a useful reliable and valid method for measuring the risk of falls.
2.3. Vitamin B12
B12 from serum samples was measured using the Architect vitamin kit (Abbott Diagnostics, Lake Forest, IL). In the previous study, the participants were divided by serum B12 concentrations above 350 pg/mL, which was found to be the protective level of neurogenesis. Individuals with a serum B12 concentration of more than 350 pg/mL were diagnosed with low B12 level.[29,30] We divided B12 concentrations into clinically relevant categories: insufficiency range (<350 pg/mL, same as <258.3 pmol/L) and sufficiency (≥350 pg/mL, same as ≥258.3 pmol/L).
2.4. Statistical analysis
The demographic characteristics of the participants based on Fried's criteria for diagnosing frailty were analyzed using t test for continuous variables and χ2 test for categorical variables. The results were expressed as mean ± standard deviation (SD) or number according to the characteristics of the variables. Univariable and multivariable analyses were performed using generalized linear regression models to examine the association between B12 level and physical functional scales. Univariable and multivariable logistic regression analyses were used to evaluate the odds ratio (OR) of frailty and sarcopenia depending on the B12 levels. Each multivariable model for multiple correlations between physical functions and other potential confounder variables such as age, sex, education periods, location of residence, body mass index, alcohol use, current smoker, depression, marital status, annual income, depression, osteoarthritis,[31] rheumatoid arthritis (RA), osteoporosis, diabetes mellitus, cardiovascular, and pulmonary diseases were fully adjusted. The collected data were analyzed using SPSS 23.0 (IBM, Inc, Chicago, IL) software, and a P value of <.05 was considered significant.
3. Results
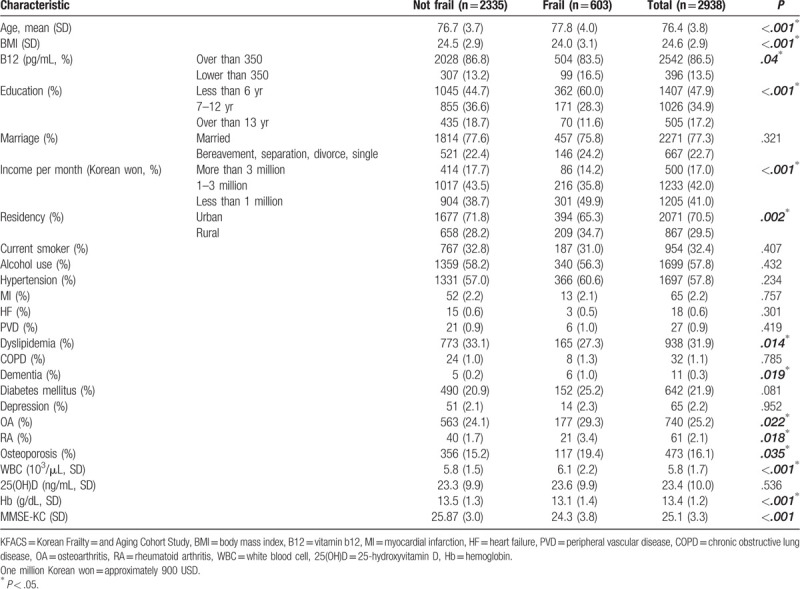
The baseline characteristics of KFACS participants evaluated based on the CHS frailty index developed by Fried are presented in Table 1. Of the 2938 participants, 603 (20.5%) met the frailty criteria, while 2335 (79.5%) were classified as having a robust or prefrail status (not-frail group). The mean ages were 77.8 (SD = 3.7) years for the frail group and 76.7 (SD = 4.0) for the not-frail group. The frail group's mean age was considered to be significantly high (P < .001). The BMI values of the frail group and not-frail group were 24.0 and 24.5, respectively, of which that of the frail group was significantly lower (P < .001). Based on a B12 concentration of 350 pg/mL, the insufficiency group showed a high proportion of participants with frail status. When the 2 groups were compared by education level, the frail group had a higher probability of having a lower education level. In terms of monthly income, the frail group had lesser income than the not-frail group. In addition, the not-frail group had a higher proportion of individuals living in urban areas. In terms of comorbidities, the frail group had higher proportion of individuals with dementia, OA,[31] RA, and osteoporosis (Table 1).
Table 1.
Baseline characteristics of KFACS participants by frailty status.
The results of the frailty, sarcopenia, and physical function tests of the 2 B12 groups are shown in Table 2. Frailty was more prevalent in the B12 insufficiency group, while the prevalence of sarcopenia did not differ between the 2 groups. The B12 sufficiency group had a total SPPB score of 10.84, while the insufficiency group had a total SPPB score of 10.63, which was higher in the sufficiency group. No statistical significance was observed in HGS, a marker of muscle strength, between the 2 groups. The 4-m gait speed of the B12 sufficiency group was 3.82 seconds, while that of the B12 insufficiency group was 4.04 seconds, which showed that the B12 sufficiency group was faster. The B12 sufficiency group also showed better TUG test results (Table 2).
Table 2.
Frailty, sarcopenia, and physical functions of KFACS participants by vitamin B12 level.
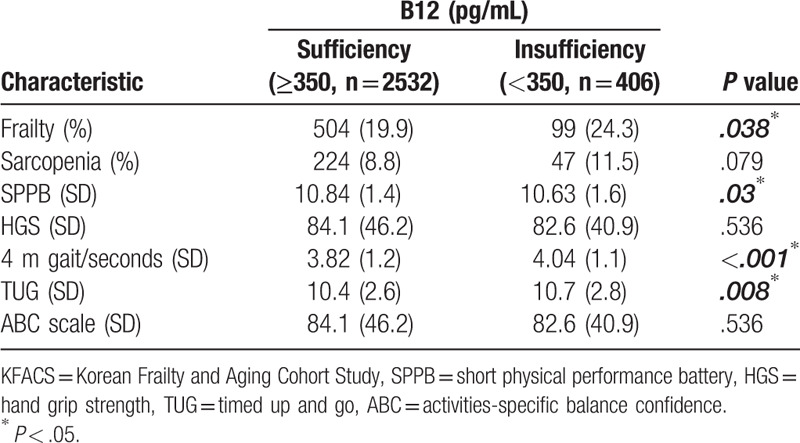
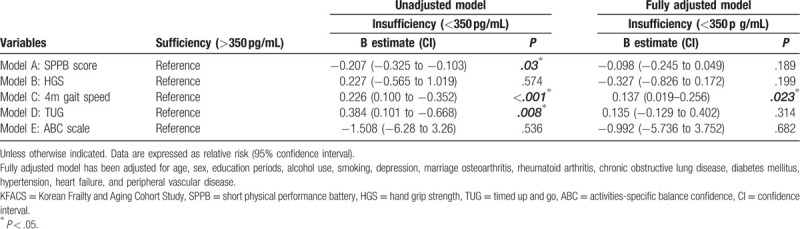
Table 3 shows the results of the logistic regression analysis of frailty and sarcopenia according to B12 level. In the unadjusted model, the incidence of frailty was high in patients with a B12 level below 350 pg/mL (OR = 1.298). However, in the model fully adjusted for age, sex, education period, alcohol use, smoking, depression, marital status, OA, RA, chronic obstructive pulmonary disease, diabetes mellitus,[32] hypertension, peripheral vascular disease, and heart failure, these associations were attenuated. In the linear regression model analysis of continuous variables such as physical function scores and parameters, the B12 sufficiency group showed better results in SPPB, 4-m gait speed, and TUG tests. However, these results were not statistically significant in the fully adjusted model (Table 4.).
Table 3.
Logistic regression analysis of frailty and sarcopenia in KFACS by vitamin B12 level.
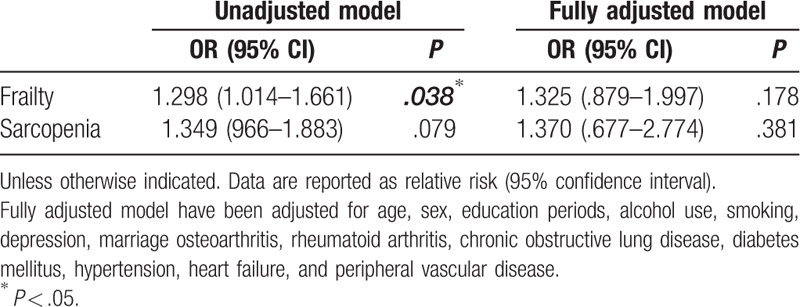
Table 4.
Linear regression model analysis of physical function in KFACS participants by vitamin B12 level.
4. Discussion
Frailty is an aging-related syndrome characterized by physiological functional decline and adversely affects health.[4] The risk factors of frailty are not only age but also medical, environmental, educational, and psychological background, and chronic comorbidities.[6,8,33,34] Various age-related diseases are related to frailty; hence, the assessment of frailty requires a multidisciplinary approach.[35,36] Because both frailty and B12 insufficiency are highly prevalent in the older population, the relationship between them is well known in previous studies.
Lack of B12 impairs peripheral nerve production and thus causes motor and sensory disturbance as well as balance problems. However, the direct relationship between frailty and B12 remains controversial. Oberlin et al's study showed that among the 3015 participants in the National Health and Nutrition Examination Surveys, those aged 60 years or more with B12 insufficiency (<350 pg/mL) or methylmalonic acid [37] (>0.21 μmol/L) reported a disability risk odds ratio of 1.6 in the activities of daily living, mobility, and activity.[38] Matteini et al[20] reported a 1.66 to 2.33 greater risk of frailty in HGS, endurance, physical activity, and gait speed in a study of 703 community-dwelling Caucasian women aged 70 to 79 years. Besides, the length of stay in the rehabilitation hospital after obtaining the fracture was longer in patients with low B12 level, suggesting that the neuromuscular structure and function are further declined in these patients.[37]
However, other studies reported that frailty had no correlation with B12 level. In a study of 335 older outpatients, the relationship between Fried criteria for frailty and B12 level of less than 400 pg/mL was evaluated. However, shrinking, weakness, slowness, and low activity were not correlated in all categories.[19] In Balboa-Castillo et al's study of 1643 community-dwelling individuals aged more than 65 years, the correlation between frailty by Fried and intake of 10 kinds of vitamins (vitamin A, thiamine, riboflavin, niacin, pyridoxine (B6), B12, C, D, E, and folates) was examined. In a 3.5-year follow-up, the lower intake of vitamins B6, C, E, and folates was associated with a higher risk of frailty, but B12 was not correlated.[31] Swart et al evaluated the effect of a 2-year daily oral supplementation of 500 ug of B12 in the intervention group (1454 patients) and control group (1452 patients). Physical performance including gait speed, HGS, and fall risk were also measured. Both groups showed a decrease in physical performance, HGS, and fall risk during the follow-up period, but no difference was observed between the groups.[39]
As a result of these contradictory results, this study examined the sarcopenia and frailty status based on the B12 level. Sarcopenia is a combination of the word “Sarco,” which is a Greek word for muscle, and “penia,” meaning loss. Sarcopenia is marked by a decrease in muscle mass due to aging.[40] Physiological changes in older adults vary widely, one of which is sarcopenia due to a decrease in skeletal muscle mass. Sarcopenia directly causes a reduction in muscle strength, which results in a reduction in various physical functions and eventually an increase in the risk of mortality. Sarcopenia, which refers to a decrease in skeletal muscle and decreased muscle strength, is considered to be an important link leading to frailty.[41] In this study, B12 insufficiency showed no correlation with sarcopenia and HGS, suggesting that B12 affects physical performance, psychological aspects, and sociological aspects, which is essential in the development of frailty rather than the development of skeletal muscle or strength of the body. Gait speed, SPPB score, and TUG, which measures physical performance, showed a significant correlation with B12 without adjusting for confounder parameters. However, these results did not increase the incidence in both the frailty and physical functions in a multivariable analysis adjusted for all possible confounders except for gait speed. Interestingly, in the 4-m gait speed test, when all variables were corrected, a sufficient group was found to be significantly faster.
The gait speed test is a quick and inexpensive method for measuring functional capacity in older adults, with high interrater and test–retest reliability and high predictive value.[42] The slowing of gait with aging is a result of the overall biological phenomenon and the integrated performance of numerous organ systems.[3] B12 insufficiency may cause myelin disorders in the central nervous system such as the brain and peripheral nervous system that could affect the gait speed. In previous studies, gait speed in itself has widely considered a simple indicator of the physical function and estimator of the frailty of the elderly population.[43,44] A gait speed lower than 1.0 m/s predicts frailty and has been associated with disability, hospitalization, and even mortality.[45] These results are consistent with our findings. In this study, B12 insufficiency was associated with low gait speed, and it is a factor that increases the incidence of frailty. However, this association was not shown based on the results of the participants’ SPPB and TUG tests, which are considered to be more variable because these sophisticated tests can assess turning ability, balance, proprioception, vision, and cognitive ability, as well as gait speed.
There are several limitations to this study. This study is cross-sectional in nature. However, the result of this large cohort study with almost 3000 participants is meaningful because it is the first study to examine the relationship between the B12 level and frailty. However, further prospective study or randomized control studies are warranted to confirm our findings. Second, the patients’ nutritional status and B12 supplements were not considered in this study. It is important to examine these factors to determine their correlation with frailty. As B12 is a commercial multivitamin supplement, it was meaningful to investigate whether the participants were taking it or not. Third, since this study was conducted in older adults, the results may vary depending on the patient's fatigue status or body condition when measuring the patient's physical function. Hence, our results may not be accurate. Forth, the results of the evaluation of other vitamin B groups (MMA and homocysteine) were missing. Although both are elevated in patients with B12 deficiency, there is no consensus of the B12 deficiency level use homocysteine or MMA. B12, pyridoxine (B6), and folic acid (B9) act together to lower homocysteine concentration in the blood. Elevated homocysteine levels are a risk factor for DNA damage. B9 supplement, especially when taken in high doses, can hide the symptoms of a B12 deficiency. Hence, further studies are warranted to evaluate these correlations.
This study is a large cross-sectional cohort study of the older Korean population to identify their B12 level and its correlation correlation with frailty, sarcopenia, and physical function. When the variables were not adjusted, low B12 based on 350 pg/mL had a significant correlation with the incidence of frailty by Fried criteria and low gait speed, SPPB, and TUG test results. The correlations were attenuated in the fully adjusted model when the potential confounder variables were adjusted except gait speed. These results suggest that insufficient B12 level does not directly increase the incidence of frailty, sarcopenia, or low physical performance. Adjusting the confounder is essential because aging can cause frailty and B12 insufficiency. Therefore, to evaluate the cause of frailty, multifactorial assessments should be performed. The mechanism of frailty is very complex. Along with aging itself, decrease functions in neuroendocrine, immune, nutritional status, physical activity, as well as various comorbidity could be a cause of frailty. B12, a cofactor in DNA synthesis and involved in the metabolism of every cell in the human body, could be one of the causes of frailty. In this study, low gait speed was itself still statistically significantly associated with B12 insufficiency after fully adjusted model. Because low gait speed has widely considered a simple indicator of the physical function of the elderly population, B12 insufficiency might be associated with low physical function. However, this study showed that a decrease in B12 does not directly increase frailty.
5. Conclusion
In this cross-sectional study, low B12 increased the incidence of frailty and affected physical performance, but it was not a direct risk factor when considering age, sex, personal history, and presence of comorbidities, which are various confounders of frailty. Frailty is caused by several factors rather than a single factor, and B12 is considered to be one of these factors.
Author contributions
Conceptualization: Yunsoo Soh
Data curation: Chang Won Won
Investigation: Yunsoo Soh
Interpretation of Data: Yunsoo Soh and Chang Won Won
Methodology: Yunsoo Soh
Supervision: Yunsoo Soh and Chang Won Won
Writing: original draft: Yunsoo Soh
Writing, review, and editing: Yunsoo Soh
Conceptualization: Yunsoo Soh.
Data curation: Chang Won Won.
Formal analysis: Chang Won Won.
Investigation: Yunsoo Soh.
Methodology: Yunsoo Soh.
Resources: Chang Won Won.
Supervision: Yunsoo Soh, Chang Won Won.
Writing – original draft: Yunsoo Soh.
Writing – review & editing: Yunsoo Soh.
Footnotes
Abbreviations: ABC test = activities-specific balance confidence test, B12 = vitamin B12, CHS = cardiovascular health study, HGS = hand grip strength, KFACS = Korean Frailty and Aging Cohort study, SPPB = short physical performance battery, TUG = timed up and go.
How to cite this article: Soh Y, Won CW. Association between frailty and vitamin B12 in the older Korean population. Medicine. 2020;99:43(e22327).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3153). This manuscript acquired the editorial certificate from “Editage” by Cactus (https://online.editage.co.kr/).
The authors have no conflicts of interest to disclose.
References
- [1].Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med 2007;120:748–53. [DOI] [PubMed] [Google Scholar]
- [2].Topinková E. Aging, disability and frailty. Ann Nutr Metab 2008;52: suppl 1: 6–11. [DOI] [PubMed] [Google Scholar]
- [3].Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–25. [DOI] [PubMed] [Google Scholar]
- [4].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Sci Med Sci 2001;56:M146–57. [DOI] [PubMed] [Google Scholar]
- [5].Khandelwal D, Goel A, Kumar U, et al. Frailty is associated with longer hospital stay and increased mortality in hospitalized older patients. J Nutr Health Aging 2012;16:732–5. [DOI] [PubMed] [Google Scholar]
- [6].Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. Jo Am Geriatr Soc 2005;53:1321–30. [DOI] [PubMed] [Google Scholar]
- [7].Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70:1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. J Am Geriatr Soc 2012;60:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- [10].Hendee W. American-medical-association white paper on elderly health-report of the council-on-scientific-affairs. Arch Intern Med 1990;150:2459–72. [PubMed] [Google Scholar]
- [11].Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- [12].Irizarry M, Gurol M, Raju S, et al. Association of homocysteine with plasma amyloid β protein in aging and neurodegenerative disease. Neurology 2005;65:1402–8. [DOI] [PubMed] [Google Scholar]
- [13].Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog Neurobiol 2009;88:203–20. [DOI] [PubMed] [Google Scholar]
- [14].Langan RC, Zawistoski KJ. Update on vitamin B 12 deficiency. Am Fam Physician 2011;83:1425–30. [PubMed] [Google Scholar]
- [15].Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci 1995;50:M211–5. [DOI] [PubMed] [Google Scholar]
- [16].Wuehr M, Schniepp R, Schlick C, et al. Sensory loss and walking speed related factors for gait alterations in patients with peripheral neuropathy. Gait Posture 2014;39:852–8. [DOI] [PubMed] [Google Scholar]
- [17].Tuttle LJ, Bittel DC, Bittel AJ, et al. Early-onset physical frailty in adults with diabesity and peripheral neuropathy. Can J Diabetes 2018;42:478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf 2013;4:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dokuzlar O, Soysal P, Isik AT. Association between serum vitamin B12 level and frailty in older adults. North Clin Istanb 2017;4:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matteini AM, Walston JD, Fallin M, et al. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging 2008;12:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging 2007;28:171–8. [DOI] [PubMed] [Google Scholar]
- [22].Senanarong V, Vannasaeng S, Poungvarin N, et al. Endogenous estradiol in elderly individuals: cognitive and noncognitive associations. Arch Neurol 2002;59:385–9. [DOI] [PubMed] [Google Scholar]
- [23].Hwang HS, Kwon IS, Park BJ, et al. The validity and reliability of Korean frailty index. J Korean Geriatr Soc 2010;14:191–202. [Google Scholar]
- [24].Kim S, Kim M, Won CW. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean frailty and aging cohort study. J Am Med Dir Assoc 2018;19: 40.e1-L 45.e1. [DOI] [PubMed] [Google Scholar]
- [25].Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–2. [DOI] [PubMed] [Google Scholar]
- [26].Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- [27].Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903. [PubMed] [Google Scholar]
- [28].Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1995;50:M28–34. [DOI] [PubMed] [Google Scholar]
- [29].Werder SF, Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat 2010;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Werder SF. Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat 2010;6:159–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Balboa-Castillo T, Struijk EA, Lopez-Garcia E, et al. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018;47:872–9. [DOI] [PubMed] [Google Scholar]
- [32].Angela Garcia M, Alicia Paris-Pombo M, Evans L, et al. Is low-dose oral cobalamin enough to normalize cobalamin function in older people? J Am Geriatr Soc 2002;50:1401–4. [DOI] [PubMed] [Google Scholar]
- [33].Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc 2007;55:1216–23. [DOI] [PubMed] [Google Scholar]
- [34].Evenhuis HM, Hermans H, Hilgenkamp TI, et al. Frailty and disability in older adults with intellectual disabilities: results from the healthy ageing and intellectual disability study. J Am Geriatr Soc 2012;60:934–8. [DOI] [PubMed] [Google Scholar]
- [35].Levers MJ, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs 2006;56:282–91. [DOI] [PubMed] [Google Scholar]
- [36].Chen C-Y, Wu S-C, Chen L-J, et al. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch Gerontol Geriatr 2010;50:S43–7. [DOI] [PubMed] [Google Scholar]
- [37].O’Leary F, Flood VM, Petocz P, et al. B vitamin status, dietary intake and length of stay in a sample of elderly rehabilitation patients. J Nutr Health Aging 2011;15:485–9. [DOI] [PubMed] [Google Scholar]
- [38].Oberlin BS, Tangney CC, Gustashaw KA, et al. Vitamin B12 deficiency in relation to functional disabilities. Nutrients 2013;5:4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Swart KM, Ham AC, van Wijngaarden JP, et al. A randomized controlled trial to examine the effect of 2-year vitamin B12 and folic acid supplementation on physical performance, strength, and falling: additional findings from the B-PROOF study. Calcif Tissue Int 2016;98:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–1S. [DOI] [PubMed] [Google Scholar]
- [41].Bauer JM, Sieber CC. Sarcopenia and frailty: a clinician's controversial point of view. Exp Gerontol 2008;43:674–8. [DOI] [PubMed] [Google Scholar]
- [42].Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci 2013;68:39–46. [DOI] [PubMed] [Google Scholar]
- [43].Castell M-V, Sánchez M, Julián R, et al. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Family Pract 2013;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shimada H, Makizako H, Doi T, et al. Incidence of disability in frail older persons with or without slow walking speed. J Am Med Dir Assoc 2015;16:690–6. [DOI] [PubMed] [Google Scholar]
- [45].Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2005;53:1675–80. [DOI] [PubMed] [Google Scholar]


