Abstract
The ratio of triglyceride (TG)/high-density lipoprotein cholesterol (HDL-C) has been proposed as an easily obtainable atherogenic marker and high TG/HDL-C ratio is associated with insulin resistance. This study investigated the associated between a high TG/HDL-C ratio and cardiovascular mortality in patients with ST-segment elevation myocardial infarction (STEMI), with or without diabetes mellitus (DM).
Between January 2005 and December 2014, 1661 patients with STEMI underwent primary percutaneous coronary intervention in our hospital. Of these, 289 were classified into group 1 (with both DM and a high TG/HDL-C ratio), 295 into group 2 (with DM, but without a high TG/HDL-C ratio), 501 into group 3 (without DM, but a high TG/HDL-C ratio), and 576 into group 4 (without DM or a high TG/HDL-C ratio).
Older age, longer chest pain to reperfusion time, poor hemodynamic condition, and higher prevalence of multiple vessel coronary artery disease were noted in those with DM. Poor outcomes including higher 30-day and 1-year cardiovascular mortality and all-cause mortality rates were noted in those with DM but without a high TG/HDL-C ratio. Patients with DM but without a high TG/HDL-C ratio had a Hazard ratio of 3.637 for cardiovascular mortality relative to those without DM, but without a high TG/HDL-C ratio.
Even though a high TG/HDL-C ratio is associated with insulin resistance, patients with or without DM, but with a high TG/HDL-C ratio had better 30-day and 1-year outcomes.
Keywords: cardiovascular mortality, diabetes mellitus, high triglyceride/high-density lipoprotein cholesterol ratio, insulin resistance, ST-segment elevation myocardial infarction
1. Introduction
Cardiovascular disease (CVD) is the most common cause of death and primary source of disease burden in developing and developed countries.[1] Dyslipidemia is a prominent risk factor for CVD.[2] Lowering low-density lipoprotein cholesterol (LDL-C) with a statin is important in both primary and secondary intervention settings.[3] On the other hand, increased high-density lipoprotein cholesterol (HDL-C) is associated with a decrease in CVD, with an effect predominantly observed in patients with low HDL-C.[4] Although the role of high LDL-C and low HDL-C in CVD development has been widely accepted, the role of hypertriglyceridemia remains controversial. As serum triglyceride (TG) levels are inversely correlated with in-hospital death and adverse late outcomes in patients with ST-segment elevation myocardial infarction (STEMI), a high serum TG level can be regarded as benign and not a target for aggressive therapy.[5]
The TG/HDL-C ratio has been proposed as an easily obtainable atherogenic marker[6] and has been proposed as a predictor of insulin resistance.[7] A high TG/HDL-C ratio is also correlated with LDL phenotype B,[8] and small HDL particles,[9] and is also associated with increased arterial stiffness and impaired heart rate recovery after exercise.[10] In children, the TG/HDL-C ratio was found to be positively associated with systolic and diastolic blood pressure and metabolic syndrome.[11] The cut-off for the TG/HDL-C ratio varied among different ethnic groups and was reported to be 3.0 for non-Hispanic whites and Mexican Americans and 2.0 for non-Hispanic blacks.[12] Cardiometabolic risk factors were more adverse in men and women whose TG/HDL-C ratio exceeded 3.5 and 2.5, respectively.[13] In the acute phase of myocardial infarction (MI), the relationship between the TG/HDL-C ratio and cardiovascular outcomes is controversial. One study in patients with acute MI reported that a low TG level was associated with high in-hospital mortality. Another study in patients with acute coronary syndrome reported that a high TG/HDL ratio was a powerful independent predictor of all-cause mortality and a risk factor for cardiovascular events. Due to this gap in knowledge, the present study aimed to explore the relationship between the TG/HDL-C ratio and STEMI, and to determine the influence of insulin resistance in such patients.
2. Materials and methods
2.1. Patients and groups
Between January 2005 and December 2014, 1661 patients with STEMI underwent primary percutaneous coronary intervention (PCI) in our hospital and were enrolled in the STEMI registry. A TG/HDL-C ratio higher than 3.5 in men and 2.5 in women was defined a high ratio 13. All patients underwent a fasting lipid profile within the first 3 days of hospitalization. Of these 1661 patients, 289 were classified into group 1 (with both DM and a high TG/HDL-C ratio), 295 into group 2 (with DM, but without a high TG/HDL-C ratio), 501 into group 3 (without DM, but with a high TG/HDL-C ratio), and 576 into group 4 (without DM or a high TG/HDL-C ratio). The baseline characteristics and cardiovascular mortality were compared among the 4 groups. The Institutional Review Committee on Human Research at our institution approved the study protocol.
2.2. Definitions
Our MI criteria were in accordance with the most recent universal definitions.[14] Advanced heart failure (HF) was graded as greater than III, according to the New York Heart Association Classification. Based on the Kidney Disease Improving Global Outcomes definition, acute kidney injury (AKI) was defined as an absolute increase in serum creatinine of at least 0.3 mg/dL within 48 hours or a 50% increase in serum creatinine from baseline within 7 days, or a urine volume of less than 0.5 mL/kg/h for at least 6 hours.[15] Target vessel revascularization (TVR) was defined as any repeat PCI or coronary artery bypass graft for lesions with stenosis ≧70%, and a target vessel was defined as the entire major coronary vessel proximal and distal to the target lesion, including upstream and downstream branches and the target lesion itself.[16] Cardiovascular mortality was defined as death related to an MI, cardiac arrhythmia, or HF. All-cause mortality was defined as death from any cause. A major adverse cardiac event (MACE) included an MI, TVR, and cardiovascular mortality.
2.3. Study endpoints
The primary endpoints of our study were recurrent MI, TVR, and cardiovascular mortality during the 30-day and 1-year follow-up period. The secondary endpoints were all-cause mortality, regardless of cause, during the 30-day and 1-year follow-up period.
2.4. Statistical analysis
Data were expressed as the mean ± standard deviation for continuous variables, or as counts and percentages for categorical variables. Continuous variables were compared using an independent t test or the Mann-Whitney U test. Categorical variables were compared using a chi-square statistic. Multivariate Cox regression analysis included a hazard ratio (HR) < 0.100 for 1-year cardiovascular mortality in univariate Cox regression analyses. Multivariate Cox regression analyses on 1-year cardiovascular mortality were performed to determine the HR between groups. The patient without DM, but a high TG/HDL-C ratio was set as an HR of 1. A Kaplan-Meier curve was calculated for 1-year cardiovascular mortality in all groups. All statistical analyses were performed using SPSS 22.0 (IBM. Corp., Armonk. NY, USA). A P-value <.05 was statistically significant.
3. Results
In STEMI patients, significantly higher TG/HDL-C ratio was noted in patients with DM (DM vs non-DM; 4.08 ± 3.65 vs 3.62 ± 2.88; P = .012).
3.1. Baseline characteristics of study groups
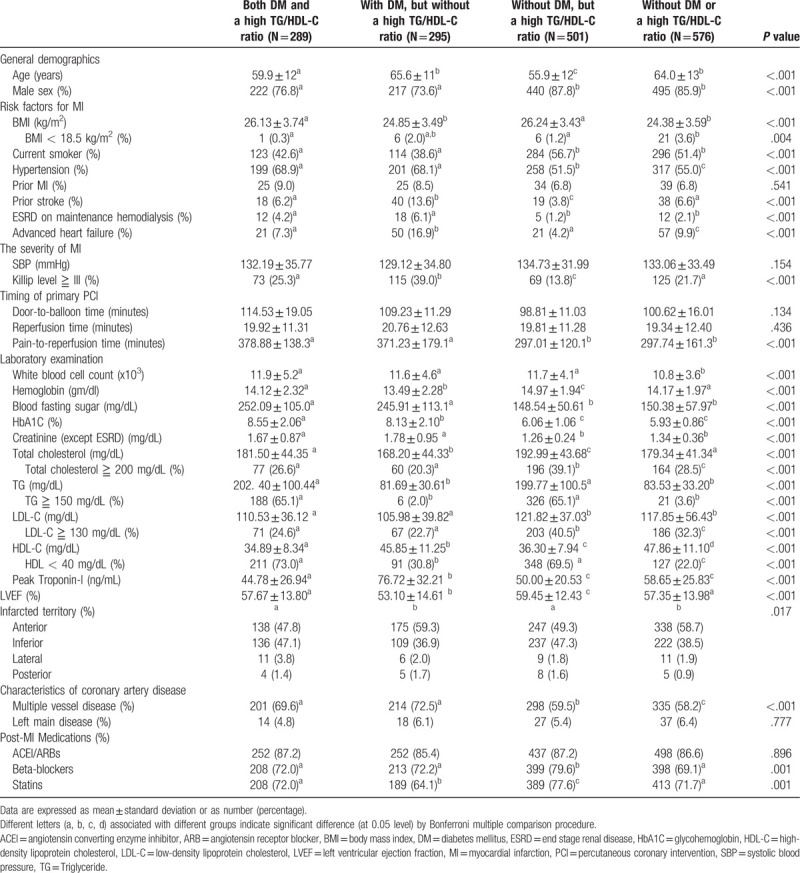
Baseline characteristics of the 4 groups are listed in Table 1. The average age of groups with a high TG/HDL-C ratio was greater than that of groups without a high TG/HDL-C ratio. The prevalence of males was greater in groups without DM. The comorbidities in the 4 groups showed significant differences, except for the prevalence of prior MI. The prevalence of Killip class ≥III was greater in those with DM, but without a high TG/HDL-C ratio. Longer chest pain-to-reperfusion time was observed in those with DM. Laboratory results showed significant differences between the 4 groups. Lower levels of total cholesterol and LDL-C were noted in those with DM patients, but without a high TG/HDL-C ratio. Higher HDL-C levels were noted in groups without a high TG/HDL-C ratio. Higher peak troponin-I levels and a worse left ventricular ejection fraction were noted in patients with DM, but without a high TG/HDL-C ratio. Higher prevalence of anterior wall infarction was noted in groups without a high TG/HDL-C ratio. Higher prevalence of multiple vessel disease was noted in patients with DM, but without a high TG/HDL-C ratio. More intensive guideline-based treatment was applied in patients without DM, but with a high TG/HDL-C ratio.
Table 1.
Baseline characteristics of DM patients with or without a high TG/HDL-C ratio and non-DM patients with or without a high TG/HDL-C ratio.
3.2. Angiographic characteristics of study groups
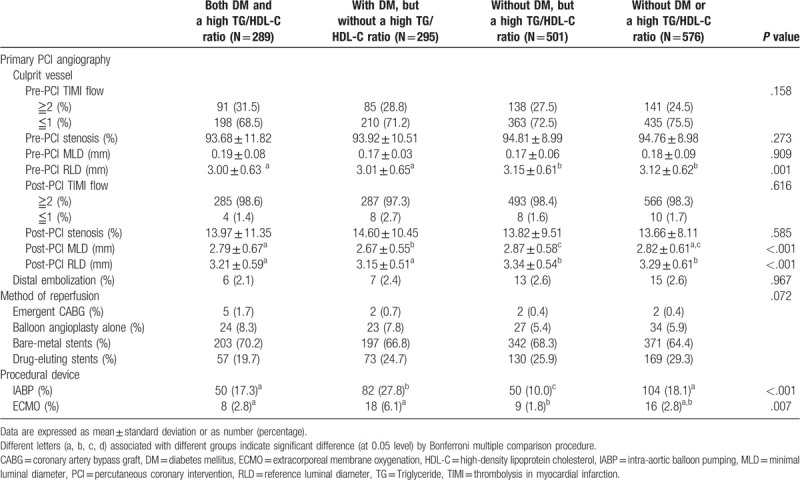
Angiographic characteristics of the 4 groups are listed in Table 2. Larger pre-PCI reference luminal diameter (RLD) was noted in groups without DM. Smaller post-PCI minimal luminal diameter (MLD) was noted those with DM, but without a high TG/HDL-C ratio. Larger post-PCI RLD was noted in groups without DM. The method of reperfusion showed no significant difference among the 4 groups. Those with DM, but without a high TG/HDL-C ratio, had higher prevalence of mechanical support.
Table 2.
Angiographic characteristics of DM patients with or without a high TG/HDL-C ratio and non-DM patients with or without a high TG/HDL-C ratio.
3.3. Thirty-day and one-year clinical outcomes
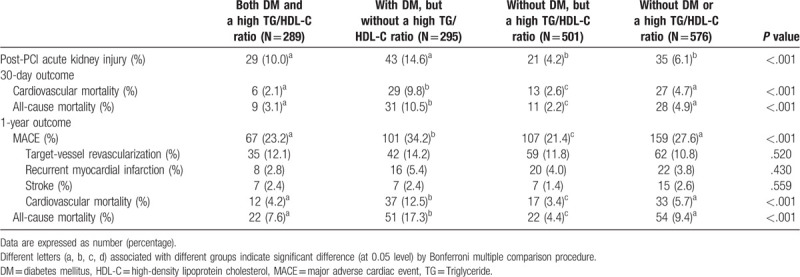
Clinical outcomes are listed in Table 3. A higher incidence of post-PCI AKI was noted in those with DM. A higher incidence of 30-day cardiovascular mortality and all-cause mortality was noted in those with DM, but without a high TG/HDL-C ratio. Higher 1-year MACE, cardiovascular mortality, and all-cause mortality rates were noted in those with DM, but without a high TG/HDL-C ratio.
Table 3.
Clinical outcomes of DM patients with or without a high TG/HDL-C ratio and non-DM patients with or without a high TG/HDL-C ratio.
The Kaplan-Meier curve for 1-year cardiovascular mortality showed the poorest result in those with DM, but without a high TG/HDL-C ratio (log rank P < .001) (Fig. 1).
Figure 1.
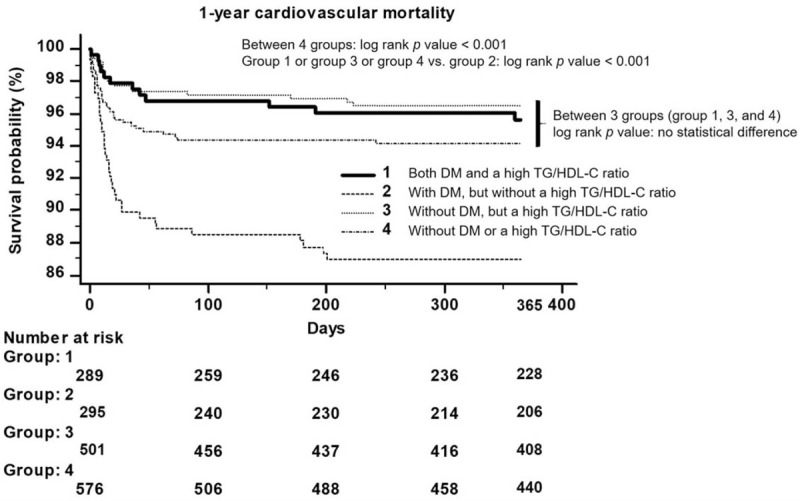
Kaplan-Meier curve for 1-year cardiovascular mortality: In ST-segment elevation myocardial infarction, patients with diabetes mellitus, but without a high triglyceride/high-density lipoprotein cholesterol ratio, had a worse clinical outcome than the other 3 groups (log rank P < .001).
3.4. Multivariate Cox regression analysis for 1-year cardiovascular mortality
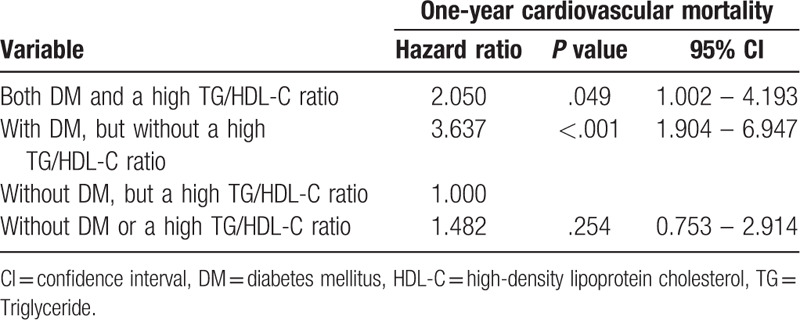
Multivariate Cox regression for 1-year cardiovascular mortality in the 4 groups is shown in Table 4. Those without DM, but a high TG/HDL-C ratio, were set as an HR of 1.000. Those with DM, but with a high TG/HDL-C ratio, had an HR of 2.050 (P = .049; 95% confidence interval [CI] = 1.002–4.193). Those with DM, but without a high TG/HDL-C ratio had an HR of 3.637 (P < .001; 95% CI = 1.904–6.947). Those without DM patients or a high TG/HDL-C ratio had an HR of 1.482 (P = .254; 95% CI = 0.753–2.914).
Table 4.
Hazard ratio of 1-year cardiovascular mortality in DM patients with or without a high TG/HDL-C ratio when comparing with non-DM patients with or without a high TG/HDL-C ratio.
4. Discussion
Dyslipidemia is defined as elevated plasma concentration of lipid (TG, total cholesterol, and LDL-C) and as decreased plasma concentration of HDL-C.[17] Strong scientific evidence indicates that there is a significant association between the incidence of CVD and high levels of LDL-C and low levels of HDL-C.[4,18] In addition, high levels of TG are associated with an increase in LDL-C particles and increased cardiovascular risk.[19] Many clinical studies have attempted to identify a marker of atherogenic dyslipidemia that can better predict the risk of CVD, and the atherogenic dyslipidemia index reflects the balance between atherogenic and antiatherogenic factors.[20–22] The TG/HDL-C ratio is a strong predictor of the risk of atherosclerosis and CVD,[23] as well as insulin resistance.[7,13] However, few studies have focused on the effect of the TG/HDL-C ratio in STEMI. In addition, no studies have focused on the effect of the TG/HDL-C ratio in patients with and without DM.
In patients with acute MI, two-thirds had low HDL-C levels, greater than three-fourths had total cholesterol level below 200 mg/dL, and greater than three-fourths had LDL-C level below 130 mg/dL.[24] After STEMI, low HDL-C was associated with significantly higher risk of in-hospital mortality.[25] However, serum TG levels were inversely correlated with in-hospital mortality and adverse late outcomes in patients with STEMI treated with primary PCI; thus a high serum TG level can be a benign factor.[5] This lipid paradox was noted in STEMI patients, and high TG may not be a treatment target in the acute phase.[26] However, 1 study showed that a high TG/HDL-C ratio may be independently associated with MACEs in female revascularized STEMI patients, but not in male patients.[27] Nicholls et al also found lowering the TG/HDL-C ratio is associated with a beneficial impact on the progression of coronary atherosclerosis in DM patients for long-term care.[28] In our study, patients with a low TG/HDL-C ratio, with and without DM, had worse outcomes and those without DM, but with a high TG/HDL-C ratio had better outcomes than other groups. Therefore, the value of TG/HDL-C presented the lipid paradox in the acute phase.
Lipids may be crucial for cell survival during the acute phase of AMI in the presence of life-threatening ischemia and a fulminant reactive inflammatory response, when cells and cell membranes are vulnerable.[29] Physiologically, LDL-C is a critical component of cell and hormones, and an LDL-C level < 30 mg/dL is reportedly associated with an increase in psychiatric and hepatobiliary disorders.[30] Another large population study also revealed that patients with non-STEMI, but with a history of hypercholesterolemia had lower in-hospital mortality.[31] This lipid paradox has also been found in the elderly, as well as those with rheumatoid arthritis, or HF, or stroke.[31–35] The lipid paradox was also observed in those with AMI who were also at high risk of malnutrition.[36] Aggressive lipid-lowering therapy is still recommended for patients with AMI, but improvement of suboptimal nutritional status may be more beneficial than strict LDL-C control when treating patients at high risk of malnutrition.[35]
In one previous study, a high mortality rate (17.8% at 1-year follow-up period) was noted in patients with DM and STEMI.[37] In our study, those with DM, but without a high TG/HDL-C ratio had the worst clinical outcomes (one-year CV mortality: 12.5%; and one-year all-cause mortality: 17.3%). On the other hand, STEMI patients with a low TG/HDL ratio both with and without DM, had worse outcomes. However, based on current dyslipidemia guidelines, appropriate intensity of statin therapy should be used to reduce high atherosclerotic cardiovascular risk, especially in patients with prior MI.[3] In the acute phase, lipids still have a critical role in cell membrane synthesis and cell survival. In addition, the lipid profile reflects nutritional status. Even though a high TG/HDL ratio indicates insulin resistance, an inverse effect is observed in STEMI patients with or without DM. Thyroid functions also play an important impact on the TG level and TG/HDL ratio.[37] In acute illness including STEMI, most patients may present abnormal thyroid function and difficultly interpret.[38,39] Therefore, the relationship between thyroid profile and lipid profile in STEMI patients may need a large cohort study to explore.
As a limitation, this was a retrospective study from a single medical center. However, the current strategy for lipid control has only focused on lowering the serum LDL-C level and has not considered nutritional status and acuity of illness. In our STEMI registry, the thyroid profile was not regularly checked, so we could not provide this relationship between TG/HDL-C ratio and thyroid profile. Our study demonstrated that a high TG/HDL-C ratio was not a treatment target in the acute phase of MI in patients with or without DM. TG/HDL-C ratio is an atherogenic marker that correlated to insulin resistance, blood pressure, and metabolic syndrome. This value also presented the lipid paradox in the STEMI population during the acute phase.
5. Conclusions
Even though a high TG/HDL-C ratio is associated with insulin resistance, patients with or without DM, but with a high TG/HDL-C ratio, had better 30-day and 1-year outcomes.
Author contributions
Conceptualization: Huang-Chung Chen.
Data curation: Huang-Chung Chen, Wei-Chieh Lee.
Formal analysis: Wei-Chieh Lee.
Investigation: Wei-Chieh Lee.
Methodology: Wei-Chieh Lee.
Project administration: Wei-Chieh Lee.
Resources: Wei-Chieh Lee, Hsiu-Yu Fang, Chih-Yuan Fang, Chien-Jen Chen, Cheng-Hsu Yang, Chiung-Jen Wu.
Software: Wei-Chieh Lee.
Writing – original draft: Wei-Chieh Lee.
Writing – review & editing: Wei-Chieh Lee.
Footnotes
Abbreviations: AKI = acute kidney injury, CI = confidence interval, CVD = cardiovascular disease, DM = diabetes mellitus, HDL-C = high-density lipoprotein cholesterol (HDL-C), HF = heart failure, HR = hazard ratio, LDL-C = low-density lipoprotein cholesterol, MACE = major adverse cardiac event (MACE), MI = myocardial infarction, MLD = minimal luminal diameter, PCI = percutaneous coronary intervention, RLD = reference luminal diameter, STEMI = ST-segment elevation myocardial infarction, TG = triglyceride, TVR = target vessel revascularization.
How to cite this article: Chen HC, Lee WC, Fang HY, Fang CY, Chen CJ, Yang CH, Wu CJ. Impact of high triglyceride/high-density lipoprotein cholesterol ratio (insulin resistance) in ST-segment elevation myocardial infarction. Medicine. 2020;99:43(e22848).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].A Gaziano A Gaziano, Bitton A, Anand S, et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 2010;35:72–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- [3].Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. circulation 2019;139:e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mahdy Ali K, Wonnerth A, Huber K, et al. Cardiovascular disease risk reduction by raising HDL cholesterol – current therapies and future opportunities. Br J Pharmacol 2012;167:1177–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng YT, Liu TJ, Lai HC, et al. Lower serum triglyceride level is a risk factor for in-hospital and late major adverse events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention- a cohort study. BMC Cardiovasc Disord 2014;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vera Bittner B, Johnson D, Zineh I, et al. The TG/HDL cholesterol ratio predicts all cause mortality in women with suspected myocardial ischemia: a report from the women's ischemia syndrome evaluation (WISE). Am Heart J 2009;157:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Linthout S, Spillmann F, Schultheiss HP, et al. High-density lipoprotein at the interface of type 2 diabetes mellitus and cardiovascular disorders. Curr Pharm Des 2010;16:1504–16. [DOI] [PubMed] [Google Scholar]
- [8].Hanak V, Munoz J, Teague J, et al. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol 2004;94:219–22. [DOI] [PubMed] [Google Scholar]
- [9].Jia L, Long S, Fu M, et al. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism 2006;55:1141–8. [DOI] [PubMed] [Google Scholar]
- [10].Urbina EM, Khoury PR, Coy EC, et al. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics 2013;131:e1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quijada Z, Paoli M, Zerpa Y, et al. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes 2008;9:464–71. [DOI] [PubMed] [Google Scholar]
- [12].Li C, Ford ES, Meng YX, et al. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol 2008;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC, et al. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res 2013;54:2795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618–51. [DOI] [PubMed] [Google Scholar]
- [15].Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. [DOI] [PubMed] [Google Scholar]
- [16].Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. [DOI] [PubMed] [Google Scholar]
- [17].Fodor G. Primary prevention of CVD: treating dyslipidaemia. BMJ Clin Evid 2008;6:2008. [PMC free article] [PubMed] [Google Scholar]
- [18].Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL-cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep 2012;14:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guérin M, Le Goff W, Lassel TS, et al. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol 2001;21:282–8. [DOI] [PubMed] [Google Scholar]
- [20].Nwagha UI, Igweh JC. Atherogenic Index of Plasma: a significant indicator for the onset of Atherosclerosis during menopause in hypertensive females of South East Nigeria. J Coll Med 2005;10:67–71. [Google Scholar]
- [21].Nwagha UI, Ikekpeazu EJ, Ejezie FE, et al. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci 2010;10:248–52. [PMC free article] [PubMed] [Google Scholar]
- [22].Mudhaffar SK. Atherogenic index of plasma (AIP) as a parameter in predicting cardiovascular risk in males compared to the conventional dyslipidemic indices (Cholesterol Ratios) karbala. J Med 2013;6:1506–13. [Google Scholar]
- [23].Dobiásová M. AIP--atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek 2006;52:64–71. [PubMed] [Google Scholar]
- [24].González-Pacheco H, Vargas-Barrón J, Vallejo M, et al. Prevalence of conventional risk factors and lipid profiles in patients with acute coronary syndrome and significant coronary disease. Ther Clin Risk Manag 2014;10:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ji MS, Jeong MH, Ahn YK, et al. Impact of low level of high-density lipoprotein-cholesterol sampled in overnight fasting state on the clinical outcomes in patients with acute myocardial infarction (difference between ST-segment and non-ST-segment-elevation myocardial infarction). J Cardiol 2015;65:63–70. [DOI] [PubMed] [Google Scholar]
- [26].Cheng KH, Chu CS, Lin TH, et al. Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit Care Med 2015;43:1255–64. [DOI] [PubMed] [Google Scholar]
- [27].Wan GX, Xia WB, Ji LH, et al. Triglyceride to high density lipoprotein cholesterol ratio may serve as a useful predictor of major adverse coronary event in female revascularized ST-elevation myocardial infarction. Clin Chim Acta 2018;485:166–72. [DOI] [PubMed] [Google Scholar]
- [28].Nicholls SJ, Tuzcu EM, Wolski K, et al. Lowering the triglyceride/high-density lipoprotein cholesterol ratio is associated with the beneficial impact of pioglitazone on progression of coronary atherosclerosis in diabetic patients: insights from the PERISCOPE (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) study. J Am Coll Cardiol 2011;57:153–9. [DOI] [PubMed] [Google Scholar]
- [29].Stancu CS, Toma L, Sima AV. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res 2012;349:433–46. [DOI] [PubMed] [Google Scholar]
- [30].Everett BM, Mora S, Glynn RJ, et al. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dl) with rosuvastatin 20 mg daily (from JUPITER). Am J Cardiol 2014;114:1682–9. [DOI] [PubMed] [Google Scholar]
- [31].Wang TY, Newby LK, Chen AY, et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin Cardiol 2009;32:E22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schatz IJ, Masaki K, Yano K, et al. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet 2001;358:351–5. [DOI] [PubMed] [Google Scholar]
- [33].Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 2011;70:482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Güder G1 Frantz S, Bauersachs J, Allolio B, et al. Reverse epidemiology in systolic and nonsystolic heart failure: cumulative prognostic benefit of classical cardiovascular risk factors. Circ Heart Fail 2009;2:563–71. [DOI] [PubMed] [Google Scholar]
- [35].Markaki I, Nilsson U, Kostulas K, et al. High cholesterol levels are associated with improved long-term survival after acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:e47–53. [DOI] [PubMed] [Google Scholar]
- [36].Lu YW, Lu SF, Chou RH, et al. Lipid paradox in patients with acute myocardial infarction: Potential impact of malnutrition. Clin Nutr 2019;38:2311–8. [DOI] [PubMed] [Google Scholar]
- [37].Qin K, Zhang F, Wu Q, et al. Thyroid hormone changes in euthyroid patients with diabetes. Diabetes Metab Syndr Obes 2020;13:2533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lakdasa D. Premawardhana. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb) 2017;27:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hafe MV, Neves JS, Vale C, et al. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr Connect 2019;8:R76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


