Abstract
Compared to the general population, Persons Living With HIV (PLWH) have higher rates of tobacco use and an increased risk of morbidity from tobacco-related diseases. We conducted a single arm pilot study of the real-world feasibility of integrating a smoking cessation decisional algorithm within routine clinic visits to engage non-treatment seeking smokers in smoking cessation therapies. Smokers had an initial study visit during routine care followed by phone contacts at one and three months. Participants completed a baseline survey, followed by the algorithm which resulted in a recommendation for a smoking cessation medication, which was prescribed during the visit. Follow-up phone surveys assessed changes in smoking behavior and use of cessation medications at 1 and 3 months. Participants’ (N=60) self-reported smoking decreased from a baseline average of 14.4 cigarettes/day to 7.1 cigarettes/day at 3 months (p=0.001). Nicotine dependence (FTND) decreased from 5.6 at baseline to 3.6 at 3 months (p<0.001). Twenty-seven (45%) made a 24-hour quit attempt and 39 (65%) used cessation medication. Insurance prior-authorization delayed medication receipt for 7 participants, and insurance denial occurred for one. The algorithm was successful in engaging participants to use cessation medications and change smoking behaviors.
Keywords: decisional aid, smoking cessation, HIV
Introduction
Compared to the general population, people living with HIV and AIDS (PLWH) have higher rates of tobacco use, decreased rates of treatment utilization, decreased rates of tobacco cessation, and increased risk of morbidity from tobacco related diseases. Compared to general population of adults, smoking rates among PLWH are 2 to 3 times higher, with estimates that smokers account for 40—70% of PLWH with 85% having a lifetime history of ever smoking (Smoking, 2018; Calvo, Laguno, Martinez & Martinez, 2015; Burkhalter, Springer, Chhabra, Ostroff & Rapkin, 2005). Medical advances in HIV treatment have resulted in near normal life expectancies for PLWH, and as a result smoking-related mortality is accounting for more years of life lost than HIV-associated mortality (Reddy et al., 2016). PLWH who smoke are at greater risk of mortality and negative health outcomes than smokers without HIV infection as well as compared with PLWH who do not smoke (Calvo et al., 2015; O’Cleirigh et al., 2014). PLWH who smoke have higher incidence of lung disease including COPD and bacterial pneumonia, increased inflammation resulting in cardio-metabolic and renal diseases, as well as increased risks in developing cancer (Calvo et al., 2015; O’Cleirigh et al., 2014; Lifson et al., 2010). Additionally, HIV-related morbidity is associated with smoking in PLWH, with a pro-inflammatory state and increased reactive oxygen species from nicotine metabolism resulting in poorer virologic response to antiretroviral therapy (ART), increased immune activation, microbial translocation, and disease progression. PLWH who smoke also report lower quality of life than those who do not smoke (Calvo et al., 2015; Cropsey et al., 2016; Feldman et al., 2006; Ande et al., 2015; Earla, Ande, McArthur, Kumar & Kumar, 2013). Smoking is a modifiable risk factor for these diseases and several cessation strategies have been tested and are FDA approved, including among PLWH (Fiore MC et al., 2008).
There are many barriers to smoking cessation among PLWH, including those at the patient level. PLWH who smoke have high rates of unemployment, poverty, drug and alcohol use, mental illness, and social isolation, all which have been shown to impair cessation success (Burkhalter et al., 2005; Cropsey et al., 2016; Mdodo et al., 2015; Shuter et al., 2011). PLWH who smoke often have increased pill burden for treatment of other co-morbidities, which further undermines compliance with cessation pharmacotherapies. In addition to patient-level barriers there are in-clinic barriers that limit cessation service delivery among providers who manage PLWH. For example, providers may focus on treatment of active conditions and these competing clinical care goals distract from other goals related to preventative health and smoking cessation (Crothers et al., 2007). Likewise, patients put less importance on prevention which impairs cessation discussions, perceiving that HIV has greater impact on health than smoking (Feldman et al., 2006; Crothers et al., 2007; Calvo-Sanchez & Martinez, 2014). Additionally, providers report lack of confidence and knowledge for smoking cessation interventions. HIV providers were less likely to identify current smoking status and only half as likely to be confident in their ability to influence cessation compared with non-HIV providers (Crothers et al., 2007). Only 23% of providers responding to a survey study from the HIV Medicine Association reported formal training or education on smoking cessation topics (Shuter et al., 2011).
In addition to provider and patient level barriers, system barriers exist that undermine cessation as well. These may include the various payer sources, ranging from private or government sponsored insurance plans to federal programs for uninsured PLWH, each of which have different medication formularies, approval processes, tiers of coverage, and copays which are difficult for providers and patients to navigate and have impacted the ability to obtain treatment (Chew, Steinberg, Thomas, Swaminathan, Hodder, 2014). Delays in receiving the medication may result in waning enthusiasm and high out of pocket costs may deter patients from obtaining the appropriate medications.
Altogether, these individual, provider, and system-level barriers to cessation among PLWH are particularly significant because interest in quitting among smokers with HIV is comparable to the general population. Up to 82% of PLWH report lifetime quit attempt with 60–70% contemplating smoking cessation (Reddy et al., 2016; Calvo-Sanchez & Martinez, 2014; Benard et al., 2007; Shahrir et al., 2015). PLWH demonstrate improved health behaviors following HIV diagnosis, including stopping or reducing smoking, and have a predisposition to consider medical advice by HIV providers which supports the practice of HIV providers focusing on smoking cessation efforts (Calvo-Sanchez & Martinez, 2014; Benard et al., 2007; Collins et al., 2001).
Our clinic serves 1200 patients with HIV annually, without regard to ability to pay. In a clinic-wide smoking survey in 2010, 40% of patients reported current smoking, and 85% had lifetime a quit attempt and 49% expressing an interest in speaking to a clinician about quitting (Bean, Richey, Williams, Wahlquist, Kilby, 2016). We used a recently published a cessation algorithm that provides concise guidance for treatment selection, designed to prioritize important factors when prescribing pharmacotherapy. The algorithm considers treatment options for tobacco use, including all FDA-approved options (bupropion, varenicline, and nicotine replacement therapy (NRT)) and combinations of treatment. This prior study offered and provided medications as part of algorithm delivery, which conflates the decisional support tool (algorithm) itself with pharmacotherapy (Cropsey et al., 2015). For this study we were interested in real-world feasibility of the use of the algorithm in a busy HIV clinic setting to assess changes in smoking behavior and use of cessation medications. Our secondary objective was to assess implementation and utilization barriers, including cost and insurance, and quantify these barriers to obtaining the recommended therapy.
Methods
This was a single arm pilot study to evaluate the real-world impact of the smoking cessation algorithm among 60 participants. Institutional Review Board approval was obtained prior to any recruitment of patients. Patients were approached about participation in the study if they: presented for management of HIV infection, were age 18 or older, were English-speaking, and reported daily tobacco use (5+ cigarettes per day, for 25+ days within prior month), and not currently making a quit attempt. Enrollment was not based on motivation to quit, so all PLWH who smoked were approached for participation. Patients were excluded if they were pregnant or nursing, or had cognitive impairment that prevented informed consent.
Each participant had an initial visit and two follow-up phone contacts at one and three months, receiving up to $160 in total compensation. After informed consent, patients completed a computer-based survey, followed by completion of the algorithm questions to determine optimal pharmacotherapy. Surveys included the Abstinence-Related Motivational Engagement (ARME) and contemplation ladder, both of which are standard measures of motivation to quit smoking, as well as the Fagerstrom Test for Nicotine Dependence (FTND), a standardized measure of nicotine dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Additional questions regarding smoking history and behaviors were also assessed. The algorithm (Cropsey et al., 2015) was made into a computer based decision tool that utilized guideline recommendations, manufacturer package insert medication use guidance, and patient related factors. Participants answered questions using the decisional tool, which took into account the branching in the previously published algorithm (Cropsey et al., 2015) to reach a medication recommendation (see Figure 1).
Figure 1.
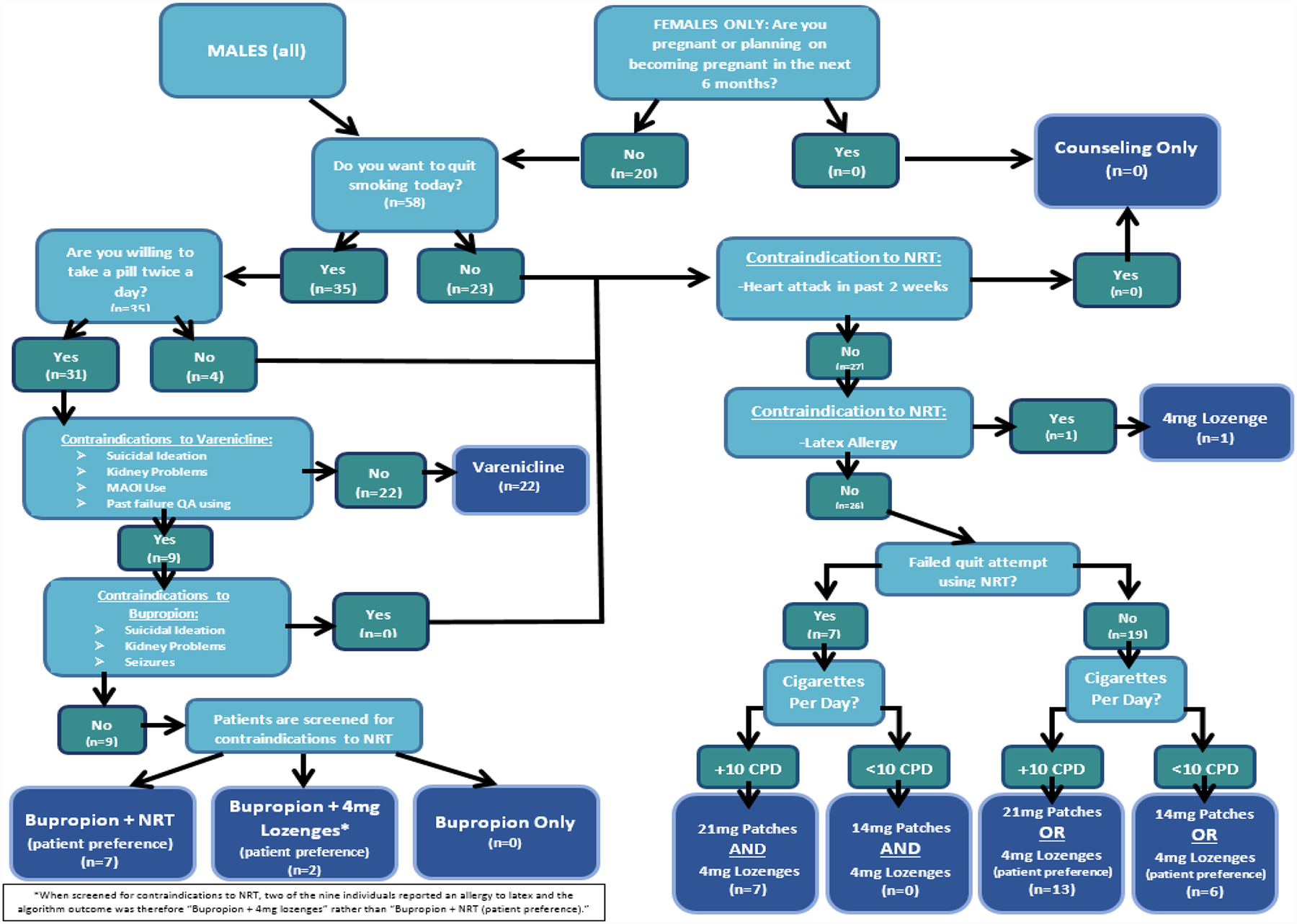
Algorithm Schematic
Following completion of the algorithm, the pharmacist provided standard cessation counseling, inclusive of the recommended medication option. The pharmacist retained ability to deviate from algorithm for any reason (see results below). Following prescription from the patient’s medical provider, medication was obtained using the existing standard of care mechanisms based in part on payer/insurance support, with anticipated variation in coverage of the pharmacotherapy options for smoking cessation reflective of the variance in insurance type. This included co-payment structure, drug formulary, and prior-authorization and/or medical exemptions.
Follow-up telephone surveys lasted about 30 minutes and assessed prospective outcomes, including use of and time to obtaining the medication, copay cost, barriers to receiving the preferred medication, changes in the use patterns of tobacco use, and quit attempts.
Data collection was initiated November 2015 and continued until study completion in October 2016. Demographic, clinical, medication, insurance, laboratory, and socioeconomic data were obtained from the electronic medical record and other institutional data sources. No patients withdrew from the study or were lost to follow-up, all were included in the analysis. Statistical software (SPSS 24) was used for analysis, and included paired t-tests and ANOVA for continuous variables and chi squared for dichotomous variables.
Results
Baseline characteristics are displayed in Table 1. Participants (n=60) were mostly African American (72%) men (67%) with average age of 48 years with well-controlled HIV infection on antiretroviral therapy (Table 1). Participants had significant substance and alcohol use and as well as high rates of depression (Table 1). Tobacco related characteristics at baseline are displayed in Table 2. Participants reported an average of 14.4 cigarettes/day at study entry and had an average score of 5.6 on FTND, both demonstrating moderate nicotine dependence (Table 2). At three months the average cigarettes/day dropped to 7.1 (p< 0.001) and the FTND to 3.6 (p< 0.001). Measures of motivation to quit also changed significantly. The ARME increased from 3.8 at baseline to 4.4 at 3 months (p<0.001) and the contemplation ladder for the motivation to quit in the next month increased from 6.4 to 7.5 (p=0.01). Additionally, 27 (45%) participants reported a 24-hour quit attempt. We separately examined whether outcomes differed according to baseline motivation. No significant differences were seen in cigarettes reduction, incidence rates of 24-hour quit attempt, or medication use were seen when stratified by motivation to quit at in the next month or within 6 months (low 0–7 on contemplation ladder versus high 8–10) (Table 3).
Table 1.
Participant baseline characteristics
| Age (years, range) | 48 (22–63) |
| Male (n, %) | 40 (67%) |
| African-American (n, %) | 43 (72%) |
| Insurancea | |
| Medicaid | 6(10%) |
| Medicare | 16(27%) |
| Ryan White | 20 (33%) |
| Private | 18 (30%) |
| AIDS Drugs Assistance Program Participant | 28(47%) |
| CD4 Count (mean, range) | 622 (9–1820) |
| HIV VL (copies/mL) | |
| VL < 40 (n, %) | 42 (70%) |
| VL < 200 (n, %) | 49 (82%) |
| On ART (n, %) | 58 (97%) |
| Depression | |
| On Chart Review | 40% |
| CES-D (mean) | 16.3 |
| CES-D ≥ 16b | 45% |
| Co-morbid anxiety | |
| On Chart Review | 15% |
| Harmful Alcohol Use | |
| On Chart Review | 20% |
| AUDIT (mean) | 7.1 |
| AUDIT ≥ 8c | 23% |
| Illicit Drug Use | |
| On Chart Review | 18% |
| DAST (mean) | 2.3 |
| DAST ≥ 6d | 15% |
Insurance listed is patient’s primary insurance
CES-D ≥ 16 correlates with clinical depression
Indicates harmful alcohol consumption
Indicates substance use disorder
VL= Viral Load
CES-D= Center for Epidemiologic Studies-Depression
AUDIT=Alcohol Use Disorders Identification Test
DAST=Drug Abuse Screening Test
Table 2.
Participant Smoking Related Characteristics at Baseline and 3 Months
| Baseline | 3 months | P value | |
|---|---|---|---|
| Cigarettes/day (mean) | 14.4 | 7.1 | <0.001 |
| Nicotine dependence (FTND) mean | 5.6 | 3.6 | <0.001 |
| Next month | 6.4 | 7.5 | 0.01 |
“intent to quit on a scale of 1 to 10,” mean value
FTND=Fagerstrom Test for Nicotine Dependence
ARME=Abstinence-Related Motivational Engagement
Table 3:
Quit Attempts, Decrease in Cigarettes per Day and Medication Use by Motivation to Quit
| Lowa | Higha | P value | |
|---|---|---|---|
| Motivation to Quit within a month | N= 36 | N=24 | |
| Quit Attempts | 42% | 63% | 0.11 |
| Decrease in Cigs/day | 6.1 | 9.1 | 0.14 |
| Medication Use | 58% | 75% | 0.19 |
| Motivation to Quit within six months | N=17 | N=43 | |
| Quit Attempts | 35% | 56% | 0.15 |
| Decrease in Cigs/day | 8.0 | 5.7 | 0.30 |
| Medication Use | 53% | 70% | 0.22 |
High Motivation is 8–10 and Low Motivation is 0–7 on the contemplation ladder for intent to quit smoking. Intent to quit is on a self-rated scale of 1 to 10, with 10 being the highest intent.
Medications recommended by the algorithm were mostly varenicline (38.3%), but also bupropion + NRT (15%) and NRT (46.7%). Medications actually prescribed by provider were similar, mostly varenicline (31.7%), bupropion + NRT (10%), bupropion (3.3%), NRT (45%), and the remaining 10% refused pharmacotherapy. Uninsured patients received medication assistance through the AIDS Drug Assistance Program (ADAP). Additionally, some insured patients received co-pay assistance through ADAP if eligible. A prior authorization was required for 3 patients with Medicare, 2 with Medicare/ADAP, 1 with Medicaid, and 1 with private/ADAP, and ultimately resulted in at least a 14-day delay to start medication in each case. The only patient to not receive prescribed therapy was a patient with Medicaid who was prescribed varenicline but ultimately received bupropion after a prior authorization denial. Thirty-nine (65%) of participants used the cessation medication, demonstrating engagement in medication use. Only 3 patients utilized other treatments such as calling the quitline. No serious adverse events were reported with use of medications during the intervention and follow-up period.
Discussion
The results of this study highlight a proactive approach that treats smokers in a challenging HIV clinic setting, increasing treatment engagement even among those with little motivation to quit. We demonstrated a significant reduction in smoking, and a high percentage of quit attempts. It is the second study to demonstrate successful use of a smoking algorithm to increase use of medications and counseling to PLWH regardless of motivation to quit. In the study published by Cropsey et al. (2015), participants were randomized to algorithm-guided treatment versus treatment as usual, combined with an initial 20 minute in-person counseling session and free provision of cessation medication. This study documented improved smoking outcomes, including increased quit attempts (50% vs. 38%), increased use of medications for smoking cessation (81% vs. 23%), and reduction in average cigarettes per day (10 vs. 6) (Cropsey et al., 2015). However, this previous study offered free cessation medication and our current study was interested in determining the utility of the algorithm in standard practice, where medication would be paid for by insurance or other third party payers. This study demonstrated that most smokers were able to get smoking pharmacotherapy, with 65% using cessation medications, 45% of patients making a quit attempt, and significant reduction in cigarettes/day over a 3-month follow-up. The current real-world application of a smoking algorithm supports a proactive approach and algorithm use, by itself, to improve outcomes.
The proactive use of the algorithm demonstrated engagement in smoking cessation therapy regardless of motivation to quit. This is important as standard smoking cessation practice generally states that patients must demonstrate motivation to quit smoking prior to offering an active intervention such as provision of smoking pharmacotherapy (Fiore MC et al., 2008). However, more recent studies, even with persons living with HIV, demonstrated that motivation to quit smoking was not necessary prior to offering cessation treatment (Cropsey et al, 2013). Participants who were offered smoking pharmacotherapy showed improvement over usual care in decreasing cigarettes per day, decreased physical nicotine dependence, decrease smoking urge and also found an overall decrease in physical nicotine dependence regardless of motivation to quit (Cropsey et al, 2013).
Our intervention demonstrated the real-world application of this algorithm model in clinical practice. In our study, the proactive algorithm was implemented with high feasibility in a way that did not disrupt clinic flow or efficiency. The algorithm was able to be completed by the patients and did not require face-to-face time with the provider; thus had no anticipated impact on clinical efficiency. As demonstrated in this study, the algorithm could be implemented by clinic staff with counseling provided by a clinical pharmacist, in addition to being utilized by the health care provider if desired. Additionally, we were able to establish that the algorithm effectively assessed and provided recommendations for smoking cessation medications to be subsequently prescribed. The use of the algorithm bypassed any barriers due to lack of confidence in cessation medication utilization and simultaneously improved smoking cessation offering for PLWH who smoke. In addition to overcoming clinic barriers, the study demonstrates that system barriers including insurance, or lack thereof, did not affect the implementation of algorithm use in the clinic. All patients prescribed therapy were able to access the prescribed therapy except for a single patient.
There were two notable limitations to this study affecting the interpretation of results. As noted, there was no control arm as all participants were offered the algorithm. However, the algorithm was found to have benefit in another randomized study when compared to standard of care (Cropsey et al., 2015).This feasibility study sought to confirm the success of algorithm implementation in a real world setting without offering free medication, and thus, was able to use the initial randomized study to validate that the improvement in smoking outcomes were not due to the free provision of pharmacotherapy. A second limitation to this study was that it used self-reported outcomes and only followed patients for 3 months after the intervention. To limit the bias of self-report, the study used previously validated scales to assess self-reported smoking outcomes. Future studies should examine the real-world use of pharmacotherapy and intervention delivery in a randomized controlled trial.
The study had several important strengths. We did not identify any limitations to applying the algorithm to the varied payer mix seen in the population of patients seen in the clinic. The patients were able to access medications in almost all situations. We demonstrated that the algorithm could be offered to all patients, regardless of comorbid medical conditions or motivation to quit. In fact, the outcomes demonstrated no differences for patients regardless of motivation (low or high). Among this varied group we saw significant decreases in cigarette use and increases in medication use at 3 months without any reported serious adverse effects.
People living with HIV and AIDS have higher tobacco use and worse tobacco related outcomes. Importantly, PLWH now lose more years to tobacco use than to HIV-related diseases, making smoking cessation critically important for all PLWH. The smoking cessation algorithm tested herein was successful in proactively engaging patients to use cessation medications and led to meaningful changes in smoking behavior. Real world implementation of this algorithm using a computer program addressed both provider education and clinic time barriers to the universal delivery of cessation services. The algorithm is an inexpensive and effective intervention to proactively address smoking cessation in people living with HIV.
Acknowledgements
This work was supported through the Southern Consortium of the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) (UG1 DA 013727). We thank the staff and providers involved at the Medical University of South Carolina for their tireless efforts to improve the care of patients. We also thank the individual participants from the trial. MCB and LER: project design, analysis, and manuscript draft. KLC, LH and MJC: project design and manuscript revision.
Footnotes
Declaration of Interest
Funding and Declarations of Interest for all authors: This work was supported through the Southern Consortium of the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) (UG1 DA 013727). The findings and conclusions herein are those of the authors and do not necessarily represent the official position of NIDA or CTN. All authors report no grant funding outside the support listed above for the submitted work.
References
- Ande A, McArthur C, Ayuk L, Awasom C, Achu PN, Njinda A, … Kumar S (2015). Effect of Mild-to-Moderate Smoking on Viral Load, Cytokines, Oxidative Stress, and Cytochrome P450 Enzymes in HIV-Infected Individuals. PLOS ONE, 10(4), e0122402. doi: 10.1371/journal.pone.0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean MC, Richey LE, Williams K, Wahlquist AE, & Kilby JM (2016). Tobacco Use Patterns in a Southern US HIV Clinic. Southern Medical Journal, 109(5), 305–308. doi: 10.14423/smj.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, … The Groupe D’epidemiologie Clinique Du Sida En Aqutaine. (2007). Tobacco Addiction and HIV Infection: Toward the Implementation of Cessation Programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care And STDs, 21(7), 458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10(5), 360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, & Rapkin BD (2005). Tobacco use and readiness to quit smoking in low‐income HIV‐infected persons. Nicotine & Tobacco Research, 7(4), 511–522. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- Calvo M, Laguno M, Martinez M, Martinez E (2015). Effects of tobacco smoking on HIV-infected individuals. AIDS Review, 17(1), 47–55. [PubMed] [Google Scholar]
- Calvo-Sánchez M, & Martinez E (2014). How to address smoking cessation in HIV patients. HIV Medicine, 16(4), 201–210. doi: 10.1111/hiv.12193. [DOI] [PubMed] [Google Scholar]
- Chew D, Steinberg MB, Thomas P, Swaminathan S, & Hodder SL (2014). Evaluation of a Smoking Cessation Program for HIV Infected Individuals in an Urban HIV Clinic: Challenges and Lessons Learned. AIDS Research and Treatment, 2014, 1–8. doi: 10.1155/2014/237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL, Kanouse DE, Gifford AL, Senterfitt JW, Schuster MA, McCaffrey DF, … Wenger NS (2001). Changes in health-promoting behavior following diagnosis with HIV: Prevalence and correlates in a national probability sample. Health Psychology, 20(5), 351–360. doi: 10.1037/0278-6133.20.5.351. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, Willig J, … Carpenter MJ (2013). A pilot study of Screening, Brief Intervention, and Referral for Treatment (SBIRT) in non-treatment seeking smokers with HIV. Addictive Behaviors, 38(10), 2541–2546. doi: 10.1016/j.addbeh.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Jardin BF, Burkholder GA, Clark CB, Raper JL, & Saag MS (2015). An Algorithm Approach to Determining Smoking Cessation Treatment for Persons Living With HIV/AIDS. JAIDS Journal Of Acquired Immune Deficiency Syndromes, 69(3), 291–298. doi: 10.1097/qai.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Willig JH, Mugavero MJ, Crane HM, McCullumsmith C, Lawrence S, … Centers for AIDS Research Network of Integrated Clinical Systems. (2016). Cigarette Smokers are Less Likely to Have Undetectable Viral Loads. Journal of Addiction Medicine, 10(1), 13–19. doi: 10.1097/adm.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Goulet JL, Rodriguez-Barradas MC, Gibert CL, Butt AA, Braithwaite RS, … Justice AC (2007). Decreased Awareness of Current Smoking Among Health Care Providers of HIV-positive Compared to HIV-negative Veterans. Journal of General Internal Medicine, 22(6), 749–754. doi: 10.1007/s11606-007-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earla R, Ande A, McArthur C, Kumar A, & Kumar S (2013). Enhanced Nicotine Metabolism in HIV-1-Positive Smokers Compared with HIV-Negative Smokers: Simultaneous Determination of Nicotine and its Four Metabolites in Their Plasma Using a Simple and Sensitive Electrospray Ionization Liquid Chromatography-Tandem Mass Spectrometry Technique. Drug Metabolism and Disposition, 42(2), 282–293. doi: 10.1124/dmd.113.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts DH, … Anastos K (2006). Association of Cigarette Smoking With HIV Prognosis Among Women in the HAART Era: A Report From the Women’s Interagency HIV Study. American Journal of Public Health, 96(6), 1060–1065. doi: 10.2105/ajph.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, … Wewers ME (2008). Treating tobacco use and dependence. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service. [Google Scholar]
- Gritz ER, Vidrine DJ, Lazev AB, Amick III BC, & Arduino RC (2004). Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine & Tobacco Research, 6(1), 71–77. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction, 86(9), 1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Lifson AR, Neuhaus J, Arribas JR, Van den Berg-Wolf M, Labriola AM, & Read TRH (2010). Smoking-Related Health Risks Among Persons With HIV in the Strategies for Management of Antiretroviral Therapy Clinical Trial. American Journal of Public Health, 100(10), 1896–1903. doi: 10.2105/ajph.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks J, & Skarbinski J (2015). Cigarette Smoking Prevalence Among Adults With HIV Compared With the General Adult Population in the United States. Annals of Internal Medicine, 162(5), 335. doi: 10.7326/m14-0954. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, Gordon JR, & Safren SA (2014). The Unique Challenges Facing HIV-Positive Patients Who Smoke Cigarettes: HIV Viremia, Art Adherence, Engagement in HIV care, and Concurrent Substance Use. AIDS and Behavior, 19(1), 178–185. doi: 10.1007/s10461-014-0762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, … Walensky RP, (2016). Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. Journal of Infectious Diseases, 214(11), 1672–1681. doi: 10.1093/infdis/jiw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrir S, Tindle HA, McGinnis KA, Fiellin DA, Goulet J, Akgün KM, … Crothers K (2015). Contemplation of smoking cessation and quit attempts in human immunodeficiency virus–infected and uninfected veterans. Substance Abuse, 37(2), 315–322. doi: 10.1080/08897077.2015.1062458. [DOI] [PubMed] [Google Scholar]
- Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M, & Moadel AB (2011). Provider Beliefs and Practices Relating to Tobacco Use in Patients Living with HIV/AIDS: A National Survey. AIDS and Behavior, 16(2), 288–294. doi: 10.1007/s10461-011-9891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons VN, Heckman BW, Ditre JW, & Brandon TH (2010). A measure of smoking abstinence-related motivational engagement: Development and initial validation. Nicotine & Tobacco Research, 12(4), 432–437. doi: 10.1093/ntr/ntq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoking and HIV. (2017, May 15). Retrieved from https://www.hiv.gov/hiv-basics/staying-in-hiv-care/other-related-health-issues/smoking.


