Abstract
Rationale:
Nasopharyngeal carcinoma (NPC) is one of the most common malignancies in Southern China. Although combined chemotherapy with radiotherapy has been widely used in treating locally advanced lesions, relapse and metastases remain the primary cause of treatment failure, and are associated with an extremely poor prognosis. Therefore, more efficient and milder therapies are needed.
Patient concerns:
Herein, we report a patient with advanced NPC with intracranial metastases who showed progression during conventional treatment.
Diagnoses:
Nonkeratinizing undifferentiated nasopharyngeal carcinoma (stage IV).
Interventions:
After the completion of initial chemoradiotherapy and targeted therapy, metastases to brain occurred during follow-up. Ex vivo-cultured allogeneic NK cell infusion was offered.
Outcomes:
Although the intracranial metastases did not decrease 10 months after the NK cell treatment, they decreased significantly at 31 months after the treatment and partially disappeared. The tumor response indicated partial response. Furthermore, all of the intracranial metastases continued to decrease at about 42 months after treatment.
Lessons:
The brain metastases of NPC are rare with poor prognosis. Radiotherapy in NPC can disrupt the blood–brain barrier, which may contribute to the metastases of brain. This case report will provide rationale for NK cell infusion following regular chemoradiotherapy.
Keywords: case report, chemoradiotherapy, intracranial metastases, nasopharyngeal carcinoma, NK cell transfer
1. Introduction
Nasopharyngeal carcinoma (NPC), previously known as lymphoepithelioma, is one of the most common head and neck cancers, with endemic distribution in Southern China, Southeast Asia, Africa, and the Middle East.[1] The rate varies from a minuscule value of <1 per 100,000 individuals in nonendemic areas to a high value of 25 to 30 and 15 to 20 males and females per 100,000 individuals in endemic areas, respectively.[2] NPC can be categorized into 3 histological types: type I, keratinizing type (20%–25%); type II, nonkeratinizing differentiated type (10%–15%); type III, nonkeratinizing undifferentiated (60%–65%).[3] The mainstay of treatment for NPC is radiotherapy in locoregional lesions as the nonkeratinizing variety is highly radiosensitive. Chemotherapy is preferred concomitantly with radiation in advance stages, whereas conventional chemotherapy and radiotherapy only have limited efficacy in NPC patients with late stage disease.
During recent decades, significant strides have been made in the fields of immunology and immunotherapy. Following the achievements of adjuvant immunotherapy using cytokine-induced killer (CIK) cells in the treatment of hepatocellular carcinoma (HCC), natural killer (NK) cell therapy has been discussed as a promising candidate for the next important advance. Since the discovery of NK cells, a great deal of research followed, which elucidated a critical role of NK cells in supporting the whole immune system, identified their association with many human diseases, and even attempted to use NK cells as a form of therapy.[4] Of note, it was demonstrated in more recent studies that NK cells can identify and selectively kill cancer stem cells,[5–7] suggesting that by targeting quiescent and nonproliferating cancer stem cells, NK cell-based therapy may become an effective method to prohibit relapse and metastasis.[8]
Until recent, 92% of clinical studies used NK cells from peripheral blood, either donor- (79% of recruiting trials) or patient-derived (13% of recruiting trials).[9] Alternatives are the use of NK cell lines, or the differentiation of NK cells from umbilical cord blood (UCB) or pluripotent stem cells.[10–12] Notably, UCB offers unique advantages, many of which are directly applicable to NK cell-directed alloreactivity. The ease of collection of UCB and cryopreservation makes them readily available as an off-the-shelf source for NK cell immunotherapy.[13,14] Besides, the presence of almost a log fewer T-cells in UCB compared to other graft sources,[15–19] most of which are naive,[20–22] minimizes the risk of graft versus host disease.[23–26] More importantly, NK cells reconstitute more rapidly after cord blood transplantation than peripheral blood haploidentical stem-cell transplantation.[27,28] In view of this, we developed ex vivo expansion techniques that can induce cord blood mononuclear cells to directly differentiate into high cytotoxic NK (Fig. 1) using a cocktail of cytokines and interleukin-2. The culture method of high cytotoxic NK cells will be introduced in another article. Here, we report a case of NPC with recurrence and intracranial metastasis, who received our NK cell immunotherapy inducing long-term tumor control.
Figure 1.

(A) Dot plots from one representative experiment depicting NK cell (CD3-CD56+) purity. (B) Dot plots from one representative experiment depicting NK cell (CD16+CD56+) purity. (C and D) Expression of activating receptors NKG2D and NKp30 on the expanded NK cells in the in vitro expansion protocol was evaluated by flow cytometry method. € The dot plots from one representative experiment illustrating CD107a surface expression on the NK cells after stimulation by the target cell line.
2. Case presentation
Written informed consent was obtained from the patient. A 48-year-old male was diagnosed with nonkeratinizing undifferentiated NPC with stage IV (cT4, cN1, cM0) based on the criteria of 8th AJCC/UICC edition in 3/2012. The patient was treated with concurrent paclitaxel/nedaplatin (TP)-based chemoradiotherapy (RCT) (03/2012–05/2012) with a total radiation dose of 73 Gy and the target therapy of nimotuzumab. Magnetic resonance imaging (MRI) examination after treatment (May 19, 2012) showed obvious reduction in tumor size. On July 21, 2014, the patient was readmitted to the hospital with a decreased vision of right eye and facial paralysis and diagnosed as recurrent NPC. Subsequently, the patient was treated with chemotherapy of gemcitabine/cisplatinum (GP) regimens and 2 cycles of intensity-modulated radiotherapy (IMRT) with a total radiation dose of 67 Gy and 66 Gy (July, 2014–September, 2014). After the completion of chemoradiotherapy, the tumor response indicated much better than before. For the next follow-up, there was no significant change between the MRI examination of nasopharynx on March, 2015 and December, 2014. Unfortunately, he was found to have intracranial metastases on March, 2016 by MRI scanning (Fig. 2). So the patient began to receive GP chemotherapy for 3 cycles and capecitabine afterwards to maintain chemotherapy due to intolerance. Then, NK cell treatment started on July, 2016, using ex vivo-generated NK cells from UCB, at a dose of 2 × 109 CD56+/CD3−cells, intravenously, 3 times a year, up to now. Six months after NK therapy, MRI examination (January, 2017) showed nearly no change of the intracranial metastases (Fig. 2). Two years later, MRI examination (October, 2018) showed that all the intracranial metastases had begun to decrease significantly, and some metastases had disappeared (Fig. 2). Until the recent MRI examination on September, 2019, the intracranial metastases had continued to decrease (Fig. 2). At his last follow-up, about 3 years after initiating NK cell treatment, he was observed to be in a very good condition, without evidence of disease progression (V0, diagnosis; V1, MRI after RCT; V2, recurrence; V3-V4, MRI after RCT;V5, intracranial metastasis;V6, NK cell therapy after chemotherapy intolerance; V7–V10, MRI after NK cell therapy; Fig. 3).
Figure 2.
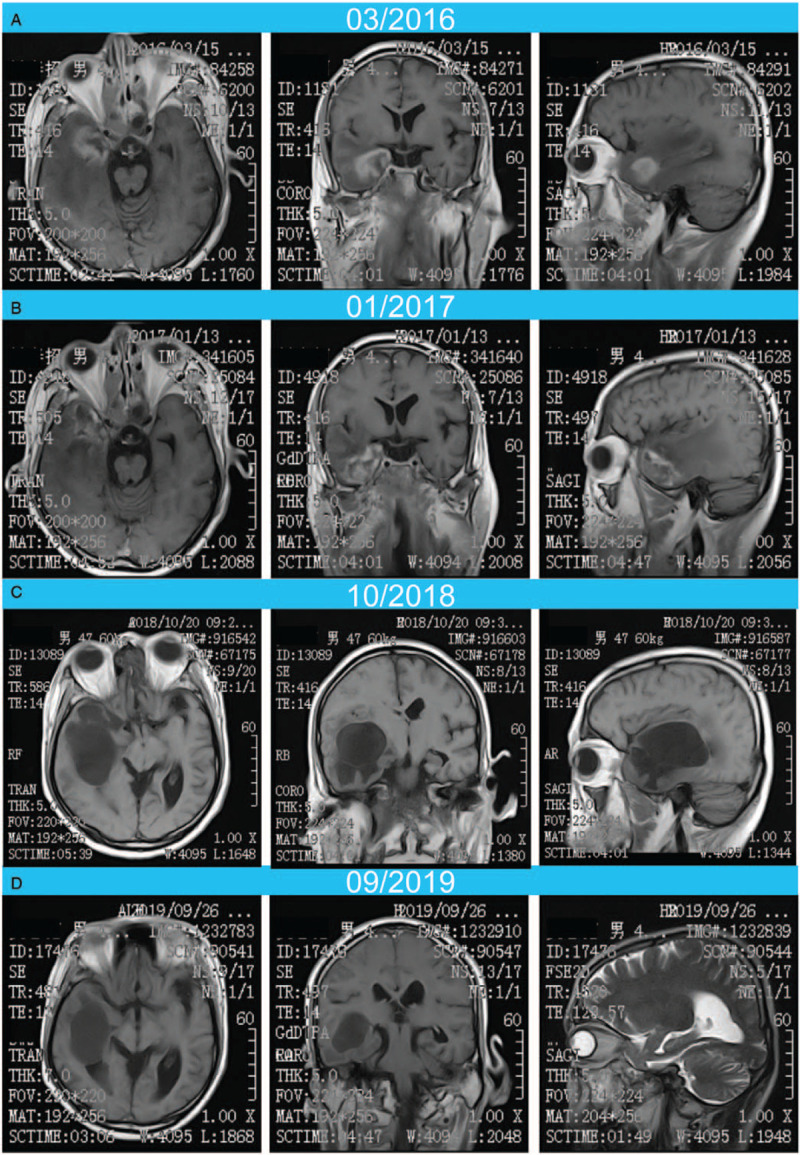
(A) The intracranial metastases before NK cell treatment (March, 2016); (B) the intracranial metastases 6 months after NK cell treatment (January, 2017); (C) the intracranial metastases about 2 years after NK cell treatment (February 26, 2018); (D) the intracranial metastases about 3 years after NK cell treatment (January 18, 2019).
Figure 3.
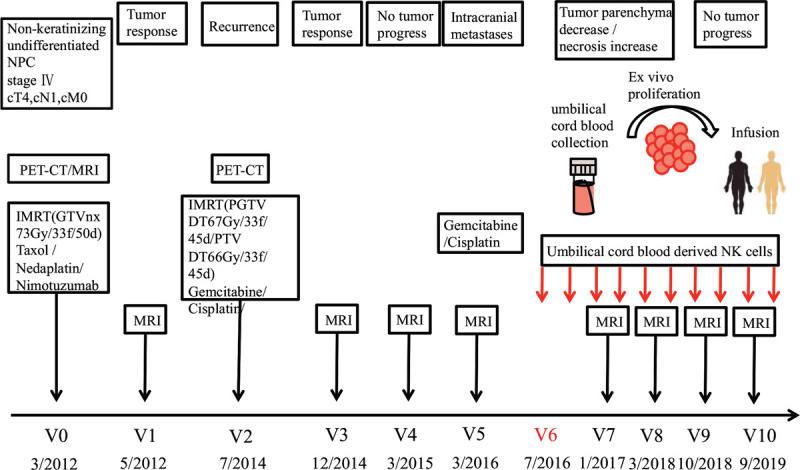
Schematic representation of the clinical history, therapy, and visits of a patient diagnosed with nonkeratinizing undifferentiated nasopharyngeal carcinoma (stage IV, cT4, cN1, cM0) in March, 2012 (V0). After concurrent RCT (paclitaxel /nedaplatin, 73Gy), a partial tumor response was evidenced by MRI scanning (V1). Two years later (V2, 7/2014), the patient received concurrent RCT (gemcitabine/cisplatinum, 67 Gy and 66 Gy), the state of the disease recurrence was evidenced much better than before by MRI scanning (V3). Three months later (V4), MRI scanning showed no tumor progression. On March, 2016, the MRI examination of brain indicated intracranial metastases (V5). On July, 2016 (V6), the patient began to receive NK cell treatment on a 3-yearly basis. MRI scanning after NK therapy (V7–V10) revealed that the intracranial metastases was gradually shrinking or even disappearing.
3. Discussion
The rate of distant metastases occurrence is higher in locally advanced NPC, and the most common sites are the bone, lung, and liver.[29] Central nervous system (CNS) metastasis of NPC is an extremely rare occurrence, although direct invasion to the skull base is not infrequent in patients at a locally advanced stage. Therefore, studies by far have failed to discuss its treatment and prognosis systematically, leaving only a few case reports.[30,31] With the development of immune therapy, the treatment effects on NPC need to be urgently explored. In this report, we present 1 unique case of NPC with recurrence and metastasis to the right temporal lobe. At the same time, we also attempt to adopt NK cell therapy after the failure of conventional therapy.
Concurrent chemoradiotherapy with or without adjuvant chemotherapy, provides a benefit in overall survival and has become the standard treatment for locoregionally advanced NPC, although with acute toxicities.[32,33] Nimotuzumab has marketing approval for the treatment of locoregionally advanced NPC,[34,35] and the addition of induction chemotherapy to concurrent chemoradiotherapy and nimotuzumab could obtain the best survival benefits.[36] The patient in our study received 2 cycles of chemotherapy consisted of paclitaxel and nedaplatin (TP) followed by radical IMRT combined with concurrent nimotuzumab after being diagnosed with advanced NPC and yielded promising objective response and local control. But unfortunately, there was local recurrence after 2 years. He then received concurrent chemoradiotherapy once again and had been sustained partial response by follow-up and imaging evaluation until March, 2016, intracranial metastases appeared. It has been reported that radiotherapy may destroy the blood–brain barrier in NPC patients.[37] Qin et al[38] reported in a retrospective study that irradiation with a 2-Gy-fraction dose resulted in maximal opening of the blood-brain barrier for over half a year. Moreover, Chan et al[39] observed blood–brain barrier disruption by MRI in 89% of radiotherapy-treated, NPC patients, even 2 to 10 years after radiotherapy. Consistent with these reports, we reasoned that disruption of the blood–brain barrier might occur following radiotherapy in this patient, which resulted in intracranial metastases.
Chemotherapy with/without targeted therapy is the main treatment for NPC with distant metastasis, according to expert opinion.[40] However, the treatment strategy for brain metastasis remains controversial. The prospect of these patients diagnosed with CNS was dismay. Most of patients in the reports suffered from exacerbated neurological deficits and succumbed finally.[30,41] The patient in our study received a combined chemotherapy consisting of 3 cycles of gemcitabine and cisplatinum (GP). Due to intolerance to this chemotherapy regimen, GP was replaced with capecitabine to maintain chemotherapy.
Considering the uncertainty of chemotherapy and the intolerance, the patient turned to NK cell-based immunotherapy for a try. Previous studies exploring different allogeneic NK cell products showed promising antitumor activity in various cancers. However, for advanced NPC, this has not been clearly demonstrated yet. Here, we present the first study investigating our allogeneic UCB-NK cell product, which is highly activated and exhibits profound cytotoxic potential, in this NPC patient following intravenous infusion. In this case, we found that the brain metastases did not decrease at 6 months’ time, yet they decreased significantly about 2 years after treatment and some disappeared. Moreover, all of the brain metastases continued to decrease at about 3 years after treatment. Therefore, we think that NK cell therapy has late-onset and lasting antitumor effects in this patient. The toxicities were very mild, and the treatment was very well tolerated. At his last follow-up, he was observed to be in a very good condition.
4. Conclusion
In conclusion, our findings have interesting implications for current efforts to develop therapeutic strategies for NPC followed with recurrence and intracranial metastases and also suggest that NK cell therapy may be a promising option for the treatment of NPC after conventional radiotherapy and chemotherapy. However, caution should be paid to the possibility of the adverse effect of acute graft-versus-host disease for patients receiving allogeneic NK. The optimal dose of NK-cells and the follow-up treatment remain to be clarified. This is a report of one case; further well-designed and randomized studies with larger numbers of cases are needed to fully evaluate this strategy.
Author contributions
The cell assay was done by Yuan-yuan Jin, Sen Zou and Zheng-yang Sun; Wen-zhuo Yang, Chun-tao Wu and Zhao-yong Yang conducted the clinical trial; The manuscript was prepared by Yuan-yuan Jin and Zhao-yong Yang.
All authors discussed the results and commented on the manuscript.
Footnotes
Abbreviations: CNS = central nervous system, IMRT = intensity-modulated radiotherapy, MRI = magnetic resonance imaging, NK = natural killer, NPC = nasopharyngeal carcinoma, RCT = chemoradiotherapy, UCB = umbilical cord blood.
How to cite this article: Jin Yy, Yang Wz, Zou S, Sun Zy, Wu Ct, Yang Zy. Chemoradiotherapy combined with NK cell transfer in a patient with recurrent and metastatic nasopharyngeal carcinoma inducing long-term tumor control: A case report. Medicine. 2020;99:43(e22785).
Y-yJ and W-zY contributed equally to this work.
The authors report no conflicts of interest.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Du T, Xiao J, Qiu Z, et al. The effectiveness of intensity-modulated radiation therapy versus 2D-RT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. PLoS One 2019;14:e0219611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011;30:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286–93. [DOI] [PubMed] [Google Scholar]
- [4].Suen WC, Lee WY, Leung KT, et al. Natural killer cell-based cancer immunotherapy: a review on 10 years completed clinical trials. Cancer Invest 2018;36:431–57. [DOI] [PubMed] [Google Scholar]
- [5].Luna JI, Grossenbacher SK, Murphy WJ, et al. Targeting cancer stem cells with natural killer cell immunotherapy. Expert Opin Biol Ther 2017;17:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kaur K, Cook J, Park SH, et al. Novel strategy to expand super-charged NK cells with significant potential to lyse and differentiate cancer stem cells: differences in NK expansion and function between healthy and cancer patients. Front Immunol 2017;8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaur K, Topchyan P, Kozlowska AK, et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology 2018;7:e1426518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Oh S, Lee JH, Kwack K, et al. Natural killer cell therapy: a new treatment paradigm for solid tumors. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cany J, Dolstra H, Shah N. Umbilical cord blood-derived cellular products for cancer immunotherapy. Cytotherapy 2015;17:739–48. [DOI] [PubMed] [Google Scholar]
- [10].Spanholtz J, Tordoir M, Eissens D, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One 2010;5:e9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Suck G, Odendahl M, Nowakowska P, et al. NK-92: an ’off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother 2016;65:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. Transl Res 2010;156:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Munoz J, Shah N, Rezvani K, et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med 2014;3:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program 2012;2012:215–22. [DOI] [PubMed] [Google Scholar]
- [15].Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 2007;369:1947–54. [DOI] [PubMed] [Google Scholar]
- [16].Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004;351:2265–75. [DOI] [PubMed] [Google Scholar]
- [17].Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004;351:2276–85. [DOI] [PubMed] [Google Scholar]
- [18].Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood 2009;113:1631–8. [DOI] [PubMed] [Google Scholar]
- [19].Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood 2007;109:1322–30. [DOI] [PubMed] [Google Scholar]
- [20].Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol 2005;141:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Park KD, Marti L, Kurtzberg J, et al. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood 2006;108:1770–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nomura A, Takada H, Jin CH, et al. Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response. Exp Hematol 2001;29:1169–76. [DOI] [PubMed] [Google Scholar]
- [23].Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Konuma T, Kato S, Ooi J, et al. Comparable long-term outcome of unrelated cord blood transplantation with related bone marrow or peripheral blood stem cell transplantation in patients aged 45 years or older with hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2014;20:1150–5. [DOI] [PubMed] [Google Scholar]
- [25].Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010;11:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen YB, Aldridge J, Kim HT, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant 2012;18:805–12. [DOI] [PubMed] [Google Scholar]
- [27].Cooley S, McCullar V, Wangen R, et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood 2005;106:4370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nguyen S, Kuentz M, Vernant JP, et al. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia 2008;22:344–52. [DOI] [PubMed] [Google Scholar]
- [29].Shen LJ, Wang SY, Xie GF, et al. Subdivision of M category for nasopharyngeal carcinoma with synchronous metastasis: time to expand the M categorization system. Chin J Cancer 2015;34:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ngan RK, Yiu HH, Cheng HK, et al. Central nervous system metastasis from nasopharyngeal carcinoma: a report of two patients and a review of the literature. Cancer 2002;94:398–405. [DOI] [PubMed] [Google Scholar]
- [31].Kaidar-Person O, Kuten J, Atrash F, et al. Brain metastasis of nasopharyngeal carcinoma: a case report and literature review. Case Rep Med 2012;2012:405917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188–98. [DOI] [PubMed] [Google Scholar]
- [33].Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 2006;64:47–56. [DOI] [PubMed] [Google Scholar]
- [34].Liu ZG, Zhao Y, Tang J, et al. Nimotuzumab combined with concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a retrospective analysis. Oncotarget 2016;7:24429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhai RP, Ying HM, Kong FF, et al. Experience with combination of nimotuzumab and intensity-modulated radiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Onco Targets Ther 2015;8:3383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yao JJ, Zhang LL, Gao TS, et al. Comparing treatment outcomes of concurrent chemoradiotherapy with or without nimotuzumab in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Biol Ther 2018;19:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sandor N, Walter FR, Bocsik A, et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS One 2014;9:e112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Qin D, Ou G, Mo H, et al. Improved efficacy of chemotherapy for glioblastoma by radiation-induced opening of blood-brain barrier: clinical results. Int J Radiat Oncol Biol Phys 2001;51:959–62. [DOI] [PubMed] [Google Scholar]
- [39].Chan YL, Leung SF, King AD, et al. Late radiation injury to the temporal lobes: morphologic evaluation at MR imaging. Radiology 1999;213:800–7. [DOI] [PubMed] [Google Scholar]
- [40].Chen YP, Wang YQ, Li WF, et al. Critical evaluation of the quality and recommendations of clinical practice guidelines for nasopharyngeal carcinoma. J Natl Compr Canc Netw 2017;15:336–44. [DOI] [PubMed] [Google Scholar]
- [41].Shen C, Ying H, Lu X, et al. Nasopharyngeal carcinoma with central nervous system metastases: two case reports and a review of the literature. Medicine (Baltimore) 2017;96:e9175. [DOI] [PMC free article] [PubMed] [Google Scholar]


