Abstract
Protein aggregation is among the most challenging new frontiers in protein chemistry as well as in molecular medicine and has direct implications in protein misfolding. This study investigated the role of sugar molecules (glucose, fructose, sucrose, and the mixture of glucose and fructose) in protecting the structural integrity of α-lactalbumin (α-LA) against aggregation. The research focused here is the inhibitory capabilities of sugars against α-LA fibril formation investigated employing diverse multispectroscopic and microscopic techniques. The aggregation was induced in α-LA thermally with a change in concentration. UV–vis spectroscopy, ThT binding assay, Trp fluorescence, Rayleigh scattering, and turbidity assay depicted synchronized results. Further, transmission electron microscopy (TEM) complemented that a mixture of glucose and fructose was the best inhibitor of α-LA fibril formation. Inhibition of α-LA aggregation by sugar osmolytes is attributed to the formation of hydrogen bonds between these osmolytes, as evidenced by the molecular docking results. This hydrogen bonding is a key player that prevents aggregation in α-LA in the presence of sugar osmolytes. This study provides an insight into the ability of naturally occurring sugar osmolytes to inhibit fibril formation and can serve as a platform to treat protein misfolding and aggregation-oriented disorders.
1. Introduction
Alpha-lactalbumin (α-LA), a whey protein, is a small globular protein having a molar mass of 14,200 Da (Figure S1). It is a Ca2+ metallo binding milk protein that contains four disulfide bonds and no free thiol (−SH) group. α-LA constitutes approximately 22% of the total protein and approximately 36% of the whey proteins in human milk and approximately 3.5% of the total protein and approximately 17% of the whey proteins in bovine milk.1 It has essential amino acid composition that is comparatively rich in tryptophan, lysine, cysteine, and the branched-chain amino acids (BCAAs) leucine, isoleucine, and valine.2 Due to a unique amino acid profile, α-LA plays an essential role in diverse activities. α-LA has therapeutic applications in diseases like cancer,3 sarcopenia,4 and many more.5 It also has the potential to be used as a nutritional supplement to support neurological function and sleep in adults. An essential characteristic of α-LA is its ability to bind metal cations; the protein has a single vital calcium-binding site and also has several zinc-binding sites.6 Thus, the importance of α-LA in human health has been explored for different research goals across the globe. Many literature studies report folding and unfolding of α-LA.7,8
Protein aggregation is one of the most challenging and appealing frontiers in protein chemistry and molecular medicine. It is a phenomenon by which a conformation change occurs due to its misfolding, which further results in polymerization of aggregates or systematized fibrils.9 It is comprehensively formed mainly due to the cluster of particles that are mostly subjected to β-sheet structures. Even after 50 years of research on protein aggregation, there are still many unanswered questions.10 Frieden briefly summarized that despite much extensive literature, the mechanism of protein folding and aggregation is poorly understood. An enormous number of human pathological diseases are associated with protein aggregation,11 which marks attention not only in the field of medical sciences but also in biochemistry, biophysics, and biotechnology. Many pathological manifestations like Parkinson’s disease due to α-synuclein protein, Alzheimer’s disease due to Aβ peptides and tau protein, Huntington disease due to Huntingtin (polyQ expansion) fibril formation, and type II diabetes due to fibrillation of a pro-islet amyloid polypeptide fragment type of emphysema and many other neurological disorders require solution through the understanding of protein misfolding and aggregation.12
Sugars as osmolytes are small molecules amassed by cells in response to extreme environmental stress conditions such as (extreme temperature, pH, and pressure).13−23 Sugars (small molecules) and their polymers (macromolecules) act as chemical chaperons and stabilize the protein.14,15,19,21−23 They act as a stress-protectant and provide the stability to proteins in in vitro and in vivo conditions. These force folding of the protein molecules against denaturing stress conditions both in vivo and in vitro, thereby favoring their compact conformation, i.e., native (N) state, over an extended one, i.e., denatured (D) state. Osmolytes affect the thermodynamic equilibrium between two-end states of the protein, i.e., native and denatured state, and shift it toward the native state of the protein. It is a well-known fact that osmolytes induce folding of peculiar proteins and hence stabilize them,15,24 and they are hence used as therapeutic agents in protein misfolding-oriented diseases. Some studies reported that sucrose, trehalose, and proline reduce/inhibit aggregation in proteins like lysozyme and therapeutic proteins like insulin.25 A study reported a low trehalose concentration stabilized α-synuclein folding much better than at high concentration by blocking in vitro α-synuclein’s polymerization, thus highlighting the therapeutic potential of trehalose in the management of neurodegenerative disorders.26 They are one of the most potent stabilizers for many proteins protecting them from denaturation or aggregation.27 Many aromatic compounds are found to inhibit the formation of the amyloid but are questionable during in vivo studies owing to their toxic effects in clinical trials.28 Although osmolytes are not protein-specific in general, however, in certain cases, osmolytes may destabilize or aggregate a specific protein.25 Further, different osmolytes may have a different magnitude of stabilization, which may be protein-dependent.25,29 Hence, there is a need for a “selective osmolyte therapy” because different osmolytes have varying effects on different proteins. Sugars or polyols, which are naturally happening osmolytes, are generally inhibitors for collagen fibrils.30 The effect of sucrose on the Aβ peptide having an inhibitory effect has been reported, which results in perturbation of aggregation as the initiation of α helical conformation.31 Water replacement theory says that sugars stabilize proteins due to the replacement of H bonds between protein and water, which eventually maintain the native conformation of the protein.32 According to the literature, the influence of osmolytes on protein function can occur through osmolyte-induced perturbation of the water structure.33 Earlier, we have reported that sugar stabilizes α-LA and lysozymes as a function of size; i.e., disaccharide stabilizes both of these proteins better than monosaccharides (glucose and fructose).15 Further, we had demonstrated that the thermal stability of both the proteins in the presence of mixtures of glucose and fructose had a greater stabilizing effect on each of the two proteins than equal weight/volume concentrations of sucrose.14
In this study, we investigated the effect of disaccharide (sucrose) and its monomers (glucose and fructose) and a mixture of glucose and fructose on the aggregation of α-LA. No literature precisely explored the effect of osmolytes on α-LA fibrillation merely and solely on a thermal basis. This research has a novel approach toward aggregating proteins thermally with concentration-dependent factors without any indulgence of chemicals for arbitrary effects for processing in vivo work in the future. Many studies reported that α-LA in its apo (calcium depleted) form induces maximum aggregate formation near its pI (4.0–5.0 pH); thus, all our studies were carried out with sodium acetate buffer (pH 4.5), near its pI.34
2. Results
2.1. Light Scattering Measurements Using UV–Vis Spectroscopy
2.1.1. Heat-Induced Concentration-Dependent Aggregation Profile of α-LA
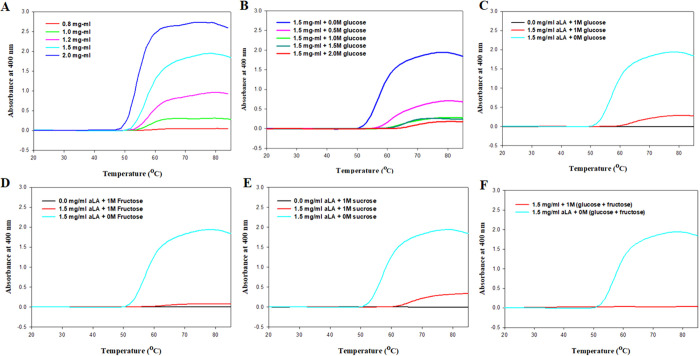
Fibrillation in apo-α-LA was thermally induced from 20 to 85 ° C in a Peltier connected to a UV–vis spectrophotometer water bath. Figure 1A shows the aggregation profile of apo-α-LA at pH 4.5 monitored by observing the changes in light scattering intensity at 400 nm (I400) as a function of temperature at different concentrations of α-LA (0.8, 1.0, 1.2, 1.5, and 2.0 mg mL–1). It is clear from the figure that at lower protein concentrations (up to 0.8 mg mL–1), there is no increase in the intensity of light scattering (20–85 °C); a concentration-dependent increase in light scattering intensity was observed with the maximum increase occurring for 1.5 and 2.0 mg mL–1. Equation 1 was employed to evaluate the dependence of I400 on temperature for varying protein concentrations. All the parameters obtained using this equation, viz., If (light scattering at the final plateau line), Tagg (the temperature at 50% maximal light scattering), and Ti (the temperature at which an increase in light scattering intensity starts) are listed in Table 1. It is quite evident from the listed values in Table 1 that there is an increase in If with increasing concentration of α-LA, implying an increase in the degree of aggregation. Additionally, it was found that Ti decreases with increasing concentration of α-LA implicative of the fact that the native state of the protein is being destabilized earlier at higher protein concentration, leading to the early onset of aggregate formation. Thus, it can be said that at higher α-LA concentration, aggregation initiation occurs at lower temperatures, for example, aggregation starts at 50.55 °C for 2.0 mg mL–1 α-LA instead of 56.27 °C for 0.8 mg mL–1 α-LA. It is visualized that 1.5 and 2 mg mL–1 α-LA show the best aggregation but owing to extensive aggregation, 2 mg mL–1 α-LA was ruled out, and for further studies investigating the effect of sugar osmolytes on α-LA aggregation, 1.5 mg mL–1 α-LA will be used.
Figure 1.
(A) Temperature-dependent aggregation profile of α-LA in the range 0.8–2.0 mg mL–1. (B) Thermal aggregation of α-LA in the presence of increasing concentration of glucose at pH 4.5. (C) Thermal aggregation of α-LA (1.5 mg mL–1) in the presence of glucose at pH 4.5. (D) Thermal aggregation of α-LA (1.5 mg mL–1) in the presence of fructose at pH 4.5. (E) Thermal aggregation of α-LA (1.5 mg mL–1) in the presence of sucrose at pH 4.5. (F) Thermal aggregation of α-LA (1.5 mg mL–1) in the presence of glucose + fructose at pH 4.5.
Table 1. Different Parameters Obtained for Temperature-Dependent α-LA Aggregation at Varying Concentrations of α-LAa.
| conc (mg mL–1) | If | 2b | Tagg (°C) | Ti (Tagg – 2b) (°C) |
|---|---|---|---|---|
| 0.8 | 0.04 | 4.08 | 60.35 | 56.27 |
| 1.0 | 0.29 | 3.5 | 58.32 | 54.82 |
| 1.2 | 0.92 | 5.5 | 59.32 | 53.83 |
| 1.5 | 1.89 | 5.2 | 58.22 | 53.02 |
| 2.0 | 2.68 | 3.96 | 54.51 | 50.55 |
I = absorbance at any time t; I0 = initial absorbance value or absorbance at t = 0; If = maximum absorbance; Tagg = temperature at which there is 50% maximal light scattering; b = constant; Ti = temperature at which aggregation begins, given by Tagg – 2b.
2.1.2. Effect of Sugar Osmolytes on Light Scattering Intensity at 400 nm (I400)
Initially, different concentrations of glucose were used (Figure 1B) to find the optimal concentration of the osmolyte that significantly inhibits the aggregation of α-LA. Figure 1B shows the aggregation profiles of α-LA as a function of temperature in the presence of increasing concentrations of glucose (0–2 M). It was quite apparent from the figure that 1 M or above, the concentration of glucose led to a significant decrease in scattering at 400 nm, i.e., significant inhibition in aggregation. Further, upon increasing the concentration of glucose to 2 M, there were no substantial changes as compared to 1 M. Thus, for all further experiments investigating the effect of osmolytes on α-LA aggregation, the concentration of osmolytes used was 1 M. Further, the experimental work was performed with other monosaccharides and disaccharides fructose and sucrose. Figure 1C-F shows aggregation profiles of apo-α-LA in the presence of different sugar osmolytes (1 M) at pH 4.5 monitored by observing the changes in light scattering intensity at 400 nm (I400) as a function of temperature. It is apparent from the figure that the maximum absorbance was observed for the native aggregated protein in the absence of sugar osmolytes. However, in the presence of sugar osmolytes, there was an observed decrease in absorbance, implying that sugar osmolytes are inhibiting protein aggregation. Figure 1C shows the aggregation profile of α-LA in the presence of 1 M glucose as a function of temperature. It is apparent from the figure that 1 M glucose significantly reduces the light scattering of α-LA (1.5 mg mL–1), thereby inhibiting aggregate formation.
Similarly, Figure 1D shows the effect of 1 M fructose on the light scattering of 1.5 mg mL–1 α-LA in which a significant reduction in scattering was observed like 1 M glucose. Figure 1E,F show the effect of sucrose and mixture of glucose and fructose, respectively, on light scattering caused by 1.5 mg mL–1 α-LA. It is evident from the figure that a significant reduction in light scattering was observed for both sucrose and mixture, thus implying the inhibition of aggregate formation of α-LA by these sugar osmolytes. Table 2 lists the comparison between values of If, Tagg, and Ti for aggregated α-LA in the absence and presence of different osmolytes. There was a clear decrease in If values in the presence of osmolytes, suggesting the inhibitory effect of osmolytes on α-LA aggregation. It is very visible that the mixture (glucose + fructose) has the least If value, thus suggesting that maximum reduction of light scattering is caused by it and hence it stands out as the best in terms of inhibiting the aggregate formation of α-LA.
Table 2. Variation in Light Scattering Parameters of 1.5 mg mL–1α-LA in the Absence and Presence of Different Osmolytesa.
| name | If | 2b | Tagg (°C) | Ti (Tagg – 2b) (°C) |
|---|---|---|---|---|
| α-LA (1.5 mg mL–1) | 1.89 | 5.2 | 58.22 | 53.02 |
| α-LA (1.5 mg mL–1) + glucose | 0.2855 | 6.16 | 66.22 | 60.06 |
| α-LA (1.5 mg mL–1) + fructose | 0.078 | 6.53 | 63.68 | 57.15 |
| α-LA (1.5 mg mL–1) + sucrose | 0.32 | 5.39 | 67.64 | 62.24 |
| α-LA (1.5 mg mL–1) + (G + F) | 0.047 | ND | ND | ND |
ND = not determined.
2.2. Fluorescence-Based Studies
2.2.1. Impact of Sugars on Thioflavin T Assay
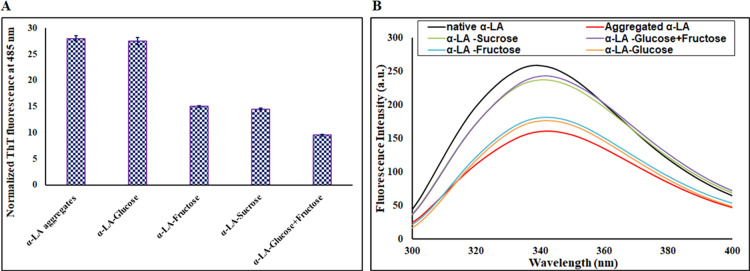
Thioflavin T is a specific dye that determines the cross β-sheet structure responsible for forming amyloids or sometimes the amorphous aggregates in protein. When ThT binds with amyloid fibrils, it displays strong fluorescence in the range of 480–490 nm upon excitation at 450 nm.35,36 An increase in ThT fluorescence intensity, which is several folds as compared to the native state of the protein, implies the formation of aggregates, as ThT is known to bind extensively to the β-sheet. Thus, ThT is a dye that is extensively used to characterize aggregates. It was found that heated α-LA solution (1.5 mg mL–1) showed a very high ThT fluorescence (nearly 10-fold increase) at 485 nm as compared to native α-LA, confirming the existence of aggregates. To investigate the effect of sugar osmolytes on α-LA aggregation in terms of ThT fluorescence, ThT fluorescence was performed in the presence of different sugar osmolytes. The sugar osmolytes caused a significant decrease in the ThT intensity of the α-LA solution, suggesting the inhibition of α-LA aggregation by sugar osmolytes. Figure 2A shows the effect of different osmolytes on α-LA aggregation. The presence of different osmolytes causes a reduction in ThT fluorescence to a different extent. The presence of 1 M glucose showed only a 2% decrease in ThT intensity as compared to the α-LA aggregates solution without sugar osmolytes. However, the ThT intensity was reduced up to 46, 48, and 66% in the presence of fructose, sucrose, and their mixture (glucose + fructose), respectively. Thus, all these observations imply that α-LA aggregation is inhibited in the presence of sugar osmolytes with the mixture (glucose + fructose) acting as the most potent inhibitor of α-LA aggregation while glucose being the least. Hence, it can be suggested that α-LA aggregation, which is implicated in many diseases, can be considerably prevented using sugar osmolytes.
Figure 2.
(A) ThT fluorescence intensity of aggregated α-LA in the absence and presence of 1 M sugar osmolytes. Error bars represent the standard errors of the mean estimated from at least three individual measurements. (B) Trp fluorescence intensity of native α-LA and aggregated α-LA in the absence and presence of 1 M sugar osmolytes.
2.2.2. Trp Fluorescence
Human α-LA contains three tryptophan residues, which are located at strategic positions in the protein.37 The global transition of native proteins to aggregates is also associated with significant changes in the microenvironment around Trp residues. Figure 2B shows the Trp fluorescence emission spectra of native α-LA, aggregated α-LA in the absence and presence of different sugar osmolytes (1 M). Native α-LA when excited at 295 nm shows its characteristic maximum emission at around 337 nm. There was a visible decrease in Trp fluorescence of aggregated alpha solution as compared to native α-LA coupled with a redshift of nearly 10 nm. This decrease in fluorescence along with a 10 nm red shift suggests the burial of the Trp residue shifting to a more polar environment.38 The aggregated α-LA solution in the presence of sugar osmolytes showed a decrease in Trp fluorescence with the mixture (glucose + fructose) restoring Trp fluorescence intensity to near that of the native form and thus acting as the best inhibitor. In the presence of glucose, the changes were not that significant, suggesting it to be the least potent in preventing aggregation of α-LA.
2.2.3. Rayleigh Scattering
Rayleigh–Tyndall scattering is a technique that reveals information regarding the scattering caused by the samples in different directions. The radiation can occur from an individual molecule or due to Tyndall scattering that results from colloidal suspension. Determination of light scattering is a very sensitive probe for the detection of protein aggregation;39 henceforth, light scattering was monitored for native α-LA and aggregated α-LA in the presence of different sugar osmolytes. The degree of light scattering by native α-LA and aggregated α-LA in the absence and presence of different osmolytes (1 M) is depicted in Figure 3A. Native α-LA showed the least light scattering while aggregated α-LA showed a prominent increase in light scattering suggestive of aggregate formation.40 α-LA in the presence of different sugar osmolytes (1 M) showed a significant decrease in light scattering, with the mixture (glucose + fructose) restoring the intensity to near that of native α-LA. Glucose was the least effective in reducing the light scattering, and the mixture (glucose + fructose) was found to be the most potent inhibitor of α-LA aggregation. These observations are in accordance with earlier experiments confirming the fact that mixture (glucose + fructose) behaves as the most potent inhibitor of α-LA aggregation.
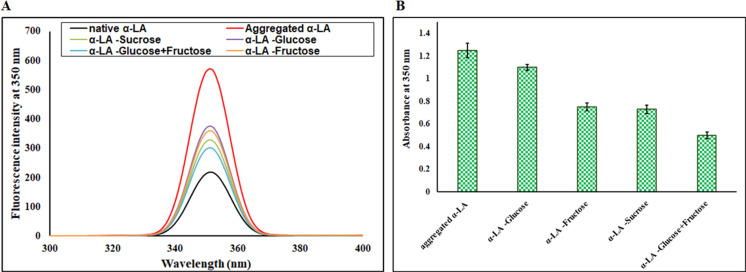
Figure 3.
(A) Rayleigh scattering analysis of native α-LA and aggregated α-LA in the absence and presence of sugar osmolytes. (B) Turbidity measurements (absorbance at 350 nm) for aggregated α-LA in the absence and presence of sugar osmolytes. Error bars represent the standard errors of the mean estimated from at least three individual measurements.
2.3. Turbidity Assay
Turbidity refers to the haziness or cloudiness of a fluid caused by individual particles.38 The aggregation tendency of α-LA in the absence and presence of different sugar osmolytes (1 M) was monitored spectrophotometrically at 350 nm by a change in absorbance. Most proteins show negligible absorbance at 350 nm; hence, absorbance at 350 nm is used to characterize aggregate formation. A high absorbance at 350 nm corresponds to aggregate formation due to scattering caused by larger aggregated particles.41 It is evident from Figure 3B that aggregated α-LA (1.5 mg mL–1) showed a very high absorbance at 350 nm, confirming aggregate formation. Further, in the presence of sugar osmolytes, there was a visible decrease in absorbance at 350 nm. The maximum decrease in absorbance (nearly 60%) was found to be for the mixture (glucose + fructose) followed by sucrose, fructose, and glucose. Thus, the mixture (glucose + fructose) was found to be the best inhibitor of α-LA aggregate formation with glucose being the least reactive. The turbidity assay observations are in line with light scattering, ThT binding, and Trp fluorescence studies, confirming that the mixture (glucose + fructose) is the best inhibitor preventing aggregate formation in α-LA.
2.4. Dynamic Light Scattering (DLS) Measurements
DLS is a technique that is employed to monitor protein aggregation. DLS presents particle size information from the fluctuations of scattered light, a result of the Brownian motion of particles in fluids.42Figure 4 gives an insight of the particle size, with aggregated α-LA showing maximum particle size, confirming the aggregates’ presence. Further, the presence of sugar osmolytes caused a reduction in particle size with the least size obtained for the mixture (glucose + fructose) followed by fructose, sucrose, and glucose. These observations imply that the mixture (glucose + fructose) is the best inhibitor of α-LA aggregate formation.
Figure 4.
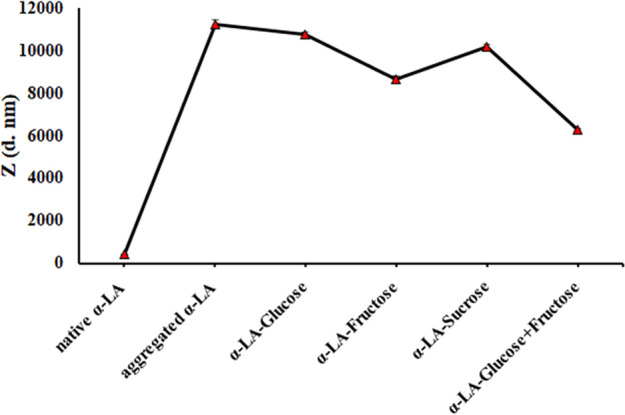
DLS measurements of 1.5 mg mL–1 α-LA in the absence and presence of different sugar osmolytes.
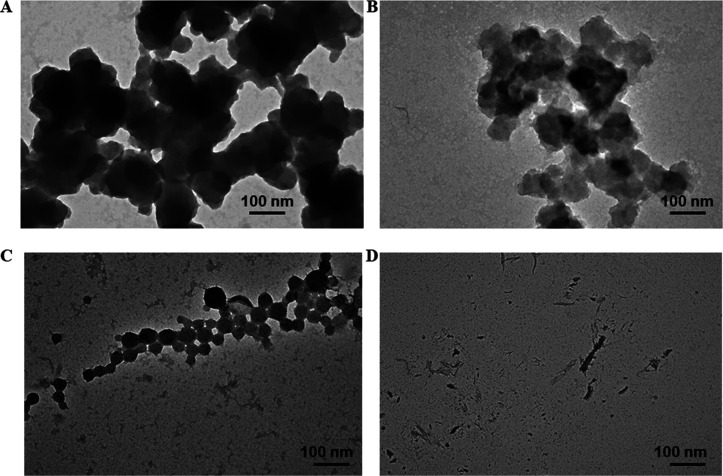
2.5. Transmission Electron Microscopy
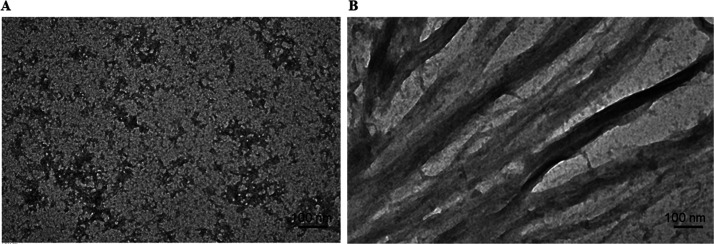
Transmission electron microscopy43 was used to gain an insight into α-LA aggregation and its inhibition by osmolytes morphologically. Figure 5A,B shows TEM images of native α-LA and aggregated α-LA (1.5 mg mL–1), respectively. The presence of abundant ribbon-like fibrils, as evidenced by Figure 5B, is an indicator of aggregated proteins. The existence of sugar osmolytes, namely, glucose, fructose, sucrose, and their mixture (glucose + fructose) converts the large aggregates into short globular and spherical aggregates. These results are in line with other spectroscopic results, validating the fact that the presence of sugar osmolytes inhibits α-LA aggregation. Figure 6A–D depicts TEM images of α-LA in the presence of sugar osmolytes, and it is evident that the presence of sugar osmolytes leads to the transition of α-LA fibrils to spherical aggregates. These TEM observations can be related to literature studies that report the sugars inhibiting fibrillation and the formation of amorphous and globular aggregates instead of fibrils.44 Elsewhere, it has been reported that sugars form hydrogen bonds with proteins leading to restriction of unfolding, thereby inhibiting fibrillation. Another study reported fructose as the inhibitor of fibril formation as it forms stronger hydrogen bonds, thereby inhibiting the fibrillation process.45
Figure 5.
TEM images of (A) native α-LA and (B) α-LA fibrils.
Figure 6.
TEM images of α-LA solutions in the absence and presence of sugar osmolytes after subjecting to thermal aggregation (20–80 °C). (A) α-LA–glucose, (B) α-LA–fructose, (C) α-LA–sucrose, and (D) α-LA–glucose + fructose.
2.6. Molecular Docking Analysis
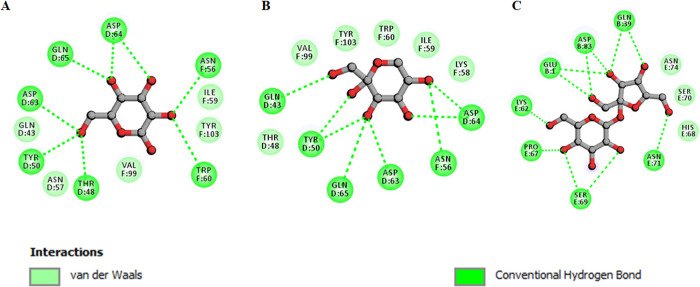
Molecular docking shows that sucrose binds with α-LA with a significant binding affinity of −5.8 kcal/mol. Fructose and glucose showed a binding affinity of −5.4 and −5.3 kcal/mol, respectively. Earlier in vitro experiments showed that the presence of sugar osmolytes prevents α-LA aggregation; meanwhile, herein, molecular docking analysis depicted the ability of sugar osmolytes to form H bonds with the protein. Table 3 depicts binding affinities, pKi, and ligand efficiency (LE) for different ligands with α-LA. All these parameters depict binding of sugar osmolytes with α-LA. Figure 7 depicts the two-dimensional diagram of α-LA residues interacting with sugar osmolytes. It is evident from the figure that all the three osmolytes, viz., glucose, sucrose, and fructose, form a significant number of hydrogen bonds (green color). Thus, this molecular docking observation validated the fact that sugar osmolytes form several H bonds with α-LA and this H bonding might play a key role in the prevention of α-LA aggregation. Hence, molecular docking further complemented the in vitro observations and provided an insight into the interaction between sugar osmolytes and α-LA.
Table 3. List of Sugar Osmolytes and Their Docking Parameters with α-LA.
| compound | affinity (kcal/mol) | pKi | ligand efficiency (kcal/mol/non-H atom) | torsional energy |
|---|---|---|---|---|
| sucrose | –5.8 | 4.25 | 0.25 | 4.04 |
| fructose | –5.4 | 3.96 | 0.45 | 1.86 |
| glucose | –5.3 | 3.89 | 0.44 | 1.86 |
Figure 7.
Two-dimensional diagram depicting interacting residues for (A) α-LA–glucose, (B) α-LA–fructose, and (C) α-LA–sucrose.
3. Discussion
Protein aggregation is a severe problem for the food and health industry. Comprehending the job of natural osmolytes in balancing distress conditions and diseases requires a thorough knowledge of their mode of action and mechanism. In brief, the hypothesis confers a precise mechanism in a case with osmolytes theory like the preferential exclusion hypothesis and their observed effects induced by osmolytes.46 The therapeutic applications of osmolytes on protein folding or fibrillation with no stimulating side effects are the things that make them one step ahead of the crowd. A general outlook of the defense mechanism against harsh cellular damage and stress conditions is necessary to understand. Although it is necessary to maintain the required pH during the physiological condition in cell organelles and organisms,47,48 here, acidic pH was maintained to investigate the effect of sugar osmolytes on the thermally induced concentration-dependent aggregation of α-LA. The selection of the working pH being at or near its pI is justified because of fact since the protein is prone to maximum aggregation under these conditions.49 Osmolytes would be of immense significance for application in a large number of human diseases. Since osmolytes are accumulated under different disease conditions, identifying specific osmolytes concerning upregulation or downregulation in the cell under particular diseases will be useful for the selective use of osmolytes against the disease.46,50 Osmolytes provide stability to the folded, functional shape of the protein and change the folding balance away from aggregation or protein degradation. They are often known as chemical chaperones. Brain osmolytes improve the speed of Aβ aggregation, interact with neighboring water molecules more rapidly, and prevent the aggregation/misfolding of proteins by providing stability.29,51
Proteins conform to their native structure and are forestalled from structural instability via the selective binding of several small molecules such as substrates, ligands, enzymes, or chemical inhibitors.52−55 Some drugs under clinical trials, for example, transthyretin, which is a natural ligand that is also a structural analog of thyroxine, inhibit tetramer dissociation of TTR and consequently familial amyloid polyneuropathy.56,57 Hampering and blockage of aggregation or amyloid arrangement has likewise been seen in acylphosphatases where specific binding of inorganic phosphate (Pi), a severe competitive inhibitor of acylphosphatases, obstructs the development of the early oligomers and their transformation into protofibrils.52−54,58,59 Polyglutamine-containing proteins, as observed, are stabilized by trehalose, a disaccharide that also inhibits their aggregation.
Sugars are known to be good inhibitors for protein misfolding and therefore protect it in vivo from the proteotoxic environment to maintain the normal functionality of the protein.60 Hydrogen bonding, to a great extent, plays a significant role in preventing the amount of aggregation development. The intracellular osmolyte concentrations vary significantly under physiological conditions. This study advocates that a mixture of osmolytes works better than individual osmolytes or their polymer. Sucrose is a disaccharide composed of glucose and fructose. The inhibitory effect of glucose, fructose, their polymer, i.e., sucrose, and a mixture of glucose and fructose on the aggregation behavior of apo-α-LA was investigated thoroughly in this study using UV–vis spectroscopy, high light scattering intensity measurements at 400 nm (I400), ThT binding using ThT fluorescence, and Trp fluorescence measurements.
Further, DLS and TEM measurements are performed for an inclusive investigation of results. All these experiments demonstrated that sucrose, its monomers, and the mixture of its monomers prevent protein aggregation. However, in the presence of different sugar osmolytes, aggregation is prevented to different extents. Glucose, sucrose, and fructose showed the presence of spherical and globular aggregates; sizeable aggregates have been observed for glucose followed by fructose and sucrose. However, a mixture of glucose and fructose completely inhibited the fibrillation of α-LA, as is evident in the TEM image of native α-LA. Molecular docking studies show that hydrogen bonds may form between the protein and osmolytes.
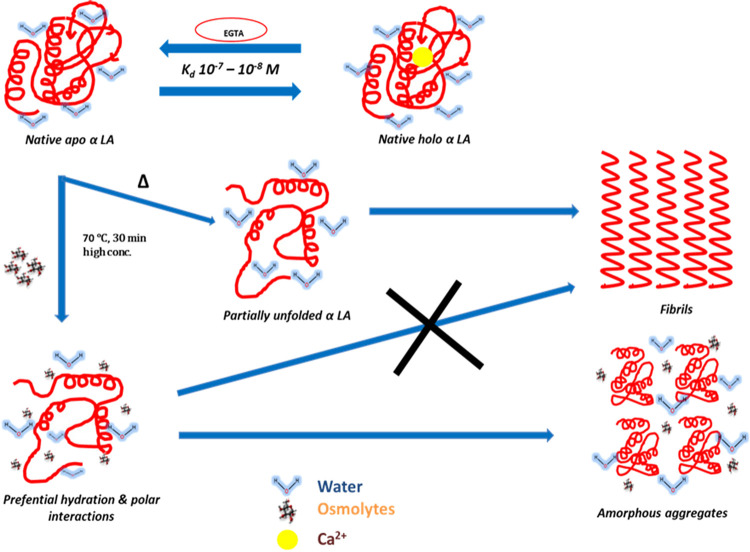
Generally, osmolytes stabilize the native conformation of a protein against heat-induced denaturation by a phenomenon called volume exclusion. According to the volume exclusion hypothesis, protein strength in aqueous solutions of osmolytes is a consequence of a sensitive balance between the preferential hydration of solutes (proteins included), the preferential exclusion of osmolytes from the protein surface, and the osmolyte binding to the protein surface. In either case, the osmolyte is excluded from the surface of the protein, and there is no direct interaction between the protein and osmolyte. There is an unfavorable interaction between osmolyte and denatured protein, which shifts the equilibrium toward native conformation. However, the case is different for protein aggregation. Due to aggregation, protein comes together and creates a crowding-like condition (Figure 8). Here, the osmolyte forms a hydrogen bond with the protein, as evidenced by docking in this investigation, which inhibits protein aggregation. Thus, it can be said that glucose, fructose, and sucrose lead to inhibition of fibrils by forming hydrogen bonds with the protein, resulting in the reduction of aggregates. One may conclude that the mixture of glucose and fructose forms a more significant number of hydrogen bonds as compared to single osmolytes and hence is the most potent inhibitor of α-LA fibrillation, eventually inhibiting the fibrillation process. Thus, it can be noted and understood that these osmolytes stabilized proteins by forming hydrogen bonds, thereby preventing aggregation.44,45
Figure 8.
Schematic representation of the mechanism of inhibition of α lactalbumin fibrillation by osmolytes.
4. Conclusions
In this study, the inhibitory potential of naturally occurring sugar osmolytes was investigated against α-LA aggregation. This study is a first of its kind where heat induced aggregation of α-LA was investigated at high concentration (1.5 mg mL–1). UV–vis spectroscopy, ThT binding assay, Trp fluorescence, Rayleigh scattering, and turbidity assay depicted that the presence of a mixture of glucose and fructose inhibits α-LA aggregation in the most efficient manner followed by sucrose, fructose, and to a very minimal extent glucose. Further, TEM analysis was used to validate the in vitro observations and it clearly showed that a mixture of glucose and fructose acts most proficiently in inhibiting α-LA aggregation. TEM analysis suggested that the presence of glucose, sucrose, and fructose caused inhibition of fibrillation thereby leading to the formation of globular aggregates. However, the presence of a mixture of glucose and fructose completely inhibited the fibril formation as well as globular aggregate formation, evidenced by the TEM image of native α-LA–glucose + fructose. The inhibition of aggregation by sugar osmolytes is attributed to the hydrogen bonding capability of sugar osmolytes with the protein. This H bonding with the protein prevents fibril formation. It can be said that the mixture of glucose and fructose forms more hydrogen bonds as compared to single osmolytes and hence is the best inhibitor to prevent fibril formation in α-LA. Also, it can be said that the presence of sugar osmolytes stabilizes the native conformation of the protein and hence prevents aggregate formation, with the mixture of glucose and fructose stabilizing the native conformation to the maximum extent and, in a way, being the most potent inhibitor. This study highlights that naturally occurring sugar molecules may play a key role in preventing protein aggregation and may lead to providing a natural solution to diseases caused by protein misfolding and to problems caused by protein aggregation in the food industry. There is a pressing need to develop strategies to prevent the amyloid formation in nutritional proteins. One such approach is to bind sugar molecules to proteins and prevent them from aggregating to maintain their food quality.
5. Materials and Methods
5.1. Materials
Commercially lyophilized preparation of holo-α-LA (from bovine milk) and sugar osmolytes (glucose, sucrose, and fructose) were purchased from Sigma Aldrich Co. (St. Louis, MO, USA). All other chemicals required were obtained from Merck, Germany. Millipore filters with a pore size of 0.22 um were bought from Millipore Corp. Parafilm used was obtained from Bemis Company, Inc., and Whatman filter papers were procured from Whatman International Ltd. Double distilled and deionized water from a Milli-Q UF-Plus purification system was used for the preparation of all buffers. Figure S1 depicts the structure of all the osmolytes investigated in this work.
5.2. Dialysis of the Protein and Analytical Procedures
The required amount of holo-α-LA powder was dissolved in a 0.1 M KCl solution. The apo form of α-LA was prepared by adding 5 mM EGTA to the solution of holo-α-LA (Ca2+-bound). This solution was dialyzed against several concentrations of 0.1 M KCl solution at pH 7.0 and 4 °C, and dialysis tubes were essentially prepared according to the procedure described by McPhie.61 The protein was then filtered through a Millipore filter with a pore size of 0.22 μm.
5.3. Fibril Formation
α-LA concentration was determined experimentally using a molar absorption coefficient (M–1 cm–1) value of 29,210 at 280 nm for α-LA.62 α-LA solution was preincubated with sugar osmolytes and subjected to heat-induced aggregation. Under similar conditions, α-LA without sugar osmolytes was also incubated and heated in the range of 20–80 °C. The protein concentration was varied from 1.5 mg mL–1, while the concentration of sugar osmolytes was fixed at 1.0 M. In the case of a mixture of glucose and fructose, 0.5 M glucose was mixed with 0.5 M fructose.
5.4. Light Scattering Measurements
Temperature-dependent α-LA aggregation was followed by monitoring the light scattering intensity, i.e., the apparent absorbance at 400 nm of the sample solutions (pH 4.5) in a Jasco V-660 UV/vis spectrophotometer equipped with a Peltier-type temperature controller. Each sample was heated from 20 to 85 °C. Necessary blanks were subtracted. Each measurement was repeated at least three times.
The sigmoidal dependence of the light scattering intensity on temperature was analyzed using eq 1
| 1 |
where I is the light scattering intensity at temperature T, and Tagg is the temperature at 50% maximal light scattering, I0 and If represent the initial baseline and final plateau line, respectively, and b is a constant.
The temperature at which aggregation (increase in light scattering intensity) begins (Ti) is given by Tagg – 2b.63
5.5. Preparation of Stock Solution of Glucose, Fructose, and Sucrose (Sugar Osmolytes)
The required amount of glucose, fructose, and sucrose was dissolved in 0.05 M sodium acetate buffer at pH 4.5, and the solution was filtered through Whatman filter paper no. 1. The highest solubility of glucose was 4 M. Fructose with the highest solubility of 5 M and sucrose of 2.5 M were made as a stock solution. The refractive index measurements were done in an Abbes refractometer. The concentration of sugar osmolytes solution was determined from the refractive indices.15
5.6. Thioflavin T (ThT) Assay
ThT spectra were taken in a Jasco FP-6200 spectrofluorometer in a 1 cm quartz cell at 25 ± 0.1 °C, with both excitation–emission slits set at 10 nm, in the medium range. Samples were scanned at an excitation wavelength of 440 nm, keeping the scanning range from 450 to600 nm. The concentration of thioflavin T was determined using an extinction coefficient e of 36 mM–1 cm–1 at 412 nm.64 To remove the insoluble particles, the solution was filtered with 0.22 μM Millipore filters. Necessary blanks were subtracted. The final concentration of α-LA was 1.5 mg mL–1, and ThT was taken in a ratio of 1:10.65
5.7. Tryptophan (Trp) Fluorescence
Fluorescence spectra were recorded on a Jasco FP-6200 spectrofluorometer (Tokyo, Japan) in a 1 cm path length quartz cell at 25 ± 0.1 °C. The protein was excited at 295 nm with an emission spectrum recorded in the range of 300 to 400 nm. In the series of experiments, the emphasis was made on incubating a constant amount of thermally aggregated α-LA (1.5 mg mL–1) in the absence and presence of 1 M osmolytes (glucose, sucrose, fructose, and the mixture of glucose and fructose).
5.8. Rayleigh Scattering
Rayleigh scattering measurement was performed on a Jasco FP-6200 spectrofluorometer (Tokyo, Japan) in a 1 cm path length quartz cell. The excitation wavelength was 350 nm, and emission was recorded in the range of 300–400 nm.66 Both excitation and emission slit widths were fixed at 10 nm. Fluorescence intensities at 350 nm were plotted. The final concentration of α-LA was 1.5 mg mL–1 in the presence and absence of different sugar osmolytes.
5.9. Dynamic Light Scattering (DLS) Measurements
Dynamic light scattering (DLS) measurements were done on a Malvern zetasizer, nano ZS. DLS was operated for measurement of protein hydrodynamic radii. The concentration of 1.5 mg mL–1 of protein was filtered by syringe filters (0.22 μm, Millipore), with and without 1 M concentration of sugar osmolytes. The wavelength was fixed at 689 nm, maintaining an angle of 90° for all the measurements. With a scan run of three cycles at a time, taking 180 s for each sample, data were noted as the acquired time taken for each sample.
5.10. Transmission Electron Microscopy
Transmission electron microscopy43 is a tool that provides an insight into the morphology of aggregates.67 α-LA samples (1.5 mg mL–1) subjected to thermal aggregation in the presence and absence of different osmolytes were prepared, placed on 400-mesh copper grids, covered with a carbon-stabilized Formvar film, and air-dried. Removal of excess fluid was done after 2 min followed by the staining of the grid using uranyl acetate. Samples were then air-dried and scanned in a TECNAI G2 20S-TWIN transmission electron microscope operating at an accelerating voltage of 80 kV.
5.11. Molecular Docking Study
A DELL workstation with a 4x 2.13 GHz processor, a 64 GB RAM, and a 2 TB hard disk running on an Ubuntu 14.04.5 LTS operating system was used for molecular docking analysis. For carrying out docking coupled with visualization, online resources such as the Protein Data Bank (PDB) and PubChem were used to retrieve the three-dimensional coordinates of α-LA and glucose, sucrose, and fructose. Bioinformatics tools AutoDock Vina,68 Discovery studio,43 and PyMOL69 were employed for docking and visualization purposes.
Atomic coordinates of α-LA were taken from PDB (PDB ID: 1F6S) and subsequently preprocessed in SPDBV70 and AutoDock Tools.71 Subsequently, co-crystal ligands were removed from the PDB coordinates file. Sugar osmolytes were obtained from the PubChem database in the processed three-dimensional format, and docking was performed as per previous reports.72
Values of the inhibition constant,73 the negative decimal logarithm of inhibition constants, were calculated from ΔG, generated from the docking study using the following formula.
where ΔG is the binding affinity (kcal mol–1), R (gas constant) is cal*(mol*K)–1, T (room temperature) is 298.15 K, and Ki,pred is the predicted inhibitory constant.
Ligand efficiency (LE) is a commonly applied parameter for selecting favorable ligands by comparing the values of average binding energy per atom.73 The following formula was applied to calculate LE:
where LE is the ligand efficiency (kcal mol–1 non-H atom–1), ΔG is the binding affinity (kcal mol–1), and N is the number of non-hydrogen atoms in the ligand.
5.12. Statistical Analysis
All the experiments were performed in triplicate, and the data is presented as average ± standard deviation. All spectra presented are the average of three replicates.
Acknowledgments
S.B. acknowledges ICMR-SRF Fellowship (45/28/2019-Bio/BMS). F.A. is grateful to the Indian National Science Academy for the award of Senior Scientist Position. A.S. is thankful to DST-SERB for the N-PDF grant (2017/000745). The authors are greatly indebted to Jamia Millia Islamia, New Delhi for providing excellent facilities to carry out the work and acknowledge the support from the FIST Program (SR/FST/LSI-541/2012). M.A.K. thanks the University of Jeddah for the financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04062.
-
•
Figure S1. alpha-lactalbumin (α-LA), structure showing Ca2+-binding metallo milk protein that contains four disulfide bonds and no free thiol (−SH) group, and sugar osmolytes (mono & disaccharide) taken for the study (PDF)
Author Contributions
All the authors contributed to this manuscript. All authors have read and agreed to publish the current version of the manuscript. S.B., A.S, and A.I. are responsible for the conceptualization; S.B. and A.S. are responsible for the methodology; S.B., A.S., and M.A.K. are responsible for the software; A.I. and M.I.H. are responsible for the validation; S.B. and A.S. are responsible for the formal analysis; S.B., A.I., and A.S. are responsible for the investigation; M.A.K. is responsible for the resources; S.B., A.S, and A.I. are responsible for the data curation; S.B., A.S., and A.I. are responsible for the writing of the original draft while A.I., M.I.H., and F.A. are responsible for the review and editing; A.S. and S.B. are responsible for the visualization; A.I. and M.I.H supervised the project; A.I. is in charge of project administration; and M.A.K is responsible for funding acquisition.
This research work was supported by the grant from the Indian Council of Medical Research (ICMR)BIC/12(16)/2014.
The authors declare no competing financial interest.
Supplementary Material
References
- Heine W. E.; Klein P. D.; Reeds P. J. The importance of α-lactalbumin in infant nutrition. The Journal of nutrition 1991, 121, 277–283. 10.1093/jn/121.3.277. [DOI] [PubMed] [Google Scholar]
- Krissansen G. W. Emerging health properties of whey proteins and their clinical implications. Journal of the American College of Nutrition 2007, 26, 713S–723S. 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- Fang B.; Zhang M.; Ge K. S.; Xing H. Z.; Ren F. Z. α-Lactalbumin-oleic acid complex kills tumor cells by inducing excess energy metabolism but inhibiting mRNA expression of the related enzymes. J. Dairy Sci. 2018, 101, 4853–4863. 10.3168/jds.2017-13731. [DOI] [PubMed] [Google Scholar]
- Layman D. K.; Lönnerdal B.; Fernstrom J. D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. 10.1093/nutrit/nuy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.; Ling P.-R.; Blackburn G. Review of infant feeding: key features of breast milk and infant formula. Nutrients 2016, 8, 279. 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov E. A.; Shnyrov V. L.; Kalinichenko L. P.; Kuchar A.; Reyzer I. L.; Berliner L. J. Binding of Zn (II) ions to α-lactalbumin. J. Protein Chem. 1991, 10, 577–584. 10.1007/BF01025709. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M.; Kuwajima K.; Mitani M.; Sugai S. Evidence for identity between the equilibrium unfolding intermediate and a transient folding intermediate: a comparative study of the folding reactions of. alpha.-lactalbumin and lysozyme. Biochemistry 1986, 25, 6965–6972. 10.1021/bi00370a034. [DOI] [PubMed] [Google Scholar]
- Wijesinha-Bettoni R.; Dobson C. M.; Redfield C. Comparison of the denaturant-induced unfolding of the bovine and human α-lactalbumin molten globules. J. Mol. Biol. 2001, 312, 261–273. 10.1006/jmbi.2001.4927. [DOI] [PubMed] [Google Scholar]
- Morris A. M.; Watzky M. A.; Finke R. G. Protein aggregation kinetics, mechanism, and curve-fitting: a review of the literature. Biochim. Biophys. Acta, Proteins Proteomics 2009, 1794, 375–397. 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Ganesh C.; Zaidi F. N.; Udgaonkar J. B.; Varadarajan R. Reversible formation of on-pathway macroscopic aggregates during the folding of maltose binding protein. Protein Sci. 2001, 10, 1635–1644. 10.1110/ps.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C. M. Protein aggregation and its consequences for human disease. Protein Pept. Lett. 2006, 13, 219–227. 10.2174/092986606775338362. [DOI] [PubMed] [Google Scholar]
- Stefani M. Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim. Biophys. Acta, Mol. Basis Dis. 2004, 1739, 5–25. 10.1016/j.bbadis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Ishrat M.; Hassan M. I.; Ahmad F.; Islam A. Sugar osmolytes-induced stabilization of RNase A in macromolecular crowded cellular environment. Int. J. Biol. Macromol. 2018, 115, 349–357. 10.1016/j.ijbiomac.2018.04.073. [DOI] [PubMed] [Google Scholar]
- Beg I.; Minton A. P.; Islam A.; Hassan M. I.; Ahmad F. Comparison of the thermal stabilization of proteins by oligosaccharides and monosaccharide mixtures: Measurement and analysis in the context of excluded volume theory. Biophys. Chem. 2018, 237, 31–37. 10.1016/j.bpc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Beg I.; Minton A. P.; Islam A.; Hassan M. I.; Ahmad F. The ph dependence of Saccharides’ influence on thermal denaturation of two model proteins supports an excluded volume model for stabilization generalized to allow for intramolecular electrostatic interactions. J. Biol. Chem. 2017, 292, 505–511. 10.1074/jbc.M116.757302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen K.; Parray Z. A.; Ahamad S.; Ahmad F.; Ahmed A.; Alamery S. F.; Hussain T.; Hassan I.; Islam A. Interactions Under Crowding Milieu: Chemical-Induced Denaturation of Myoglobin is Determined by the Extent of Heme Dissociation on Interaction with Crowders. Biomolecules 2020, 10, 490. 10.3390/biom10030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parray Z. A.; Ahmad F.; Alajmi M. F.; Hussain A.; Hassan M. I.; Islam A. Formation of molten globule state in horse heart cytochrome c under physiological conditions: Importance of soft interactions and spectroscopic approach in crowded milieu. Int. J. Biol. Macromol. 2020, 148, 192–200. 10.1016/j.ijbiomac.2020.01.119. [DOI] [PubMed] [Google Scholar]
- Shahid S.; Hasan I.; Ahmad F.; Hassan I.; Islam A. Carbohydrate-Based Macromolecular Crowding-Induced Stabilization of Proteins: Towards Understanding the Significance of the Size of the Crowder. Biomolecules 2019, 9, 477. 10.3390/biom9090477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid S.; Ahmad F.; Hassan M. I.; Islam A. Mixture of Macromolecular Crowding Agents Has a Non-additive Effect on the Stability of Proteins. Appl. Biochem. Biotechnol. 2019, 188, 927–941. 10.1007/s12010-019-02972-9. [DOI] [PubMed] [Google Scholar]
- Bashir S.; Sami N.; Bashir S.; Ahmad F.; Hassan M. I.; Islam A., Management of Insulin Through Co-Solute Engineering: A Therapeutic Approach. In Frontiers in Protein Structure, Function, and Dynamics; Springer, 2020, pp. 283–315, 10.1007/978-981-15-5530-5_12. [DOI] [Google Scholar]
- Parray Z. A.; Shahid S.; Ahmad F.; Hassan M. I.; Islam A. Characterization of intermediate state of myoglobin in the presence of PEG 10 under physiological conditions. Int. J. Biol. Macromol. 2017, 99, 241–248. 10.1016/j.ijbiomac.2017.02.084. [DOI] [PubMed] [Google Scholar]
- Shahid S.; Hassan M. I.; Islam A.; Ahmad F. Size-dependent studies of macromolecular crowding on the thermodynamic stability, structure and functional activity of proteins: in vitro and in silico approaches. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 178–197. 10.1016/j.bbagen.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Shahid S.; Ahmad F.; Hassan M. I.; Islam A. Relationship between protein stability and functional activity in the presence of macromolecular crowding agents alone and in mixture: An insight into stability-activity trade-off. Arch. Biochem. Biophys. 2015, 584, 42–50. 10.1016/j.abb.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Santoro M. M.; Liu Y.; Khan S. M. A.; Hou L. X.; Bolen D. W. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry 1992, 31, 5278–5283. 10.1021/bi00138a006. [DOI] [PubMed] [Google Scholar]
- Macchi F.; Eisenkolb M.; Kiefer H.; Otzen D. E. The effect of osmolytes on protein fibrillation. Int. J. Mol. Sci. 2012, 13, 3801–3819. 10.3390/ijms13033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzza P.; Hussain R.; Biondi B.; Calderan A.; Tessari I.; Bubacco L.; Siligardi G. Effects of trehalose on thermodynamic properties of alpha-synuclein revealed through synchrotron radiation circular dichroism. Biomolecules 2015, 5, 724–734. 10.3390/biom5020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.-H.; Chi M.-C.; Lin M.-G.; Lin L.-L.; Wang T.-F. Beneficial effect of sugar osmolytes on the refolding of guanidine hydrochloride-denatured trehalose-6-phosphate hydrolase from Bacillus licheniformis. BioMed Res. Int. 2015, 2015, 806847. 10.1155/2015/806847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger C.; Sidhu A. Differential cytotoxicity of dopamine and H2O2 in a human neuroblastoma divided cell line transfected with α-synuclein and its familial Parkinson’s disease-linked mutants. Neurosci. Lett. 2003, 342, 124–128. 10.1016/S0304-3940(03)00212-X. [DOI] [PubMed] [Google Scholar]
- Kushwah N.; Jain V.; Yadav D. Osmolytes: A Possible Therapeutic Molecule for Ameliorating the Neurodegeneration Caused by Protein Misfolding and Aggregation. Biomolecules 2020, 10, 132. 10.3390/biom10010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova N.; Chi S. L.; Leikin S. Sugars and polyols inhibit fibrillogenesis of type I collagen by disrupting hydrogen-bonded water bridges between the helices. Biochemistry 1998, 37, 11888–11895. 10.1021/bi980089+. [DOI] [PubMed] [Google Scholar]
- Ueda T.; Nagata M.; Monji A.; Yoshida I.; Tashiro N.; Imoto T. Effect of Sucrose on Formation of the β-Amyloid Fibrils and D-Aspartic Acids in Aβ 1—42. Biol. Pharm. Bull. 2002, 25, 375–378. 10.1248/bpb.25.375. [DOI] [PubMed] [Google Scholar]
- Amani M.; Barzegar A.; Mazani M. Osmolytic effect of sucrose on thermal denaturation of pea seedling copper amine oxidase. Protein J. 2017, 36, 147–153. 10.1007/s10930-017-9706-1. [DOI] [PubMed] [Google Scholar]
- Guo F.; Friedman J. M. Osmolyte-induced perturbations of hydrogen bonding between hydration layer waters: correlation with protein conformational changes. J. Phys. Chem. B 2009, 113, 16632–16642. 10.1021/jp9072284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S.; Singh L. R. Macromolecular crowding induces holo α-lactalbumin aggregation by converting to its apo form. PLoS One 2014, 9, e114029 10.1371/journal.pone.0114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H. III Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: Detection of amyloid aggregation in solution. Protein Sci. 1993, 2, 404–410. 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi A.; Amani S.; Alam M. T.; Naeem A. Aggregation as a consequence of glycation: insight into the pathogenesis of arthritis. Eur. Biophys. J. 2016, 45, 523–534. 10.1007/s00249-016-1119-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty S.; Ittah V.; Bai P.; Luo L.; Haas E.; Peng Z.-y. Structure and dynamics of the α-lactalbumin molten globule: fluorescence studies using proteins containing a single tryptophan residue. Biochemistry 2001, 40, 7228–7238. 10.1021/bi010004w. [DOI] [PubMed] [Google Scholar]
- Amani S.; Shamsi A.; Rabbani G.; Naim A. An insight into the biophysical characterization of insoluble collagen aggregates: implication for arthritis. J. fluoresc. 2014, 24, 1423–1431. 10.1007/s10895-014-1424-x. [DOI] [PubMed] [Google Scholar]
- Shamsi A.; Ahmed A.; Bano B. Glyoxal induced structural transition of buffalo kidney cystatin to molten globule and aggregates: Anti-fibrillation potency of quinic acid. IUBMB Life 2016, 68, 156–166. 10.1002/iub.1471. [DOI] [PubMed] [Google Scholar]
- Santiago P. S.; Carvalho F. A. O.; Domingues M. M.; Carvalho J. W. P.; Santos N. C.; Tabak M. Isoelectric Point Determination for Glossoscolex paulistus Extracellular Hemoglobin: Oligomeric Stability in Acidic pH and Relevance to Protein– Surfactant Interactions. Langmuir 2010, 26, 9794–9801. 10.1021/la100060p. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Petty S.; Trojanowski A.; Knee K.; Goulet D.; Mukerji I.; King J. Formation of amyloid fibrils in vitro from partially unfolded intermediates of human γC-crystallin. Invest. Ophthalmol. Visual Sci. 2010, 51, 672–678. 10.1167/iovs.09-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Reid J. C.; Yang Y.-P. Utilizing dynamic light scattering as a process analytical technology for protein folding and aggregation monitoring in vaccine manufacturing. J. Pharm. Sci. 2013, 102, 4284–4290. 10.1002/jps.23746. [DOI] [PubMed] [Google Scholar]
- Biovia D. S.Discovery studio modeling environment; Elsevier, Release: 2017. [Google Scholar]
- Pandey N. K.; Ghosh S.; Dasgupta S. Fructose restrains fibrillogenesis in human serum albumin. Int. J. Biol. Macromol. 2013, 61, 424–432. 10.1016/j.ijbiomac.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Fung J.; Darabie A. A.; McLaurin J. Contribution of simple saccharides to the stabilization of amyloid structure. Biochem. Biophys. Res. Commun. 2005, 328, 1067–1072. 10.1016/j.bbrc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- Rabbani G.; Choi I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. 10.1016/j.ijbiomac.2017.12.100. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987, 46, 233–244. 10.1111/j.1574-6968.1987.tb02463.x. [DOI] [Google Scholar]
- Casey J. R.; Grinstein S.; Orlowski J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50. 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Majhi P. R.; Ganta R. R.; Vanam R. P.; Seyrek E.; Giger K.; Dubin P. L. Electrostatically driven protein aggregation: β-lactoglobulin at low ionic strength. Langmuir 2006, 22, 9150–9159. 10.1021/la053528w. [DOI] [PubMed] [Google Scholar]
- Khan S.; Mueed Z.; Deval R.; Rai P. K.; Prajapati D. K.; Poddar N. K.. Role of osmolytes in amyloidosis. In Synucleins-Biochemistry and Role in Diseases; IntechOpen, 2019. [Google Scholar]
- Katyal N.; Agarwal M.; Sen R.; Kumar V.; Deep S. Paradoxical Effect of Trehalose on the Aggregation of α-Synuclein: Expedites Onset of Aggregation yet Reduces Fibril Load. ACS Chem. Neurosci. 2018, 9, 1477–1491. 10.1021/acschemneuro.8b00056. [DOI] [PubMed] [Google Scholar]
- Loo T. W.; Clarke D. M. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev. Mol. Med. 2007, 9, 1–18. 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- Rochet J.-C. Novel therapeutic strategies for the treatment of protein-misfolding diseases. Expert Rev. Mol. Med. 2007, 9, 1–34. 10.1017/S1462399407000385. [DOI] [PubMed] [Google Scholar]
- Papp E.; Csermely P.. Chemical chaperones: mechanisms of action and potential use. In Molecular Chaperones in Health and Disease ;Springer, 2006; pp. 405–416, 10.1007/3-540-29717-0_16. [DOI] [PubMed] [Google Scholar]
- Ray S. S.; Nowak R. J.; Brown R. H.; Lansbury P. T. Small-molecule-mediated stabilization of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants against unfolding and aggregation. Proc. Natl. Acad. Sci. 2005, 102, 3639–3644. 10.1073/pnas.0408277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroy G. J.; Lai Z.; Lashuel H. A.; Peterson S. A.; Strang C.; Kelly J. W. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc. Natl. Acad. Sci. 1996, 93, 15051–15056. 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M.; Wiseman R. L.; Sekijima Y.; Green N. S.; Adamski-Werner S. L.; Kelly J. W. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc. Chem. Res. 2005, 38, 911–921. 10.1021/ar020073i. [DOI] [PubMed] [Google Scholar]
- Soldi G.; Plakoutsi G.; Taddei N.; Chiti F. Stabilization of a native protein mediated by ligand binding inhibits amyloid formation independently of the aggregation pathway. J. Med. Chem. 2006, 49, 6057–6064. 10.1021/jm0606488. [DOI] [PubMed] [Google Scholar]
- Tanaka M.; Machida Y.; Nukina N. A novel therapeutic strategy for polyglutamine diseases by stabilizing aggregation-prone proteins with small molecules. J. Mol. Med. 2005, 83, 343–352. 10.1007/s00109-004-0632-2. [DOI] [PubMed] [Google Scholar]
- Welch W. J.; Brown C. R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1996, 1, 109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie P., [4] Dialysis. In Methods in enzymology; Elsevier, 1971; Vol. 22, pp. 23–32. [Google Scholar]
- Sugai S.; Yashiro H.; Nitta K. Equilibrium and kinetics of the unfolding of α-lactalbumin by guanidine hydrochloride. Biochim. Biophys. Acta Protein Struct. 1973, 328, 35–41. 10.1016/0005-2795(73)90327-9. [DOI] [PubMed] [Google Scholar]
- Nielsen L.; Khurana R.; Coats A.; Frokjaer S.; Brange J.; Vyas S.; Uversky V. N.; Fink A. L. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry 2001, 40, 6036–6046. 10.1021/bi002555c. [DOI] [PubMed] [Google Scholar]
- Xue C.; Lin T. Y.; Chang D.; Guo Z. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. 10.1098/rsos.160696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S. A.; Ecroyd H.; Kee T. W.; Carver J. A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- Naeem A.; Amani S. Deciphering structural intermediates and genotoxic fibrillar aggregates of albumins: a molecular mechanism underlying for degenerative diseases. PLoS One 2013, 8, e54061. 10.1371/annotation/04fa421f-3dfe-466a-8c21-17a7bab1494c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M.; Healy J.; Vasudevamurthy M.; Lassé M.; Puskar L.; Tobin M. J.; Valery C.; Gerrard J. A.; Sasso L. Stability and cytotoxicity of crystallin amyloid nanofibrils. Nanoscale 2014, 6, 13169–13178. 10.1039/C4NR04624B. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrodinger L. The PyMOL molecular graphics system. Version 2010, 1, 0. [Google Scholar]
- Guex N.; Peitsch M. C. SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Morris G. M.; Huey R.; Lindstrom W.; Sanner M. F.; Belew R. K.; Goodsell D. S.; Olson A. J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi A.; Ahmed A.; Khan M. S.; Al Shahwan M.; Husain F. M.; Bano B. Understanding the binding between Rosmarinic acid and serum albumin: In vitro and in silico insight. J. Mol. Liq. 2020, 113348. 10.1016/j.molliq.2020.113348. [DOI] [Google Scholar]
- Hopkins A. L.; Groom C. R.; Alex A. Ligand efficiency: a useful metric for lead selection. Drug Discovery Today 2004, 9, 430. 10.1016/S1359-6446(04)03069-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.