Abstract
Using Kendall's coefficient of concordance (KCC-) and Coherence (Cohe-) regional homogeneity (ReHo) to explore the alterations of brain local functional connectivity in acute and remitting relapsing-remitting multiple sclerosis (RRMS), and its clinical relevance.
18 acute RRMS, 26 remitting RRMS and 20 healthy controls received resting-state functional magnetic resonance imaging scanning. After data preprocessing and ReHo (KCC-ReHo and Cohe-ReHo) calculation, analysis of variance and followed post hoc analysis was used to compare the KCC-ReHo or Cohe ReHo maps across groups.
After analysis of variance analysis, regions with significant among-group differences detected by the 2 ReHo analysis were overlapped, these overlapped regions located in the left superior frontal gyrus (SFG), right SFG, left cuneus and right middle occipital gyrus (P < .01, Gaussian random field theory correction). Followed post hoc tests showed that, compared with healthy controls,
-
(1)
increased ReHo in left cuneus and right middle occipital gyrus only detected in acute RRMS not in remitting RRMS;
-
(2)
acute and remitting RRMS both demonstrated decreased ReHo in the SFG and medial frontal gyrus (MeFG).
Except for the overlapped location, the regions location of altered ReHo detected by the 2 ReHo analysis also present difference. Further, correlation analysis showed disease duration was negatively correlated with the KCC-ReHo (when K = 7, 19) of left SFG (r = -0.633, P = .006; r = -0.620, P = .008), left MeFG (r = -0.608, P = .010; r = -0.555, P = .022), the KCC-ReHo (when K = 27) of left and right SFG (r = -0.551, P = .022; r = -0.603, Ρ = .010), and the Cohe-ReHo of right MeFG (r = -0.608, P = .010) in acute RRMS; meanwhile, the KCC-ReHo (when K = 7, 19) of right SFG (r = -0.590, P = .013; r = -0.599, P = .011) was negatively related to expanded disability status scale scores in the acute RRMS.
Both acute and remitting RRMS patients has disease-related brain dysfunction, interestingly, relative to remitting RRMS, the acute RRMS patients mobilized more brain regions involving visual information processing in an attempt to maintain functional stability. In addition, our results also provide a methodological consideration for future ReHo analysis.
Keywords: acute relapsing-remitting multiple sclerosis, Kendall's coefficient of concordance- and Coherence- regional homogeneity, Regional Homogeneity, remitting relapsing-remitting multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease with characterized by extensive and diffuse inflammatory demyelination in the central nervous,[1] relapsing-remitting multiple sclerosis (RRMS) is the most common type. The clinical symptoms of MS are complex and varied, the common symptoms including the cognitive deterioration, visual and sensorimotor impairment.[2–4] Meanwhile, higher order visual processing and perception is 1 of the most frequently affected cognitive characteristics in MS,[5] even in patients without severe cognitive deterioration or significant visual problems.[6,7] It is widely believed that the neurocognitive and visual information processing problems were caused by diffuse brain lesions. Conventional magnetic resonance imaging (MRI) currently serves as an important tool to detect the MS-related lesions, however the ability to predict clinical status is low and difficultly show the gray matter lesions.[8]
With the developing of MRI, multimodal MRI studies have capability to reveal abnormalities that can’t be detected by conventional MRI in normal-appearing white matter, cortex, and deep gray matter nuclei at the earliest stages of MS.[9] These advanced MRI technologies play a leading role in the diagnosing and assessing MS, contributing to our further understanding of the underlying the abnormal functional and structural alter that cause the neurological symptoms of MS.[10,11]
Recently, resting-state functional MRI (rs-fMRI) has become a best-studied tool for measuring the intrinsic dynamics of the human brain of physiological and pathological conditions.[12] In rs-fMRI, functional connectivity is widely used to reflect the neuronal intrinsic activity level in functional communication between regions. Previous studies had shown the MS patients’ impairment of the integrity in inter-network coupling and intra-network functional connectivity in the salience network,[13] default mode network,[14,15] sensorimotor network,[16,17] and visual network.[18] Another study[19] revealed that different immune pathogenic processes occur as the disease stage changes, which may lead to different cognitive impairment. Moreover, a previous rs-fMRI study[20] observed the several regions with significant reductions in gray matter density in MS patients with acute lesion compared to the MS patients with chronic lesion. Together these studies revealed the changes in MS at different stages from pathology and imaging perspectives.
Nevertheless, previous most studies of MS focused on the impairment in the remission phase of RRMS, it's little know whether the local property of intrinsic brain activity is different between acute phase and remitting phase of RRMS, and how spontaneous brain activity evolves from acute RRMS to Remitting RRMS.
Regional homogeneity (ReHo) is a sensitive method to measure the local functional connectivity of a given voxel and its neighboring voxels in different conditions.[21] This method is more reliable in test–retest analysis and less affected by global nuisances compared with other method, such as amplitude of low-frequency fluctuation.[22] Therefore, we hypothesize that the local property of intrinsic brain activity of MS in 2 disease stage (acute and remitting phase) would be different, and this discrepancy would explain the different clinical manifestation varying from different disease status. In order to confirm this hypothesis, first, 2 ReHo methods for rs-fMRI based on Kendall's coefficient of concordance (KCC) and coherence (Cohe), proposed by Zang et al[23] and Liu et al[21] respectively, were used to explore the differences of regional spontaneous activity in the whole brain among the healthy controls (HCs), acute RRMS and remitting RRMS group. Another reason to use both parameters is that Cohe-ReHo is superior to KCC-ReHo,[21] but Cohe-ReHo has not been exploring the neurological mechanism of MS. Secondly, a post hoc analysis was performed to compare the ReHo index between each paired of groups. Finally, associations between ReHo and clinical variables were evaluated. This study may enrich our understanding of the possible imaging mechanisms that MS patients with different disease states accompany by different clinical manifestations.
2. Materials and methods
This study was approved by the Medical Research Ethics Committee and the Institutional Review Board of the First Affiliated Hospital of Nanchang University. All participants provided an informed consent form.
2.1. Participants
We recruited 44 patients with clinically definite MS at the First Affiliated Hospital of Nanchang University according to 2017 revision of the McDonald's criteria.[24] Subsequently, we divided the MS patient group into acute phase and remission phase. The acute MS patients mainly met the several criteria: the first acute attack or functional impairment, lasting at least 24 hours. The remitting MS patients met the criteria:
-
(1)
the Expanded Disability Status Scale (EDSS) score < 2.5, which reflecting a relatively minimally disabled stage[25];
-
(2)
had not experienced a relapse and no disease-modifying medications (eg, corticosteroids or immune suppressants) treatment in the 3 months preceding the MRI measurement.
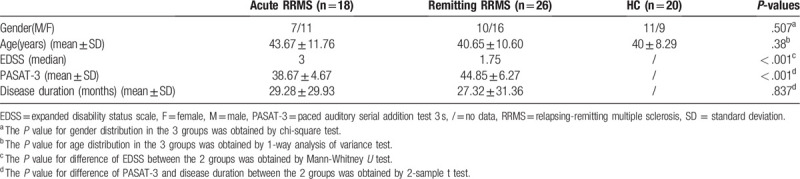
Neurological or psychiatric symptoms not attributable to MS were defined as exclusion criteria. Well-matched (age and gender) HC were enrolled from the local community. Healthy subjects had no history of neurological or psychiatric disease. Finally, a total of 18 acute RRMS, 26 remitting RRMS and 20 HC participated in the study (Table 1). Both acute and remitting RRMS underwent clinical assessments, which included EDSS and the Paced Auditory Serial Addition Test 3 s (PASAT-3).
Table 1.
Demographics and clinical characteristics of healthy controls and RRMS patients.
2.2. MR imaging acquisition
A Trio 3.0-Tesla Siemens scanner (Siemens, Munich, Germany) was used to gather the MRI data. All subjects were instructed to lay down on the bench with foam pads, wear earplugs and put their heads to the head coil. During this process, they were asked to keep their eyes closed and awake. Each participant obtained the 3D-T1-weighted images, rs-fMRI images and T2-weighted images using the following sequences:
-
(1)
High-resolution 3D-T1-weighted images: repetition time (TR)/echo time (TE) = 1900 ms/2.26 ms, field of view (FOV) = 215 mm × 230 mm, matrix = 240 × 256, thickness/gap = 1.0/0 mm and 176 sagittal slices,
-
(2)
rs-fMRI scanning using an echo planar imaging sequence with the following parameters: TR/TE = 2000/30 ms, matrix = 64 × 64, FOV = 210 × 210 mm, 30 interleaved axial slices with a slice thickness of 4 mm, slice thickness = 1.2 mm, and 240 time points which lasted about 8 min.
-
(3)
T2-weighted turbo spin-echo imaging: TR/TE = 5100/117 ms, number of excitations = 3, echo train length = 11, matrix = 416 × 416, FOV = 240 × 240 mm, slice = 22, slice thickness = 6.5 mm, orientation = axial.
2.3. Data preprocessing
Rs-fMRI data preprocessing was conducted using the Data Processing and Analysis of Brain Imaging (DPABI v4.2, http://rfmri.org/dpabi) toolbox, running on the MATLAB platform (The MathWorks Inc., Natick, MA). The main preprocessing steps included: the first ten volumes were discarded for signal equilibrium and subjects’ adaptation, the remaining 230 time points were left for further analysis. Then slice timing, head realignment. After head motion correction, the rs-fMRI data were spatial normalized to standard Montreal Neurological Institute template along with resampling to 3 × 3 × 3 mm3 isotropic voxels. During scanning, if the head motion of the paticipant was over 2.0 mm maximum translation in any direction (x,y,z) or over 1.0 degree of maximum rotation about 3 axes, the subjects would be excluded. Meanwhile, linear detrending, nuisance linear regression with the 6 head movement parameters, the white matter and the cerebrospinal fluid signals and temporal bandpass filtering (0.01–0.08 HZ) were conducted on fMRI data.
2.4. ReHo analysis
(1) The KCC-ReHo Analysis: the procedures for KCC-ReHo analysis were performed by DPABI using the rs-fMRI data. KCC was calculated to measure the local synchronization of a given voxel to its nearest neighbor voxels (6, 18, 26) in a voxel-wise way with the Eq. (1).[23]
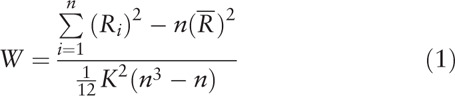 |
where W is the KCC for a given voxel, ranging from 0 to 1, a higher ReHo index correlates to greater similarity between the local activity of a given voxel and that of its neighbors; Ri is the sum rank of ith time point; 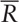 = ((n+1)K)/2 represent the mean for Ris; K is the number of voxels (1 center voxel plus the number of its neighbors) within measured cluster, which represent different neighboring states between voxels. Voxels are adjacent to each other in 3 ways: vertices, edges or faces, that is, the number of voxels around the center voxel may be 26, 18 or 6, so K = 27,19 or 7. Different neighboring states between voxels may result in different time series in the minimum volume unit for calculating ReHo, hence, in consideration of the effect of different K values on ReHo results, in our study, we select 3 values of K (K = 7, 19, 27) to generated different ReHo maps; n is the number of ranks (here n = 240 time points). The calculated KCC value was assigned to the center voxel of this cluster.
= ((n+1)K)/2 represent the mean for Ris; K is the number of voxels (1 center voxel plus the number of its neighbors) within measured cluster, which represent different neighboring states between voxels. Voxels are adjacent to each other in 3 ways: vertices, edges or faces, that is, the number of voxels around the center voxel may be 26, 18 or 6, so K = 27,19 or 7. Different neighboring states between voxels may result in different time series in the minimum volume unit for calculating ReHo, hence, in consideration of the effect of different K values on ReHo results, in our study, we select 3 values of K (K = 7, 19, 27) to generated different ReHo maps; n is the number of ranks (here n = 240 time points). The calculated KCC value was assigned to the center voxel of this cluster.
(2) The Cohe-ReHo Analysis: the data analysis was performed using the Resting-State fMRI Data Analysis Toolkit plus V1.2 (REST plus V1.2, http://restfmri.net/forum/RESTplusV1.2), and the specific process of algorithm for calculating Cohe-ReHo was described in the previous study.[21] In brief, included 3 following steps. Firstly, Welch's modified periodogram averaging methods were utilized to estimate the power spectrums and cross spectrum for any 2 times series in a given cluster. Secondly, the coherence of the 2 times series above-mentioned across low-frequency (0.01–0.08 Hz) band with their band-averaged estimates of the cross spectrum and power spectrums was evaluated. Finally, the given cluster's Cohe-ReHo was calculated by averaging coherence coefficient of the cluster.
Through calculating the KCC-ReHo and Cohe-ReHo value of every voxel in the whole brain, an individual KCC-ReHo and Cohe-ReHo map was obtained for each subject in a voxel-wise way. Then, the standardized KCC-ReHo and Cohe-ReHo maps were generated via Fisher's r-to-z standardization within a whole brain mask. Finally, the spatial smoothing was performed using a 6-mm full-width at half maximum Gaussian filter on the standardization KCC-ReHo and Cohe-ReHo maps.
2.5. Statistical analysis
For each KCC- or Cohe-ReHo map, 1-way analysis of variance (ANOVA) was conducted to explore the group differences across the 3 groups using statistical analysis of DPABI base on SPM12. The resultant F value map was corrected using Gaussian random field theory (2-tailed, voxel-wise P < .01, cluster-level P < .05). If different result was presented, the regions showed significant among-group differences at ANOVA were considered as regions of interest (ROIs). Additionally, the KCC- and Cohe-ReHo values were extracted from these ROIs within each subject. Subsequently, a post hoc test (P < .001, Bonferroni correction) of these quantitative KCC- and Cohe-ReHo values was performed using SPSS 13.0 (SPSS Inc., Chicago, IL) to investigate the differences between paired subgroups (acute RRMS vs HCs, remitting RRMS vs HCs and acute RRMS vs remitting RRMS).
To examine the clinical correlation, we conducted a partial correlation analysis with age and gender as covariates (P < .05) to evaluate the relationship between ReHo value (KCC-ReHo or CoHe-ReHo value) extracted from the regions with significant group differences and clinical variables (EDSS, PASAT-3 and disease duration) of acute and remitting RRMS by using SPSS software.
3. Results
3.1. Demographic and clinical data
Demographics and clinical data for MS patients and healthy subjects were summarized in Table 1. There were no significant differences in gender (P = .507) and age (P = .38) among the 3 groups. The EDSS and PASAT-3 scores between the acute RRMS and remitting RRMS showed significant difference (P < .01). The difference of the disease duration between the 2 patient groups didn’t reach the statistical significance (P = .837).
3.2. KCC-ReHo or Cohe-ReHo differences among the 3 group
ANOVA was used to determine the regions in which the KCC-ReHo or Cohe-ReHo index was significantly altered among the acute RRMS, remitting RRMS and HCs. Regions with significant differences among the 3 groups detected by the 2 ReHo analysis were overlapped (Table 2 and Fig. 1A-D), these overlapped regions located in: the left superior frontal gyrus (SFG), the right SFG, the left cuneus and the right middle occipital gyrus (MOG). The inconsistently altered region detected by 2 ReHo methods are detailed in the Table 2 (P < .01, Gaussian random field correction).
Table 2.
Regions with significant differences in regional homogeneity among the acute RRMS patients, remitting RRMS and healthy controls groups (voxel-level P < .01, GRF correction, cluster-level P < .05).
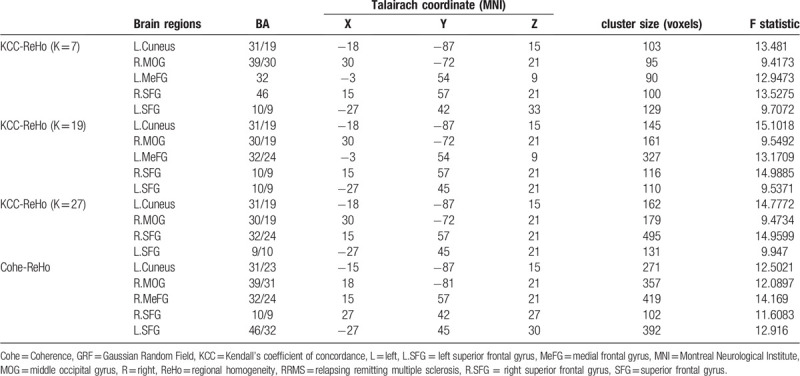
Figure 1.
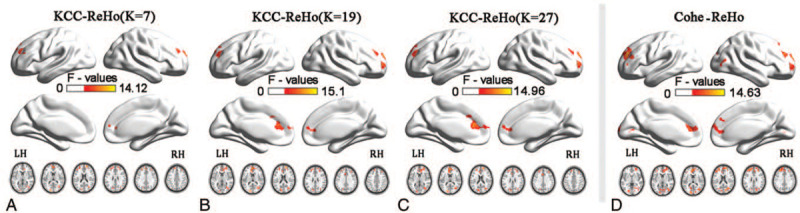
Analysis of ANOVA map of KCC- and Cohe- regional homogeneity among patients with acute RRMS, remitting RRMS, and healthy controls (voxel-level P < .01, GRF correction, cluster-level P < .05). A-C show the spatial patterns of the KCC-ReHo's group comparisons and D shows the spatial patterns of the Cohe-ReHo's group comparisons. GRF = Gaussian random field, ReHo = regional homogeneity.
3.3. KCC-ReHo or Cohe-ReHo differences between each pair of groups
We employed the post hoc analysis to revealed the detailed KCC-ReHo or Cohe-ReHo alterations between each pair of groups (acute RRMS vs HCs, remitting RRMS vs HCs and acute RRMS vs remitting RRMS), and details were as follows:
-
(1)
compared to HCs, the alterations were only detect in acute RRMS instead of remitting RRMS: the increased KCC-ReHo (when K = 7, 19, 27) (Fig. 2A-C) and Cohe-ReHo values (Fig. 2D) of acute RRMS were detected in the left cuneus and right MOG (P < .001);
-
(2)
compared to HCs, the similar alterations were detect in acute and remitting RRMS patients: both KCC ReHo (when K = 7, 19, 27) (Fig. 2A-C) and Cohe-ReHo analysis (Fig. 2D) reported that the acute and remitting RRMS similarly exhibited the decrease ReHo values in the left and right SFG (P < .001). Additionally, the acute and remitting RRMS both exhibited the decreased KCC-ReHo (when K = 7,19) in the left medial frontal gyrus (MeFG) (P < .001) (Fig. 2A-B) and decreased Cohe-ReHo in the right MeFG (P < .001) (Fig. 2D).
-
(3)
compared to remitting RRMS, the acute RRMS exhibited lower KCC-ReHo (Fig. 2A-C) and Cohe-ReHo in the right MOG (P < .001) (Fig. 2D).
Figure 2.
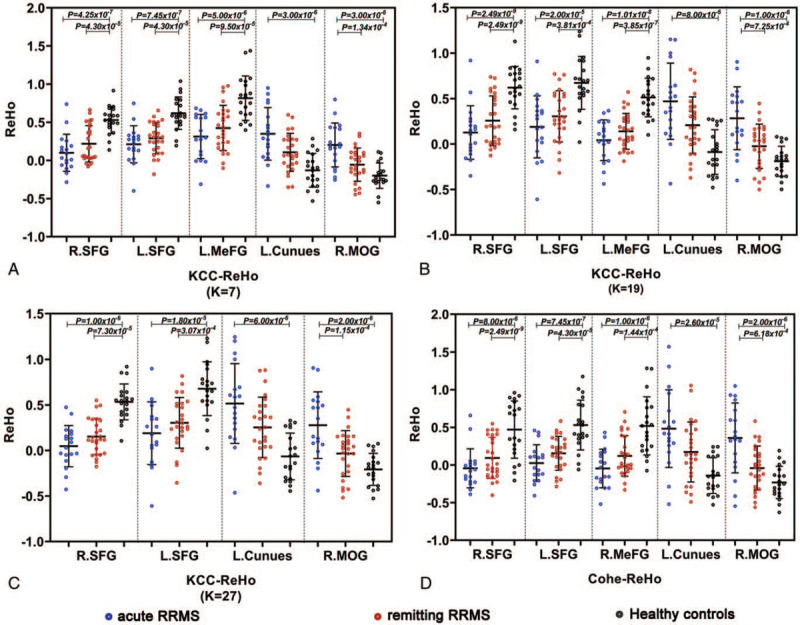
Scatter diagram of the regional homogeneity index among the acute RRMS, remitting RRMS patients and HC groups in the identified brain regions (P < .001, Bonferroni correction). The blue dots represent the acute RRMS group, the red dots represent the remitting RRMS group, and the black dots represent the HC groups. L = left, MeFG = medial frontal gyrus, MOG = middle occipital gyrus, R = right, SFG = superior frontal gyrus.
3.4. Correlations between the altered KCC-ReHo or Cohe-ReHo and clinical assessments
The correlation analysis showed that there were significantly negative correlations between the disease duration and the KCC-ReHo (when K = 7, 19) from the regions of left SFG (r = -0.633, P = .006; r = -0.620, P = .008) (Fig. 3B) or left MeFG (r = -0.608, P = .010; r = -0.555, P = .022) (Fig. 3C); between the disease duration and the KCC-ReHo (when K = 27) from the regions of left or right SFG (r = -0.603, P = .010; r = -0.551, P = .022) (Fig. 3B); between the disease duration and the Cohe-ReHo from the regions of right MeFG (r = -0.608, P = .010) (Fig. 3C). Additionally, significantly negative correlation was observed between the EDSS and the KCC-ReHo (when K = 7,19) from the regions of right SFG (r = -0.590, P = .013; r = -0.599, P = .011) (Fig. 3A). Briefly, correlations between abnormal ReHo values and clinical assessments only detected in acute RRMS patients when compared with HCs (Fig. 3A-C and see Table, Supplemental Digital Content 1, which illustrates the correlations between altered ReHo and clinical variables in the acute RRMS patients compared with HCs).
Figure 3.
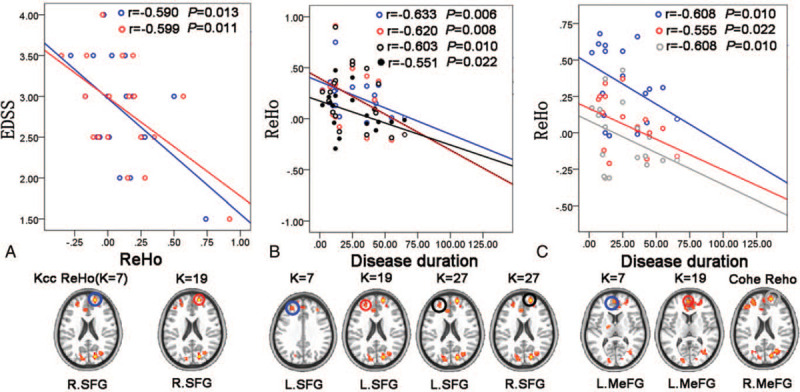
Associations between altered ReHo and clinical variables in the acute RRMS patients (P < .05). Results based on KCC-ReHo analysis (when K=7) are uniformly shown in blue; results based on KCC-ReHo analysis (when K=19) are in red; results based on KCC-ReHo analysis (when K=27) are in black (particularlly, the black solid circle and solid line represent the L.SFG, and the black hollow circle and dotted line represent the R.SFG) and results based on Cohe-ReHo analysis are uniformly shown in gray. EDSS = expanded disability status scale, L = left, MeFG = medial frontal gyrus, R = right, ReHo = regional homogeneity, SFG = superior frontal gyrus.
While no correlations were found between any of the clinical indices (disease duration, PASAT-3 and EDSS) and altered KCC- or Cohe-ReHo in remitting RRMS patients when compared with HCs (see Table, Supplemental Digital Content 2, which illustrates the correlations between altered ReHo and clinical variables in the remitting RRMS patients compared with HCs) and no correlations were found between the clinical indices and altered KCC- or Cohe-ReHo in acute RRMS patients when compared with remitting RRMS (see Table, Supplemental Digital Content 3, which illustrates the correlations between altered ReHo and clinical variables in the acute RRMS patients compared with remitting RRMS).
4. Discussion
In this study, we investigated the local functional connectivity property of the MS during the acute and remission phase using KCC-ReHo and Cohe-ReHo analyses. Our researches demonstrated that increased KCC-ReHo and Cohe-ReHo of the right MOG and left cuneus only occurred in the MS of acute phase rather than in the remission phase. MS patients have similar alteration in acute and remission phase: ReHo of the SFG and MeFG decreased compared with HCs. Furthermore, the correlations between ReHo (KCC-ReHo or Cohe-ReHo) and clinical variables (such as EDSS, disease duration) were only observed in acute RRMS.
4.1. More regional synchronized activity brain regions were mobilized/recruited in acute RRMS
In this research, patients with acute RRMS showed more widely spread KCC-ReHo or Cohe-ReHo values abnormalities in several brain regions, that is, the increased ReHo scores of the right MOG and left cuneus were only observed in the acute RRMS, indicating these changes are specific for acute RRMS patients. The MOG and the cuneus contributed to visual information processing and communication with the cerebral cortex,[26,27] indicating that the acute RRMS mobilized/recruited more brain regions involved in visual information processing. In a previous study, a visual function impairment-associated amplitude of low-frequency fluctuations enhancement in MOG was demonstrated in RRMS patients.[28] Another RRMS studies detected that the medial visual component functional connectivity significantly increased in left cuneus has influenced on visual processing speed in MS.[29] Furthermore, the Optical Coherence Tomography has detected the reduced retinal nerve fiber layer thickness which was associated with visual functional dysfunction occur in the multiples sclerosis, even in the patients absence of Optic neuritis,[30,31] implying that MS has the subclinical damage in visual pathway.
Taken together, it might be speculated that the acute RRMS mobilized/recruited more brain regions could be interpreted as an adaptive plasticity process, and this potential role is to compensate for the potential visual impairment of MS, to maintain functional stability. In early stages of lesion or the acute phase, axons and neurons are in part preserved, however, with maturation of the lesions and chronicity of the disease, substantial axonal loss is seen and the susceptibility of the target tissue for neurodegeneration increased.[32] Furthermore, previous study has observed significant heterogeneity with ReHo alterations of MS, usually, the brain lesion of MS initially causes an increase in connectivity, followed by a subsequent decrease before reaching a plateau.[33] Hence, it should be interpreted with caution about the brain local connectivity alteration present in our study that increased ReHo of the MOG and cuneus only detected in acute RRMS rather than remitting RRMS, although related to acute RRMS, these brain regions seems to have returned to normal in remission phase, while we can’t affirm curtly that the local brain functional activity of these brain area recovery in remitting phase.
4.2. Similar alteration of regional synchronized activity in acute and remitting RRMS
In our present study, we observed the similar alterations in acute and remitting RRMS compared to HCs, that is, the acute and remitting RRMS patients both showed decreased KCC-ReHo (when k = 7,19) or Cohe-ReHo values in the SFG and MeFG. The bilateral prefrontal cortices regulate several cognitive processes, mainly associated with executive functions, working memory and making decision.[34,35] A previous study reported a significant hypoperfusion area was found for MS in the superior frontal gyrus, suggesting superior frontal gyrus has tissue damage.[36] The similar alteration was also reported in other previous perfusion study,[37] which showed a progressive cerebral blood flow and cerebral blood volume deficits present in the superior frontal gyrus for RRMS patients. Another study using echo planar spectroscopic imaging showed abnormal metabolite such as decreased NAA and Glx were predominantly tested in gray matter within prefrontal cortices in MS patients.[38] In addition, several study has reported that neuronal damage in the prefrontal cortices was correlates with cognitive dysfunction.[39] So we speculate that the decreased KCC-ReHo or Cohe-Reho in the present study may be attributable to brain dysfunction, such as impaired cognitive function.
4.3. Correlations between clinical indices and abnormal KCC-ReHo or Cohe-ReHo in acute RRMS
Significant correlation was only detected in the acute RRMS patients, the KCC-ReHo of the left SFG, right SFG and the left MeFG, while the Cohe-ReHo of the right MeFG was inversely correlated with the disease duration. Together, these results suggest that decreased local connectivity is associated with prolonged disease duration, in other words, MS patients with a shorter history of disease showed greater compensatory capacity in these regions compared to those with a longer history. In addition, the associations were observed between altered KCC-ReHo and the EDSS. This correlation was inconsistent with the previous published studies, as these studies reported that the EDSS is widely used instrument to evaluating the functional systems of the central nervous system, which is usually associated with sensorimotor networks.[17,40] The exact causes for these differences were unknown, but it may be a spurious correlation that caused by the brain dysfunction.
4.4. Methodological considerations in KCC-ReHo and Cohe-ReHo
ReHo signifies the temporal synchrony of BOLD signals between an individual voxel and those of its neighboring voxels that participate in the execution of related functions in a given region. Zang and colleagues[23] applied the KCC (K = 7, 19, 27) algorithms and later Liu and colleagues[21] applied the coherence-based algorithms to measure the local synchronization of rs-fMRI signal. In our study, a large proportion of brain regions (SFG, MOG and cuneus) of aberrant ReHo and its coordinates in MS patients detected by 2 ReHo analysis were similar, while the KCC-ReHo is more sensitive to detect the clinical relevance of ReHo than the Cohe-ReHo. Our result show different with the previous study reported by Liu et al,[21] whose result suggested Cohe-ReHo is superior to KCC-ReHo owing to the Cohe-ReHo been not susceptible to random noise induced by phase delay among the time courses. In addition, previous studies mostly use KCC-ReHo (set K = 27) to detect the abnormal spontaneous brain activity regions in all kinds of diseases,[41,42] and little know that what difference result will be happened compared to K = 27 if K = 7 or K = 19. In present study, as for KCC-ReHo analysis, when K = 7 or K = 19, consistent alterations in spatial distribution were demonstrated and have the advantage to reveal more difference in the MeFG than K = 27, suggested that K = 7 or 19 is superior to K = 27 for KCC-ReHo analysis in MS patients. However, in order to deeper elucidate the sensitivity and specificity of these methods in MS patients, it's necessary to carry out further studies.
5. Conclusions
In this study, based on 2 ReHo methods (KCC- and Cohe-ReHo), we revealed the disease-related brain local functional connectivity alterations in both acute and remitting RRMS patients, while the acute RRMS patients mobilized more brain regions in an attempt to maintain functional stability. In addition, our study also provides a methodological consideration for future ReHo analysis.
5.1. Limitations
Our study presented some limits. First, previous rs-fMRI study has demonstrated contradictory results of increased and decreased connectivity in MS, and inconsistent changes in local brain functional connectivity occurred as the disease stage changes was found in our study. In future studies, the effects of disease stages should be considered when investigating the alteration brain connectivity of MS. Second, future studies should be designed to add longitudinal data to obtain more information about the ReHo alteration trends throughout the disease cycle. Thirdly, the incidence of MS in China is low, previous study showed the national incidence of MS as 0.288 in adults per 100,000,[43] so the sample size collected in this study is small. This may have an impact on the reliability of our conclusions. While the subjects’ numble in this study was within the range of the sample size estimation method.[44,45] Finally, the population of this study was limited to RRMS patients with low EDSS scores (EDSS < 2.5), so we can recruit more remitting RRMS with higher EDSS scores to lower this limitation in further study.
Author contributions
Conceptualization: Yanyan Zhu, Fuqing Zhou.
Data curation: Muhua Huang, Yao Wang.
Formal analysis: Yanyan Zhu, Yanlin Zhao.
Investigation: Yanlin Zhao, Yixiu Pei, Lei Wang, Ting He.
Methodology: Yanyan Zhu, Yixiu Pei.
Supervision: Fuqing Zhou, Xianjun Zeng.
Validation: Yanyan Zhu, Yao Wang, Xianjun Zeng.
Writing – original draft: Yanyan Zhu.
Writing – review & editing: Yanyan Zhu, Fuqing Zhou.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, Cohe = Coherence, FOV = field of view, HCs = healthy controls, KCC = Kendall's coefficient of concordance, MeFG = medial frontal gyrus, MOG = middle occipital gyrus, MRI = magnetic resonance imaging, MS = Multiple sclerosis, PASAT-3 = paced auditory serial addition test 3s, ReHo = regional homogeneity, RRMS = relapsing-remitting multiple sclerosis, Rs-fMRI = resting-state functional magnetic resonance imaging, SFG = superior frontal gyrus, TE = echo time, TR = repetition time.
How to cite this article: Zhu Y, Huang M, Zhao Y, Pei Y, Wang Y, Wang L, He T, Zhou F, Zeng X. Local functional connectivity of patients with acute and remitting multiple sclerosis: a Kendall's coefficient of concordance- and coherence-regional homogeneity study. Medicine. 2020;99:43(e22860).
This study was supported by the National Science Foundation of China (81771808 and 81560284), Jiangxi Provincial key research and development program (20192BBGL70034), the Distinguished Young Scholars of Jiangxi Province (20171BCB23089) and the Graduate Innovation Fund of Jiangxi Province (YC2019-S114).
The datasets generated during the current study are available from the corresponding author on reasonable request (fq.chou@yahoo.com).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019;26:27–40. [DOI] [PubMed] [Google Scholar]
- [2].Campbell J, Rashid W, Cercignani M, et al. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J 2017;93:143–7. [DOI] [PubMed] [Google Scholar]
- [3].Manogaran P, Walker-Egger C, Samardzija M, et al. Exploring experimental autoimmune optic neuritis using multimodal imaging. Neuroimage 2018;175:327–39. [DOI] [PubMed] [Google Scholar]
- [4].Zackowski KM, Smith SA, Reich DS, et al. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain 2009;132:1200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parmenter BA, Testa SM, Schretlen DJ, et al. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2010;16:6–16. [DOI] [PubMed] [Google Scholar]
- [6].Regan D, Simpson T. Multiple sclerosis can cause visual processing deficits specific to texture-defined form. J Neurology 1995;45:809–15. [DOI] [PubMed] [Google Scholar]
- [7].Vleugels L, Lafosse C, van Nunen A, et al. Visuoperceptual impairment in multiple sclerosis patients diagnosed with neuropsychological tasks. Mult Scler 2000;6:241–54. [DOI] [PubMed] [Google Scholar]
- [8].Filippi M, Preziosa P, Rocca MA. Multiple sclerosis. Handb Clin Neurol 2016;135:399–423. [DOI] [PubMed] [Google Scholar]
- [9].Lommers E, Simon J, Reuter G, et al. Multiparameter MRI quantification of microstructural tissue alterations in multiple sclerosis. Neuroimage Clin 2019;23:101879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Giorgio A, De Stefano N. Advanced structural and functional brain MRI in multiple sclerosis. Semin Neurol 2016;36:163–76. [DOI] [PubMed] [Google Scholar]
- [11].Cogliati Dezza I, Zito G, Tomasevic L, et al. Functional and structural balances of homologous sensorimotor regions in multiple sclerosis fatigue. J Neurol 2015;262:614–22. [DOI] [PubMed] [Google Scholar]
- [12].Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- [13].Bonavita S, Sacco R, Esposito S, et al. Default mode network changes in multiple sclerosis: a link between depression and cognitive impairment? Eur J Neurol 2017;24:27–36. [DOI] [PubMed] [Google Scholar]
- [14].Zhou F, Ying Z, Gong H, et al. Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PLoS One 2014;9:e101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang M, Zhou F, Wu L, et al. Synchronization within, and interactions between, the default mode and dorsal attention networks in relapsing-remitting multiple sclerosis. Neuropsychiatr Dis Treat 2018;14:1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bisecco A, Nardo FD, Docimo R, et al. Fatigue in multiple sclerosis: the contribution of resting-state functional connectivity reorganization. Mult Scler 2018;24:1696–705. [DOI] [PubMed] [Google Scholar]
- [17].Pinter D, Beckmann CF, Fazekas F, et al. Morphological MRI phenotypes of multiple sclerosis differ in resting-state brain function. Sci Rep 2019;9:16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cooray GK, Sundgren M, Brismar T. Mechanism of visual network dysfunction in relapsing-remitting multiple sclerosis and its relation to cognition. Clin Neurophysiol 2020;131:361–7. [DOI] [PubMed] [Google Scholar]
- [19].Berger T. Immunological processes related to cognitive impairment in MS. Acta Neurol Scand 2016;134:34–8. [DOI] [PubMed] [Google Scholar]
- [20].Droby A, Yuen KS, Muthuraman M, et al. Changes in brain functional connectivity patterns are driven by an individual lesion in MS: a resting-state fMRI study. Brain Imaging Behav 2016;10:1117–26. [DOI] [PubMed] [Google Scholar]
- [21].Liu D, Yan C, Ren J, et al. Using coherence to measure regional homogeneity of resting-state fMRI signal. Front Syst Neurosci 2010;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li Z, Kadivar A, Pluta J, et al. Test-retest stability analysis of resting brain activity revealed by blood oxygen level-dependent functional MRI. J Magn Reson Imaging 2012;36:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22:394–400. [DOI] [PubMed] [Google Scholar]
- [24].Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17:162–73. [DOI] [PubMed] [Google Scholar]
- [25].Kurtzke Some contributions of the department of veterans affairs to the epidemiology of multiple sclerosis. Mult Scler 2008;14:1007–12. [DOI] [PubMed] [Google Scholar]
- [26].Lai CH, Wu YT. Decreased regional homogeneity in lingual gyrus, increased regional homogeneity in cuneus and correlations with panic symptom severity of first-episode, medication-naive and late-onset panic disorder patients. Psychiatry Res 2013;211:127–31. [DOI] [PubMed] [Google Scholar]
- [27].Teng C, Zhou J, Ma H, et al. Abnormal resting state activity of left middle occipital gyrus and its functional connectivity in female patients with major depressive disorder. BMC Psychiatry 2018;18:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schoonheim MM, Geurts J, Wiebenga OT, et al. Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler 2014;20:1058–65. [DOI] [PubMed] [Google Scholar]
- [29].DeLuca J, Genova HM, Hillary FG, et al. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci 2008;270:28–39. [DOI] [PubMed] [Google Scholar]
- [30].Kallenbach K, Frederiksen J. Optical coherence tomography in optic neuritis and multiple sclerosis: a review. Eur J Neurol 2007;14:841–9. [DOI] [PubMed] [Google Scholar]
- [31].Sergott RC, Frohman E, Glanzman R, et al. OCT in MS Expert Panel,. The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci 2007;263:3–14. [DOI] [PubMed] [Google Scholar]
- [32].Lassmann H. Pathology and disease mechanisms in different stages of multiple sclerosis. J Neurol Sci 2013;333:1–4. [DOI] [PubMed] [Google Scholar]
- [33].Tewarie P, Steenwijk MD, Brookes MJ, et al. Explaining the heterogeneity of functional connectivity findings in multiple sclerosis: an empirically informed modeling study. Hum Brain Mapp 2018;39:2541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris 2013;107:471–82. [DOI] [PubMed] [Google Scholar]
- [35].Henri-Bhargava A, Stuss DT, Freedman M. Clinical assessment of prefrontal lobe functions. Continuum (Minneap Minn) 2018;24:704–26. [DOI] [PubMed] [Google Scholar]
- [36].Lagana MM, Mendozzi L, Pelizzari L, et al. Are cerebral perfusion and atrophy linked in multiple sclerosis? evidence for a multifactorial approach to assess neurodegeneration. Curr Neurovasc Res 2018;15:282–91. [DOI] [PubMed] [Google Scholar]
- [37].Vitorino R, Hojjat SP, Cantrell CG, et al. Regional frontal perfusion deficits in relapsing-remitting multiple sclerosis with cognitive decline. AJNR Am J Neuroradiol 2016;37:1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Donadieu M, Le Fur Y, Lecocq A, et al. Metabolic voxel-based analysis of the complete human brain using fast 3D-MRSI: proof of concept in multiple sclerosis. J Magn Reson Imaging 2016;44:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cassiano MT, Lanzillo R, Alfano B, et al. Voxel-based analysis of gray matter relaxation rates shows different correlation patterns for cognitive impairment and physical disability in relapsing-remitting multiple sclerosis. Neuroimage Clin 2020;26:102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhuang Y, Zhou F, Gong H. Intrinsic functional plasticity of the sensorimotor network in relapsing-remitting multiple sclerosis: evidence from a centrality analysis. PLoS One 2015;10:e0130524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang X, Luo J, Zhong Z, et al. Abnormal regional homogeneity in patients with obsessive-compulsive disorder and their unaffected siblings: a resting-state fMRI Study. Front Psychiatry 2019;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu Z, Lai J, Zhang H, et al. Regional homogeneity and functional connectivity analysis of resting-state magnetic resonance in patients with bipolar II disorder. Medicine (Baltimore) 2019;98:e17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tian D-C, Zhang C, Yuan M, et al. Incidence of multiple sclerosis in china: a nationwide hospital-based study. Lancet Regional Health -Western Pacific 2020;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sami W, Alrukban MO, Waqas T, et al. Sample size determination in health research. J Ayub Med Coll Abbottabad 2018;30:308–11. [PubMed] [Google Scholar]
- [45].Malone HE, Nicholl H, Coyne I. Fundamentals of estimating sample size. Nurse Res 2016;23:21–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


