Abstract
Background:
The present study aimed to investigate the prognostic value of the neutrophil-to-lymphocyte ratio (NLR) in distal cholangiocarcinoma (DCC) following radical surgery.
Methods:
The clinicopathological data of 59 patients with DCC were retrospectively reviewed. Patients were treated by radical surgery and diagnosed by postoperative pathology at the Second Affiliated Hospital of Kunming Medical University (Yunnan, China), between July 2015 and December 2017. The optimal cut-off value for the NLR was determined by generating receiver operating characteristic (ROC) curves. Kaplan–Meier survival analysis and Cox proportional hazards models were used to determine the risk factors and independent risk factors influencing the prognosis of patients with DCC.
Results:
According to the ROC curve, the optimal cut-off value for the NLR was 2.933. The results of Kaplan–Meier survival analysis and the Cox proportional hazards model showed that carbohydrate antigen 125, NLR, perineural, vascular and fat invasion, regional lymph node metastasis, and the American Joint Committee on Cancer stage were risk factors for DCC; the only independent risk factor to affect the prognosis of DCC patients was the NLR.
Conclusions:
The preoperative NLR plays an important guiding role in evaluating the prognosis of patients with DCC, and an increase in the NLR is associated with poor patient prognosis.
Keywords: distal cholangiocarcinoma, neutrophil-to-lymphocyte ratio, independent risk factor, overall survival, prognosis
1. Introduction
Cholangiocarcinoma is a malignant tumor originating from the biliary epithelium. It is a frequently fatal malignancy associated with poor patient prognosis, and accounts for∼3% of all gastrointestinal tumors. Based on anatomical location, cholangiocarcinoma is broadly categorized as intrahepatic or extrahepatic cholangiocarcinoma. The World Health Organization has further subdivided extrahepatic cholangiocarcinoma into hilar cholangiocarcinoma (HCC) and distal cholangiocarcinoma (DCC), which are separated by their location in the cystic duct outside of the liver (at the confluence of the right and left ducts for HCC, and at the point closest to the intestine for DCC). At present, ∼60% to 70% of extrahepatic cholangiocarcinomas are hilar in nature, and DCC accounts for the remaining 30% to 40%.[1] Currently, the only curative treatment for DCC is surgical resection. However, as the early symptoms are not obvious, the majority of patients miss the opportunity to undergo surgery, and only one third are suitable for radical resectionat the time of diagnosis. Furthermore, the median survival time after radical surgery is reported to be only 24 mouths, with a 5-year survival rate of <10%.[2] The poor prognosis after surgery is primarily due to tumor recurrence and metastasis; therefore, it is necessary to identify effective prognostic indicators to predict postoperative survival in patients with DCC, thus guiding the selection of treatment methods to improve patient prognosis.
Lymph node metastasis, perineural and vascular invasion, and tumor size are among the factors closely associated the progression and prognosis of malignant tumors.[3,4] However, patient prognosis is not only associated with the biological characteristics of the tumor, but also the physical condition of the host. An increasing number of studies have demonstrated that the inflammatory response plays a key role in the changes to the microenvironment of normal tissue, and thus is an important factor for tumor occurrence and development.[5,6] Several biomarkers have been identified as prognostic risk factors for various types of cancer, including interleukin-6 (IL-6) in ovarian cancer,[7] C-reactive protein (CRP) in hepatocellular carcinoma,[8] CRP and albumin (ALB) ratio in cervical cancer,[9] aspartate aminotransferase (AST) and the neutrophil ratio in intrahepatic cholangiocarcinoma,[10] and the platelet-to-lymphocyte ratio (PLR) in breast cancer.[11] The present study aimed to investigate the potential correlation between the NLR and the prognosis of DCC patients after radical surgery.
2. Methods
2.1. Patients and analytical factors
A retrospective study was conducted using a database of 59 DCC patients who underwent radical resection at the Second Affiliated Hospital of Kunming Medical University (Yunnan, China), between July 2015 and December 2017; 33 men and 26 women were included, with a mean age of 57.5 years (range, 37–76 years). Factors analyzed included sex, age, ALB, alanine transaminase (ALT), AST, total bilirubin (TB), direct bilirubin (DB), NLR, PLR, carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), Ki 67, perineural invasion, vascular invasion, fat invasion, total lymph node metastasis, regional lymph node metastasis, and American Joint Committee on cancer (AJCC) stage. The cut-off values for ALB (35 g/L), ALT (40 U/L,) AST (40 U/L), CEA (5 ng/L), CA125 (35 U/ml) and CA19-9 (35 U/ml) according to the normal biological levels. The cut-off value for Ki 67 was set as 20% according to the research of Rijken et al,[12] and the AJCC stage was classified using the guidelines outlined in the eighth edition of the AJCC staging manual.
2.2. Inclusion and exclusion criteria
All hematological examination data were obtained before admission, prior to the administration of antibiotics, hepatoprotective drugs, or preoperative drainage for obstructive jaundice. The inclusion criteria included:
-
1.
Complete clinical information; and
-
2.
that patients underwent radical surgery and were diagnosed with DCC by postoperative pathology.
The exclusion criteria included:
-
1.
Incomplete clinical data;
-
2.
patients undergoing palliative surgery only;
-
3.
patients with a pre- or postoperative history of radiotherapy and chemotherapy; and
-
4.
patients diagnosed with ampulla carcinoma.
2.3. Follow-up
According to the inclusion and exclusion criteria, a total of 59 patients were included in this study. All patients were followed up by telephone or outpatient appointment, and the follow-up deadline was July 1, 2018.
2.4. Methods
The research database was established using the 2010 version of Office Excel, and statistical analyses were performed using SPSS version 19.0. Kaplan–Meier analysis was used to determine the risk factors for DCC, and those identified by univariate analysis were also subjected to multivariate analysis. Hazard ratios and 95% confidence intervals (95% CI) were estimated using forward stepwise Cox proportional hazard model analysis. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut–off values for the NLR, PLR, T, and DB. The patients were then assigned to high or low NLR groups according to the NLR cut-off value Categorical variables between the different NLR groups were compared using Pearsons Chi-Squared test, and P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. General patient data and oncological characteristics
The data of 33 male (55.93%) and 26 female (44.07%) patients were used in the present study. The mean patient age was 57.5 years (range, 37–76 years). During the follow-up period, a total of 22 patients died; the mean postoperative time and median survival time of the 59 patients were 11.92 and 12 months, respectively, and the 1- and 3-year survival rates were 63.1% and 50.9%, respectively. Detection of preoperative serum tumor markers revealed an increase in CEA in 52 patients, 51 patients with increased CA125 and 15 with increased CA19-9. In the postoperative pathological examination, 27 patients exhibited Ki67 > 20%, perineural invasion occurred in 30 patients, vascular invasion in 14 patients, fat invasion in 17 patients, total lymph node metastasis in 20 patients and regional lymph node metastasis in 16 patients. According to the eighth edition of the AJCC DCC staging system, 50 were I/II stage and 9 were III/IV stage patients.
3.2. ROC curve analysis
The NLR was calculated using the following formula: NLR = neutrophil count/lymphocyte count. According to the ROC curve analysis results, the optimal cut-off value for the NLR was 2.933 when predicting postoperative prognosis. The area under the ROC curve for survival status was 0.671, with a 95% CI of 0.532 to 0.810 (P = .029) (Fig. 1). Subsequently, all patients were divided into high (NLR > 2.933, n = 31) and low (NLR ≤2.933, n = 28) NLR groups. Using the same method, the optimal cut-off values for PLR, TB and DB were determined to be 269.921, 175.5, and 126.4, respectively. The ROC curves are displayed in Fig. 1.
Figure 1.

ROC analysis was performed to determine the optimal cut-off value of NLR, PLR, TB, DB in patients with DCC after radical surgery.
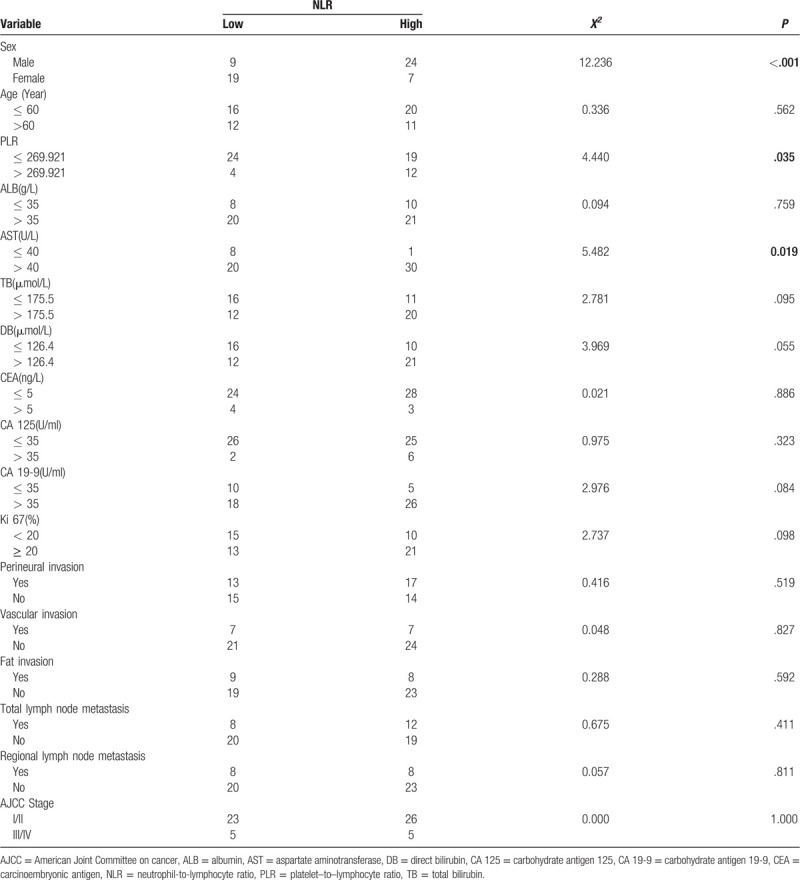
3.3. Relationship between the NLR and clinicopathological characteristics in DCC
Pearsons Chi-Squared test was used to determine potential statistical differences in the distribution of categorical variables between the NLR groups. The results indicate that sex (P = .000), AST (P = .019) and PLR (P = .035) were statistically different between the two NLR groups (Table 1).
Table 1.
Relationship between neutrophil-to-lymphocyte ratio and clinicopathological characteristics in 59 distal cholangiocarcinoma patients.
3.4. Univariate and multivariate survival analyses
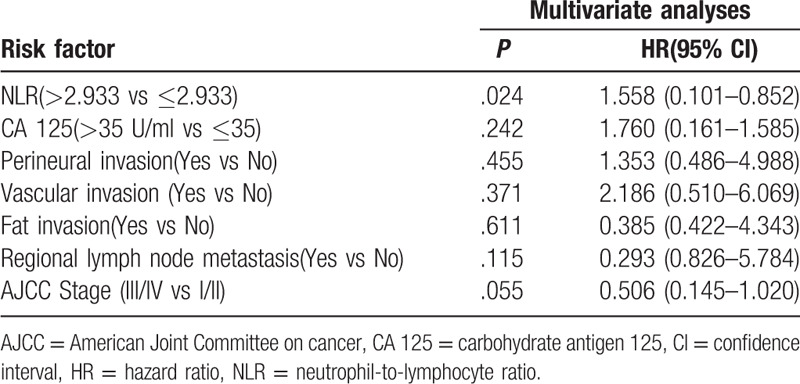
Univariate analysis revealed that the NLR (P = .023), CA125 (P = .008), perineural invasion (P = .023), vascular invasion (P = .003), fat invasion (P = .023), regional lymph node metastasis (P = .037) and AJCC stage (P = .006) were all risk factors for the overall survival of DCC patients following radical surgery (Figs. 2 and 3, Table 2). Multivariate analysis revealed that only the NLR (P = .024) was an independent risk factor for the prognosis of patients with DCC (Table 3).
Figure 2.
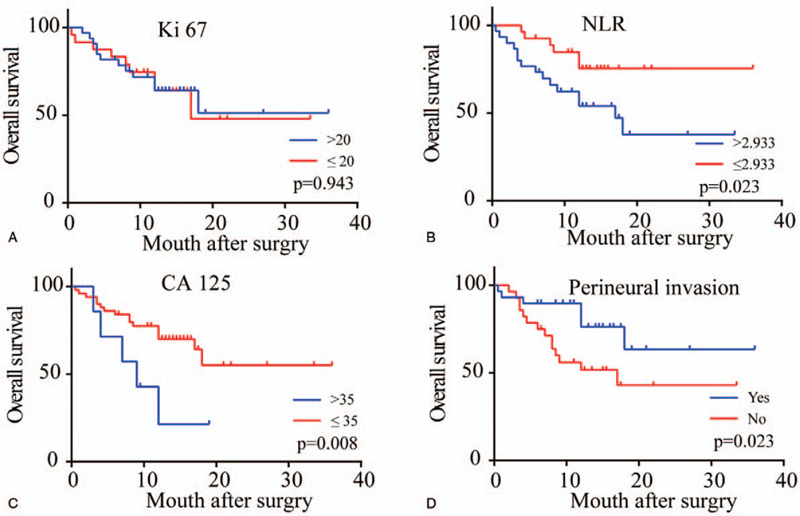
Kaplan–Meier survival curves of patients with DCC after radical surgery by Ki 67 (A), NLR (B), CA 125 (C), perineural invasion (D). P values were obtained by log-rank tests.
Figure 3.

Kaplan–Meier survival curves of patients with DCC after radical surgery by vascular invasion (A), fat invasion (B), regional lymph node metastasis (C), AJCC stage (D). P values were obtained by log-rank tests.
Table 2.
Univariate analysis of factors associated with overall survival in 59 distal cholangiocarcinoma patients.
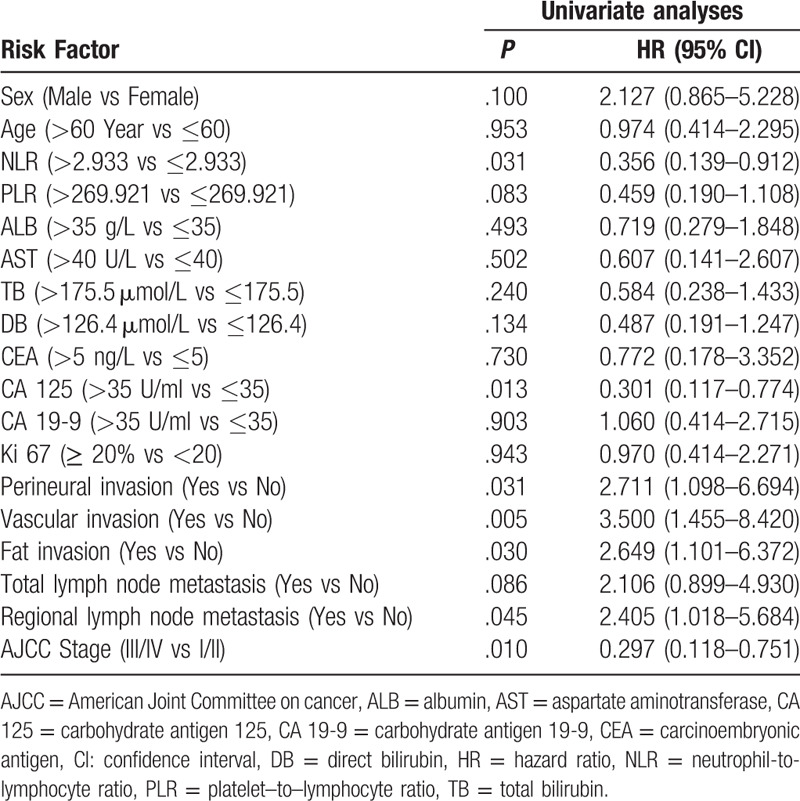
Table 3.
Multivariate analysis of factors associated with overall survival in 59 distal cholangiocarcinoma patients.
4. Discussion
An increasing number of studies has indicated that a high preoperative NLR is associated with poor patient outcome in various types of cancer, including pancreatic cancer, renal cancer, non-small cell lung cancer, gastric cancer, hepatocellular carcinoma, and various other solid tumors.[6,13–16] Using univariate and multivariate analysis, the present study investigated the relationship between multiple clinicopathological factors and the prognosis of DCC patients. It was ultimately confirmed that a high preoperative NLR (>2.933) is an independent risk factor for the overall survival rate of patients with DCC. Although numerous studies have determined that regional lymph node metastasis, perineural invasion, vascular invasion, and tumor differentiation have prognostic value in predicting the progression of malignant tumors, these indicators can only be assessed following radical surgery.[17] The NLR has the advantages of being economical and conveniently accessible, but can also be obtained preoperatively, thus has great practical value in a clinical setting.
As one of the 10 characteristics of malignant tumors, the inflammatory response has attracted a great deal of attention. In 1863, Rudolf Virchow et al[18] identified a large degree of inflammatory cell infiltration in tumor tissues, and first proposed that chronic inflammation was the origin of malignant tumors. A large number of studies have confirmed that the inflammatory response serves an important role in the occurrence, development and metastasis (as well as other stages) of tumorigenesis. Inflammation can promote tumorigenesis through gene mutation, genomic instability, and epigenetic modification. It can also activate the tissue repair response and induce the proliferation of precancerous cells.[19] Inflammation also stimulates angiogenesis, leads to immunosuppression, and promotes the formation of tumor microenvironments that ultimately result in metastasis.[20]
Neutrophils are white blood cells that play important roles in the inflammatory response. They have strong antibacterial phagocytic functions and are an important barrier against exogenous infection.[21] Neutrophils play opposing roles in the process of tumorigenesis and development. On the one hand, neutrophils destroy tumor cells by directly releasing antibacterial and cytotoxic substances, or immune mediators that activate the relevant anti-tumor cells. On the other hand, neutrophils also release cytotoxic substances that cause DNA damage in epithelial cells and activate oncogenes.[22] Neutrophil infiltration into precancerous lesions is mediated by chemokine receptor 2 ligands. Once activated, precancerous tissue neutrophils release reactive oxygen and nitrogen species, and proteases, which collectively promote tissue carcinogenesis. Nitric oxide synthase, arginase 1 and matrix metalloproteinase-9 released by neutrophils activate the immune escape mechanism whilst inhibiting the anti-tumor properties of CD8+ T lymphocytes, which ultimately results in tumor metastasis.[23,24]
Lymphocytes are an important immune barrier against tumor cells. Karin et al[25] established a tumor-susceptible mouse model lacking both B and T lymphocytes. They demonstrated that B lymphocytes were necessary for establishing chronic inflammation, and that activated B lymphocytes may regulate cancer development by altering the levels of circulating cytokines and/or chemokines. T lymphocytes not only destroy tumor cells directly, but can also induce apoptosis of target cells by Fas-FasL binding on the surface of tumor cells. Furthermore, Tumor-infiltrating CD4+ T lymphocytes induce apoptosis by activating CD8+ T lymphocytes and releasing cytotoxic factors.[26]
Neutrophils can inhibit adaptive immune responses and reduce the burden on activated lymphocytes.[27] Therefore, a high NLR may indicate an increase in neutrophil-dependent inflammatory responses, and a corresponding decrease in the lymphocyte-mediated antitumor immune response. Neutrophil-dependent responses can promote tumor invasiveness and thus a poor patient prognosis.[6] Therefore, the NLR is an important indicator of the balance between the inflammatory and immune responses.
At present, the applications of the NLR are not limited to the prognostic evaluation of malignant tumor patients. Patients with laryngeal squamous cell carcinoma who present with a preoperative NLR > 2.5 showed a higher risk of developing pharyngocutaneous fistula in the postoperative setting of total laryngectomy (P = .007).[28] A study of Julia et al[29] demonstrated that the NLR has important clinical significance for the identification of sepsis and bacteraemia in patients with burn injuries (P = .000). Furthermore, Jeong-ju et al[30] highlighted that a high NLR is an independent risk factor for poor prognosis in patients with primary cholangitis (P < .01), and Lou et al[31] found that an NLR > 11 had important predictive value for the weaning failure of ICU patients (P < .001). Zhou et al[32] has revealed that NLR > 6.5 often indicates the occurrence of a strangulated inguinal hernia in patients with inguinal hernia (P < .001), and Ali et al[33] suggest that a preoperative NLR > 5 is an independent predictive marker of 30 day morbidity in ruptured abdominal aortic aneurysms (P = .02).
In the present study, a systematic review of the preoperative hematological and pathological indices of 59 DCC patients revealed that the preoperative NLR was an independent risk factor affecting the overall survival rates of DCC patients. However, the study had several limitations. Firstly, bias when selecting cases was inevitable in a single-center retrospective study. Secondly, due to objective factors, procalcitonin, CRP, IL-6, and other important inflammatory indicators were not assessedin this study. Therefore, further prospective studies are required to confirm the results of the present study.
5. Conclusions
The results of the present study reveal that a preoperative NLR > 2.933 is an independent risk factor for poor prognosis in patients with DCC. As an economical and convenient preoperative indicator, the NLR can be used to preliminarily estimate the prognosis of patients, and to formulate adjuvant treatment and follow-up plans. Many studies have indicated that a high NLR is associated with the poor prognosis of malignant tumor patients; however, whether clinical intervention to reduce the preoperative NLR has clinical significance in improving long-term prognosis remains to be elucidated.
Author contributions
Data curation: Lianmin Wang.
Formal analysis: Fengming Ji, Lixin Liu.
Funding acquisition: Hao Zou.
Methodology: Qiang Kang, Yang Ke, Ya Zhu.
Project administration: Hao Zou.
Software: Shifeng Xiong.
Visualization: Naiqiang Zhang.
Writing – original draft: Fengming Ji, Qiang Kang, Lianmin Wang.
Writing – review & editing: Yuehua Li, Hao Zou.
Footnotes
Abbreviations: ALB = albumin, AST = aminotransferase, AJCC = American Joint Committee on cancer, CEA = carcinoembryonic antigen, CA125 = carbohydrate antigen 125, CA19-9 = carbohydrate antigen 19-9, 95% CI = 95% confidence intervals, CRP = C-reactive protein, DCC = distal cholangiocarcinoma, HCC = hilar cholangiocarcinoma, IL-6 = interleukin-6, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, ROC = receiver operating characteristic.
How to cite this article: Ji F, Kang Q, Wang L, Liu L, Ke Y, Zhu Y, Zhang N, Xiong S, Li Y, Zou H. Prognostic significance of the neutrophil-to-lymphocyte ratio with distal cholangiocarcinoma patients. Medicine. 2020;99:43(e22827).
FJ, QK, and LW contributed equally to this work.
This study was supported by the Training Program Foundation of Medical Reserve Talent for Health and Family Planning Commission of Yunnan Province (grant no.H-201604); Yunnan Provincial Department of Science and Technology-Kunming Medical University Joint Funding for Applied Basic Research(2018FE001(-232)); Graduate innovation Foundation of Kunming Medical University (2018D002).
The study was confirmed by the China Ethics Committee of Registering Clinical Trials (Ethical review number: Chi ECRCT20190065).
All authors approved the final manuscript for publication.
Relevant data can be provided if necessary. Data were available to FJ and HZ.
The authors disclose no conflicts.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Esnaola NF, Meyer JE, Karachristos A, et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer 2016;122:1349–69. [DOI] [PubMed] [Google Scholar]
- [3].Bilici A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol 2014;20:10802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bagante F, Spolverato G, Weiss M, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new eighth edition AJCC staging system. J Gastrointest Surg 2018;22:52–9. [DOI] [PubMed] [Google Scholar]
- [5].Fruchtenicht AVG, Poziomyck AK, Reis AMD, et al. Inflammatory and nutritional statuses of patients submitted to resection of gastrointestinal tumors. Revista do Colegio Brasileiro de Cirurgioes 2018;45:e1614. [DOI] [PubMed] [Google Scholar]
- [6].Ventriglia J, Petrillo A, Huerta Alvaro M, et al. Neutrophil to lymphocyte ratio as a predictor of poor prognosis in metastatic pancreatic cancer patients treated with nab-paclitaxel plus gemcitabine: a propensity score analysis. Gastroenterol Res Pract 2018;2018:2373868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Isobe A, Sawada K, Kinose Y, et al. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PloS One 2015;10:e0118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elbaz S, Mousa N, Besheer T, et al. Malondialdehyde and C-reactive protein as prognostic markers ofhepatocellular carcinoma. Br J Biomed Sci 2020;77:94–6. [DOI] [PubMed] [Google Scholar]
- [9].He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer 2018;9:1877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu L, Wang W, Zhang Y, et al. Declined preoperative aspartate aminotransferase to neutrophil ratio index predicts poor prognosis in patients with intrahepatic cholangiocarcinoma after hepatectomy. Cancer Res Treat 2018;50:538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cuello-Lopez J, Fidalgo-Zapata A, Lopez-Agudelo L, et al. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PloS One 2018;13:e0207224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rijken AM, Umezawa A, van Gulik TM, et al. Prognostic value of cell proliferation (Ki-67 antigen) and nuclear DNA content in clinically resectable, distal bile duct carcinoma. Ann Surg Oncol 1998;5:699–705. [DOI] [PubMed] [Google Scholar]
- [13].Ogata T, Satake H, Ogata M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget 2018;9:34520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aguiar-Bujanda D, Duenas-Comino A, Saura-Grau S, et al. Neutrophil to lymphocyte ratio as a prognostic factor in european patients with epidermal growth factor receptor-mutant non-small cell lung cancer treated with tyrosine kinase inhibitors. Oncol Res Treat 2018;41:755–61. [DOI] [PubMed] [Google Scholar]
- [15].Zhang LX, Wei ZJ, Xu AM, et al. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg 2018;56:320–7. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta-analysis. Int J Surg 2018;55:73–80. [DOI] [PubMed] [Google Scholar]
- [17].Wellner UF, Shen Y, Keck T, et al. The survival outcome and prognostic factors for distal cholangiocarcinoma following surgical resection: a meta-analysis for the 5-year survival. Surg Today 2017;47:271–9. [DOI] [PubMed] [Google Scholar]
- [18].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [19].Oh D, Pyo JS, Son BK. Prognostic roles of inflammatory markers in pancreatic cancer: comparison between the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Gastroenterol Res Pract 2018;2018:9745601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. nature reviews. Cancer 2013;13:759–71. [DOI] [PubMed] [Google Scholar]
- [21].Jackaman C, Tomay F, Duong L, et al. Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev 2017;36:105–16. [DOI] [PubMed] [Google Scholar]
- [22].Zhang X, Zhang W, Yuan X, et al. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol 2016;49:857–67. [DOI] [PubMed] [Google Scholar]
- [23].Ocana A, Nieto-Jimenez C, Pandiella A, et al. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer 2017;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature reviews. Cancer 2016;16:431–46. [DOI] [PubMed] [Google Scholar]
- [25].de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005;7:411–23. [DOI] [PubMed] [Google Scholar]
- [26].Tan DW, Fu Y, Su Q, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: a meta-analysis. Sci Rep 2016;6:33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buettner S, Spolverato G, Kimbrough CW, et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 2018;164:411–8. [DOI] [PubMed] [Google Scholar]
- [28].Aires FT, Dedivitis RA, Kulcsar MAV, et al. Neutrophil-to-lymphocyte ratio as a prognostic factor for pharyngocutaneous fistula after total laryngectomy. Acta otorhinolaryngologica Italica 2018;38:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fuss J, Voloboyeva A, Poliovyj V. Prognostic value of using neutrophil-lymphocyte ratio in patients with burn injury for the diagnosis of sepsis and bacteraemia. Polski przeglad chirurgiczny 2018;90:13–6. [DOI] [PubMed] [Google Scholar]
- [30].Yoo JJ, Cho EJ, Lee B, et al. Prognostic value of biochemical response models for primary biliary cholangitis and the additional role of the neutrophil-to-lymphocyte ratio. Gut Liver 2018;12:714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luo Z, Zheng Y, Yang L, et al. Neutrophil/lymphocyte ratio is helpful for predicting weaning failure: a prospective, observational cohort study. J Thor Dis 2018;10:5232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou H, Ruan X, Shao X, et al. Clinical value of the neutrophil/lymphocyte ratio in diagnosing adult strangulated inguinal hernia. Int J Surg 2016;36:76–80. [DOI] [PubMed] [Google Scholar]
- [33].Kordzadeh A, Malietzis G, Browne T, et al. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): a retrospective cohort study. Int J Surg 2015;15:45–8. [DOI] [PubMed] [Google Scholar]


