Abstract
Gestational diabetes mellitus (GDM) is a kind of chronic inflammatory condition with carbohydrate metabolism disorder. Interleukin-1beta (IL-1β) plays an important role in inflammatory response, but its role in GDM development remains unknown. The aim of this study was to analyze the association between Interleukin 1beta (IL1B) rs1143623 and rs16944 polymorphisms and susceptibility to GDM.
In total, 300 pregnant women with GDM and 261 healthy pregnant women were included in the study. In both groups, single nucleotide polymorphism (SNP) rs1143623 and rs16944 were analyzed by using snapshot technology. IL-1β serum values were determined by ELISA.
Serum IL-1β levels involvement in GDM development. According to the results, we found the association between the IL1B rs1143623 polymorphism and susceptibility to GDM. In further analysis, IL1B rs1143623 GG genotype had a higher level of total cholesterol (TCHO) and lower level of high density lipoprotein (HDL) in GDM patients compared with the CC/GC genotypes. However, there were no statistically significant difference between the GDM and healthy control groups in terms of rs16944 polymorphism.
Our results indicated that rs1143623 in IL1B gene may lead to GDM in the southwest of china. However, no significant difference was found between GDM and rs16944. The rs1143623 genotype may significantly impact the fat metabolism, especially the levels of TCHO and HDL. We believe that our findings will contribute to understanding of the etiology and possible novel prognostic markers for GDM.
Keywords: gestational diabetes mellitus, interleukin 1beta, fat metabolism, single nucleotide polymorphism
1. Introduction
Gestational diabetes mellitus (GDM) is a global health problem caused by carbohydrate metabolism disorder and affects approximately 17% of gestational women worldwide.[1,2] It has been reported that chronic inflammatory responses have a strong correlation with the occurrence of the GDM, and the pathophysiological process is not only involved in insulin resistance but also in disruption of insulin secretion. Chronic inflammation has been suggested as a contributor in development of pancreatic inflammatory diseases, such as insulin resistance.[3] To date, the molecular mechanisms of chronic inflammation in normal pregnancy women and GDM patients are not yet fully elucidated.
Cytokine is one of the inflammatory mediators that are considered to be the risk factors leading to the chronic inflammatory process in GDM. Interleukin-1beta (IL-1β) is a kind of pro-inflammatory cytokine encoded by IL1B gene. Increased levels of IL-1β protein have been reported to enhance the intensity of the inflammatory response, and therefore leading to the damages in pancreatic beta cells through nitric oxide production, and also inhibiting insulin release in pancreatic islets.[4] Recent studies suggest that the polymorphism of IL1B gene not only affect the protein level of IL-1β, but also changes susceptibility to diabetes mellitus in patients.[5–7] Previous study found that the IL1B polymorphism rs1143623 may affect the glucose-lowing efficacy of metformin in type 2 diabetes mellitus patients.[5] Furthermore, IL1B polymorphism rs16944 is associated with multiple inflammatory diseases such as chronic periodontitis, coronary artery lesions and chronic osteomyelitis.[8–10] Therefore, it is conceivable that IL1B polymorphism rs1143623 and rs16944 potentially have the closely related to inflammatory diseases, such as GDM.
As far as we know, IL1B gene polymorphism rs1143623 and rs16944 in the development of GDM has not been previously studied. In this study, our aim is to demonstrate any correlation between IL1B gene polymorphism and the occurrence of GDM in gestational women, which we suspect to play a key role in the inflammation of GDM. In addition, we also investigate the serum values of IL-1β protein and fat metabolism in GDM patients. Our study might provide novel biomarkers for predicting GDM in clinical patients.
2. Materials and methods
2.1. Patients
A total of 300 pregnant women diagnosed with GDM and 261 healthy pregnant women with no complaints were included in this study. Patients as well as controls were recruited from West China Second University Hospital, Sichuan University, during January 2019 to December 2019. The gestational ages were comparable between the 2 groups. For the purpose of GDM screening, fasting blood glucose (FBG) test was performed firstly during the time period between 24∼28 weeks of pregnancy. GDM can be diagnosed with FBG ≥5.8 mmol/L for 2 or more times. For those pregnant women whose FBG < 5.8 mmol/L, they were subjected to the 75 gram oral glucose tolerance test (OGTT). The cut-off values were 5.3 mmol/L for fasting blood glucose (FBG), 10.0 mmol/L for 1 hour measurement after load and 8.6 mmol/L for 2 hours measurement after load. The pregnant women with 2 or more than 2 blood glucose value measurement over the limit were diagnosed to GDM. Those with a previous history of diabetes, hypertension, chronic inflammatory systemic disease and the cases of multiple pregnancies were excluded from the study. The patient body mass index (BMI) was calculated using the formula BMI = weight (kg)/(height2) (m2). Serum lipid and glucose levels were measured by a Siemens automated biochemical analyzers. Serum IL-1β values in all participants with GDM or not were detected by commercial ELISA kits (Absin, Shanghai, China). This study was approved by the Medical Ethics Committee of West China Second University Hospital, Sichuan University. All patients signed informed consents of this study before blood analysis.
2.2. DNA isolation
Fresh peripheral blood was collected into tubes containing ethylenediaminetetraacetic acid (EDTA), and then the genomic DNA (gDNA) was isolated using the commercial kit (Tiangen Biotech, Beijing, China) following the manufacturers protocol.
2.3. SNP genotyping
Firstly, the extracted DNA should be diluted to working concentration (5∼10 ng/μl). Then multiple PCR experiments were performed. Each 20 μl reaction consists of 1x HotStar Taq buffer, 3.0 Mm Mg2+, 0.3 mM dNTP, 1U HotStar Taq polymerase (Qiagen), 1 μl DNA and 1 μl multiplex PCR primers (2 μM). The following is the specific information of primers which synthesized by Shanghai Sangon Biotech. rs1143623F: 5′-AAACCTTGCTCCTCCTGGTTCC-3′, IL1β- rs1143623R: 5′-TGCTTGGAGAGCAAGTCCATGA-3′, rs16944F: 5′-TGTGGGACAAAG, TGGAAGACACA-3′, rs16944R: 5′-CAGGAGCCTGAACCCTGCATAC-3′. All reactions were run on an ABI 2720 Thermal Cycler (Thermo Fisher Scientific, USA) using the following cycling parameters: 95°C for 2 minutes; 11 cycles of 94°C for 20 second, 60°C for 40 second (−0.5°C/cycle) and 72°C for 90 second; 24 cycles of 94°C for 20 second, 59°C for 30 second and 72°C for 90 second; and a final elongation step of 72°C for 2 minutes. Next, following the protocol of ABI PRISM Snapshot Multiplex Kit, 10 μl PCR products were treated with 5U enzyme SAP and 2U enzyme exonuclease I, bathing at 37°C for 1 hour, and then inactivate at 75°C for 15 minutes. Target SNPs were analyzed using real-time PCR system with following primers: rs1143623SR: 5′-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT CACAGAGGCTCACTCCCTTG-3′, rs16944SR: 5′-TTTTTTTCTGCAATTGACAGAGAGCTC C-3′. All reactions were run on an ABI 3730XL DNA Analyzer (Thermo Fisher Scientific, USA) and analysis with GeneMapper 4.1 (AppliedBiosystems, Foster City, CA, USA).
2.4. Statistical analysis
The SPSS statistical software was used to analysis. For the analysis of distribution of genotypes and alleles between the GDM patients and control group, Chi-Squared test and Mann–Whitney U test were used when appropriate. The OR and 95% confidence interval (CI) was performed for the analysis of IL1B genotype/haplotype differences between patients with GDM and healthy controls. Differences were considered to be statistically significant when P < .05. Genotype frequencies were distributed in accordance with the Hardy–Weinberg equilibrium.
3. Results
3.1. Serum IL-1β level involvement in GDM development
The serum IL-1β level in the GDM patients and healthy controls are present in Figure 1. Significantly higher level of serum IL-1β was observed in GDM patients with those in the healthy control group (n = 23/group, P = .023). Therefore, serum IL-1β level involvement in GDM development.
Figure 1.
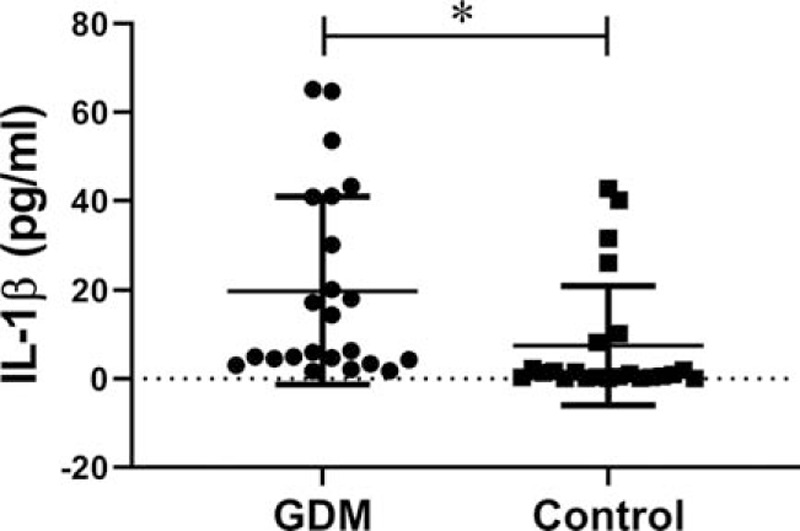
Serum IL-1β level in the GDM patients and healthy controls. The serum IL-1β levels in GDM patients are significantly higher than those of healthy controls (n = 23/group, P = .023). These data indicate that the serum IL-1β level is involved in the occurrence and development of GDM (∗P < .05, GDM vs Control).
3.2. Clinical characteristics of women with and without GDM
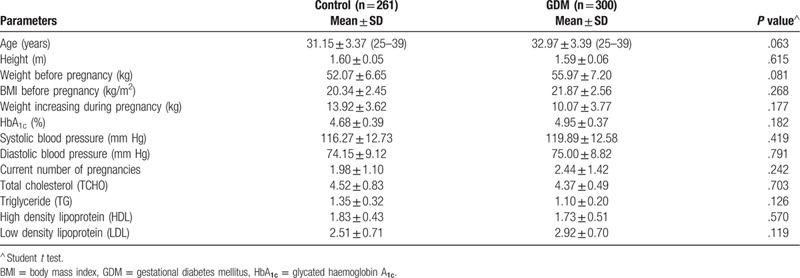
The clinical characteristics of women with and without GDM are summarized in Table 1. Totally, 300 GDM patients and 261 healthy controls were recruited in this study. The mean ages were 31.15 ± 3.37 years old for GDM patients (range 25–39) and 32.97 ± 3.39 years old for healthy controls (range 25–39). Of the GDM patients, the BMI before pregnancy is 20.34 ± 2.45 kg/m2 and the healthy control group is 21.87 ± 2.45 kg/m2. As we can see, the gestational age, height, weight before pregnancy, BMI before pregnancy, weight increasing during pregnancy, glycated haemoglobin A1c (HbA1c), blood pressure and current number of pregnancy between the 2 groups were roughly comparable.
Table 1.
Clinical parameters of women with and without GDM.
3.3. Distribution of IL1B genotypes in women with and without GDM
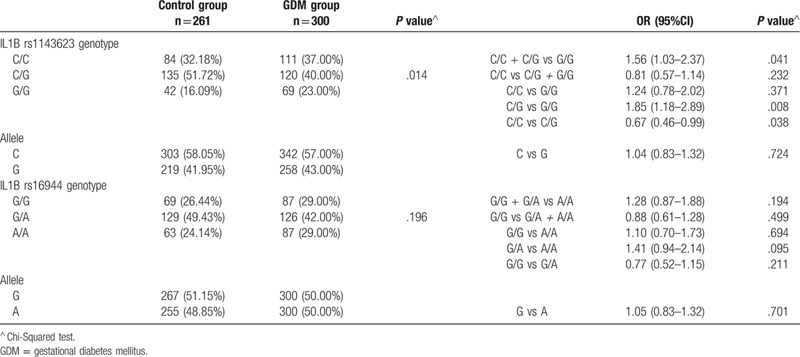
The genotype frequencies in women with and without GDM are shown in Table 2. The distributions of the studied genotypes rs1143623 and rs16944 were in HWE (P > .05). There were no statistically significant differences between the GDM patients and controls in terms of IL1B polymorphism rs16944, whereas there was a statistically significant association between the IL1B polymorphism rs1143623 and GDM. In this study, we compared the distribution of studied genotypes between women with and without GDM. Among women with GDM, we observed the increased frequency of the IL1B rs1143623 GG genotype compared with healthy control group (23% vs 16.09%). Additionally, among women with GDM, a prevalence of GG genotype was observed (CC+CG vs GG; P = .041; OR = 1.56, 95% CI, 1.03–2.37).
Table 2.
Distribution of IL1B genotypes in women with and without GDM.
3.4. Clinical parameters of women with GDM according to IL1B rs1143623 genotype
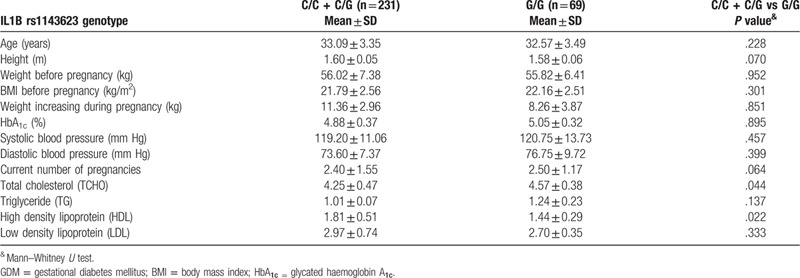
We also examined the association between the rs1143623 polymorphisms and clinical parameters, such as age, height, body weight before pregnancy, body mass index (BMI) before pregnancy, weight increasing during pregnancy, glycated hemoglobin A1c (HbA1c), systolic blood pressure, diastolic blood pressure and current number of pregnancy. According to the results, no statistically significant difference of above parameters has been observed. However, the IL1B rs1143623 GG genotype was found to be associated with increased expression of total cholesterol (TCHO) and high density lipoprotein (HDL) (TCHO, 4.25 ± 0.47 vs 4.57 ± 0.38, P = .044; HDL, 1.81 ± 0.51 vs 1.44 ± 0.29, P = .022.) (Table 3).
Table 3.
Clinical parameters of women with GDM stratified according rs1143623 genotype.
4. Discussion
In this study, we investigated the association between IL1B polymorphisms and its protein expression in patients with GDM in Chinese Han population from southwest of China. The results imply a significant association for IL1B rs1143623 polymorphism and GDM patients, however the IL1B rs16944 polymorphism has no coloration to the occurrence and development of GDM. GDM is characterized by a chronic inflammatory condition, that induced by various pro-inflammatory and anti-inflammatory cytokines. IL-1β is an important cytokine that plays an important role in the induction and maintenance of the inflammatory response in pancreas tissue. The role of IL-1β protein in the pathogenesis of GDM has been investigated in both animal models and in clinical studies.[11,12] In our study, we also find a significant increased expression of serum IL-1β protein in patients with GDM. IL-1β is involved in the tissue destruction associated with GDM due to its pro-inflammatory properties. It is over-expressed in the pancreas tissue and peripheral blood of patients with GDM, where it appears to play an important role in the regulation of inflammatory factors such as interleukins, chemokines and adhesion molecules. Previous studies have indicated that the expression of IL-1β protein in GDM patients is related to the degree of disease activity.[11,12] Increased serum levels of IL-1β in patients with GDM correlated with several clinical disease parameters. In addition, some studies suggest that IL1B rs1143623 involved in regulation of fasting and postprandial lipid metabolism.[13] It is reported that the carriers of IL1B rs1143623 polymorphisms have an increased postprandial lipemia and that elderly homozygotes with rare allele also have an increased level of fasting triglyceride (TG).[13] In our study, we find that IL1B rs1143623 polymorphism has the fat metabolism regulation properties, especially in the regulation of total cholesterol (TCHO) and high density lipoprotein (HDL) levels. GG genotype pregnant women are more likely to develop gestational diabetes mellitus with the increase of THCO and the decrease of HDL. These findings are consistent with previous reports.[14,15] The combination of increased TCHO and HDL levels allows us to hypothesize that these patients may have a higher inflammatory status and may over respond to the pro-inflammatory stimulus that represents a fatty meal.
In conclude, the results of this study show no association between IL1B rs16944 polymorphisms and GDM in Chinese population located in southwest of China. Additionally, we observed that the IL1B rs1143623 polymorphism is closely related to the susceptibility to GDM of women in Chinese Han population from southwest of China, accompanied by an increased level of THCO and decreased level of HDL.
Although this study is statistically convincing, there were still some intrinsic limitations. First, a small number of patients are included in this study, thus a larger study is needed. Second, further experiments are needed to investigate the molecular mechanism of IL1B rs16944 polymorphisms in regulating fat metabolism.
Acknowledgments
The authors express great thanks to National Natural Science Foundation of China and the Department of Science and Technology of Sichuan Province.
Author contributions
Conceptualization: Ting Liu, Li Chang.
Data curation: Jia-min Deng, Yan-ling Liu.
Formal analysis: Ting Liu, Jia-min Deng.
Project administration: Li Chang, Yong-mei Jiang.
Visualization: Ting Liu, Jia-min Deng.
Writing – original draft: Ting Liu, Jia-min Deng.
Writing – review & editing: Li Chang, Yong-mei Jiang.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, EDTA = ethylenediaminetetraacetic acid, FBG = fasting blood glucose, FBG = fasting blood glucose, GDM = gestational diabetes mellitus, gDNA = genomic DNA, HbA1c = glycated haemoglobin A1c, HDL = high density lipoprotein, IL1B = Interleukin 1beta, IL-1β = Interleukin-1beta, OGTT = oral glucose tolerance test, PCR = polymerase chain reaction, SNP = single nucleotide polymorphism, TCHO = total cholesterol, TG = triglyceride.
How to cite this article: Liu T, Deng Jm, Liu Yl, Chang L, Jiang Ym. The relationship between gestational diabetes mellitus and interleukin 1beta gene polymorphisms in southwest of China. Medicine. 2020;99:43(e22679).
This study was generously supported by a grant from the National Natural Science Foundation of China (No.81801628) and the Key Research and Development Projects of Sichuan Science and Technology Department (No.2019YFS0315).
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Coustan DR. Recurrent GDM and the development of type 2 diabetes have similar risk factors. Endocrine 2016;53:624–5. [DOI] [PubMed] [Google Scholar]
- [2].Wang Y, Luo BR. The association of body composition with the risk of gestational diabetes mellitus in Chinese pregnant women: a case-control study. Medicine 2019;98:e17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen G, Hohmeier HE, Newgard CB. Expression of the transcription factor STAT-1 alpha in insulinoma cells protects against cytotoxic effects of multiple cytokines. J Biol Chem 2001;276:766–72. [DOI] [PubMed] [Google Scholar]
- [4].Corbett JA, Sweetland MA, Wang JL, et al. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A 1993;90:1731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiao D, Zhang SM, Li X, et al. IL-1B rs1143623 and EEF1A1P11-RPL7P9 rs10783050 polymorphisms affect the glucose-lowing efficacy of metformin in Chinese overweight or obese Type 2 diabetes mellitus patients. Pharmacogenomics 2015;16:1621–9. [DOI] [PubMed] [Google Scholar]
- [6].Patel R, Dwivedi M, Mansuri MS, et al. Association of Neuropeptide-Y (NPY) and Interleukin-1beta (IL1B), Genotype-Phenotype Correlation and Plasma Lipids with Type-II Diabetes. PloS one 2016;11:e0164437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Saxena M, Srivastava N, Banerjee M. Cytokine gene variants as predictors of type 2 diabetes mellitus. Curr Diabetes Rev 2018;14:307–19. [DOI] [PubMed] [Google Scholar]
- [8].Hong SJ, Kang SW, Kim SK, et al. Lack of association between interleukin-1beta gene polymorphism (rs16944) and chronic periodontitis: from a case-control studies to an updated meta-analysis. Dis Markers 2018;2018:8287026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu LY, Qiu X, Deng QL, et al. The IL-1B gene polymorphisms rs16944 and rs1143627 contribute to an increased risk of coronary artery lesions in southern Chinese children with kawasaki disease. J Immunol Res 2019;2019:4730507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yao ZL, Lin QR, Hu YJ, et al. Interleukin-1 beta gene polymorphism rs16944 may associate with increased susceptibility to extremity chronic osteomyelitis in Chinese han population. Biomed Res Int 2019;2019:7483537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schulze F, Wehner J, Kratschmar DV, et al. Inhibition of IL-1beta improves glycaemia in a mouse model for gestational diabetes. Sci Rep 2020;10:3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, et al. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol 2013;69:545–57. [DOI] [PubMed] [Google Scholar]
- [13].Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, et al. Interleukin 1B variant -1473G/C (rs1143623) influences triglyceride and interleukin 6 metabolism. J Clin Endocrinol Metabolism 2011;96:E816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tu WJ, Guo M, Shi XD, et al. First-trimester serum fatty acid-binding protein 4 and subsequent gestational diabetes mellitus. Obstetrics Gynecol 2017;130:1011–6. [DOI] [PubMed] [Google Scholar]
- [15].Wang J, Li Z, Lin L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine 2019;98:e15320. [DOI] [PMC free article] [PubMed] [Google Scholar]


