Abstract
The objectives of this study were to describe the prevalence and characteristics of radiographic lesions of the hands, and calcifications of the spine on computer tomography scans (CT-scans), and to investigate the relationships between radiographic and CT-scan abnormalities and clinical features in a population of patients with systemic sclerosis (SSc).
Subjects underwent X-ray examination of the hands, and thoracic or thoraco-abdominal and pelvic CT scan or lumbar CT scan in the year. Structural lesions on hand X ray was scored and spinal calcifications were evaluated in the anterior, intracanal and posterior segments. Intra and inter-reliability was tested for radiography and CT- scan. Prognostic factors considered were interstitial pulmonary lesions on the CT scan, pulmonary arterial hypertension (PAH) and death.
This study involved 77 SSc patients, 58 (75%) with limited cutaneous SSc (lcSSc) and 19 (25%) with diffuse SSc (dSSc). The prevalences of radiographic lesions of the hand were 28.6% for periarticular calcifications and 26% for calcinosis. On CT scan, 64 (83%) patients exhibited at least 1 calcification. Spine calcifications were depicted in 80.5%, 27.3%, and 35.1% at the anterior, intracanal and posterior segments respectively. Calcifications were mainly localized on thoracic spine. Inter reader reliabilities were good for hands and moderate for spine respectively. Spine calcifications and periarticular calcifications in the hands were associated (P = .012). Calcinosis in the hands was related to PAH (P = .02). Posterior calcification segment and foraminal calcifications were associated with interstitial lung disease (ILD) (P = .029) and death (P = .001).
More than 80% of systemic sclerosis patients presented spine calcifications. A significant association between hands and spinal calcifications were confirmed and some localization in the posterior segment considered as a bad prognostic factor.
Keywords: calcifications, systemic sclerosis
1. Introduction
Systemic sclerosis (SSc) is a multi system auto-immune disease involving activation of lymphocytes, fibroblasts, and endothelial cells, and secretion of vasoconstriction mediators (endothelin), profibrosing agents (transforming growth factor β (TGFβ), growth factors (connective transforming growth factors (CTGF)), and pro-inflammatory cytokines (IL17 and TNFα). Initial endothelial dysfunction is followed by an inflammatory phase and then fibrosis.[1]
The classical clinical description distinguishes 2 forms: diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc). Both are associated with degradation in the quality of life.[2] Life expectancy is reduced by 10% to 20% in lcSSc patients and by 20% to 40% in dcSSc patients.[5–7] Death is related to PAH (pulmonary arterial hypertension) and pulmonary fibrosis.[4] Clinically, hands are frequently affected (24%–94%)[1,8–13] with concomitant Raynauds syndrome, digital ulcers and necrosis combined with major functional disability due to tendon and joint lesions, and digital flexor retractions.
Regarding structural damage, hand radiography classically demonstrates the following lesions: resorption of phalangeal tufts; calcifications; digital retractions; erosions and demineralisation. Acro-osteolysis and calcinosis are associated with the presence of digital ulcers, evidence of the severity of vascular damage.[13]
In contrast, damage to the axial skeleton is less well understood and has only recently been reported in a few case reports secondary to nerve root or medullary compression, in some cases justifying emergency surgery, as was observed in one of our patients.[15–23] Most of these cases concerned cervical locations.[14–16,18,21–28] The lumbar spine was less often affected,[17,19,22,29] and the thoracic spine even more rarely.[30] In 2009, Ogawa et al showed, in a series of 41 SSc patients, a 58.5% prevalence of dorsal intraspinal and paraspinal calcifications, of which 29% were observed to be intracanal on thoracic computer tomography scans (CT-scans) during follow-up. Moreover, a relationship was found between the presence of these calcifications and Raynauds syndrome, calcinosis cutis and digital ulcerations.
The primary objective was, therefore, to describe the prevalence and characteristics of radiographic lesions of the hands and spine observed on thoracic, thoracic abdominal pelvic (TAP) or lumbar CT scans, and its reliability (intra and inter readers) in a population of 77 SSc patients followed at Nancy University Hospital. The secondary objective was to determine the association between these imaging findings (axial and peripheral) and clinical features including ILD, PAH, and death.
2. Methods
2.1. Population
This was a single center descriptive and retrospective study in patients treated at Nancy University Hospital since January 2000 for limited or diffuse cutaneous SSc. The Department of Medical Information approved the request for all medical records encoded M34.0, M34.1, M34.8, and M34.9 between January 2000 and April 2014 according to the International Classification of Diseases.
Patients were included if they met the ACR/EULAR (American College of Rheumatology/European League Against Rheumatism) 2013 systemic sclerosis classification criteria (sclerodactyly, pulpar scarring or digital ulcerations; telangiectasia; abnormal nailfold capillaroscopy; PAH and/or interstitial lung disease; Raynauds syndrome; anti-centromere, anti Scl-70 and anti-RNA polymerase III antibodies). Patients fulfilled the classification criteria if they had a score of 9 points or more.
They also had to have had a hand X-ray and chest or TAP or lumbar CT scan with not more than 12 months between the 2 imaging techniques.
Data were collected on: demographic characteristics (age, sex); clinical factors (duration of disease progression, history, joint pain, sclerodactyly (modified Rodnan score) Raynauds syndrome, telangiectasia, dyspnoea (NYHA), digital ulcers (DU), and gastro-intestinal symptoms); laboratory test parameters (antinuclear antibodies (ANA), anti Scl-70 and anti-centromere antibodies, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), lipid and phospho-calcium profiles); and capillaroscopy and therapeutic issues. Also of interest were echocardiographic parameters including left ventricular ejection fraction (LVEF) and the estimation of PAPs by tricuspid regurgitant velocity (TRV) (tricuspid valve insufficiency flow rate), which were evaluated by an experienced cardiologist. Pulmonary hypertension was diagnosed if the estimation of [PAPs>35 mm Hg by the TRV >2.8 m/second and then confirmed by right heart catheterisation: PAPm ≥ 25 mm Hg and PCWP≤15 mm Hg (pre-capillary pulmonary hypertension).[31] Pulmonary involvement was determined with functional measurement in terms of obstructive and restrictive defects and decreased diffusing capacity of the lungs for carbon monoxide DLCO (<80%), and with a specific examination of the CT scan by an experienced radiologist who classified interstitial lung disease (ILD) lesions into 3 categories: definite usual interstitial pneumonia (UIP), probable UIP and non-specific interstitial pneumonia (NSIP).
2.2. Evaluation of calcifications and structural lesions on hand X-rays
Bilateral frontal posterior-anterior projection X-rays of the hands were performed in the radiology department of Nancy University Hospital. X-rays were read using OsiriX (OsiriX v6.5.1–64 bits) software, and calcification was identified radiologically by 2 rheumatologists blinded to clinical and laboratory test data. Three radiological patterns of lesions were dectected:[10,13] joint pattern (space narrowing, erosions), bone pattern (resorption), and soft tissues pattern (calcifications, flexion contracture). Each radiological finding was scored for the presence or the absence of lesion.
2.3. Evaluation of axial calcifications on CT scans
All CT scans were performed at Nancy University Hospital. For the TAP multiple row detector array scans, the field of exploration included the thoracic, abdominal and pelvic regions. The acquisition diameter was 50 cm with a 512 × 512 matrix. Slices were 1.25 mm thick in the axial acquisition mode. These examinations were performed with injection of contrast material unless contra-indicated (52 of 77 CT scans were injected). The mean dose received was estimated to be between 193 and 500 mGy.cm for a chest scan and between 1068 and 2718 mGy.cm for a TAP scan. TAP was performed in 52 cases and involved spinal exploration from the lower edge of C7 to the upper edge of S1 vertebrae. In 24 patients who underwent thoracic CT scan, the spinal exploration was performed from C7 to L1 vertebrae. Lastly, in 1 patient no TAP or chest scan was performed, but we had a scan of the lumbar spine. Readings were performed on OsiriX (OsiriX v6.5.1–64 bits) software initially on the axial sections in bone windows when blinded to the patients characteristics. Sagittal and coronal reconstruction was performed in the software with the aim of obtaining a multiplanar assessment. Each discovertebral level was scored for the presence of calcification, defined as its presence on at least 2 contiguous slices and confirmed in one of the 2 other planes and its topography (anterior (anterior, central or posterior discovertebral space); intracanal; and posterior segments (costotransverse, interspinous, and transverse process)).
The intra and inter-reader reliabilities to classify patients with hands calcifications was performed on 29 radiographies. Intra and inter reader reliabilities to classify patients with spine calcification was performed on 38 and 30 CT-scans. Intra-reader exercise was performed with a delay of 2 weeks, for both radiography and CT-scan.
2.4. Statistical analysis
Statistical analysis was performed with SAS 9.3 software (SAS Institute Inc, Cary, NC). Qualitative variables were described as populations and percentages and quantitative variables by means and their standard deviation. Intergroup comparisons were performed by Chi-Squared tests for qualitative variables and Student tests for quantitative variables.
We used the Cohen's kappa to analyze the intra and inter readers agreement to classify at the patient level the presence of hand and spine calcifications.
The risk α is taken as 0.05. The software used is IBM SPSS Statistics V22.
2.5. Ethics approval and consent to participate
All data were available from usual care in patients with scleroderma. The ethical committee of Nancy hospital agreed with this study (referral file number 166). This study was designed in accordance with the general ethical principles outlined in the Declaration of Helsinki, patients gave their consent to use their medical data when they were care at the university hospital.
3. Results
3.1. Patient characteristics
One hundred SSc patients were identified in the database, from which we selected a final population of 77 who complied with the ACR/EULAR 2013 criteria and had undergone X-ray profiling of the hands and a CT scan (chest = 24; TAP n = 52; 1 lumbar scan).
The population had a mean age of 56.9 years (+/-14.3) and was very predominantly female (88.3%). Fifty eight (75%) exhibited a limited cutaneous form and 19 (25%) a diffuse cutaneous form; 83% of the patients suffered from joint pain. Radiography and CT scan were performed or mean disease duration of 5.8 years with no significant difference between limited and diffuse SSc. All patient characteristics were as displayed in Table 1. The mean modified Rodnan score was higher in diffuse forms vs limited SSc (P < .0001). Interstitial lung disease (ILD) was observed in 52.6% of the cases of diffuse cutaneous SSc vs 24.1% of the cases of limited cutaneous SSc (P = .02); NSIP was seen in 31.6% of dcSSc cases vs 12.1% of lcSSc cases (P = .04) and a restrictive syndrome in 26.3% vs 5.2%, respectively (P = .02). There was no significant difference between the 2 groups in PAH, death or the presence of DU.
Table 1.
Clinical and therapeutic characteristics of the 77 patients with systemic sclerosis.
3.2. Evaluation of calcifications and structural hand lesions on radiography
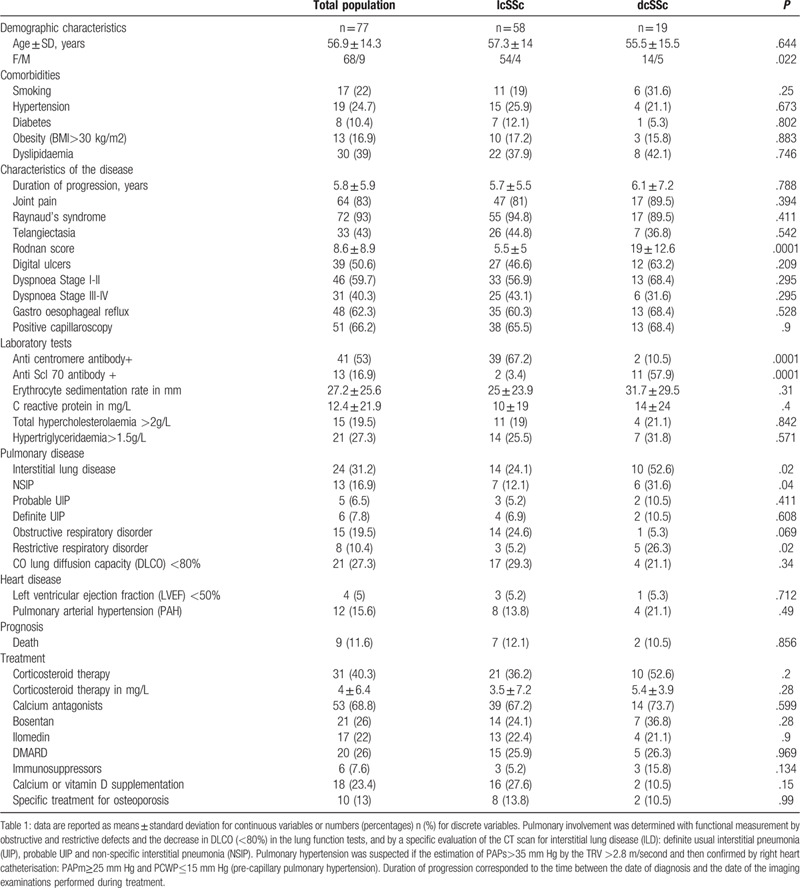
Radiographic characteristics of structural lesions were as illustrated in Figure 1, and their prevalences in the total population and the different forms of SSc were as presented in Table 2. Only the flexion attitude was statistically significantly higher at 50% in diffuse cutaneous SSc patients compared to 18.2% in limited cutaneous SSc patients (P = .005). Periarticular calcifications were more present in limited forms (P = .006). The distributions of the structural lesions were distal phalanx 20%, DIPs joints 45%, PIPs joints 18.9%, MCPs joints 13.3% and wrist 2.4%.
Figure 1.
A periarticular (white arrow) calcifications, B Sub-cutaneous (yellow arrow) calcifications and C: Sub-cutaneous calcifications in patients treated for SSc on posterior-anterior projection X-rays of the hands.
Table 2.
Prevalence of structural lesions on hands in the total population (n = 77) and in the lcSSc (n = 55) and dcSSc (n = 22) groups.

3.3. Evaluation of axial calcifications on CT scan
Of the 77 patients, 64 (83%) exhibited at least 1 calcification of the spine; 62 (80.5%) had lesions of the anterior segment which could be divided into calcifications of the anterior longitudinal ligament n = 58 (75.3%), intra-disc calcification, n = 40 (51.9%) and calcifications of the posterior longitudinal ligament (PLL), n = 29 (37.7%). Intracanal lesions were observed in 21 patients (27.3%) including nine foraminal localizations with a pseudotumoral appearance. Posterior spinal lesions were present in 27 patients (35.1%) (Fig. 2). Finally, 13 (16.9%) patients presented calcifications in all 3 segments: anterior, canal and posterior.
Figure 2.
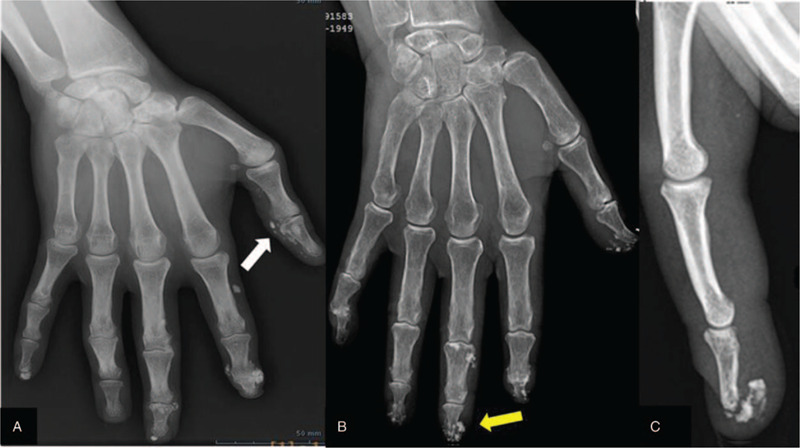
TAP bone window of axial plan with sagittal plane reconstruction (OsiriX software) presented 4 cases of different calcifications patterns for the 3 segments of the spine (yellow arrow on the sagittal section represents the level of the axial section) A: multiple intracanal calcifications extending from L2 vertebra to the middle third of the sacrum and confirmed on the axial section (A1); B: pseudo tumoral intracanal calcification (25 mm of diameter) localized between L4 and L5 vertebra with a foraminal development (yellow arrow) and right posterior inter-apophyseal joint deposits (white arrow) clearly visible on the axial section (B1); C: anterior intervertebral disc deposits (T2-T3,T3-T4, T9-T10 and T11-12) intra-spinal disc deposit (T4-T5 and T9-T10) and posterior intervertebral disc (T4-T5) calcifications. Noted on the axial section (C1) the voluminous rounded calcification located in the central part of the intervertebral disc (T9-T10). D: pseudo tumoral peri-spinous calcification and pseudo tumoral calcification of the right L5-S1 facet joint on the sagittal and axial sections.
On the 77 CT scans performed, a total of 435 calcifications was counted, 330 (75.9%) calcifications of the anterior segment with 207 (47.6%) calcifications in the anterior longitudinal ligament, 87 intervertebral disc (20%) and 36 (8.3%) in the posterior longitudinal ligament, 43 intracanal (10%) and 62 calcifications of the posterior segment (14.3%). The location of the calcifications for the 3 segments studied (anterior, canal and posterior) for each disco-vertebral unit is displayed in Figure 3 A. The distribution of the lesions in the spine is homogenous for intracanal and posterior lesions. There is a clear predominance of anterior calcic deposits in the thoracic segment and notably for T8-T9 disco-vertebral unit (Fig. 3A). An average of 6.8 calcifications per patient was calculated with a more frequently rounded pattern observed in the 3 segments of the spine. The pseudo-tumoral calcic deposits were depicted only in the intracanal and posterior segment while linear calcifications were observed only in the anterior longitudinal ligament.
Figure 3.
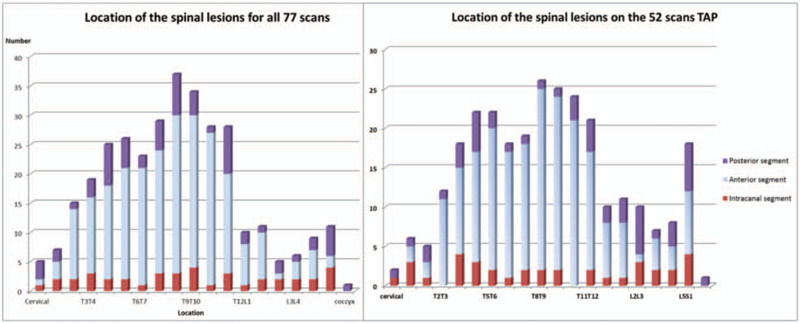
(A) Location of the spinal lesions for all 77 thoracic and TAP scans, and (B), location of the spinal lesions on the 52 TAP scans.
For the 52 patients who had a TAP CT scan, a total of 285 calcifications was counted, 201 (70.5%) calcifications in the anterior segment, 36 intracanal calcifications (12.6%) and 48 calcifications of the posterior segment (16.8%). The location of the calcifications for the 3 segments studied (anterior, canal, and posterior) for each disco-vertebral unit is displayed in Figure 3 B. The distribution of these calcifications in relation to the 3 segments was identical to that of the total population (Fig. 3).
3.4. Reliability
Classification of patients with calcifications in hands was excellent for intra-reader reliability (kappa = 0.917 P = .002 with 1/29 (3.4% of disagreement) and good for inter-reader reliability (kappa = 0717 P = 0001 with 4/29 (14% of disagreement). Classification of patients with calcifications on spine was good for intra-reader reliability (kappa = 0.72 P = .002 with 2/38 (5% of disagreement) and moderate for inter-reader reliability (kappa = 0429 P = 0064 with 4/30 (13% of disagreement).
3.5. Relationship between the presence of axial calcifications and radiographic structural lesions on hands
Periarticular calcifications on hand X-rays were significantly linked to the presence of spinal calcifications (all segments combined) (P = .012) and especially the anterior segment (P = .006). This relationship also tended to be significant for foraminal lesions (P = .056). There was no significant relationship for the other structural lesions on hands (joint space narrowing, erosions, and digital flexor retractions).
3.6. Axial and peripheral calcifications and structural lesions: SSc patients characteristics
With respect to radiographic lesions, age was one of the major clinical factors related to periarticular calcifications (P < .001), erosions (P < .009), narrowing (P < .001), and subluxation (P < .001). Calcinosis, acro-osteolysis and periarticular calcifications were significantly related to the disease duration of the systemic sclerosis (P = .002; P < .001 and P < .001) and were also statistically significantly related to the presence of digital ulcers (P = .002; P < .001, P = .014). Bosentan is prescribed more frequently in the presence of radiographic lesions such as calcinosis and acro-osteolysis (P < .001).
Age was also the major clinical factor related to all spinal calcification lesions (P < .05). Spinal calcifications were detected more frequently in patients with limited cutaneous SSc (P = .027).
3.7. Axial and peripheral calcifications and structural lesions: association with prognostic factors in SSc patients
With respect to radiographic lesions, only calcinosis was significantly related to PAH (P = .02). Periarticular calcifications tended to be linked to death (P = .056). No association was established for the other structural lesions in hands.
With respect to spinal calcifications, their presence in the posterior segment (P = .029) and in foraminal space (P = .041) was associated to interstitial lung disease. Foraminal calcifications were the only localization related to death (P = .001).
4. Discussion
A relationship between axial calcifications detected on CT scans (thoracic, TAP, lumbar) and peripheral structural disease observed on X-rays has been shown in a population of 77 patients with SSc. Axial lesions generally remained undetected, being observed at the onset of a dramatic clinical event such as a deficit due to nerve root or medullary compression, in some cases justifying emergency surgery, as was observed in one of our patients. Axial calcification is rarely described in the literature, being reported in a few cases where cervical lesions predominated (80% of cases),[15–17,19,22–27,30] followed by lumbar (15% of cases)[17,19,22] and lastly thoracic in 5% of cases only.[30]
This study was performed in a sample of SSc patients who were representative according to classically published data: diffuse interstitial lung disease was present in 29% of patients, with literature-reported prevalences between 16% and 90%, of which 15% progress to fibrosis.[3] In this study the disease had already progressed to ILD in 6.5%; PAH was observed in 19.5%, a frequency which classically ranges from 7.85% to 13%.[29,31–33]
The prevalence of DU was 50% compared with usual values of between 34% and 58%.[1,36] The radiographic characteristics were also in accord with those described in the literature for calcinosis, acro-osteolysis, and erosions.[7–9,11–13] Like Freire V et al,[37] we observed in hands radiography calcinosis and periarticular calcifications with a punctuate appearance in clusters, sometimes linear in the articular capsule and/or ligaments and in certain cases, true lobulated pseudo-tumoral calcifications. As described by Avouac et al [13] and Johnstone et al [38] we confirmed the combination of calcinosis, acro-osteolysis and periarticular calcifications with the presence of digital ulcers, which is more related to vascular disease. As suggested by Motegi and al[39] the development of spinal calcinosis may be associated with peripheral vasculopathy. With respect to erosions and joint space narrowing, these lesions were mainly observed on DIP and PIP joints whereas the MCP joints were spared, a classical finding usually observed in digital osteoarthritis and psoriatic arthritis.
In our study, axial calcifications were evaluated in the 3 spinal segments from thin axial slices (1.25 mm), enabling simultaneous multiplanar assessment. We took into account the spatial location of the calcifications in 2 contiguous slices and its presence detected in one of the other 2 planes. As for hands, we described 3 types of calcifications: pseudo tumoral forms mainly observed in the foraminal space with extension in the paraspinal space; smaller calcifications, punctuate and rounded, in intracanal and spinal disc and thin linear calcic deposits located in the ligament structures. In agreement with Ogawa et al[40] we confirmed a prevalence of 83% (64 patients) of spinal calcifications in the total population. These calcifications were significantly more frequent in the limited cutaneous form. The occurrence in order of frequency is first the anterior segment (80.5%), then the posterior segment (35.1%) and finally the intracanal sites (27.3%). In the anterior segment, the calcifications were mainly observed in the anterior longitudinal ligament with fewer in the spinal disc and even fewer in the posterior longitudinal ligament. Like Ogawa et al we found intracanal calcifications in nearly one-third of patients. Our work also precisely detected the most frequently diseased anatomical region: that is the thoracic spine at the discovertebral unit T8-T9, a predominance that was maintained when the study concerned only the 52 TAP scans. The other locations which should be viewed are the discovertebral units T9-T10 and L5-S1. Compared with the findings so far reported where the cervical and then lumbar segments are affected, our series showed that thoracic spine involvement is probably underestimated, with a prevalence of the same order of magnitude as the lumbar spine. These locations suggest a possible link between calcifications and osteoarthritis, with stress and repetitive microtraumas having a role in predisposing to the formation of these calcifications as suggested by Scharer and Smith.[41] Concerning clinical phenotype and the presence of axial calcification, we observed a relationship between digital periarticular calcifications and the presence of spinal calcifications notably in the foraminal localization and posterior segment. This relationship suggested a predisposition to a generalized process of calcification without clear pathophysiological mechanisms identified.
Concerning prognosis, our study showed for the first time that foraminal calcifications and posterior segment calcifications are related to the presence of interstitial lung disease and associated with death. Ogawa et al did not detect this relationship.[40] With respect to the other prognostic factors, calcinosis on hand radiography was significantly linked to PAH and periarticular calcifications tended to be related to death.
Calcinosis and periarticular calcifications classically correspond to deposits of calcium hydroxyapatite. In this study we were able to eliminate other aetiologies of calcification. None of our 77 patients exhibited phospho-calcium disorders. With respect to the risk of developing calcium deposits due to dyslipidaemia, 19.5% of our SSc population had dyslipidaemia, but no relationship was found with the presence of calcifications on X-ray and/or CT scan. The effect of age could not, of course, be excluded. The limits of the study are the lack of a full spinal examination (cervical, thoracic and lumbar) and the absence of any quantitative or semi-quantitative scoring system to evaluate the severity of the calcifications as described by Chung and al.[42] As the study was retrospective, it was not possible to obtain information about the characteristics of back pain disorders: acute or chronic; mechanical or inflammatory; with or without nervous compression. The sensitivity and specificity of spinal calcic deposits could not be evaluated in this study and should be determined in a large sample with a control group. Among our cases, 1 patient with a pseudotumoral calcification had justified emergency surgery for a nerve root compression with a dramatic issue.
5. Conclusion
To conclude we showed that calcifications were not limited to hands. More than 80% of systemic sclerosis patients presented spine calcifications. We reported 3 patterns of spinal calcifications linear, punctuated and rounded and pseudo tumoral with a predominance for the dorsal vertebrae. A significant association between hands and spinal calcifications were confirmed and some localization in the posterior segment considered as a bad prognostic factor. Rheumatologists should be aware of spinal calcic deposits because they were related to disease severity particularly those with foraminal development.
Author contributions
Data curation: Marine Fauny.
Footnotes
Abbreviations: ACR/EULAR = American College of Rheumatology/European League Against Rheumatism, ANA = Antinuclear antibodies, CRP = C-reactive protein, CT = computer tomography, dcSSc = diffuse cutaneous systemic sclerosis, DIP = distal interphalangeal, DLCO = diffusing capacity of the lungs for carbon monoxide DLCO, DU = digital ulcers, ESR = erythrocyte sedimentation rate, ILD = Interstitial Lung Disease, lcSSc = Limited cutaneous systemic sclerosis, LVEF = left ventricular ejection fraction, MCP = Metacarpophalangeal, NSIP = non-specific interstitial pneumonia, PAH = Pulmonary Arterial Hypertension, PAPm = Mean pulmonary artery pressure, PCWP = pre-capillary pulmonary hypertension, PIP = proximal interphalangeal, PLL = posterior longitudinal ligament, SSc = systemic sclerosis, TAP = thoracic abdominal pelvic, TRV = tricuspid regurgitant velocity, UIP = usual interstitial pneumonia.
How to cite this article: Bauer E, Fauny M, Tanguy M, Albuisson E, Mandry D, Huttin O, Chabot F, Deibener J, Chary-Valckenaere I, Loeuille D. Relationship between calcifications and structural lesions on hand radiography and axial calcifications on CT-scan: a retrospective study in systemic sclerosis. Medicine. 2020;99:43(e22443).
The authors declare that there is no conflict of interest regarding the publication of this paper.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Nitsche A. Raynaud, digital ulcers and calcinosis in scleroderma. Reumatol Clin 2012;8:270–7. [DOI] [PubMed] [Google Scholar]
- [2].Mouthon L, Rannou F, Bérezné A, et al. Patient preference disability questionnaire in systemic sclerosis: a cross-sectional survey. Arthritis Rheum 2008;59:968–73. [DOI] [PubMed] [Google Scholar]
- [3].Allanore Y, Avouac J, Kahan A. Systemic sclerosis: an update in 2008. Jt Bone Spine Rev Rhum 2008;75:650–5. [DOI] [PubMed] [Google Scholar]
- [4].Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- [5].Pope JE. Survival is improving in systemic sclerosis: true or false? Rheumatol Oxf Engl 2012;51:959–61. [DOI] [PubMed] [Google Scholar]
- [6].Pope JE. Musculoskeletal involvement in scleroderma. Rheum Dis Clin North Am 2003;29:391–408. [DOI] [PubMed] [Google Scholar]
- [7].La Montagna G, Sodano A, Capurro V, et al. The arthropathy of systemic sclerosis: a 12 month prospective clinical and imaging study. Skeletal Radiol 2005;34:35–41. [DOI] [PubMed] [Google Scholar]
- [8].Baron M, Lee P, Keystone EC. The articular manifestations of progressive systemic sclerosis (scleroderma). Ann Rheum Dis 1982;41:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Catoggio LJ, Evison G, Harkness JA, et al. The arthropathy of systemic sclerosis (scleroderma); comparison with mixed connective tissue disease. Clin Exp Rheumatol 1983;1:101–12. [PubMed] [Google Scholar]
- [10].Erre GL, Marongiu A, Fenu P, et al. The « sclerodermic hand »: a radiological and clinical study. Jt Bone Spine Rev Rhum 2008;75:426–31. [DOI] [PubMed] [Google Scholar]
- [11].Misra R, Darton K, Jewkes RF, et al. Arthritis in scleroderma. Br J Rheumatol 1995;34:831–7. [DOI] [PubMed] [Google Scholar]
- [12].Tuffanelli DL, Winkelmann RK. Systemic scleroderma, a clinical study of 727 cases. Arch Dermatol 1961;84:359–71. [DOI] [PubMed] [Google Scholar]
- [13].Avouac J, Guerini H, Wipff J, et al. Radiological hand involvement in systemic sclerosis. Ann Rheum Dis 2006;65:1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nakamura T, Hirakawa K, Takaoka H, et al. Dystrophic calcinosis with both a huge calcified mass in the cervical spine and calcification in the chest wall in a patient with rheumatoid overlap syndrome. Clin Rheumatol 2016;35:1403–9. [DOI] [PubMed] [Google Scholar]
- [15].Bluett J, Davies C, Harris J, et al. Cervical spine calcinosis in systemic sclerosis. J Rheumatol 2013;40:1617–8. [DOI] [PubMed] [Google Scholar]
- [16].Bisson-Vaivre A, Somon T, Alcaix D, et al. Cervical spinal calcinosis in a patient with systemic sclerosis. Diagn Interv Imaging 2013;94:645–7. [DOI] [PubMed] [Google Scholar]
- [17].Weerakoon A, Sharp D, Chapman J, et al. Lumbar canal spinal stenosis due to axial skeletal calcinosis and heterotopic ossification in limited cutaneous systemic sclerosis: successful spinal decompression. Rheumatol Oxf Engl 2011;50:2144–6. [DOI] [PubMed] [Google Scholar]
- [18].Shoji A, Tahara K, Hayashi H, et al. Severe headache complicated by vertical atlantoaxial subluxation in diffuse systemic sclerosis with crowned dens pattern calcification. Rheumatol Int 2011;31:1247–50. [DOI] [PubMed] [Google Scholar]
- [19].Durant C, Farge-Bancel D. Clinical images: Voluminous ectopic tumoral calcinosis of the spine in systemic sclerosis. Arthritis Rheum 2011;63:411. [DOI] [PubMed] [Google Scholar]
- [20].Bassett LW, Blocka KL, Furst DE, et al. Skeletal findings in progressive systemic sclerosis (scleroderma). AJR Am J Roentgenol 1981;136:1121–6. [DOI] [PubMed] [Google Scholar]
- [21].Olsen KM, Pike EJ, Chew FS. Progressive systemic sclerosis with massive paraspinal soft-tissue calcinosis. AJR Am J Roentgenol 2004;183:634. [DOI] [PubMed] [Google Scholar]
- [22].Ward M, Curé J, Schabel S, et al. Symptomatic spinal calcinosis in systemic sclerosis (scleroderma). Arthritis Rheum 1997;40:1892–5. [DOI] [PubMed] [Google Scholar]
- [23].Manelfe C, Catalaâ I, Sévely A. Case no.3. Diagnosis: cervical vertebral calcinosis associated with systemic scleroderma. J Radiol 1999;80:1704–6. [PubMed] [Google Scholar]
- [24].Teng AL, Robbin MR, Furey CG, et al. Tumoral calcinosis in the cervical spine in a patient with CREST syndrome. A case report. J Bone Joint Surg Am 2006;88:193–7. [DOI] [PubMed] [Google Scholar]
- [25].Bracard S, Thomas E, Braun M, et al. Cervical cord compression in scleroderma. One case. J Neuroradiol J Neuroradiol 1991;18:12–7. [PubMed] [Google Scholar]
- [26].Daumas A, Grob A, Faucher B, et al. Unusual cause of neck pain in systemic sclerosis. Rev Med Interne 2013;34:719–20. [DOI] [PubMed] [Google Scholar]
- [27].Lima IVS, Galrão LA, Maia TSL, et al. Spinal cord compression by ectopic calcinosis in scleroderma. Clin Exp Rheumatol 2005;23:704–6. [PubMed] [Google Scholar]
- [28].Sambataro D, Sambataro G, Zaccara E, et al. Tumoral calcinosis of the spine in the course of systemic sclerosis: report of a new case and review of the literature. Clin Exp Rheumatol 2015;33:S175–8. [PubMed] [Google Scholar]
- [29].Liberato ACP, Amaral LLFD, Marussi VHR. Tumoral calcinosis in the lumbar spine secondary to systemic sclerosis: a rare cause of radiculopathy in an adult with advanced disease. BJR Case Rep 2016;2:20150435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ogawa T, Ogura T, Hayashi N, et al. Tumoral calcinosis of thoracic spine associated with systemic sclerosis. J Rheumatol 2009;36:2552–3. [DOI] [PubMed] [Google Scholar]
- [31].Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- [32].Arginteanu MS, Perin NI. Paraspinal calcinosis associated with progressive systemic sclerosis. Case report. J Neurosurg 1997;87:761–3. [DOI] [PubMed] [Google Scholar]
- [33].Hachulla E, Gressin V, Guillevin L, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 2005;52:3792–800. [DOI] [PubMed] [Google Scholar]
- [34].Elhai M, Avouac J, Kahan A, et al. Systemic sclerosis: recent insights. Jt Bone Spine Rev Rhum 2015;82:148–53. [DOI] [PubMed] [Google Scholar]
- [35].Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis 2003;62:1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mihai C, Landewé R, van der Heijde D, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis 2016;75:681–6. [DOI] [PubMed] [Google Scholar]
- [37].Freire V, Bazeli R, Elhai M, et al. Hand and wrist involvement in systemic sclerosis: US features. Radiology 2013;269:824–30. [DOI] [PubMed] [Google Scholar]
- [38].Johnstone EM, Hutchinson CE, Vail A, et al. Acro-osteolysis in systemic sclerosis is associated with digital ischaemia and severe calcinosis. Rheumatol Oxf Engl 2012;51:2234–8. [DOI] [PubMed] [Google Scholar]
- [39].Motegi S-I, Sekiguchi A, Yonemoto Y, et al. Demographic and clinical characteristics of spinal calcinosis in systemic sclerosis: Possible association with peripheral angiopathy. J Dermatol 2019;46:33–6. [DOI] [PubMed] [Google Scholar]
- [40].Ogawa T, Ogura T, Ogawa K, et al. Paraspinal and intraspinal calcinosis: frequent complications in patients with systemic sclerosis. Ann Rheum Dis 2009;68:1655–6. [DOI] [PubMed] [Google Scholar]
- [41].Scharer L, Smith DW. Resorption of the terminal phalanges in scleroderma. Arthritis Rheum 1969;12:51–63. [DOI] [PubMed] [Google Scholar]
- [42].Chung L, Valenzuela A, Fiorentino D, et al. Validation of a novel radiographic scoring system for calcinosis affecting the hands of patients with systemic sclerosis. Arthritis Care Res 2015;67:425–30. [DOI] [PubMed] [Google Scholar]


