Abstract
Oral health can affect or be a manifestation of general health. Although oral health assessment has been used as a proxy for general health, few studies have reported an association between oral health status and allergic diseases. This cross-sectional study aimed to investigate the relationship between subjective oral health status and asthma/allergic rhinitis in a nationwide representative sample of Korean adults.
A total of 227,977 participants from the Korean Community Health Survey 2015 were enrolled. Participants were asked about their subjective oral health status (very good, good, normal, poor, very poor), periodontal status (mobility, swelling, calculus, bleeding), teeth brushing frequency, and scaling history within the past 12 months. Histories of physician-diagnosed asthma and allergic rhinitis throughout life were surveyed. The associations between subjective oral health status and allergic diseases were analyzed using multiple logistic regression analysis. Age, sex, economic level, educational level, region of residence, smoking, alcohol, obesity, subjective general health status, stress level, physical activity, periodontal status, teeth brushing frequency, and scaling history within the past 12 months were adjusted as covariates.
A higher prevalence of asthma (3.6%) was reported in the poor oral health group than in the good (1.8%) and normal (2.1%) groups (P < .001). Poor oral health status was significantly related to asthma, with an adjusted odds ratio (aOR) of 1.19 (95% CI = 1.07–1.33, P = .002). Although the prevalence of allergic rhinitis was not higher in the poor oral health group (13.4%) than in the good (15.4%) and normal oral health groups (15.9%), the aOR for allergic rhinitis was 1.05 (95% CI = 1.00–1.11, P = .045) in the poor oral health group after adjusting for covariates.
Subjective poor oral health status was significantly associated with asthma and allergic rhinitis in Korean adults.
Keywords: allergic rhinitis, asthma, oral health, population surveillance
1. Introduction
Oral diseases have a negative impact on general health and quality of life. The recent Global Burden of Disease 2017 study reported that the number of people with untreated oral conditions increased from 2.5 billion in 1990 to 3.5 billion in 2017.[1] Years lived with disability due to oral conditions increased by 68% worldwide.[1] Systematic reviews have reported that poor oral health and periodontal disease may be risk factors for chronic diseases such as coronary heart disease,[2] diabetes,[3] ∼asthma, and chronic∼ obstructive pulmonary disease.[4] Microbial infection, immune cross-reactivity, and inflammatory mediators are hypothesized to be contributing factors to the increased risks of these diseases.
In addition, oral health can be a manifestation of general health. Research has established that patients with diabetes may present a variety of oral manifestations, such as periodontal disease, xerostomia, caries, oral candidiasis, and delayed wound healing, and uncontrolled diabetes is associated with an increased risk of periodontal disease and oral inflammation because of excess sugars.[5] According to a study that analyzed data from the Korean National Health and Nutrition Examination Survey (KNHANES), toothache was reported more frequently by patients with depression than by those without depression, and they also frequently reported uncomfortable mastication, temporomandibular joint symptoms and periodontal bleeding.[6]
Several studies have demonstrated an agreement between subjective oral health and objective evaluations. In previous studies, individuals who reported an increased number of oral health symptoms, including dry mouth, impairment, and disabilities such as tooth loss and dental problems, assessed their oral health negatively.[7,8] The prevalence of negatively self-rated oral health was 40% higher in subjects who had periodontal disease than in those without periodontal disease in a Brazilian National Oral Health Survey (prevalence ratio = 1.4, 95% confidence interval [CI] = 1.2–1.5).[9] In a study from the KNHANES IV, self-perceived oral health status and function were strongly associated with clinical oral health status, which included the number of untreated decayed teeth (B = 0.405, P < .001); number of decayed, missing, and filled teeth (B = 0.126, P < .001); prosthesis scores (B = 0.691, P < .001); and periodontal scores (B = 0.700, P < .001), as determined by trained dentists.[10] Using self-rated oral health in population surveys is a more valid, reliable, and cost-effective measure than carrying out examinations, as it can quickly identify the state of the populations oral health and associated factors.[11]
Although assessing subjective oral health has been used as a proxy of general health, few studies have reported an association between subjective oral health status and allergic disease. We hypothesized those patients who estimated subjective oral health as poor actually have poor oral health and that it would be associated with allergic diseases. The objective of the present study was to investigate the associations of subjective poor oral health with asthma and allergic rhinitis using a nationwide representative population of Korean adults.
2. Materials and methods
2.1. Study population and data collection
This study was a cross-sectional study using data from the Korean Community Health Survey (KCHS).[12] The data were obtained from 1 nation using statistical methods based on designed sampling and adjusted weighted values. Data from the KCHS 2015 were analyzed in accordance with the guidelines and regulations provided by the Korea Centers for Disease Control and Prevention (KCDC). This study was performed in accordance with the Helsinki Declaration and approved by the Institutional Review Board of the KCDC (IRB No. 2010-02CON-22-P, 2011-05CON-04-C, 2012-07CON-01-2C, 2013-06EXP-01-3C, and 2014-08EXP-09-4CA), and written informed consent was obtained from all the participants prior to the survey.
We describe the KCHS data characteristics in the supplemental content. Of the 228,558 total participants, we excluded the following participants from this study: participants who did not report answers to allergic rhinitis questions (n = 29); participants who did not report answers to asthma questions (n = 26); participants who did not report answers to subjective oral health status questions (n = 17); participants who did not report answers to scaling questions (n = 111); the participants who did not report answers to periodontal status questions (n = 398). Ultimately, 227,977 participants were included in this study (Fig. 1).
Figure 1.
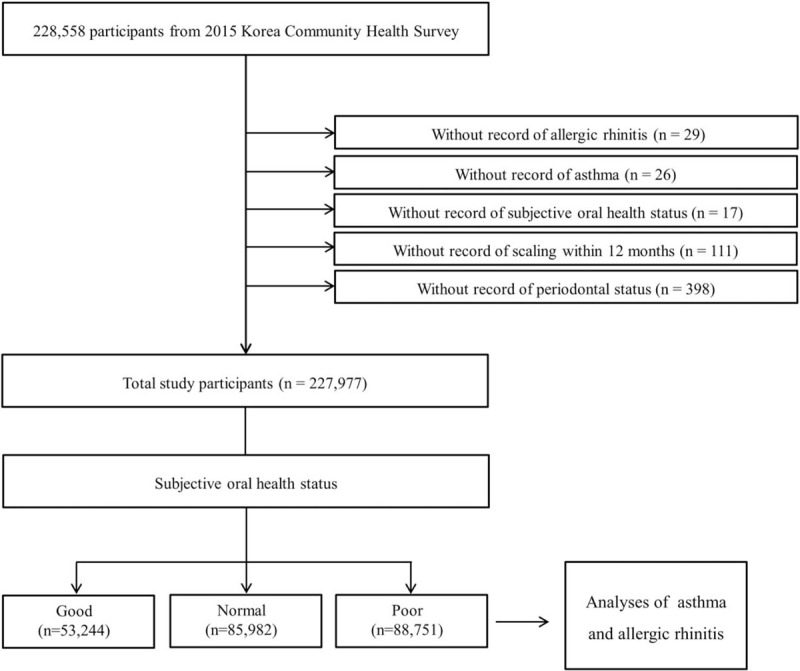
A schematic illustration of the participant selection. Among a total of 228,558 participants, those without a record of allergic rhinitis (n = 29), asthma (n = 26), subjective oral health status (n = 17), scaling history (n = 111), and periodontal status (n = 398) were excluded. Data were obtained from 227,977 participants with complete data records.
2.2. Subjective oral health status and oral hygiene-related behaviors
To measure the subjective oral health status, the participants were asked “What do you think of your oral health, including the health of your teeth and gums?”. The answer options were as follows: “very good”, “good”, “normal”, “poor”, and “very poor”. The answers were regrouped into 3 categories for simplification as follows: “good” (“very good” and “good”), “normal”, and “poor” (“poor” and “very poor”).
Participants were asked about their periodontal status. The answers to the survey were as follows: tooth mobility (without dental caries); swelling (gums swollen or with a tingling sensation); calculus (seen and/or told by self or others); bleeding (when brushing teeth or anytime); and normal (none of the above). Participants who answered “mobility”, “swelling”, “calculus”, or “bleeding” were categorized as “abnormal”, and the others were categorized as “normal”.
Participants were asked if they brushed their teeth yesterday according to the following situations: after breakfast, after lunch, after dinner, and before falling asleep. The frequency of teeth brushing was calculated as the number of times the teeth were brushed according to the following: 0 times per day, 1 time per day, 2 times per day, 3 times per day, and 4 times per day. Participants were asked if they had received dental scaling within the past 12 months. The answer options were “yes” or “no”.
2.3. Definition of asthma and allergic rhinitis
The participants were asked if they were diagnosed with asthma or allergic rhinitis at any point in their lifetime by a physician. If the participants answered “yes” to the question, then they were defined as having asthma or allergic rhinitis for each question regarding diagnosis.
2.4. Covariates
The participants were divided into 7 age groups: 19–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥80 years. Monthly income was classified into 5 groups: unknown, 1 (lowest), 2, 3, and 4 (highest). Region of residence was divided into 2 groups according to administrative district: urban (i.e., Seoul, Gyeonggi, Busan, Daegu, Incheon, Gwangju, Daejeon, Ulsan, and Sejong) and rural (i.e., Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju). Smoking status was divided into 4 groups: unknown, nonsmoker, past smoker, and current smoker. The participants were asked to report the frequency of alcohol consumption and were divided into 4 groups accordingly: unknown < 1 time a month, 1–4 times a month, and ≥2 times a week. Education level was divided into 4 groups: “unknown” (participants who refused to respond or did not know), “low” (participants who uneducated or those who finished less than elementary school), “middle” (participants who finished middle school or high school), and “high” (participants who graduated college or graduate school). Obesity was measured using body mass index (BMI, kg/m2). BMI was categorized as <18.5 (underweight), ≥18.5 to < 23 (normal), ≥ 23 to < 25 (overweight), ≥25 to < 30 (obese I), and ≥30 (obese II) based on the Asia-Pacific criteria following the Western Pacific Regional Office (WPRO) 2000.[13] Subjective health status was divided into the following: unknown, good, normal, and poor. Stress level was divided into the following 5 groups: unknown, no stress, some stress, moderate stress, and severe stress. Physical activity was divided into the following 3 groups: 0 minutes/week, 1–149 minutes/week, and ≥150 minutes/week.
2.5. Statistical analysis
The differences in the distributions of general characteristics were compared using the Rao-Scott chi-square test with sampling weights. To calculate the odds ratios (ORs) with 95% CIs for asthma and allergic rhinitis according to oral health status, crude model (simple model), model 1 (adjusted for age, sex, monthly income, education level, region of residence, smoking, alcohol consumption, obesity, subjective general health level, stress, and physical activity), and model 2 (adjusted for all the variables in model 1 plus subjective oral health, periodontal status, brushing teeth, and scaling) were analyzed using multiple logistic regression with sampling weights.
Sampling weights were used to perform complex sampling of the national survey data using PROC SURVEY. Two-tailed analyses were conducted, and P-values below 0.05 were considered significant. The results were statistically analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
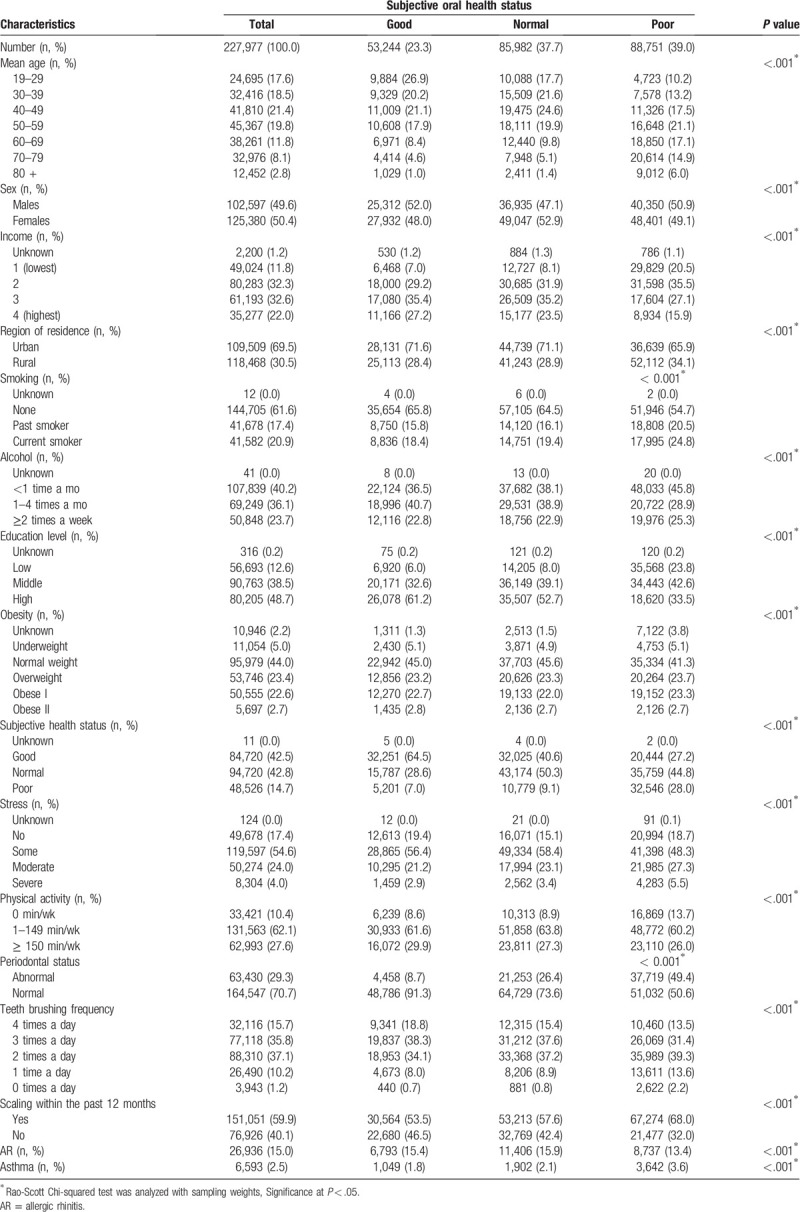
In total, 23.3%, 37.7%, and 39.0% of the participants reported their subjective oral health status as good, normal, and poor, respectively (Table 1). According to the distribution of participants, older age, female sex, low income, rural residence, smoking, alcohol consumption, low education level, obesity, poor general health status, stress, and little physical activity were associated with poor oral health (each P < .001; Table 1). Periodontal status, frequency of brushing teeth, and history of scaling within the past 12 months significantly differed across the groups with regard to subjective oral health status (each P < .001). A higher prevalence of asthma (3.6%) was reported in the poor oral health group than in the good (1.8%) and normal (2.1%) groups (P < .001), but the prevalence of allergic rhinitis was not higher in the poor oral health group (13.4%) than in the good (15.4%) or normal health groups (15.9%).
Table 1.
General characteristics of participants.
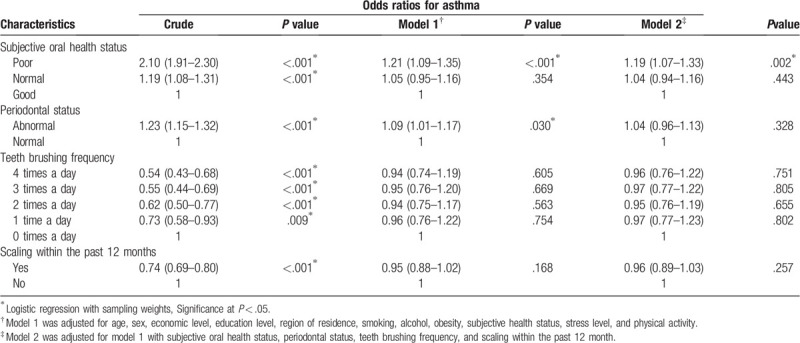
The OR for asthma in model 2 was higher in the poor oral health group than in the good oral health group (adjusted OR = 1.19, 95% CI = 1.07–1.33, P = .002; Table 2). Abnormal periodontal status was significantly associated with asthma (adjusted OR = 1.09, 95% CI = 1.01–1.17, P = .030) after adjusting for age, sex, economic level, education level, region of residence, smoking, alcohol, obesity, subjective general health status, stress level, and physical activity. However, the frequency of teeth brushing and scaling history within the past 12 months were not significantly associated with asthma in models 1 and 2.
Table 2.
Odds ratios for asthma according to subjective oral health status.
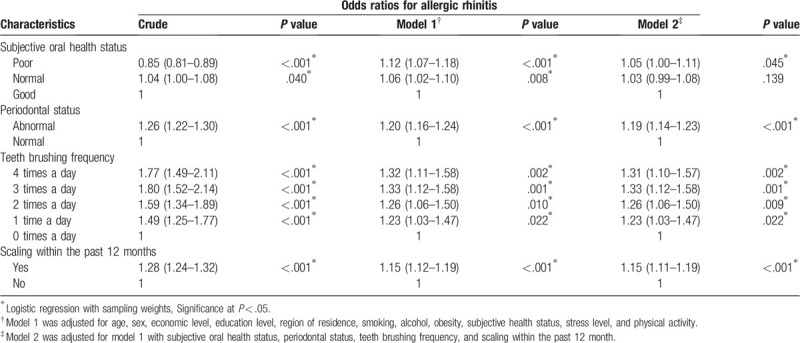
The adjusted OR for allergic rhinitis in model 2 was higher in the poor oral health group than in the good oral health group (adjusted OR = 1.05, 95% CI = 1.00–1.11, P = .045; Table 3). The adjusted OR for allergic rhinitis in model 2 was higher in participants with an abnormal periodontal status than in those with a normal periodontal status (adjusted OR = 1.19, 95% CI = 1.14–1.23, P < .001). Participants with allergic rhinitis were more likely to brush their teeth (adjusted OR = 1.31, 95% CI = 1.10–1.57, P = .002) and to have developed scaling within the last 12 months (adjusted OR = 1.15, 95% CI = 1.11–1.19, P < .001).
Table 3.
Odds ratios for allergic rhinitis according to subjective oral health status.
4. Discussion
The present study showed that participants with poor oral health had significantly higher ORs for asthma and allergic rhinitis than those with good oral health after adjusting for age, sex, economic level, education level, region of residence, smoking, alcohol, obesity, subjective general health status, stress level, physical activity, periodontal status, frequency of teeth brushing, and history of scaling.
These findings can be explained by bidirectional relationships between oral health and allergic disease. One probable mechanism involves the immune system. Interleukin (IL)-12 and IL-16 may be related to the pathogenesis of periodontal disease,[14] and IL-12 has been proposed to play a key role in allergic rhinitis.[15] Elevated gingival IgE concentrations were found in both the asthma and periodontitis groups,[16] suggesting that IgE-mediated hypersensitivity reactions may occur in the gingiva. A reduction in IL-10 and gene polymorphisms associated with IL-10 trigger the progression and worsening of periodontal disease[17] and increase susceptibility to asthma.[18]
Another possible mechanism is that poor oral health is a result of allergic disease. Patients with allergic rhinitis or asthma have a greater tendency than those without to mouth-breathe because of nasal congestion and various dentofacial abnormalities, such as anterior open bite. Mouth breathing leads to dehydration of the alveolar mucosa and an increase in gingival inflammation due to decreased epithelial resistance to bacterial plaques.[19,20]
In addition, diminished salivary production and secretion are associated with the prolonged use of β2 agonists,[21] inhaled corticosteroids,[22] and antihistamines.[23] The reduction in saliva can affect the natural process by which the mouth maintains its chemical balance and the protective function of cleaning the mouth. Reduced salivary flow provides a favorable environment for the growth and multiplication of microorganisms, such as Lactobacilli and Streptococcus mutans, causing dental caries.[24] Fexofenadine-treated patients with allergic rhinitis showed significantly lower salivary flow rates than healthy individuals in a comparative observational study in Israel.[23]
This study showed that the adjusted ORs for asthma were higher in the subjective poor oral health group and in the abnormal periodontal status group. Previous studies have reported a positive association between poor oral health and asthma, consistent with our results. A study in Finland showed that asthmatic patients had a higher (52.7%) mean periodontal status index (percentage of teeth with gingival bleeding, calculus, or periodontal pockets) than nonasthmatic controls (37.1%) (P = .05).[25] In a case-control study in India, asthmatic subjects had higher mean plaque (1.94 ± 0.73 vs 1.51 ± 0.37) and gingivitis (1.42 ± 0.31 vs 0.90 ± 0.21) scores than nonasthmatic subjects (P < .001 for each test).[19] Individuals with periodontitis had an approximately 3 fold increased risk of severe asthma than those without periodontitis (adjusted OR = 3.01–3.25) in Brazilian adults.[26] Furthermore, in a recent meta-analysis, periodontitis was associated with asthma (adjusted OR = 3.54, 95% CI = 2.47–5.07).[27]
On the other hand, there are few studies on the association between poor oral health and allergic rhinitis, and they have yielded conflicting results. The adjusted OR for allergic rhinitis was 1.21 (95% CI = 1.18–1.23) in patients with chronic periodontitis compared with controls from the Longitudinal Health Insurance Database in Taiwan.[28] In contrast to our study, in the KNHANES 2013–2015, there was a significant inverse association between allergic rhinitis and periodontal status (adjusted OR = 0.79, 95% CI = 0.66–0.95).[29] They explained that their results supported the hygiene hypothesis, but the hygiene hypothesis proposes that early life infections have preventive effects against the pathogenesis of allergies, which is more appropriate for children than adults.
Additionally, the present study showed that subjects with allergic rhinitis were more likely to brush their teeth and to develop scaling, while oral health-related behaviors were not significantly associated with asthma. A Brazilian study presented a positive association between asthma and periodontitis, as our study showed, but there was no significant difference in brushing frequency and the use of dental floss between the asthma and control groups.[26] This lack of a relationship might explain why the present study attempted to adjust for numerous possible confounders. Generally, poor oral health behaviors are known to increase poor oral health and the rate of inflammatory disease.[30] However, good oral health-related behaviors in patients with allergic rhinitis may be the result of motivation for improved health, as they consider subjective oral health to be poor and may have xerostomia caused by mouth breathing and the use of antihistamines.
The strengths of the present study include a large representative sample of Korean adults and adjustment for numerous potential covariates, including age, sex, economic level, education level, region of residence, smoking, alcohol, obesity, subjective general health status, stress level, physical activity, and oral health-related behaviors. Previous studies have shown socioeconomic inequalities in subjective oral health, and the reporting of poor oral health is associated with low socioeconomic status.[31,32] Our previous study showed that allergic rhinitis was associated with male sex (adjusted OR = 1.64, 95% CI = 1.13–2.36), younger age (adjusted OR = 1.96, 95% CI = 1.30–2.96), and a high stress level (adjusted OR = 1.55, 95% CI = 1.05–2.30).[33] Another previous study reported that current asthma was associated with current smoking in both worker (adjusted OR = 1.33, 95% CI = 1.13–1.57) and nonworker groups (adjusted OR = 2.07, 95% CI = 1.76–2.43).[34]
The present results should be interpreted with caution because of limitations. The cross-sectional study design limited the determination of causality between subjective oral health and allergic disease. The survey was based on self-reported questionnaires, which are subject to recall bias. We could not conduct objective oral health tests. The asthma information was based on a history of diagnosis by a clinician. Thus, it was possible that there were misdiagnosed or underdiagnosed patients in our cohort.
In conclusion, subjective oral health status was significantly associated with asthma and allergic rhinitis after adjusting for multiple covariates in Korean adults.
Author contributions
Conceptualization: Hyo-Jeong Lee, Bumjung Park, Min Woo Park, Hyo Geun Choi.
Data curation: Jee Hye Wee, Dae Myoung Yoo.
Formal analysis: Jee Hye Wee, Dae Myoung Yoo, Soo Hwan Byun.
Funding acquisition: Jee Hye Wee, Dae Myoung Yoo, Hyo Geun Choi.
Methodology: Dae Myoung Yoo, Soo Hwan Byun.
Software: Hyo Geun Choi.
Supervision: Bumjung Park, Min Woo Park, Hyo Geun Choi.
Validation: Min Woo Park, Hyo Geun Choi.
Visualization: Jee Hye Wee.
Writing – original draft: Jee Hye Wee, Dae Myoung Yoo.
Writing – review & editing: Soo Hwan Byun, Hyo-Jeong Lee, Bumjung Park, Min Woo Park, Hyo Geun Choi.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, IL = interleukin, KCDC = Korea Centers for Disease Control and Prevention, KCHS = Korean Community Health Survey, KNHANES = Korean National Health and Nutrition Examination Survey, OR = odds ratio, WPRO = Western Pacific Regional Office.
How to cite this article: Wee JH, Yoo DM, Byun SH, Lee HJ, Park B, Park MW, Choi HG. Subjective oral health status in an adult Korean population with asthma or allergic rhinitis. Medicine. 2020;99:43(e22967).
JHW and DMY contributed equally in this work.
This work was supported by the National Research Foundation (Choi HG, grant number NRF-2018-R1D1A1A02085328) (Wee JH, grant number NRF-2020-R1G1A1005390) and by the Hallym University Research Fund (HURF). The funding organization did not contribute to the design or conduct of this study, preparation, review, approval, or decision to submit this manuscript for publication.
The manuscript was edited for English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (3B6C-19E3-697B-3046-A35P).
The authors have no conflicts of interests to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
References
- [1].Bernabe E, Marcenes W, et al. GBD 2017 Oral Disorders Collaborators. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dental Res 2020;99:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bahekar AA, Singh S, Saha S, et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J 2007;154:830–7. [DOI] [PubMed] [Google Scholar]
- [3].Borgnakke WS, Ylöstalo PV, Taylor GW, et al. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol 2013;84: 4 Suppl: S135–52. [DOI] [PubMed] [Google Scholar]
- [4].Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol 2006;77:1465–82. [DOI] [PubMed] [Google Scholar]
- [5].Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, et al. Oral manifestations of diabetes mellitus. A systematic review. Med Oral Patol Oral Cir Bucal 2017;22:e586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Park SJ, Ko KD, Shin SI, et al. Association of oral health behaviors and status with depression: results from the K orean National Health and Nutrition Examination Survey, 2010. J Public Health Dentistry 2014;74:127–38. [DOI] [PubMed] [Google Scholar]
- [7].Locker D, Wexler E, Jokovic A. What do older adults’ global self-ratings of oral health measure? J Public Health Dent 2005;65:146–52. [DOI] [PubMed] [Google Scholar]
- [8].Kieffer JM, Hoogstraten J. Linking oral health, general health, and quality of life. Eur J Oral Sci 2008;116:445–50. [DOI] [PubMed] [Google Scholar]
- [9].Cascaes AM, Peres KG, Peres MA. Periodontal disease is associated with poor self-rated oral health among Brazilian adults. J Clin Periodontol 2009;36:25–33. [DOI] [PubMed] [Google Scholar]
- [10].Kim S-Y, Kim J-E, Kim H-N, et al. Association of self-perceived oral health and function with clinically determined oral health status among adults aged 35–54 years: a cross-sectional study. Int J Environmental Res Public Health 2018;15:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pattussi MP, Peres KG, Boing AF, et al. Self-rated oral health and associated factors in Brazilian elders. Community Dent Oral Epidemiol 2010;38:348–59. [DOI] [PubMed] [Google Scholar]
- [12]. Korean Community Health Survey. 2015. http://chs.cdc.go.kr. Accessed March 2020. [Google Scholar]
- [13].International Association for the Study of Obesity. World Health Organization. International Obesity ∼TaskForce. The Asia∼ pacific perspective: redefining obesity and its treatment. Health Communications Australia Pty Limited 2000. [Google Scholar]
- [14].Tsai C-C, Ho Y-P, Ho K-Y, et al. Interleukin-12 and interleukin-16 in periodontal disease. Cytokine 2005;31:34–40. [DOI] [PubMed] [Google Scholar]
- [15].Wei P, Kou W, Sun R, et al. Association study between interleukin-12 receptor 1/2 genes and allergic rhinitis in the Chinese Han population. European Archives of Oto-Rhino-Laryngol 2015;272:889–93. [DOI] [PubMed] [Google Scholar]
- [16].Hyyppä T. Gingival IgE and histamine concentrations in patients with asthma and in patients with periodontitis. J Clin Periodontol 1984;11:132–7. [DOI] [PubMed] [Google Scholar]
- [17].Cullinan M, Westerman B, Hamlet S, et al. Progression of periodontal disease and interleukin-10 gene polymorphism. J Periodontal Res 2008;43:328–33. [DOI] [PubMed] [Google Scholar]
- [18].Hyun M-H, Lee C-H, Kang M-H, et al. Interleukin-10 promoter gene polymorphisms and susceptibility to asthma: a meta-analysis. PloS One 2013;8:e53758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mehta A, Sequeira PS, Sahoo RC, et al. Is bronchial asthma a risk factor for gingival diseases? A control study. N Y State Dent J 2009;75:44–6. [PubMed] [Google Scholar]
- [20].Triana BEG, Ali AH, León IG. Mouth breathing and its relationship to some oral and medical conditions: physiopathological mechanisms involved. Revista Habanera de Ciencias Médicas 2016;15:200–12. [Google Scholar]
- [21].Ryberg M, Möller C, Ericson T. Saliva composition in asthmatic patients after treatment with two dose levels of a 2-adrenoceptor agonist. Archives Oral Biol 1990;35:945–8. [DOI] [PubMed] [Google Scholar]
- [22].Godara N, Godara R, Khullar M. Impact of inhalation therapy on oral health. Lung India 2011;28:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Elad S, Heisler S, Shalit M. Saliva secretion in patients with allergic rhinitis. Int Arch Allergy Immunol 2006;141:276–80. [DOI] [PubMed] [Google Scholar]
- [24].Ryberg M, Möller G, Erigson T. Saliva composition and caries development in asthmatic patients treated with 2-adrenoceptor agonists: a 4-year follow-up study. Euro J Oral Sci 1991;99:212–8. [DOI] [PubMed] [Google Scholar]
- [25].Laurikainen K, Kuusisto P. Comparison of the oral health status and salivary flow rate of asthmatic patients with those of nonasthmatic adults–results of a pilot study. Allergy 1998;53:316–9. [DOI] [PubMed] [Google Scholar]
- [26].Soledade-Marques KR, Gomes-Filho IS, da Cruz SS, et al. Association between periodontitis and severe asthma in adults: a case–control study. Oral Dis 2018;24:442–8. [DOI] [PubMed] [Google Scholar]
- [27].Gomes-Filho IS, Cruz SSd, Trindade SC, et al. Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis 2020;26:439–46. [DOI] [PubMed] [Google Scholar]
- [28].Hung SH, Tsai MC, Lin HC, et al. Allergic rhinitis is associated with periodontitis: a population-based study. J Periodontol 2016;87:749–55. [DOI] [PubMed] [Google Scholar]
- [29].Kim E-J, Choi Y-K. Allergic rhinitis and periodontitis among Korean adults: results from a nationwide population-based study (2013–2015). BMC Ear Nose Throat Disord 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kojima A, Ekuni D, Mizutani S, et al. Relationships between self-rated oral health, subjective symptoms, oral health behavior and clinical conditions in Japanese university students: a cross-sectional survey at Okayama University. BMC Oral Health 2013;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guarnizo-Herreño CC, Watt RG, Fuller E, et al. Socioeconomic position and subjective oral health: findings for the adult population in England, Wales and Northern Ireland. BMC Public Health 2014;14:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Luchi CA, Peres KG, Bastos JL, et al. Inequalities in self-rated oral health in adults. Rev Saude Publica 2013;47:740–51. [DOI] [PubMed] [Google Scholar]
- [33].Rhee C-S, Wee JH, Ahn J-C, et al. Prevalence, risk factors and comorbidities of allergic rhinitis in South Korea: the Fifth Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy 2014;28:e107–14. [DOI] [PubMed] [Google Scholar]
- [34].Kim SY, Sim S, Choi HG. Active and passive smoking impacts on asthma with quantitative and temporal relations: a Korean Community Health Survey. Sci Rep 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


