Abstract
Although sedation for bronchoscopy improves patient comfort, there is a risk of oversedation in elderly patients. Only a few studies have evaluated the efficacy and safety of sedation for bronchoscopy in elderly patients.
This study retrospectively analyzed records of 210 patients who underwent transbronchial brushing and/or biopsy under midazolam sedation at National Hospital Organization Omuta National Hospital between June 2017 and October 2019. Patients were administered 1 mg midazolam following 10 mL 4% lidocaine inhalation. When sedation was insufficient, 0.5 mg midazolam was administered additionally. Diagnostic yield, incidence of complications, amount of oxygen supplementation, decreases in percutaneous oxygen saturation (SpO2), changes in blood pressure, and degree of comfort were analyzed.
Patients were divided into the elderly (n = 102) and non-elderly (n = 108) groups. No significant differences were observed in diagnostic yield and procedure time between the 2 groups, and no severe adverse events were noted in the elderly group. The degree of comfort during bronchoscopy was significantly higher in the elderly group. In patients administered < 2 mg midazolam, the amount of oxygen supplementation and decreases in SpO2 were significantly smaller in the elderly group compared to the non-elderly group.
The risk of adverse events related to midazolam sedation in bronchoscopy does not increase with age, and sedation improves comfort during flexible bronchoscopy in elderly patients. Moreover, a total dose of midazolam <2 mg is safe for elderly patients undergoing bronchoscopy.
Keywords: bronchoscopy, complication, elderly patient, midazolam, sedation
1. Introduction
Flexible bronchoscopy is frequently performed when assessing, diagnosing, and treating patients with respiratory disease. While advances in modalities such as radial endobronchial ultrasound and navigation bronchoscopy have improved diagnostic yield and the ability to reach target lesions in the peripheral lung, bronchoscopy is generally an uncomfortable procedure.
Sedation improves patient comfort and tolerance during bronchoscopy, and increases the willingness to undergo a repeat procedure.[1,2] The use of topical anesthesia, analgesia, and sedation during bronchoscopy varies among physicians, institutions, and geographic locations. In Japan, a 2016 national survey revealed that intravenous sedatives/anesthetics are routinely administered at 49% of all facilities.[3] The British Thoracic Society guidelines recommend sedating patients undergoing bronchoscopic examination, unless contraindicated.[4] Yet, some studies have suggested that sedation should remain an option and be provided according to patient preference and comorbidities, as unsedated bronchoscopy may be tolerable in some cases.[5,6] When delivering sedatives, bronchoscopists should carefully titrate doses to avoid oversedation. Dose adjustment is recommended in patients with advanced age and liver cirrhosis, as benzodiazepines are metabolized more slowly in these patients.[7]
Only a limited number of studies have evaluated the efficacy and safety of sedation for bronchoscopy in elderly patients. Regarding the safety of bronchoscopy, some studies have reported that older patients tolerate the procedure well with no increase in complications,[8,9] whereas others found higher complication rates and mortality rates in those aged ≥80 years compared to young patients.[10] Although dose adjustment is important in elderly patients, no study to date has determined a safe and feasible midazolam dose for bronchoscopy in elderly patients. To this end, the present retrospective study aimed to determine an optimal midazolam dose for bronchoscopy in elderly patients based on changes in respiratory parameters.
2. Methods
2.1. Subjects
This study retrospectively analyzed records of 210 elderly and non-elderly patients with suspected lung cancer who underwent transbronchial brushing and/or biopsy under sedation and local anesthesia at National Hospital Organization Omuta National Hospital between June 2017 and October 2019. Exclusion criteria were respiratory failure with percutaneous oxygen saturation (SpO2) <90% (room air at rest); history of severe drug allergy to midazolam; history of myocardial infarction within 6 weeks; presence of a proximal airway lesion (located proximal to the segmental bronchus); and incomplete clinical data. Informed consent was obtained from each patient prior to bronchoscopy. This study was approved by the ethics committee of National Hospital Organization Omuta National Hospital.
2.2. Procedure, sedation, and monitoring
Patients were administered 1.0 mg midazolam following 10 mL 4% lidocaine inhalation. When sedation was insufficient, additional midazolam was administered (0.5 mg at a time) until sufficient sedation was achieved. The desired depth of sedation was ‘conscious’ sedation, where the patient remains in verbal contact. Flexible bronchoscopy was performed using BF-260 and BF-P290 videobronchoscopes (Olympus Corporation, Tokyo, Japan) after confirming sedation. In all patients, SpO2 and electrocardiogram (ECG) were continuously monitored, and intermittent blood pressure was measured. Oxygen inhalation was provided to patients with significant desaturation (SpO2 <90%). Oxygen supplementation was titrated to achieve SpO2 >90%. Following diagnostic bronchoscopy, a chest x-ray was obtained in each patient in order to check for the occurrence of pneumothorax.
Diagnostic yield, incidence of complications, amount of oxygen supplementation, decreases in SpO2, changes in blood pressure, and degree of comfort in elderly patients (≥75 years) and non-elderly patients (<75 years) were analyzed. Non-diagnostic bronchoscopic findings were validated by video-assisted thoracoscopic surgery, computed tomography (CT)-guided needle biopsy, or thoracentesis. When a patient refused to undergo these procedures, radiological follow-up was performed. The diagnostic yield was evaluated based on the final pathological diagnosis. Patients were asked to rate the degree of comfort using a questionnaire sheet provided after the bronchoscopic examination (5, great; 4, good; 3, normal; 2, uncomfortable; and 1, very uncomfortable) and the willingness to undergo re-examination (yes/no).
The following complications were examined: pneumothorax, hemorrhage (blood loss of ≥300 mL, or need for blood transfusion), pulmonary infections (pneumonia and/or pleuritis), bronchial asthma, respiratory failure (excluding those treated with oxygen administration alone), lidocaine intoxication, falls, and complications related to the circulatory system and requiring special treatment (e.g., heart failure, myocardial infarction, arrhythmia, and cerebral infarction).
2.3. Factors associated with complications
Some factors, including patient characteristics, are known to influence the risk of complications.[4] Moreover, dose adjustment is recommended in patients with advanced age and liver cirrhosis, as benzodiazepines are metabolized more slowly in these patients.[7] Accordingly, comorbidities, blood pressure, respiratory status before bronchoscopy, and dose of midazolam were considered potential confounders in this study.
2.4. Statistical analysis
The t test was used to compare normally distributed continuous variables between groups. The Wilcoxon signed-rank test was used to compare nominal variables or ordinal variables. Statistical analysis was performed using the JMP version 15.0 (SAS Institute, Cary, NC). P < .05 was considered statistically significant.
3. Results
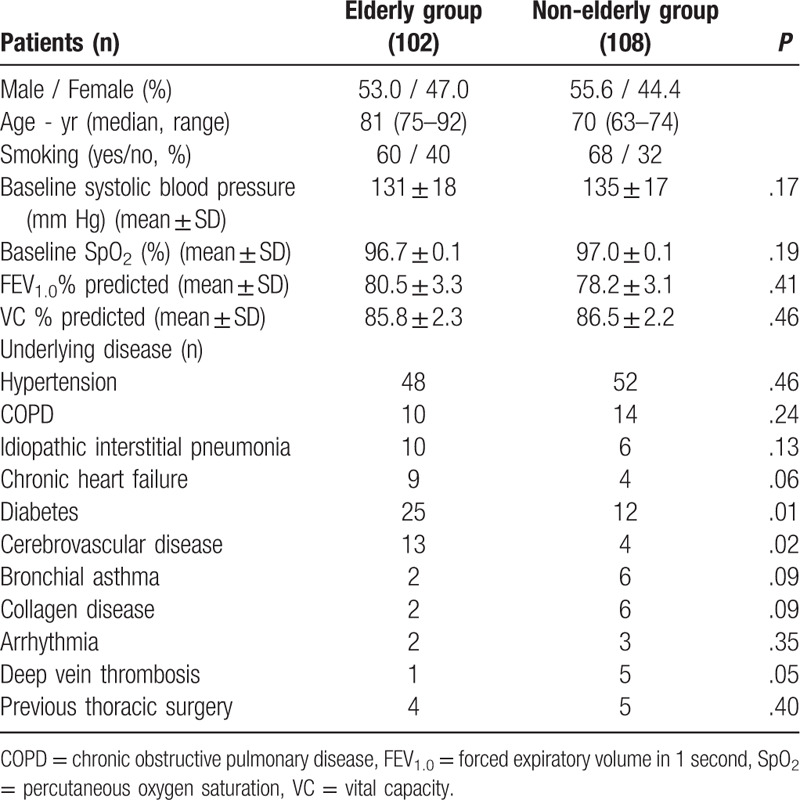
From June 2017 to October 2019, a total of 243 patients underwent bronchoscopy under local anesthesia and sedation. Twenty-four patients were excluded due to proximal airway lesions or respiratory failure. The elderly group comprised 102 patients aged ≥75 years, and the non-elderly group comprised 108 patients aged < 75 years (Fig. 1). Patient characteristics are summarized in Table 1. Median age was 81 years in the elderly group and 70 years in the non-elderly group. Baseline respiratory conditions were similar between the 2 groups. SpO2 values were 96.7 ± 0.1% and 97.0 ± 0.1% in the elderly and non-elderly groups, respectively. The percent predicted forced expiratory volume in 1 second and percent predicted vital capacity were 80.5 ± 3.3% and 85.8 ± 2.3%, respectively, in the elderly group, and 78.2 ± 3.1% and 86.5 ± 2.2%, respectively, in the non-elderly group. Underlying diseases were more common in the elderly group than in the non-elderly group.
Figure 1.
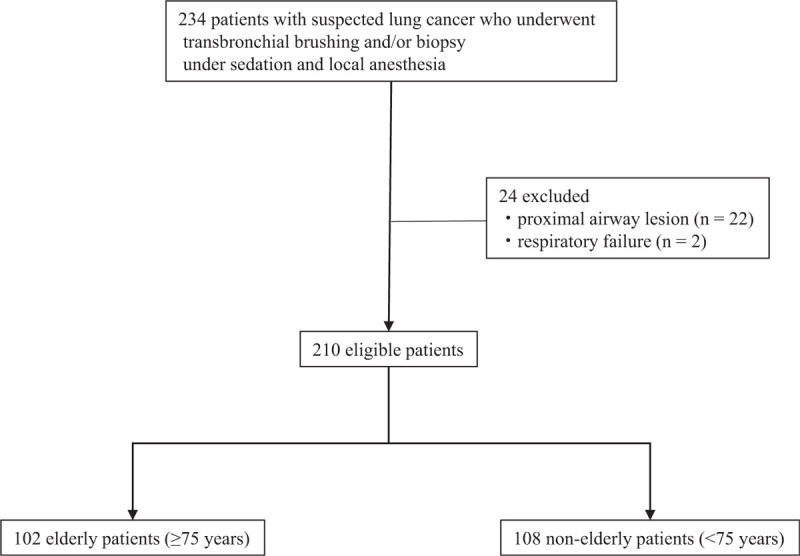
Study flow diagram.
Table 1.
Patient characteristics.
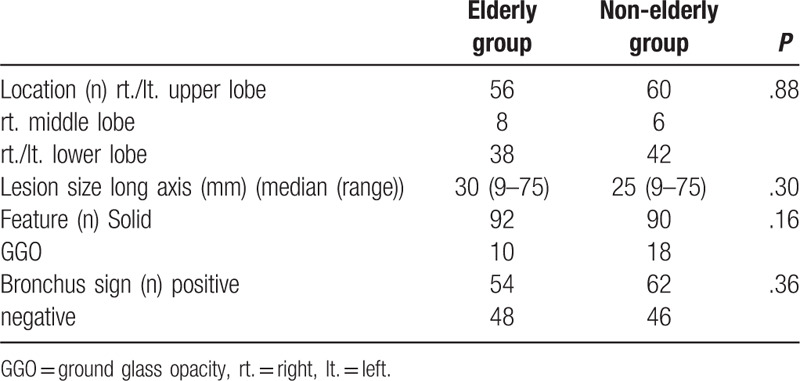
The characteristics of lesions are provided in Table 2. No significant differences were observed between the elderly and non-elderly groups in terms of location, size, structure, and bronchus sign. The most common site of lesions was the upper lobe, followed by the lower lobe, in both groups. With respect to CT appearance in the elderly group, 92 lesions were solid, 10 were ground glass opacities (GGOs), and 54 had a positive bronchus sign, with a median size of 30 mm (range, 9 – 75 mm). In the non-elderly group, 90 lesions were solid, 18 were GGOs, and 62 had a positive bronchus sign, with a median size of 25 mm (range, 9 – 75 mm).
Table 2.
Lesion characteristics.
Diagnoses of pulmonary lesions are provided in Table 3. In the elderly group, 60 (69.0%) patients had adenocarcinoma, 10 (11.5%) had squamous cell carcinoma, 12 (13.8%) had non-small cell lung carcinoma not otherwise specified, 1 (1.1%) had small cell carcinoma, 2 (2.3%) had organizing pneumonia, and 2 (2.3%) had lymphoma. In the non-elderly group, 62 (66.7%) patients had adenocarcinoma, 10 (10.8%) had squamous cell carcinoma, 10 (10.8%) had non-small cell lung carcinoma not otherwise specified, 1 (1.1%) had small cell carcinoma, 6 (6.5%) had organizing pneumonia, 2 (2.2%) had lymphoma, and 2 (2.2%) had nontuberculous mycobacteriosis. Diagnostic yields were similar between the 2 groups, at 69.0% and 66.7% in the elderly and non-elderly groups, respectively.
Table 3.
Diagnoses of pulmonary lesions.
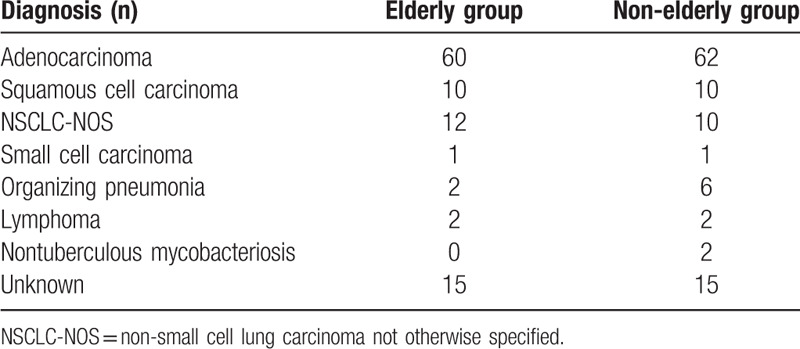
Table 4 provides the vital parameters and complications for the 2 groups. No significant differences were observed between the elderly and non-elderly groups in terms of procedure times, changes in systolic blood pressure, and maximum rate–pressure product. Both elderly and non-elderly patients recovered cognitive ability within a few minutes after bronchoscopy. None of the patients experienced falls within 24 hours post-bronchoscopy. None of the patients in the elderly group developed complications, whereas 1 patient in the non-elderly group developed pneumothorax. The dose of midazolam was lower in the elderly group (1.3 ± 0.4 mg) compared to the non-elderly group (1.7 ± 0.5 mg, P = .03). None of the patients were administered an antagonist to recover from sedation. Mean lowest SpO2 during bronchoscopy was 91.3 ± 0.3% in the elderly group and 89.4 ± 0.6% in the non-elderly group (P = .02). The decrease in SpO2 was greater in the non-elderly group (7.6 ± 0.6%) than in the elderly group (5.4 ± 0.3%, P = .01). The required amount of oxygen was also greater in the non-elderly group (2.3 ± 0.2 L/min) than in the elderly group (1.7 ± 0.1 L/minP = .03). In patients administered ≥2 mg midazolam, minimum SpO2, decrease in SpO2, and oxygen supplementation did not significantly differ between the elderly and non-elderly groups (Fig. 2).
Table 4.
Vital parameters and complications.

Figure 2.
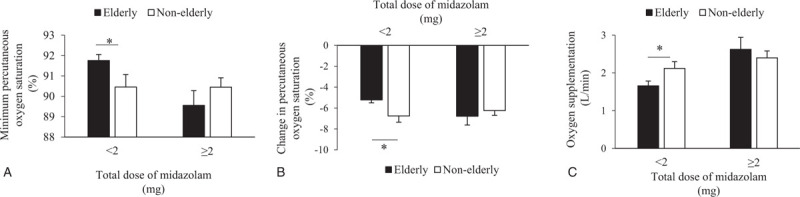
Changes in respiratory condition by dose of midazolam. ∗: P < .05.
Comfort was rated significantly higher by patients in the elderly group (3.6 ± 0.1) than those in the non-elderly group (3.2 ± 0.1, P = .02) (Fig. 3A). The willingness to undergo re-examination was similar between the 2 groups (85% and 94% in the elderly and non-elderly groups, respectively; P = .13) (Fig. 3B).
Figure 3.
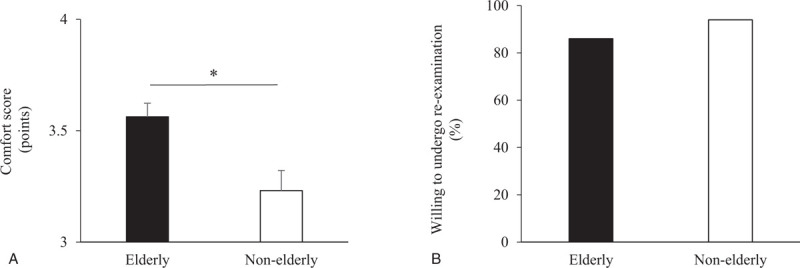
Comfort and willingness to undergo re-examination. ∗: P < .05.
4. Discussion
The present study retrospectively analyzed the efficacy and safety of bronchoscopy performed under sedation and local anesthesia in elderly patients. Diagnostic yields and the willingness to undergo re-examination by bronchoscopy performed under sedation were similar between the elderly and non-elderly groups, while the degree of comfort was higher in the elderly group than non-elderly group. These results suggest that bronchoscopy under sedation in elderly patients is as effective as it is in non-elderly patients. Moreover, although complication rates were similar between the elderly and non-elderly groups, the decrease in SpO2 and oxygen supplementation during bronchoscopy were lower in elderly patients than in non-elderly patients. These findings suggest that bronchoscopy under sedation in elderly patients is safer than in non-elderly patients.
Intravenous midazolam is a preferred agent for sedation due to its rapid onset of action and relatively short duration of effect. Patients with advanced age and impaired liver function are more prone to adverse effects, possibly due to slower benzodiazepine metabolism. Korttila et al[11] reported that patients aged ≥60 years had an extended duration of impaired coordination. In addition, the average serum benzodiazepine concentration 2 hours after sedation was reportedly 2-fold higher in patients aged ≥70 years than in those aged <40 years. These findings suggest that elderly patients are more likely to require lower doses of sedatives for bronchoscopy.[4] In fact, in the present study, the required dose of midazolam to achieve sufficient sedation was lower in the elderly group than in the non-elderly group. In addition, elderly patients did not experience cognitive impairment or falls, suggesting that using a low dose of midazolam reduces the risk of falls and cognitive impairment after bronchoscopy under sedation in elderly patients. Previous studies and guidelines stated that the initial dose of midazolam during bronchoscopy should be lower in elderly patients than in non-elderly patients and that additional administrations should be given in small doses.[4,7,12] But there was no mention of the recommended total midazolam dose for elderly patients during bronchoscopy. Ulasli et al reported that patients aged ≥65 years showed a higher incidence of respiratory depression than in those aged <65 years despite initial dose of midazolam was 1 mg during bronchscopy.[13] McLaughlin et al reported that hypoxemia occurred more frequently in octogenarians despite receiving lower doses of midazolam compared to younger patients.[14] In the present study, however, the decrease in SpO2 was lower in the elderly group than in the non-elderly group. The total dose of midazolam administered to elderly patients was lower in the present study (1.3 ± 0.4 mg) compared to the above-mentioned study (2.0 ± 0.9 mg),[14] and this difference might reflect different respiratory conditions in elderly patients. In patients administered ≥2 mg midazolam in the present study, minimum SpO2 was slightly lower in the elderly group (89.6 ± 0.7%) than in the non-elderly group (90.4 ± 0.5%) (not significant, p = .31), whereas in those administered <2 mg midazolam, minimum SpO2 was significantly lower in the non-elderly group (90.5 ± 0.6%) than in the elderly group (91.8 ± 0.3%). These findings suggest that a total dose of midazolam <2 mg is suitable for elderly patients undergoing bronchoscopy.
Many factors influence the risk of complications, including patient characteristics.[4] The rate–pressure product (heart rate × systolic blood pressure) during bronchoscopy can approach or exceed the level associated with silent myocardial ischemia, particularly in patients with hypertension.[15] Increases in systolic blood pressure and heart rate during bronchoscopy are associated with ECG changes in 15% of patients.[16] In addition, ECG changes are correlated with older age and a higher pack-year smoking history, but not with lung function or initial oxygen saturation.[16] In the present study, smoking history was similar in the 2 groups, and patients with hypertension were common in both groups (roughly 50%). Moreover, the rate–pressure product during bronchoscopy did not significantly differ between groups. While diabetes and cerebrovascular disease were more frequently observed in elderly patients than in non-elderly patients, none of the elderly patients developed severe complications defined in the protocol. These findings suggest that complication rates do not necessarily increase in patients with advanced age.
Several factors affect the diagnostic yield of bronchoscopy for peripheral pulmonary lesions. Rivera et al[17] reported a sensitivity of 34% for peripheral lesions <2 cm in diameter, while that for peripheral lesions >2 cm in diameter was 63%. A bronchus sign on CT refers to an air-filled bronchus leading to a peripheral pulmonary lesion.[18] Ali et al[19] reported an odds ratio of 3.4 (95% confidence interval, 2.4 – 5.0) for successfully diagnosing a peripheral pulmonary lesion with a bronchus sign. The present study found no significant differences in lesions between the elderly and non-elderly groups in terms of location, size, structure, and bronchus sign. The similarities in radiologic findings might explain the similar diagnostic yield and examination time in the 2 groups.
The willingness to undergo re-examination was a relevant factor in the present study, since patients undergoing bronchoscopy for oncological diagnostic purposes are often required to undergo a repeat procedure to confirm the diagnosis, examine gene mutations, and inspect new lesions. The characteristics, previous healthcare experience, and expectations of patients, as well as the type of procedure and post-procedure care, all play a role in determining the degree of patient satisfaction with bronchoscopy. Lechtzin et al[20] reported that patients who are more willing to return for a repeat bronchoscopy had a better general health status, experienced less pain with bronchoscope insertion, and were provided with better quality information about the procedure. Hirose et al[21] reported that male sex, shorter examination time, and less discomfort from coughing, pharyngeal pain, and swallowing were associated with higher patient satisfaction.
In the present study, the procedure time was similar in elderly and non-elderly groups, with elderly patients experiencing less discomfort with bronchoscopy than non-elderly patients. The high rate of willingness to undergo re-examination in both elderly and non-elderly groups suggests that sedation for bronchoscopy contributes to patient comfort regardless of age.
This study has some limitations. First, since the study was conducted at a single institution using a retrospective design, the findings are not generalizable. Second, patients with respiratory failure were excluded from the study. Consequently, all patients had normal or mild impairment of respiratory function, and thus, the present findings may apply to patients with near normal respiratory function who do not require oxygen supplementation before bronchoscopy.
5. Conclusions
The risk of adverse events related to midazolam sedation in flexible bronchoscopy did not increase with age, and sedation for bronchoscopy improved patient comfort to a higher degree in elderly patients than in non-elderly patients. Moreover, bronchoscopy under sedation with <2 mg midazolam was safer in elderly patients than in non-elderly patients. It is a novel finding that a total dose of midazolam <2 mg is suitable for elderly patients undergoing bronchoscopy. The present study will assist clinicians when adjusting midazolam dosage for elderly patients undergoing bronchoscopy. Although respiratory function declines with age, the present study did not analyze patients with respiratory failure. Further studies to assess the efficacy and safety of sedation for bronchoscopy in this patient population are warranted.
Author contributions
Conceptualization: Naotaka Noda.
Data curation: Naotaka Noda.
Formal analysis: Naotaka Noda.
Investigation: Naotaka Noda.
Methodology: Naotaka Noda.
Project administration: Naotaka Noda.
Resources: Naotaka Noda, Makiko Hara, Shinji Ise, Mizuko Ose, Miyoko Tatsuta, Aiko Nagaoka.
Supervision: Miiru Izumi, Kentaro Wakamatsu, Masayuki Kawasaki.
Validation: Naotaka Noda.
Visualization: Naotaka Noda.
Writing – original draft: Naotaka Noda.
Writing – review & editing: Naotaka Noda.
Footnotes
Abbreviations: CT = computed tomography, ECG = electrocardiogram, NSCLC-NOS = non-small cell lung carcinoma not otherwise specified, SpO2 = percutaneous oxygen saturation.
How to cite this article: Noda N, Hara M, Ise S, Ose M, Tatsuta M, Nagaoka A, Izumi M, Wakamatsu K, Kawasaki M. Comfort and safety of bronchoscopy performed under sedation and local anesthesia in elderly patients. Medicine. 2020;99:43(e22561).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files.
References
- [1].Putinati S, Ballerin L, Corbetta L, et al. Patient satisfaction with conscious sedation for bronchoscopy. Chest 1999;115:1437–40. [DOI] [PubMed] [Google Scholar]
- [2].Maltais F, Laberge F, Laviolette M. A randomized, double-blind, placebo-controlled study of lorazepam as premedication for bronchoscopy. Chest 1996;109:1195–8. DOI:10.1378/chest.109.5.1195. [DOI] [PubMed] [Google Scholar]
- [3].Horinouchi H, Asano F, Okubo K, et al. Current status of diagnostic and therapeutic bronchoscopy in Japan: 2016 national survey of bronchoscopy. Respir Investig 2019;57:238–44. [DOI] [PubMed] [Google Scholar]
- [4].Du Rand IA, Blaikley J, Booton R, et al. British thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68: Suppl 1: i1–44. [DOI] [PubMed] [Google Scholar]
- [5].Morris LG, Zeitler DM, Amin MR. Unsedated flexible fiberoptic bronchoscopy in the resident clinic: technique and patient satisfaction. Laryngoscope 2007;117:1159–62. [DOI] [PubMed] [Google Scholar]
- [6].Hatton MQ, Allen MB, Vathenen AS, et al. Does sedation help in fibreoptic bronchoscopy? BMJ 1994;309:1206–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wahidi MM, Jain P, Jantz M, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest 2011;140:1342–50. [DOI] [PubMed] [Google Scholar]
- [8].O’Hickey S, Hilton AM. Fibreoptic bronchoscopy in the elderly. Age Ageing 1987;16:229–33. [DOI] [PubMed] [Google Scholar]
- [9].Knox AJ, Mascie-Taylor BH, Page RL. Fibreoptic bronchoscopy in the elderly: 4years’ experience. Br J Dis Chest 1988;82:290–3. [DOI] [PubMed] [Google Scholar]
- [10].Rokach A, Fridlender ZG, Arish N, et al. Bronchoscopy in octogenarians. Age Ageing 2008;37:710–3. [DOI] [PubMed] [Google Scholar]
- [11].Korttila K, Saarnivaara L, Tarkkanen J, et al. Effect of age on amnesia and sedation induced by flunitrazepam during local anaesthesia for bronchoscopy. Br J Anaesth 1978;50:1211–8. [DOI] [PubMed] [Google Scholar]
- [12].Ricardo J, José, Shahzad Shaefi, et al. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev 2013;22:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sevinc Sarinc Ulasli, Ersin Gunay, Olcay Akar, et al. Diagnostic utility of flexible bronchoscopy in elderly patients. Clin Respir J 2014;8:357–63. [DOI] [PubMed] [Google Scholar]
- [14].McLaughlin CW, Skabelund AJ, Easterling ER, et al. The safety and utility of fiberoptic bronchoscopy in the very elderly. J Bronchology Interv Pulmonol 2018;25:300–4. [DOI] [PubMed] [Google Scholar]
- [15].Ouellette DR, Diaz J. Elevation of the double product during flexible bronchoscopy: effects of uncontrolled hypertension and the use of beta-blockade. J Bronchology 2008;15:73–7. [Google Scholar]
- [16].Davies L, Mister R, Spence DP, et al. Cardiovascular consequences of fibreoptic bronchoscopy. Eur Respir J 1997;10:695–8. [PubMed] [Google Scholar]
- [17].Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S–165S. [DOI] [PubMed] [Google Scholar]
- [18].Gaeta M, Pandolfo I, Volta S, et al. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR 1991;157:1181–5. [DOI] [PubMed] [Google Scholar]
- [19].Ali MS, Sethi J, Taneja A, et al. Computed Tomography Bronchus Sign and the Diagnostic Yield of Guided Bronchoscopy for Peripheral Pulmonary Lesions. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2018;15:978–87. [DOI] [PubMed] [Google Scholar]
- [20].Lechtzin N, Rubin HR, White P, Jr, et al. Patient satisfaction with bronchoscopy. Am J Respir Crit Care Med 2002;166:1326–31. [DOI] [PubMed] [Google Scholar]
- [21].Hirose T, Okuda K, Ishida H, et al. Patient satisfaction with sedation for flexible bronchoscopy. Respirology 2008;13:722–7. [DOI] [PubMed] [Google Scholar]


