Abstract
Coronavirus Disease 2019 (COVID-19) has became a major problem affecting global health security.
To assess the differences and dynamic changes of blood coagulation function in COVID-19 patients with different severity.
A total of 261 COVID-19 patients from January 24 to March 25, 2020 in Huangshi, Hubei Province were enrolled.
We designed a retrospective observational study. Clinical information, including age, blood routine and blood coagulation function, were collected. According to the Diagnosis and Treatment Guidelines for COVID-19 (seventh version) that issued by the National Health Committee of the People's Republic of China, patients were divided into 3 subgroups: 186 ordinary, 45 severe and 30 critical ones. We compared the differences in blood coagulation factors among groups.
Average age in critical group (71.47 ± 11.48 years) was the oldest of 3 subgroups. At admission, statistically differences could be observed among ordinary, severe and critical patients in D-dimer (0.18 ± 0.33, 0.63 ± 1.13 and 1.16 ± 1.58 mg/L), fibrinogen/fibrin degradation products (FDP) (3.11 ± 5.30, 9.82 ± 23.91 and 21.94 ± 40.98 μg/ml), platelet [(169 ± 62.85), (188 ± 71.56) and (117 ± 38.31) × 109/L)] and lymphocyte count [(1.18 ± 0.46), (0.82 ± 0.35) and (0.75 ± 0.39) × 109/L)], respectively (P < .05). During hospitalization, the peak values of coagulation and valley values of blood routine were monitored. There were significant differences among ordinary, severe and critical patients in D-dimer (0.26 ± 0.46, 1.39 ± 1.51 and 2.89 ± 1.68 mg/L), FDP (3.29 ± 5.52, 23.68 ± 39.07 and 56.11 ± 49.94 μg/ml), platelet [(164 ± 55.53), (171 ± 69.96) and (84 ± 57.80) × 109/L)] and lymphocyte count [(1.10 ± 0.46), (0.65 ± 0.35) and (0.55 ± 0.31) × 109/L)], respectively (P < .001). D-dimer and FDP in the course of disease in severe/critical groups showed a first upward and then downward trend.
We concluded that coagulation function indexes such as D-dimer and FDP could be served as markers to estimate COVID-19 patients condition. Close monitoring of coagulation function may be helpful for early diagnosis of severe patients and guidance of treatments.
Keywords: coagulation function, D-dimer, fibrin/fibrinogen degradation products (FDP), COVID-19, SARS-CoV-2
1. Introduction
From December 2019, patients with pneumonia of unknown cause connected to the seafood wholesale market in Wuhan, China, were discovered 1 after another.[1] Rapidly, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) isolated from the throat swab sample of patients was identified as the pathogen. Subsequently, this unique pneumonia was named Coronavirus Disease-19 (COVID-19) by World Health Organization (WHO). With its high contagious capacity, over 370,000 cases were confirmed, with 16,231 deaths all over the world until March 24, 2020.[2] COVID-19 is mainly transmitted through respiratory droplets and close contact. Fever, dry cough, and fatigue are the main clinical manifestations of this disease. A few patients are accompanied by nasal congestion, runny nose, myalgia, and diarrhea. Ordinary patients have a good prognosis, but severe patients can rapidly develop a range of complications such as acute respiratory distress syndrome (ARDS), coagulation dysfunction and multiple organ failure (MOF), which can eventually lead to death.[3]
The pathological results of the Diagnosis and Treatment Guidelines for COVID-19 (seventh version) indicate the existence of microthrombus in pulmonary vessels, liver and kidney of COVID-19 patients.[3] Microthrombus, also known as transparent thrombus, is mainly composed of fibrin, which is common in disseminated intravascular coagulation (DIC). Fibrinogen (FIB), as a protein synthesized from the liver, increases significantly when vascular damage, infection and inflammation occurs.[4] D-dimer is a biomarker of fibrin formation and degradation which is at low levels in healthy individuals but will going to be elevated in conditions with thrombosis.[5] Platelets are anuclear blood cells with activities such as aggregation, adhesion, release, and play an important role in the physiological and pathological processes like thrombosis.[6] Fibrin/fibrinogen degradation products (FDP) is a general term for various degradation products produced by the dissolution of fibrin/fibrinogen in the blood under the action of the fibrinolytic enzyme. While low levels of D-dimer and FDP can be detected in the circulation of healthy individuals, their levels increase significantly in the presence of pulmonary embolism, disseminated intravascular coagulation, myocardial infarction and sepsis.[7]
Patients with COVID-19 are characterized by a strong systemic inflammation as measured by high levels of inflammatory cytokines, which can cause “cytokine storm” during the initial stage of the disease, and thuspromoting blood coagulation.[8] Albeit a good knowledge has been obtained on the pathogenesis, clinical features and CT imaging changes of COVID-19, little is known about the derangement of blood coagulation. Our study aims to investigate the differences of FIB, FDP, D-dimer and PLT in different types of patients, as well as the dynamic changes of the above indexed in severe and critical patients, and further explore the relationship between coagulation indexes and COVID-19 progress.
2. Methods
2.1. Patients
The clinical data of 261 patients with COVID-19 confirmed in Huangshi Hospital of traditional Chinese medicine in Hubei Province from January 24 to March 25, 2020 were collected. According to the Diagnosis and Treatment Guidelines for COVID-19 (seventh version), COVID-19 patients were confirmed by positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2, or the sequencing of the virus, highly homologous with SARS-CoV-2, or detection of new coronavirus-specific antibody.[3] Clinical and laboratory information of enrolled patients could be completed without missing.
Patients infected with other common respiratory viruses, including influenza A and B virus, respiratory syncytial virus, parainfluenza virus, adenovirus were excluded from this study. At the same time, patients with abnormal coagulation function caused by underlying conditions, recently taking anticoagulant and antiplatelet drugs, and pregnant or lactating women were excluded as well.
The degrees of COVID-19 were categorized into ordinary, severe and critical, based on the Diagnosis and Treatment Guidelines for COVID-19 (seventh version).[3] In brief, ordinary patients exhibited mild clinical symptoms with or without imaging changes. Severe type was characterized by at least one of the following symptoms: respiratory frequency ≥30/minute, blood oxygen saturation at rest ≤93%, PaO2/FiO2 ratio < 300 mm Hg, and lung infiltrates >50% within 24 to 48 hours. Critical cases were those that exhibited respiratory failure with mechanical ventilation, septic shock, and/or multiple organ dysfunction/failure needed intensive care unit (ICU) monitoring.
This study was approved by the Ethics Committee of the Huangshi Hospital of traditional Chinese medicine in Wuhan Province (HSZYPJ-2020-021-01). Informed consent was obtained from all patients.
2.2. Data collection
Clinical information, such as age, gender, date of admission and course records, and laboratory information, especially blood coagulation parameters, of 261 SARS-CoV-2 infected patients at admission and during following hospitalization were obtained from medical records. Peripheral venous blood was collected from all patients within 12 hours after admission and stored in anticoagulation tubes for blood routine and coagulation function test. Platelet count, lymphocyte count and coagulation indexes such as FDP, FIB and D-dimer were tested. And during hospitalization, severe or critical patients patients were reexamined at least once a week for these 2 kinds of tests.
2.3. Statistical analysis
SPSS (version 22.0) was used to perform statistical analyses. Continuous data were expressed as means ± standard deviation (SD) or means ± standard error of the mean (SEM), and were analyzed by one-way analysis or Welch analysis of variance, depending on the result of homogeneity of variance tests. Categorical data were expressed as numbers (%) and were compared by Chi-Squared test. In all tests, P ≤ .05 was considered as statistical significance.
3. Results
3.1. Clinical characteristics of patients with COVID-19
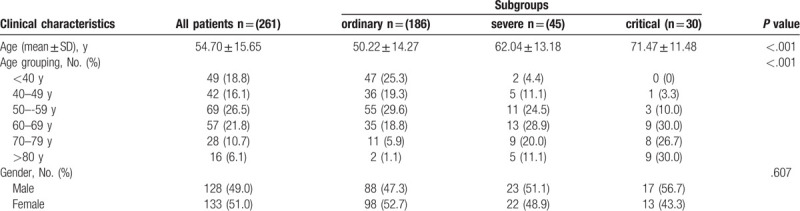
Among all the 261 COVID-19 patients, 186 (71.3%) were ordinary, 45 (17.2%) were severe, and 30 (11.5%) were critical. All patients included 128 (49%) males and 133 (51%) females, with an average age of 54.70 years old, and the majority of patients (65.1%) were over 50 years old. There were significant differences in age (P < .001) among different subgroups, of which the mean age of the critical group was the oldest (71.47 ± 11.48 years), but no significant differences were found in gender (P = .607) among the subgroups. Notably, none of the subjects in our study was taking anticoagulant drugs before blood drawing. The distribution of age within each group is presented in Table 1.
Table 1.
The distribution of baseline characteristics of COVID-19 patients.
3.2. Comparison of coagulation function among different subgroups of COVID-19 at admission
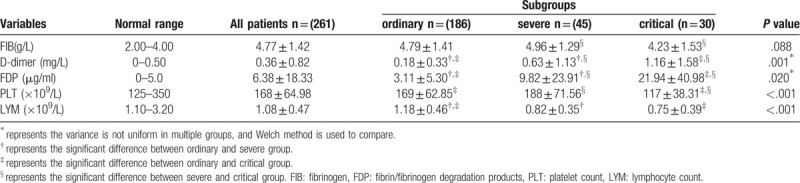
At admission, significant differences for the values of D-dimer, FDP, platelet count and lymphocyte count among 3 subgroups were observed (P < .05). Compared with ordinary (D-dimer: 0.18 ± 0.33 mg/L; FDP: 3.11 ± 5.30 μg/ml) and severe groups (D-dimer: 0.63 ± 1.13 mg/L; FDP: 9.82 ± 23.91 μg/ml), the D-dimer and FDP values in critical patients (D-dimer: 1.16 ± 1.58 mg/L; FDP: 21.94 ± 40.98 μg/ml) were found to be higher, while the platelet count [(117 ± 38.31) × 109/L] was lower (P < .05). Notably, lymphocyte counts in severe [(0.82 ± 0.35) × 109/L] and critical groups [(0.75 ± 0.39) × 109/L] were lower than that in the ordinary group [(1.18 ± 0.46) × 109/L] (P < .05) (Table 2). Unlike the parameters mentioned above, no difference was observed in the value of FIB among 3 subgroups (P = .088).
Table 2.
Comparison of coagulation function among COVID-19 patients from different subgroups at admission.
3.3. Dynamic changes of coagulation indexes during hospitalization in severe and critical patients
Blood routine examination and coagulation function were performed once a week in severe and critical patients during hospitalization, depending on the patients condition. We found the levels of FIB, PLT and LYM of ordinary patients were significantly higher than that of critical patients at discharge, while the FDP level was significantly lower. As for D-dimer, the level was significantly lower than that of severe or critical patients. In addition, the levels of D-dimer in severe and critical group showed a similar trend of first increasing and then decreasing as with FDP, and most of them were above the normal values. In severe and critical group, there were no obvious regularity in the change of FIB. The platelet count and lymphocyte count of critical patients were always at a low level, and their rising trend was not obvious as shown in Figure 1.
Figure 1.
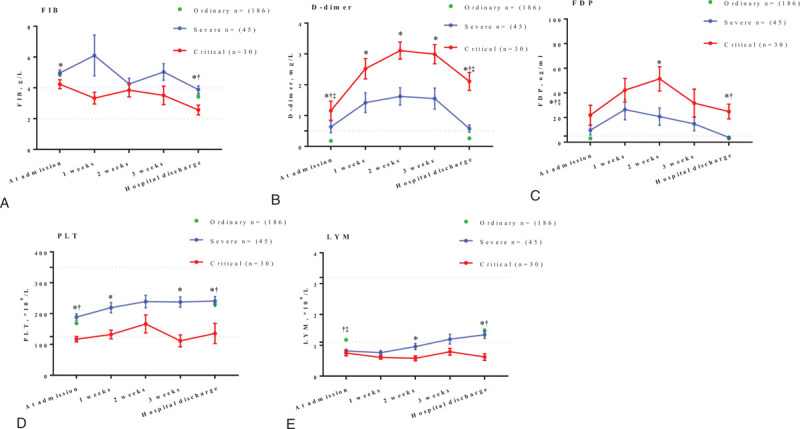
Line graph represents the dynamic changes of laboratory examination results during hospitalization. Thedata are shown as means ± SEM. Dashes indicate the range of normal values. ∗represents the significant difference between non-critical and critical group (P < .05). †represents the significant difference between ordinary and critical group (P < .05). ‡represents the significant difference between ordinary and severe group (P < .05).
3.4. Comparison of coagulation function among different degrees of COVID-19 during hospitalization
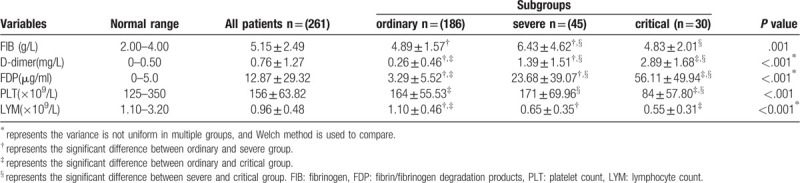
During hospitalization, blood routine examination and coagulation function were carried out once a week. The peak values of FIB, D-dimer and FDP, and the valley values of platelet and lymphocyte count were selected for further statistical analysis. Multiple comparative analysis allowed us to define that the values of FIB, D-dimer and FDP, platelet count and lymphocyte count were significantly different among 3 different groups of COVID-19 patients (P < .001). In detail, compared to ordinary patients (D-dimer: 0.26 ± 0.46 mg/L; FDP: 3.29 ± 5.52 μg/ml), the values of D-dimer and FDP were higher in severe (D-dimer: 1.39 ± 1.51 mg/L; FDP: 23.68 ± 39.07 μg/ml) or critical patients (D-dimer: 2.89 ± 1.68 mg/L; FDP: 56.11 ± 49.94 μg/ml), but the opposite trend was observed in lymphocyte count (P < .05). As concerns FIB, severe patients (6.43 ± 4.62 g/L) displayed considerably higher values than those in the other 2 groups (P < .05). Notably, the platelet count in critical patients [(84 ± 57.80) × 109/L] was significantly lower than the normal range, and was significantly different from that in ordinary [(164 ± 55.53) × 109/L] and severe groups [(171 ± 69.96) × 109/L], as shown in Table 3.
Table 3.
Comparison of coagulation function among different degrees of COVID-19 patients during hospitalization.
4. Discussion
At present, COVID-19 has become a worldwide pandemic. The number of confirmed patients in Italy, the United States, Spain, Germany and other countries has already exceeded 60,000.[2,3,9,10] Although its mortality rate is lower than SARS, its stronger infectivity still puts enormous pressure on public health.[11] Studies have shown that the development of ARDS in critical patients is extremely rapid, and the mortality rate of patients at this stage is very high.[12] Therefore, it is of great importance to monitor the laboratory examination closely in the early stage of COVID-19 and prevent further deterioration.
Interestingly, we found that the platelet count in critical patients was at a low level at admission, and the decrease was more obvious at exacerbation, while the changes in ordinary and severe patients were relatively small. It is widely recognized that platelets not only play a role in coagulation, but also are an integral part in infection and inflammatory immune response.[13] Patients with severe thrombocytopenia have higher levels of proinflammatory cytokines than those with normal platelet count.[6,14] When severe inflammation occurs, abnormal regulation of the vascular anticoagulant system may lead to blood hypercoagulability and thrombocytopenia. Taken together, thrombocytopenia has a strong correlation with severe infection and increased risk of DIC. As an indicator of disease deterioration, the platelet count must be paid enough attention. Consistent with previous studies, we observed lymphopenia in severe and critical patients, which might be caused by the translocation of lymphocytes from peripheral blood to lungs.[15,16]
In the present study, the majority of patients were elderly, and 86.7% of critical patients are over 60 years old, indicating that elderly patients are easy to worsen, in line with other report.[17] This may be related to the decline of resistance caused by the decrease of immune cells in the elderly. Similar to the reports from Han H et al,[18] the FIB values of 3 subgroups were greater at admission compared to the normal range, but the differences within different subgroups were not significant. We also found the FIB values of severe patients were significantly higher than that of critical patients during hospitalization, which may be owing to the more serious condition of critical patients, more disorder of coagulation and fibrinolysis. As a result, a large quantity of FIB was consumed, and even DIC appeared. This can cause further aggravate the patients condition.
As for the values of D-dimer and FDP in severe and critical patients, they were much higher than normal range, and show significantly difference to the ordinary group. D-dimer and FDP are sensitive indicators which can reflect the coagulation and fibrinolysis status. The abnormal increase of these 2 indexes might be caused by secondary fibrinolysis hyperactivity due to microthrombus formation in severe and critical patients. These results suggest that routine coagulation parameters were significantly correlated with the severity of COVID-19.[2,3]
What is novel in this study is the dynamic analyses of FIB, D-dimer, FDP, platelet count and lymphocyte count in severe or critical COVID-19 patients. The data in this study showed that the values of D-dimer and FDP increased significantly after admission, and then decreased gradually after reaching the peak. The dynamic changes were consistent with the progress and recovery of disease.
Many critical patients eventually develop ARDS and coagulationdys function which is one of the common causes of death in critical patients. The relationship between critical COVID-19 patients and coagulation disorders is crucial, but the mechanism that leads to coagulation disorders remains unclear. We hypothesized that the cause of coagulation disorders in COVID-19 patients may be the direct influence of coronavirus on the coagulation pathway,[19] or hypoxia induced coagulation disorder,[20,21] etc. The specific mechanism still needs to be further explored. Previous studies have shown that severe acute respiratory syndrome (SARS),[22] Middle East respiratory syndrome (MERS)[23] and community-acquired pneumonia (CAP)[24] have significantly abnormal coagulation function at the disease onset, mainly manifested as the increase of FIB, D-dimer and FDP, and the elevation degree will become more and more sign. As a marker of DIC, secondary hyperfibrinolysis and thrombosis, D-dimeris of great significance in the diagnosis of hypercoagulability.[8] Recently, it has been found that compared with survival patients, dead COVID-19 patients have higher levels of D-dimer and FDP, as with the appearance of DIC.[8,12] Therefore, close monitoring of coagulation index may be helpful for early diagnosis of severe patients and guidance of treatments.
This study still requires further refinement. As a single-center retrospective study, there might be some limitations because all the enrolled patients were from Huangshi Hospital of traditional Chinese medicine. And due to the relatively stable condition of ordinary patients, the number of coagulation function rechecks during hospitalization is less, thus the dynamic changes can not be observed. As the end point of observation was discharge, the changes of coagulation function post-discharge could not be further analyzed.
5. Conclusions
With the progress of disease, the coagulation function of patients with COVID-19 will show different degrees of abnormality. As there is no specific antiviral treatment for COVID-19 at present, it is of great importance to find and correct the coagulation dysfunction in time to reduce the formation of DIC and improve the prognosis.
Author contributions
Conceptualization: Nian Chen, Yuwen Li, Haozhi Fan, Pingping Hu, Jun Li, Chuanlong Zhu.
Data curation: Nian Chen, Yuwen Li, Haozhi Fan, Anran Tian, Hui Yuan, Zhengyi Jiang, Yunxi Yu, Lili Ruan, Pingping Hu, Ming Yue.
Formal analysis: Nian Chen, Yuwen Li, Chuanlong Zhu.
Investigation: Nian Chen, Zhengyi Jiang, Yunxi Yu, Lili Ruan, Pingping Hu.
Methodology: Nian Chen, Haozhi Fan, Yunxi Yu, Lili Ruan.
Resources: Nian Chen, Yunxi Yu.
Software: Haozhi Fan, Anran Tian, Hui Yuan, Ming Yue.
Supervision: Jun Li, Chuanlong Zhu.
Validation: Jun Li, Chuanlong Zhu.
Writing – original draft: Yuwen Li, Anran Tian, Hui Yuan, Zhengyi Jiang, Chuanlong Zhu.
Writing – review & editing: Jun Li, Chuanlong Zhu.
Footnotes
Abbreviations: COVID-19 = Coronavirus Disease 2019, SARS-CoV-2 = Severe acute respiratory syndrome coronavirus 2, WHO = World Health Organization, ARDS = acute respiratory distress syndrome, MOF = multiple organ failure, RT-PCR = real-time reverse transcriptase polymerase chain reaction, SD = standard deviation, SEM = standard error of the mean, DIC = disseminated intravascular coagulation, ICU = intensive care unit, FIB = fibrinogen, FDP = fibrin/fibrinogen degradation products, PLT = platelet, LYM = lymphocyte.
How to cite this article: Chen N, Li Y, Fan H, Tian A, Yuan H, Jiang Z, Yu Y, Ruan L, Hu P, Yue M, Li J, Zhu C. Analysis of dynamic disturbance in blood coagulation function of patients with Coronavirus Disease 2019: a retrospective observational study. Medicine. 2020;99:43(e22635).
CN, LY, and FH contributed equally to this article.
This work was supported by the National Natural Science Foundation of China (grant no. 81770591, no.81800778), the Key Medical Talents Fund of Jiangsu Province (grant no. ZDRCA2016007).
This study was approved by the Ethics Committee of the Huangshi Hospital of traditional Chinese medicine in Wuhan province (HSZYPJ-2020-021-01). Informed consent was obtained from all patients.
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Coronavirus disease (COVID-19) Situation Dashboard of World Health Organization https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.Accessed April 2, 2020. [Google Scholar]
- [3]. National health commission of China.Guideline of management of COVID-19 (version 7) http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.Date last updated: March 4 2020. [Google Scholar]
- [4].Adams RA, Passino M, Sachs BD, et al. Fibrin mechanisms and functions in nervous system pathology. Mol Interv 2004;4:163–76. [DOI] [PubMed] [Google Scholar]
- [5].Weitz JI, Fredenburgh JC, Eikelboom JW. A Test in Context: D-Dimer. J Am Coll Cardiol 2017;70:2411–20. [DOI] [PubMed] [Google Scholar]
- [6].McDonald B, Dunbar M. Platelets and intravascular immunity: guardians of the vascular space during bloodstream infections and sepsis. Front Immunol 2019;10:2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moresco RN, Junior RH, Claudio RVL, et al. Association between plasma levels of D-dimer and fibrinogen/fibrin degradation products (FDP) for exclusion of thromboembolic disorders. J Thromb Thrombolysis 2006;21:199–202. [DOI] [PubMed] [Google Scholar]
- [8].Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [10].Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020;323:1775–6. [DOI] [PubMed] [Google Scholar]
- [11].Hunter P. The spread of the COVID-19 coronavirus: Health agencies worldwide prepare for the seemingly inevitability of the COVID-19 coronavirus becoming endemic. EMBO Rep 2020;21:e50334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264–74. [DOI] [PubMed] [Google Scholar]
- [14].Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020;506:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang G, Zhang J, Wang B, et al. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res 2020;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58:1116–20. [DOI] [PubMed] [Google Scholar]
- [19].McGilvray ID, Lu Z, Wei AC, et al. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J Biol Chem 1998;273:32222–9. [DOI] [PubMed] [Google Scholar]
- [20].Sabit R, Thomas P, Shale DJ, et al. The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest 2010;138:47–51. [DOI] [PubMed] [Google Scholar]
- [21].Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res 2019;181:77–83. [DOI] [PubMed] [Google Scholar]
- [22].Wang J, Yuan J, Pu C, et al. The blood coagulation abnormity of severe acute respiratory syndrome patients. Chin J Lab Med 2004;27:499–501. [Google Scholar]
- [23].Singh SK. Middle east respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med 2016;37:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu Y, Zhang Y, Jiang F, et al. Comparison of relevant indicators of coagulation and fibrinolysis in patients with varying severity of community-acquired pneumonia. Zhonghua Yi Xue Za Zhi 2015;95:1925–9. [PubMed] [Google Scholar]


