Abstract
Video capsule endoscopy (VCE) can detect mucosal lesions in the intestine, especially in the small bowel.
Our study aims to evaluate the applications of VCE for pediatric gastrointestinal diseases.
In this retrospective study, we included all patients who underwent VCE between December 2012 and December 2018. Clinical information and VCE data were analyzed.
Among 828 patients, the completion rate was 99.6% (n = 825), with an average age of 10.2 ± 3.3 years old. A total of 459 VCE procedures showed abnormalities, and the overall diagnostic yield was 55.6%. The most common indications for VCE were abdominal pain among 505 (61.2%) patients and hematochezia (10.1%) among 83. Among the positive results of VCE, small bowel ulcers accounted for the highest percentage (57.7%), of which 164 cases were diagnosed as inflammatory bowel disease. For obscure gastrointestinal bleeding, 12 cases were diagnosed as Meckel's diverticulum. In terms of the small bowel transit time of VCE, compared with the negative group [288 (216.5, 390.3) min] and the enteritis group [277 (192.5, 374.8) min], a longer transit time was needed in the small bowel ulcer group [332.5 (240, 451.5) min, P < .01]. There were no correlations of positive VCE findings with anemia, the white blood cell count, the C-reactive protein level or the small bowel transit time according to Spearman rank analysis.
VCE is relatively well tolerated and safe in children and has great value for the diagnosis and treatment of abdominal pain, especially inflammatory bowel disease and obscure gastrointestinal bleeding.
Keywords: abdominal painl, capsule endoscopy, children, inflammatory bowel disease
1. Introduction
Video capsule endoscopy (VCE) is a noninvasive technology designed primarily to provide diagnostic imaging of the small intestine, an anatomic site that has proven particularly difficult to visualize. VCE is considered a milestone in the development of endoscopy.[1,2] The United States Food and Drug Administration approved the use of CE for the evaluation of small bowel diseases in adults in 2001. In 2004, CE was approved for children 10 years or older;[3] Both CE and patency capsules were approved for use in children older than 2 years in 2009.[4] VCE is used for the diagnosis and assessment of the extent of disease in the small bowel, such as for Crohn's disease. Further obscure gastrointestinal hemorrhage, intestinal polyposis, protein-losing enteropathies, and intestinal tumors are all indications for VCE when small bowel pathology is suspected.[5,6] VCE has addressed the shortcomings of traditional endoscopy or colonoscopy given its ability to detect disease in the small bowel and is more flexible and convenient than double-balloon enteroscopy.[7,8]
Fewer studies on VCE have been conducted in children than in adults to evaluate its tolerance and safety. In this retrospective study, 825 children who underwent VCE in the Children's Hospital of Fudan University were analyzed, and this study aims to shed new light on the clinical application of VCE.
2. Experimental methods
2.1. Patients
This study was approved by the Ethics Committee of the Children's Hospital of Fudan University. Informed consent was signed by the parents of all participants who underwent VCE. Information on the patients with digestive symptoms who were enrolled in the Department of Gastroenterology, Children's Hospital of Fudan University between December 31, 2012, and December 31, 2018, included their sex and age at the time of VCE examination. The follow-up period ended on August 31, 2019. The VCE used in the study was Jinshan OMOM Capsule Endoscopy Model JS-ME-1, with a diameter of 11.0 mm and a length of 25.4 mm.
2.2. Examination standards for capsule endoscopy
The indications for VCE in the pediatric population outlined in the ESPHGAN guidelines were followed.[3] VCE was used when a diagnosis could not be confirmed with gastroscopy or to supplement the diagnosis reached via colonoscopy. The groups were identified by their chief complaints and main clinical features.
VCE images were categorized as normal, inflammation, ulcer, polyps, lymphangiectasia, bleeding, vascular disease, protruding lesion, lymphatic follicular hyperplasia, diverticulum, parasites, and other diseases, referring to the report published previously.[9]
2.3. Intestinal preparation and testing method
Two days before VCE, oral lactulose was given 15 ml/time twice a day; 2 days before VCE, polyethylene glycol electrolyte solution was given orally to children. Two days before the examination, children were put on liquid diets. They were required to fast, abstaining from solids and liquids, on the examination day. Two hours after the capsule entered the duodenum, a small amount of solid food could be taken orally. If it was difficult for the child to swallow the capsule or the capsule did not enter the duodenum within 2 hours of real-time monitoring after it was swallowed, the capsule was transmitted to the duodenum via gastroscopy under anesthesia. A normal diet could be resumed when the capsule was excreted or at the end of the examination.
The following outcomes were measured: the transit time of the capsule through the stomach and small bowel, capsule excreting time, examination completion rate of the entire small bowel (the proportion of patients in whom the capsule entered the large intestine through the ileocecal valve after the battery was drained), capsule retention rate (the proportion of patients who did not excrete the capsule from the digestive tract after 2 or more weeks), the detection rate of small bowel disease and the type of lesion identified.
2.4. Statistical analysis
SPSS 22.0 software was used for the data analysis. Normally distributed data are expressed as the means ± standard deviations, and nonnormally distributed data are expressed as the medians and interquartile ranges. The Kruskal-Wallis statistical methods were employed to establish significance of transit time of stomach, small bowel and total time among the negative group, small bowel ulcer group and small bowel inflammation group. A P < .05 was deemed to be significant. Spearman rank correlation coefficient was used to assess the correlations of positive VCE findings with the fecal occult blood (OB) test result, anemia, white blood cell count, C-reactive protein level and small bowel transit time.
3. Results
3.1. Demographic features of patients
A total of 828 VCE patients were identified, of whom 3 patients were not able to undergo VCE due to intestinal stenosis detected during colonoscopy and small bowel MRI and were excluded (Fig. 1). Among the children who completed VCE, the diagnostic yield of VCE was 55.6% (459 patients). A total of 120 patients could not pass the capsule through the gastric antrum within the specified time and needed gastroscopic assistance.
Figure 1.
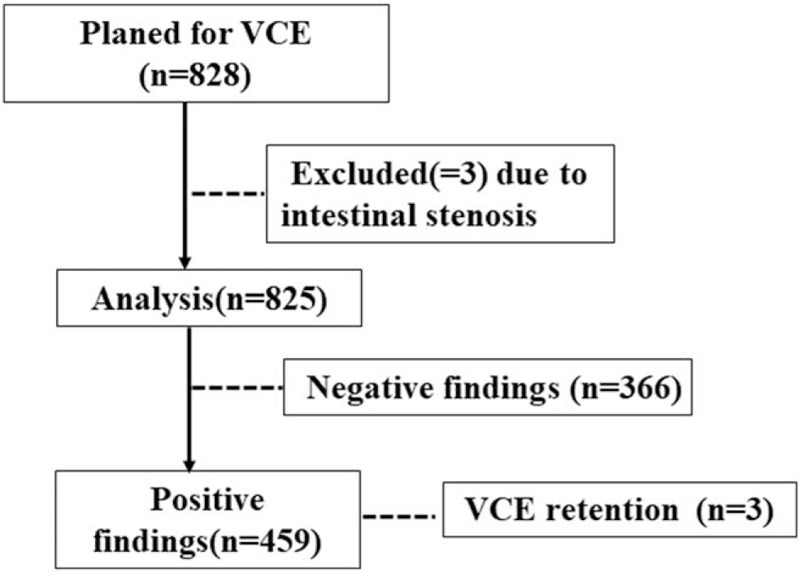
Flow chart of patient inclusion and exclusion of the diseases diagnosed in this study. VCE = video capsule endoscopy.
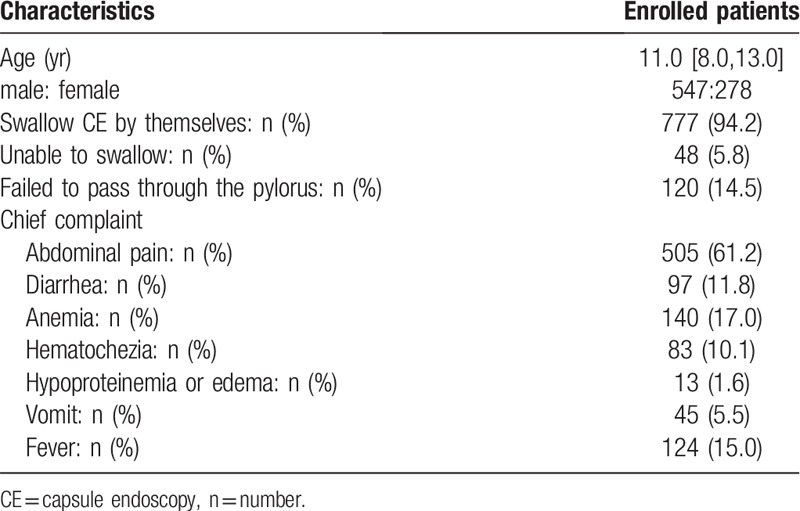
The analysis of the chief complaints during VCE showed that abdominal pain occurred in 505 patients (61.2%), accounting for the highest percentage, and hematochezia occurred in 83 patients (10.1%). In total, 137 patients had a positive fecal OB test (16.6%), and 140 patients had anemia (17.0%) (Table 1).
Table 1.
Baseline demographic and clinical characteristics of enrolled patients.
3.2. Capsule endoscopy findings
In our study, 459 VCEs (55.6%) revealed positive findings. In the 505 patients with abdominal pain as the chief complaint, 248 patients had positive VCE findings, including 147 patients in the small bowel ulcer group, 67 in the enteritis group, and 12 in the intestinal erosion group, 3 in intestinal lymphangiectasia, 2 in Meckel's diverticulum, 4 in intestinal polyps, 3 in vascular malformations, 3 in gastritis, 5 protuberant lesions, 2 intestinal bleeding; 257 patients had negative VCE results. The major positive results classified according to the VCE findings are shown below.
3.3. Small bowel ulcer
Small bowel ulcers were found in 265 patients (184 males and 81 females). With regard to the chief complaints of these patients, abdominal pain was noted in 150 patients (56.6%), diarrhea in 47 patients (17.7%), hematochezia in 23 patients (8.7%), fever in 85 patients (32.1%) and vomiting in 9 patients (3.4%).
A total of 164 patients (61.9%) in the small bowel ulcer group were diagnosed with inflammatory bowel diseases (IBD) with 150 Crohn disease (CD) and 14 ulcerative colitis (UC) (Fig. 2A, B). Seven patients were diagnosed with intestinal tuberculosis, 5 patients were diagnosed with cryptogenic multifocal ulcerating stenosing enteritis (Fig. 2C), 1 patient was diagnosed with Epstein-Barr virus infective enteritis, and 4 patients were diagnosed with Henoch-Schönlein purpura (HSP) (Fig. 2D). The diagnosis of the remaining cases were as follows,1 Behcet's disease, 40 duodenal ulcer, 1 leukemia, 2 rheumatoid arthritis,40 small bowel ulcer with diagnosis unknown
Figure 2.
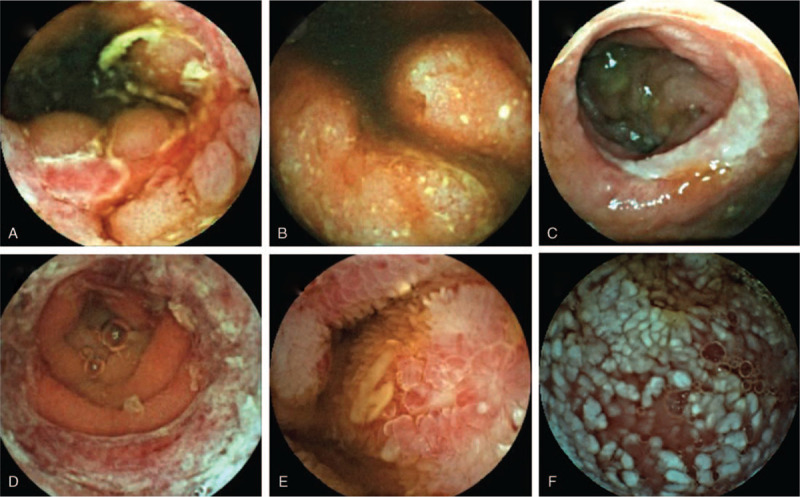
Findings of VCE. A. Image suggestive of Crohn's disease; B. Image suggestive of ulcerative colitis; C. Image suggestive of cryptogenic multifocal ulcerating stenosing enteritis; D. Image suggestive of Henoch-Schönlein purpura; E. Image suggestive of eosinophilic gastroenteritis; F. Image suggestive of intestinal lymphangiectasia. VCE = video capsule endoscopy.
3.4. Small bowel inflammation
Small bowel inflammation was found in 120 patients (82 males, 38 females). With regard to the primary complaint, abdominal pain was noted in 79 patients (65.8%), diarrhea in 13 patients (10.8%), hematochezia in 16 patients (13.3%), fever in 11 patients (9.2%), vomiting in 13 patients (10.8%) and hypoproteinemia or edema in 2 patients (1.7%).
In the patients with small bowel inflammation, there were 106 cases of enteritis and 14 cases of erosion. In total, 20 patients were diagnosed with IBD included 14 CD, 6 UC, 2 patients were diagnosed with eosinophilic gastroenteritis (Fig. 2E), and 1 patient in the small intestinal erosion group was diagnosed with HSP.
3.5. Lymphangiectasia
Eleven patients were diagnosed with intestinal lymphangiectasia in this group. In these patients, diarrhea (7/11), hypoproteinemia (5/11), edema (4/11), and abdominal pain (3/11) were the chief complaints (Fig. 2F). The average albumin level was 24.1 ± 3.0 g/L. Among these patients, 1 was diagnosed with lymphangioma and was treated with oral sirolimus regularly. Another patient died, possibly due to abdominal lymphoma.
3.6. Diverticulum
Among the patients with obscure GI bleeding and anemia, 12 patients were diagnosed with Meckel's diverticulum (Fig. 3A). The average Hb level of patients with Meckel's diverticulum was 87.9 ± 18.2 g/L. Eleven patients had their diagnoses confirmed during surgery, and the overall diagnostic yield was 91.7%.
Figure 3.
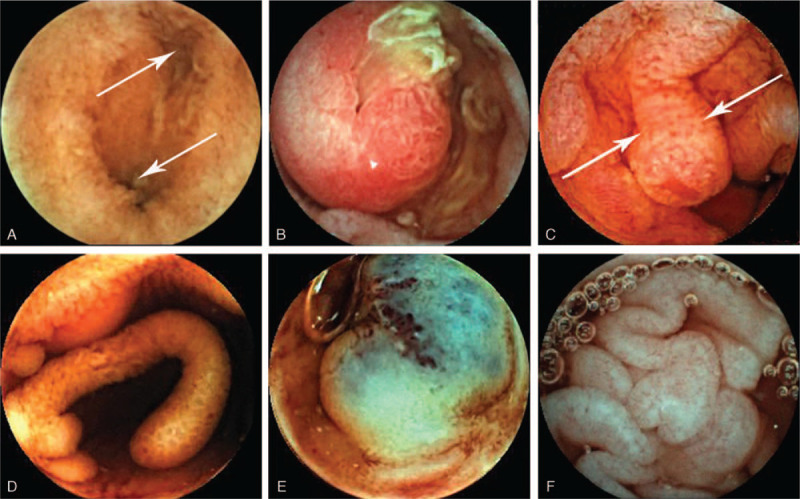
Findings of VCE. Image of the double lumen sign (2 arrows) and a diaphragm sign suggesting Meckel's diverticulum; B. Intestinal polyp indicative of Peutz-Jeghers syndrome; C. Image of juvenile polyposis syndrome (2 arrows); D. Image of small bowel pseudopolyposis; E. Blue rubber bleb nevus syndrome; F. Image suggestive of celiac disease. VCE = video capsule endoscopy.
3.7. Intestinal polyps
Fourteen patients had intestinal polyps (8 males and 6 females). Among these patients, abdominal pain was observed in 4 patients, hematochezia in 4 patients, and diarrhea in 3 patients.
Five patients were finally diagnosed with Peutz-Jeghers syndrome (Fig. 3B), 2 patients were diagnosed with juvenile polyposis syndrome (JPS) (Fig. 3C), 1 patient was diagnosed with familial adenomatous polyposis (FAP), and 4 patients were diagnosed with small bowel pseudopolyposis due to inflammatory stimulation (2 with primary immunodeficiency disease, 1 with CD, and 1 with UC) (Fig. 3D).
3.8. Vascular malformation
Vascular malformations were found in 10 patients. Hematochezia was seen in 7 patients, abdominal pain was observed in 3 patients, and anemia was noted in 2 patients.
In the vascular malformation group, angiodysplasia was found in 5 patients, and vascular protrusions were found in 4 cases. One patient was diagnosed with blue rubber bleb nevus syndrome (Fig. 3E). The lowest Hb level in the blue rubber bleb nevus syndrome patient was 3.3 g/dL, and the disease was controlled by sclerotherapy and oral sirolimus.
3.9. Rare disease
Diarrhea and abdominal swelling were the chief complaints in 2 patients. VCE showing flattened or absent intestinal villi were finally diagnosed with celiac disease (Fig. 3F). The gastroscopic examination showed flattened or absent villi in the descending part of the duodenum and terminal ileum. The test for the antibodies associated with celiac disease was positive, and the patients recovered after starting a gluten-free diet.
3.10. Normal
There were 366 patients with negative VCE findings. With regard to the chief complaint, abdominal pain accounted for 71.0% (260 patients), hematochezia accounted for 11.2% (41 patients), diarrhea accounted for 6.8% (25 patients), vomiting accounted for 5.2% (19 patients), and anemia accounted for 1.1% (4 patients). Twenty-one patients were diagnosed with IBD (14 CD, 7 UC) with the small bowel spared.
3.11. Comparison of VCE completion rate and complications
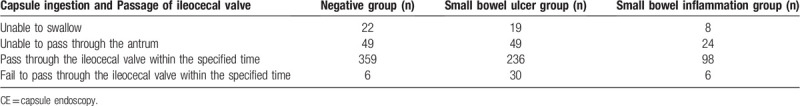
A subsequent analysis of swallowing was carried out due to the large numbers of patients in the group with negative VCE results, small bowel ulcer group and small bowel inflammation group (Table 2).
Table 2.
Examination condition of CE in Negative group, Small bowel ulcer group and inflammation group.
3.12. Comparison of the transit time of VCE
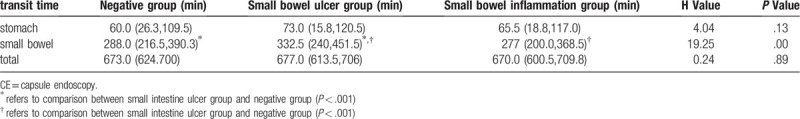
In this study, the transit time in the small bowel was 300.0 (218.0,410.5) min, the transit time in the stomach was 67.0 (29.0,122.0) min, and the total transit time was 671.0 (609.5, 703.0) min.
A comparison was made among the transit times in the patients in the negative results group, small bowel ulcer group and small bowel inflammation group due to the large numbers of cases (Table 3). The transit time in the small bowel ulcer group was significantly longer than those in the negative results group and enteritis group (P < .01). Furthermore, the transit time in the stomach in the small bowel ulcer group was no significantly difference than that in the negative results group and enteritis group (P = .13). Three patients had capsule retention because of intestinal stenosis, and abdominal surgery was needed.
Table 3.
Comparison of the transit time of CE in negative group, small bowel ulcer group and inflammation group.
There were no correlations between positive VCE findings and the white blood cell count (r = 0.026), anemia (r = 0.191), fecal OB (r = 0.164) and the small bowel transit time (r = 0.071). The C-reactive protein level (r = 0.244) had a weak correlation with an increased prevalence of positive findings.
4. Discussion
VCE is a noninvasive and convenient examination that plays an important role in the diagnosis of gastrointestinal diseases, especially suspected small bowel disease.[5,10] However, the literature regarding the use of VCE in pediatric patients is limited. Case reports have demonstrated that VCE is safe to use in children as young as 8 months or weighing as little as 7.9 kg.[11,12] The usefulness of VCE in children has been established for several small-bowel pathologies, such as Crohn's disease,[13] obscure gastrointestinal bleeding,[14] hereditary polyposis,[15] abdominal pain, and protein-losing enteropathy.[4,16] Furthermore, VCE is well suited for longitudinal monitoring of Crohn's disease activity in the small bowel.[17]
This study explored the characteristics of VCE in 825 patients enrolled at our hospital from 2012 to 2018. To the best of our knowledge, this is the largest number of VCE procedures in children reported to date. We found that abdominal pain was the main indication for VCE, followed by diarrhea, hematochezia, and so on. This is different from the results in adults, in whom obscure gastrointestinal bleeding is the main indication for VCE.[18,19] Recently, a systemic review reported that the pooled diagnostic yield of VCE for chronic abdominal pain was 20.9%;[20] Huang et al showed a 28.15% positive rate of VCE findings in the small bowel in chronic abdominal pain patients.[6] In this study, abdominal pain was the main indicator for VCE (54.1%), with 49.1% diagnostic yield in patients with chronic abdominal pain. Our diagnostic yield was higher than that reported in other groups because our hospital is a pediatric IBD center.
In recent years, the incidence of IBD in children has also increased annually; this is especially notable in CD, with 30% of cases involving the small bowel.[21] As an important tool for the exploration of small bowel diseases, VCE is of vital importance for CD patients.[22] UC is restricted to the colon; however, a few cases of UC with gastroduodenal lesions and small bowel lesions have also recently been reported.[23–25] In our study, 180 patients were diagnosed with IBD (160 CD, 20 UC). In children with IBD, VCE can be used to investigate the parts of the small bowel that cannot be observed by gastroscopy and colonoscopy. VCE can be used for the diagnosis and follow-up of IBD with small bowel lesions.
VCE is transmitted through the gastrointestinal tract passively and is excreted through gastrointestinal peristalsis. The transit time of VCE is related to the function of intestinal peristalsis and the condition of the intestinal surface. The diagnostic yield in small bowel CE is positively correlated with the small bowel transit time.[26] We found that the transit time of the capsules in the small bowel ulcer group was significantly delayed, which might be related to the rough texture and congestion of the intestinal mucosa or due to intestinal stricture and slow peristalsis.
Due to the difficulty in having young children (under 3 years old) swallow the VCE capsule, the application of VCE is limited.[12] In our study, 25.5% of the children were unable to swallow the capsule or the capsule failed to pass through the gastric antrum and gastroscopy was required. Nuutinen et al reported that CE was performed in infants with a minimum age of 8 months and a minimum weight of 8 kg.[11] In this study, children undergoing VCE were at least 2 years old and still needed endoscopic assistance.
The risk of capsule intestinal retention is another reason to limit the use of VCE. In this study, intestinal retention of VCE occurred in 3 children, and surgery was needed. In a review of 22,840 adult patients, it was found that the retention rate of the capsule was 1.2% in patients with gastrointestinal hemorrhage of unknown cause, 2.6% in patients with Crohn's disease, and 2.1% in patients with intestinal tumors.[27] The review of a study on CE in 1013 children found that 2.3% of patients retained the capsule (among 22 patients in total, 18 had intestinal retention and 4 had gastric retention). The retention rates in patients with gastrointestinal hemorrhage of unknown cause, CD and polyp disease were 1.4%, 2.2% and 1.3%, respectively.[28]
In this study, IBD, peptic ulcers, HSP, multiple intestinal polyps, vascular malformations, parasitic disease and other rare diseases were found by VCE. This shows the important value of VCE in the diagnosis of a relatively wide range of intestinal diseases, owing to the fact that VCE can expand the area of intestinal examination and address the shortcomings of colonoscopy.[29] Compared with colonoscopy, VCE has the advantages of noninvasiveness, comfort and high tolerance. Unlike CTE, MRE and CT enterography, CE does not involve radiation and has a higher level of diagnostic accuracy.[30] However, VCE also has shortcomings: biopsies cannot be performed, and repeated observations of specific lesions are not possible. Therefore, it should be selected based on the needs of the patient and the specific circumstances.
Our study had limitations. First, we could not display the detailed VCE findings of all types of diseases because of the large number of patients. Another major limitation of our study and other trials is the lack of a gold standard against which to assess the accuracy of VCE. The diagnostic yield and positive findings are crude measures. We do not know the rate of false positive or false negative results. Therefore, colonoscopy and other auxiliary examinations are necessary.
In conclusion, this single-center study reports the characteristics of VCE in Chinese children, and its safety and effectiveness make it a valuable technique in clinical practice.
Author contributions
Data curation: Jie Wu, Zifei Tang, Lingyu Lai.
Formal analysis: Yuhuan Wang, Aijuan Xue.
Methodology: Yuhuan Wang.
Project administration: Ying Huang.
Resources: Yuhuan Wang, Lingyu Lai.
Software: Aijuan Xue.
Supervision: Zhiheng Huang, Ying Huang.
Writing – original draft: Jie Wu.
Writing – review & editing: Zhiheng Huang.
Footnotes
Abbreviations: CD = Crohn disease, HSP = Henoch-Schönlein purpura, IBD = inflammatory bowel disease, OB = occult blood, UC = ulcerative colitis, VCE = video capsule endoscopy.
How to cite this article: Wu J, Huang Z, Wang Y, Tang Z, Lai L, Xue A, Huang Y. Clinical features of capsule endoscopy in 825 children: a single-center, retrospective cohort study. Medicine. 2020;99:43(e22864).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Cohen SA, Ephrath H, Lewis JD, et al. Pediatric capsule endoscopy: review of the small bowel and patency capsules. J Pediatr Gastroenterol Nutr 2012;54:409–13. [DOI] [PubMed] [Google Scholar]
- [2].Oliva S, Cohen SA, Di Nardo G, et al. Capsule endoscopy in pediatrics: a 10-years journey. World J Gastroenterol 2014;20:16603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Friedlander JA, Liu QY, Sahn B, et al. NASPGHAN capsule endoscopy clinical report. J Pediatr Gastroenterol Nutr 2017;64:485–94. [DOI] [PubMed] [Google Scholar]
- [4].Fornaroli F, Gaiani F, Vincenzi F, et al. Applications of wireless capsule endoscopy in pediatric age: an update. Acta Biomed 2018;89(9-S):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flemming J, Cameron S. Small bowel capsule endoscopy: indications, results, and clinical benefit in a University environment. Medicine (Baltimore) 2018;97:e0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang L, Huang Z, Tai Y, et al. The small bowel diseases detected by capsule endoscopy in patients with chronic abdominal pain: a retrospective study. Medicine (Baltimore) 2018;97:e0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yokoyama K, Yano T, Kumagai H, et al. Double-balloon enteroscopy for pediatric patients: evaluation of safety and efficacy in 257 cases. J Pediatr Gastroenterol Nutr 2016;63:34–40. [DOI] [PubMed] [Google Scholar]
- [8].Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008;27:146–54. [DOI] [PubMed] [Google Scholar]
- [9].Ding Z, Shi H, Zhang H, et al. Gastroenterologist-level identification of small-bowel diseases and normal variants by capsule endoscopy using a deep-learning model. Gastroenterology 2019;157:1044–54 e1045. [DOI] [PubMed] [Google Scholar]
- [10].Alkhormi A, Memon MY, Elhafi A, et al. Initial experience of video capsule endoscopy at a tertiary center in Saudi Arabia. Saudi J Gastroenterol 2018;24:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nuutinen H, Kolho KL, Salminen P, et al. Capsule endoscopy in pediatric patients: technique and results in our first 100 consecutive children. Scand J Gastroenterol 2011;46:1138–43. [DOI] [PubMed] [Google Scholar]
- [12].Oikawa-Kawamoto M, Sogo T, Yamaguchi T, et al. Safety and utility of capsule endoscopy for infants and young children. World J Gastroenterol 2013;19:8342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nemeth A, Agardh D, Wurm Johansson G, et al. Video capsule endoscopy in pediatric patients with Crohn's disease: a single-center experience of 180 procedures. Therap Adv Gastroenterol 2018;11:1756284818758929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xiao WD, Chen W, Yang H. Heterotopic pancreas within Meckel's diverticulum with obscure then massive gastrointestinal bleeding in a 12-year-old child: case report and review of the literature. J Int Med Res 2009;37:967–72. [DOI] [PubMed] [Google Scholar]
- [15].Gastineau S, Viala J, Caldari D, et al. Contribution of capsule endoscopy to Peutz-Jeghers syndrome management in children. Dig Liver Dis 2012;44:839–43. [DOI] [PubMed] [Google Scholar]
- [16].van der Reijden SM, van Wijk MP, Jacobs M, et al. Video capsule endoscopy to diagnose primary intestinal lymphangiectasia in a 14-month-old child. J Pediatr Gastroenterol Nutr 2017;64:e161. [DOI] [PubMed] [Google Scholar]
- [17].He C, Zhang J, Chen Z, et al. Relationships of capsule endoscopy Lewis score with clinical disease activity indices, C-reactive protein, and small bowel transit time in pediatric and adult patients with small bowel Crohn's disease. Medicine (Baltimore) 2017;96:e7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Juanmartinena Fernandez JF, Fernandez-Urien Sainz I, Zabalza Ollo B, et al. Gastroduodenal lesions detected during small bowel capsule endoscopy: incidence, diagnostic and therapeutic impact. Rev Esp Enferm Dig 2018;110:102–8. [DOI] [PubMed] [Google Scholar]
- [19].Perez-Cuadrado-Robles E, Zamora-Nava LE, Jimenez-Garcia VA, et al. Indications for and diagnostic yield of capsule endoscopy in the elderly. Rev Gastroenterol Mex 2018;83:238–44. [DOI] [PubMed] [Google Scholar]
- [20].Xue M, Chen X, Shi L, et al. Small-bowel capsule endoscopy in patients with unexplained chronic abdominal pain: a systematic review. Gastrointest Endosc 2015;81:186–93. [DOI] [PubMed] [Google Scholar]
- [21].Huang Z, Peng K, Li X, et al. Mutations in interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: a Chinese VEO-IBD collaboration group survey. Inflamm Bowel Dis 2017;23:578–90. [DOI] [PubMed] [Google Scholar]
- [22].Okuhira T, Yoden A, Aomatsu T, et al. Correlation of the endoscopic findings for small and large bowels in pediatric patients with established Crohn's disease. J Clin Biochem Nutr 2019;64:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ninomiya K, Hisabe T, Okado Y, et al. Comparison of small bowel lesions using capsule endoscopy in ulcerative colitis and Crohn's disease: a single-center retrospective analysis. Digestion 2018;98:119–26. [DOI] [PubMed] [Google Scholar]
- [24].Rubenstein J, Sherif A, Appelman H, et al. Ulcerative colitis associated enteritis: is ulcerative colitis always confined to the colon? J Clin Gastroenterol 2004;38:46–51. [DOI] [PubMed] [Google Scholar]
- [25].Hori K, Ikeuchi H, Nakano H, et al. Gastroduodenitis associated with ulcerative colitis. J Gastroenterol 2008;43:193–201. [DOI] [PubMed] [Google Scholar]
- [26].Westerhof J, Koornstra JJ, Hoedemaker RA, et al. Diagnostic yield of small bowel capsule endoscopy depends on the small bowel transit time. World J Gastroenterol 2012;18:1502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 2010;71:280–6. [DOI] [PubMed] [Google Scholar]
- [28].Cohen SA. The potential applications of capsule endoscopy in pediatric patients compared with adult patients. Gastroenterol Hepatol (N Y) 2013;9:92–7. [PMC free article] [PubMed] [Google Scholar]
- [29].Cohen S, Hyer W, Mas E, et al. Management of juvenile polyposis syndrome in children and adolescents: a position paper from the ESPGHAN polyposis working group. J Pediatr Gastroenterol Nutr 2019;68:453–62. [DOI] [PubMed] [Google Scholar]
- [30].Gonzalez-Suarez B, Rodriguez S, Ricart E, et al. Comparison of capsule endoscopy and magnetic resonance enterography for the assessment of small bowel lesions in Crohn's disease. Inflamm Bowel Dis 2018;24:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


