Abstract
Purpose:
The eukaryotic translation initiation factor complex 4E (eIF4E) is downstream in the mammalian target of rapamycin (mTOR) pathway. This study explored expression of eIF4E and its relationship with the PTEN/AKT and RAS/MEK/ERK pathways in non–small cell lung carcinoma (NSCLC).
Experimental Design:
The status of phosphorylated eIF4E (p-eIF4E), phosphorylated AKT (p-AKT), PTEN, phosphorylated tuberin (p-TSC2), phosphorylated mTOR (p-mTOR), phosphorylated S6 (p-S6), and phosphorylated Erk1/2 (p-Erk1/2) was studied using immunohistochemical analysis applied to a tissue microarray containing 300 NSCLCs. Staining results for each antibody were compared with clinical and pathologic features, and the relationship between staining results was explored.
Results:
Overexpression of p-eIF4E, p-AKT, p-TSC2, p-mTOR, p-S6, and p-Erk1/2 in NSCLC was found in 39.9%, 78.8%, 5.1%, 46.7%, 27.1%, and 16.6% of tumors, respectively. The phenotype of p-eIF4E correlated positively with that of p-AKT, p-TSC2, and p-S6 (P < 0.001). Overall survival in NSCLC patients was significantly shorter in cases with overexpression of p-eIF4E and p-AKT alone and in combination (log-rank P < 0.001, each). Cases with underexpression of PTEN were limited (6.4%), and this phenotype did not correlate with any clinical variable. In cluster analysis, the p-AKT/p-mTOR/p-eIF4E/p-S6–positive group had significantly shorter survival compared with the survival of all cases (P < 0.001). Multivariate analysis showed that p-eIF4E overexpression is an independent prognostic factor for NSCLC (P = 0.004).
Conclusions:
This study shows that p-eIF4E expression in addition to p-AKT predicts poor prognosis in NSCLC. Moreover, the correlation between expression of p-eIF4E with p-AKT, as well as p-TSC2 and p-S6, indicates that eIF4E activation through the AKT pathway plays an important role in the progression of NSCLC.
Lung cancer remains the leading cause of cancer-related deaths in the world; the overall 5-year survival is only 16% (1). More than 75% to 85% of lung cancers are non–small cell lung carcinoma (NSCLC) at diagnosis (2).
The AKT pathway regulates many diverse biological functions. It is activated by tyrosine kinase receptor growth factors, such as the epidermal growth factor receptor, that lead to the generation of membrane-bound phosphoinositides, which then recruit and phophorylate AKT (3-5). Phosphorylated AKT results in gain or loss of function of its downstream proteins and contributes to cell proliferation, cell survival, cell size, and response to nutrient availability (3-5). The deregulation of these proteins promotes tumorigenesis in many cancers (3-5). Investigators have recently reported frequent activation of AKT kinases in a variety of tumor types, such as brain, breast, prostate, and endometrium (6-10). They also point out that the investigation of this pathway is critical because its various proteins provide attractive targets for therapy, and several inhibitors are being developed and tested in early clinical trials (4, 11, 12).
The mammalian target of rapamycin (mTOR) has been proposed to play a central role in tumor progression in the downstream signaling of the AKT pathway (13-15). mTOR has also been recognized as a promising therapeutic target because its activation can be inhibited by rapamycin or its homologue (4, 12). However, the status of mTOR and its relationship with the proteins of the AKT pathway in lung cancer are still unclear (16-18). It is known that mTOR can be activated directly by phosphorylated AKT or indirectly through the tuberous sclerosis complex, which consists of hamartin (TSC1) and tuberin (TSC2; refs. 13, 14).
Few reports describe that the loss of heterogeneity of either TSC1 or TSC2, whose gene mutations are responsible for the multiorgan syndrome lymphoangioleiomyomatosis, can be found in atypical adenomatous hyperplasia and adenocarcinoma of lung (19, 20). Little is known about the status of TSC1 or TSC2 in NSCLC and their relationships with AKT and mTOR.
Two major signaling pathways downstream of mTOR are the ribosomal S6 kinase and the 4E-binding protein 4EBP-1, both of which have been implicated in control of protein translation (21). The phosphorylation of 4EBP-1 by mTOR results in the release of a cap-binding protein eukaryotic translation initiation factor complex 4E (eIF4E), which is held inactive when bound to the hypophosphorylated 4EBP-1 complex (14, 15, 22). It is reported that many transformed cell lines express higher levels of eIF4E (23), and a variety of human cancers, such as breast, bladder, colorectum, uterine cervix, non-Hodgkin lymphoma, and head and neck cancers, overexpress eIF4E (24). Although overexpression of phosphorylated eIF4E in lung adenocarcinoma has been reported, studies dealing with its relationship with AKT or mTOR have not been fully explored (25-30). Phosphorylation of ribosomal S6 kinase and eIF4E is controlled directly and indirectly by mTOR, but their roles in the development of lung cancer remains unclear (14, 15, 22, 31).
The RAS/MEK/ERK pathway, which is one of the epidermal growth factor receptor downstream pathways, regulates cell proliferation and cell survival as the AKT pathway does (32, 33). Erk1/2, which is one of the members of this pathway, can lead to cell proliferation and protein synthesis (32, 33). A few publications have reported Erk1/2 activation in 22% to 63% of lung cancers in vivo and its association with advanced disease (25, 34-37). However, no study has reported the relationship between phosphorylated Erk1/2 overexpression and the downstream mediators of the AKT pathway in NSCLC.
Because most of the current knowledge of the AKT pathway in NSCLC is based on cancer cell lines, this study used a tissue microarray (TMA) of human NSCLC with sufficient clinical information to investigate the expression status of different proteins in the AKT pathway and their prognostic significance.
Materials and Methods
Lung cancer tissue microarray.
Three hundred surgically resected lung cancer cases were selected from the files of the Department of Pulmonary and Mediastinal Pathology of the Armed Forces Institute of Pathology (Washington, DC). All tumor samples are from the primary lung cancer at the time of original surgical resection. No patients received neoadjuvant therapy. The clinical characteristics of the patients previously published and summarized below were updated (refs. 38, 39; Supplementary Table S1). Cases included 150 adenocarcinomas and 150 squamous cell carcinomas, along with 100 adjacent normal pulmonary tissues in the same block. The detail of the assembly of the tissue microarray and gathering of the clinical information of these patients is described in previous reports (38, 39). The survival time and outcome data were available for 252 patients, with a median follow-up of 3.4 y (range, 0.1-13.6 y). The tumors were staged according to the International Union Against Cancer's tumor-nodemetastasis classification and subclassified and graded histologically according to WHO guidelines (2, 40). Approval for use of the tissue in research was obtained from the Institutional Review Board of the Armed Forces Institute of Pathology and the Office of Human Subjects Research of the NIH.
Antibody selections.
Antibodies were selected to represent key elements of the different pathway functions. Staining was done using following antibodies: phosphorylated AKT (p-AKT; clone S473; Cell Signaling Technology; 1:100 dilution), PTEN (clone 6H2.1; Cascade BioScience; 1:75 dilution), phosphorylated TSC2 [p-TSC2; polyclonal antibody; generated by Ma et al. (41); 1:500 dilution], phosphorylated mTOR (p-mTOR; clone Ser2448; Cell Signalling Technology; 1:100 dilution), phosphorylated S6 (p-S6; clone 5G10; Cell Signalling Technology; 1:250 dilution), phosphorylated eIF4E (p-eIF4E; clone 87; BD Transduction Laboratories; 1:250 dilution), phosphorylated Erk1/2 (p-Erk1/2; clone 20G11; Cell signaling Technology; 1:100 dilution).
Immunohistochemical staining.
The sections were deparaffinized with xylene, rehydrated in graded alcohols, and rinsed in distilled water. Endogenous peroxidase was blocked with 3% H2O2 in PBS for 15 min, followed by three PBS washes. Antigen retrieval was done with high citrate buffer (DAKO Cytomation) for 30 min using a decloaking chamber (Biocare Medical) or for 15 min using a microwave oven. Sections were blocked with 10% normal serum diluted in 2% bovine serum albumin-PBS for 30 min at room temperature. The sections were incubated overnight at 4°C with the specific primary antibodies. Sections were washed three times with PBS, then incubated for 30 min with a biotinylated secondary antibody (Vector Laboratories). They were then incubated for 30 min with avidinbiotin complex (Vectastain Elite ABC Kit, Vector Laboratories). The slides were washed thrice in PBS, placed in 0.5% Triton-PBS, and then developed in 3,3-diaminobenzidine (Sigma) for 3 to 10 min. Finally, the sections were counterstained with Harris hematoxylin, dehydrated and cleared in xylene, and mounted.
Scoring and interpretation of immunohistochemistry
Before scoring, specimens with no tumor cells, specimens with questionable cells from inflammatory cells, and specimens with only necrotic tissue were excluded from analysis. The scoring system used in this study was described in previous studies (38, 39). Scoring of all phosphorylated antibodies was based on distribution and intensity of staining. Distribution was scored as 0 (0%), 1 (1-50%), and 2 (51-100%) to indicate the percentage of positive cells of interest in a single core. The intensity of the signal was scored as 0 (no signal), 1 (mild expression), 2 (intermediate expression), and 3 (strong expression). According to previous studies, alveolar epithelial cells in tissues adjacent to primary tumors were referred to as internal controls. Aberrant expressions of p-AKT, p-S6, and p-Erk1/2 were judged by cytoplasmic and/or nuclear staining, whereas aberrant expressions of p-TSC2, p-eIF4E, and p-mTOR were judged by cytoplasmic staining (Fig. 1). The distribution score and intensity score were then summed into a total score of TS0 (sum = 0), TS2 (sum = 2), TS3 (sum = 3), TS4 (sum = 4), and TS5 (sum = 5). In this study, TS0 and TS2 were regarded as a negative reaction, whereas TS3, TS4, and TS5 were regarded as overexpression of p-AKT, p-mTOR, p-TSC2, p-S6, p-eIF4E. For p-Erk1/2, only TS0 was regarded as a negative reaction, in accordance with previous papers (25, 34-37). Distribution of PTEN staining was assessed in the same way as phosphorylated antibodies. TS0 or TS2 of PTEN was regarded as underexpressed when differences with clinical variables were assessed statistically. As in our previous reports (38, 39), staining was scored independently by two individuals (A. Yoshizawa and S. Shimizu) who were blinded to the clinical information. Equivocal or borderline cases were re-examined, and final total score was reached by review with WDT.
Fig. 1.
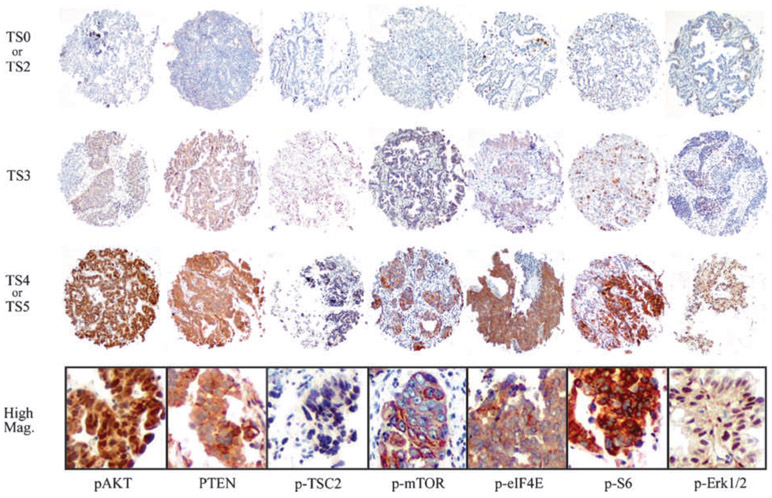
Representative examples of immunohistochemical staining by p-AKT, PTEN, p-TSC2, p-mTOR, p-eIF4E, p-S6, and p-Erk1/2 (from left column) of NSCLC specimens. The TMA cores of the first line show TS0 or TS2, those of the second line show TS3, and the third line shows TS4 or TS5 for each antibody. Diameter of tissue core, 0.6 mm. Photomicrographs of the bottom line show high magnification view of the third line TMA cores each. Original magnification, ×400.
Statistical analysis.
Pearson's χ2 test or Fisher's exact test was used to examine the association of expression of proteins with clinical characteristics such as sex, age, tumor grade, tumor size, tumor stage, and histologic subtype. To assess the correlation between any two protein expressions, Spearman's rank test was used. Univariate analysis was done using the Kaplan-Meier method to estimate survival, and log-rank tests were used to assess survival differences between groups separated by sex, age, histologic subtype, tumor grade, tumor size, tumor stage, expression of proteins, and cluster group. All P values were determined from two-sided tests. To avoid chance associations due to multiple variable correlations, we used a P value of <0.01 rather than <0.05 for significance for associations between antibody staining and clinical factors, as well as between phosphorylated proteins. Hierarchical cluster analysis was done using the Ward method. Multivariate analysis was done with a Cox regression model, including only the clinical and antibody expression markers that showed significance in univariate analysis. Tissue samples were clustered based by marker protein expression profiles. Data analysis and summary graphs were produced by the JMP statistical software package, version 8 (SAS Institute) and SPSS for Windows, version 17 (SPSS, Inc.).
Results
Relationship between clinical variables and expressions of proteins.
The 5-year survival rate for the entire cohort was 38.1%, and there was a trend for reduced survival in males compared with females (35.2% versus 44.2%; P = 0.018). Overall data characteristics, such as sex, age, tumor grade, tumor size, and stage, and which of these show significant survival differences are summarized in Supplementary Table S1. The univariate relationship between clinical variables and immunophenotypes of the analyzed pathway proteins was investigated (Supplementary Table S2), finding that the expression of p-Erk1/2 was inversely associated with tumor size in NSCLC (P = 0.010).
Status of protein expression and relationship with survival.
An assessment for p-eIF4E, p-AKT, p-TSC2, p-mTOR, p-S6, and p-Erk1/2 was available in the TMA sections for 278, 265, 274, 276, 277, and 283 cores, respectively. Identification of a positive phenotype for p-eIF4E, p-AKT, p-TSC2, p-mTOR, p-S6, and p-Erk1/2 in total NSCLC was found in 39.9% (111 of 278), 78.8% (209 of 265), 5.1% (14 of 274), 46.7% (129 of 276), 27.1% (75 of 277), and 16.6% (47 of 283) of tumors analyzed, respectively (Table 1; Fig. 1). p-mTOR overexpression was more frequently observed in squamous cell carcinoma than adenocarcinoma (P = 0.006), whereas p-Erk1/2 overexpression was more frequently observed in adenocarcinoma than squamous cell carcinoma (P = 0.002). Among these phosphorylated proteins, the 5-year survival rate was significantly lower in NSCLC patients whose tumors overexpressed p-eIF4E and p-AKT (log-rank P < 0.001 and P < 0.001, respectively), as well as in adenocarcinoma patients (log-rank P = 0.004 and P = 0.003, respectively; Table 2; Fig. 2A and B). In addition, patients with overexpression of p-AKT and p-eIF4E had significantly worse prognosis than other combinations of p-AKT and p-eIF4E (log-rank P < 0.001; Fig.2C). Overexpression of p-S6 showed a trend for association with worse prognosis (p-S6, log-rank P = 0.012) Cases with underexpression of PTEN were limited (6.4%), and these cases did not correlate with any clinical variables.
Table 1.
Protein expression by histology of NSCLC
| Antibody | P/N | NSCLC, n (%) | ADC, n (%) | SqCC, n (%) | P* |
|---|---|---|---|---|---|
| p-AKT | P | 209 (78.8) | 113 (42.6) | 96 (36.2) | 0.136 |
| N | 56 (21.2) | 24 (9.1) | 32 (12.1) | ||
| PTEN | P | 250 (93.6) | 127 (47.6) | 123 (46.1) | 0.765 |
| N | 17 (6.4) | 8 (3.0) | 9 (3.4) | ||
| p-TSC2 | P | 14 (5.1) | 11 (4.0) | 3 (1.1) | 0.058† |
| N | 260 (94.9) | 127 (46.4) | 133 (48.5) | ||
| p-mTOR | P | 129 (46.7) | 53 (19.2) | 76 (27.5) | 0.006 |
| N | 147 (53.3) | 85 (30.8) | 62 (22.5) | ||
| p-S6 | P | 75 (27.1) | 44 (15.9) | 31 (11.2) | 0.085 |
| N | 202 (72.9) | 95 (34.3) | 107 (38.6) | ||
| p-eIF4E | P | 111 (39.9) | 58 (20.9) | 53 (19.1) | 0.607 |
| N | 167 (60.1) | 82 (29.5) | 85 (30.6) | ||
| p-Erk1/2 | P | 47 (16.6) | 33 (11.7) | 14 (4.9) | 0.002 |
| N | 236 (83.4) | 108 (38.2) | 128 (45.2) |
Abbreviations: P, positive; N, negative; ADC, adenocarcinoma; SqCC, squamous cell carcinoma.
P value is for comparison of positive staining between adenocarcinoma and squamous carcinoma.
Fisher's exact test (the others were judged by χ2 test).
Table 2.
Protein expression and prognosis
| Antibody | ADC, HR (95% CI) | P | SqCC, HR (95% CI) | P | NSCLC, HR (95% CI) | P |
|---|---|---|---|---|---|---|
| p-AKT (P vs N) | 2.75 (1.44-5.94) | 0.001 | 1.57 (0.92-2.84) | 0.093 | 2.02 (1.34-3.19) | <0.001 |
| PTEN (P vs N) | 0.72 (0.34-1.86) | 0.465 | 1.61 (0.71-4.64) | 0.276 | 1.11 (0.63-2.2) | 0.72 |
| p-TSC2 (P vs N) | 1.95 (0.81-3.95) | 0.122 | 1.11 (0.06-5.26) | 0.888 | 1.82 (0.81-3.47) | 0.131 |
| p-mTOR (P vs N) | 1.09 (0.71-1.67) | 0.669 | 0.99 (0.65-1.54) | 0.999 | 1.01 (0.75-1.36) | 0.913 |
| p-S6 (P vs N) | 1.68 (1.06-2.60) | 0.025 | 1.36 (0.82-2.17) | 0.221 | 1.5 (1.07-2.06) | 0.016 |
| p-eIF4E (P vs N) | 1.8 (1.18-2.74) | 0.006 | 1.55 (1.00-2.39) | 0.048 | 1.68 (1.24-2.27) | <0.001 |
| p-ERK1/2 (P vs N) | 1.12 (0.68-1.78) | 0.621 | 1.3 (0.63-2.41) | 0.443 | 1.2 (0.81-1.73) | 0.346 |
NOTE: Univariate analysis.
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Fig. 2.
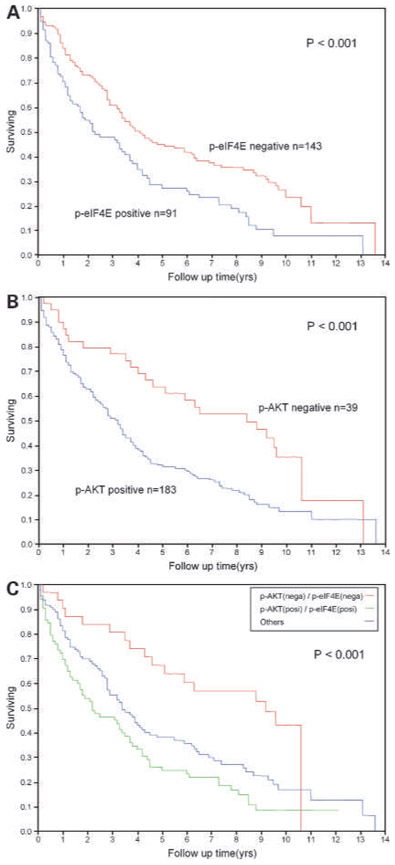
Kaplan-Meier curve comparing overall survival for AKT, p-eIF4E, and various combinations of AKT and p-eIF4E: worse survival for p-eIF4E–positive patients (blue line) compared with p-eIF4E–negative patients (red line; A), worse survival for p-AKT–positive patients (blue line) compared with p-AKT–negative patients (red line; B), and worse survival for patients with a combination of p-AKT and p-eIF4E–positive tumors (green line; n = 83) compared with those with a combination of p-AKT– and p-eIF4E–negative tumors (red line; n = 31) and the other patients (blue line; n = 108; C).
Using multivariate analysis with a Cox regression model that included sex, stage, p-eIF4E, p-AKT, and p-S6, we found that only p-eIF4E and stage were independent prognostic factors (P = 0.006 and P = 0.005, respectively; Table 3). p-AKT only showed a trend.
Table 3.
Multivariate survival analysis
| HR (95% CI) | P | |
|---|---|---|
| Sex (male vs female) | 1.64 (1.06-2.65) | 0.023* |
| Stage (I and II vs III and IV) | 1.85 (1.21-2.77) | 0.005 |
| p-AKT (P vs N) | 1.83 (1.11-3.22) | 0.016* |
| p-eIF4E (P vs N) | 1.66 (1.15-2.39) | 0.006 |
| p-S6 (P vs N) | 1.35 (0.92-1.97) | 0.126 |
Abbreviations: HR, hazard ration; 95% CI, 95% confidence interval.
P > 0.01.
Correlations between phosphorylated proteins.
The correlation between the expressions of semiquantitative biomarkers was explored using the Spearman's rank test (Supplementary Table S3). In this analysis, significant differences were found in many pairs of proteins, and its Spearman's coefficients (r) were all positive values. Expression of p-AKT and p-Erk1/2 correlated with expression of p-TSC2 (r = 0.202, P = 0.001; r = 0.252, P < 0.001) and p-S6 (r = 0.248, P <0.001; r = 0.360, P <0.001). Expression of p-eIF4E strongly correlated with expression of p-AKT, p-TSC2, and p-S6 (r = 0.375, P < 0.001; r = 0.208, P < 0.001; r = 0.366, P < 0.001). Expression of mTOR correlated with expression of p-S6 (r = 0.156, P = 0.010), although no correlation was observed between p-mTOR and p-TSC2 or p-AKT expression (P = 0.078 and P = 0.513, respectively).
Correlations between clusters grouped by expression of the proteins and survival.
Hierarchical clustering analysis was done using p-AKT, PTEN, p-mTOR, p-TSC2, p-S6, p-eIF4E1, and p-Erk1/2 long scores (Fig. 3). Fifteen groups emerged from the analysis, which had similar protein expression patterns according to the dendrogram. Among these groups, group 9 (median survival time, 0.7 years), group 10 (median survival time, 2.2 years), and group 11 (median survival time, 1.2 years) had relatively lower survival rates compared with the overall survival rates of the other groups (log-rank; group 9, P = 0.006; group 10, P = 0.010; group 11, P = 0.040), whereas group 3 (median survival time, 9.4 years) had relatively higher survival rates compared with the overall survival rates of the other groups (log-rank P = 0.019; Supplementary Fig. S1; Supplementary Table S4). All tumors in groups 9, 10, and 11 showed a positive p-AKT and p-mTOR phenotype. Furthermore, all tumors in groups 9 and 10 showed positive phenotype for p-S6 and p-eIF4E, and all tumors in groups 10 and 11 showed a positive p-Erk1/2 phenotype. Conversely, all tumors in group 3 showed a negative phenotype for p-AKT, p-TSC2, p-eIF4E, p-S6, and p-Erk1/2, although they showed a positive phenotype for PTEN and p-mTOR.
Fig. 3.
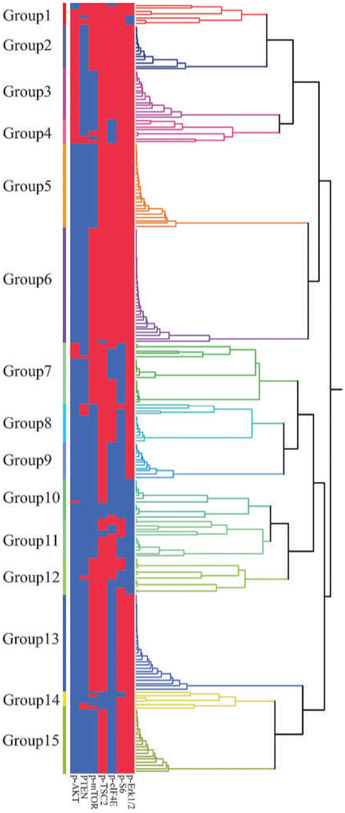
Hierarchical cluster analysis for a seven-protein immunophenotype. Each row is a different tumor, and each column is a different immunohistochemical stain. Blue bars, marker positivity; red bars, negative staining for the marker. MST, median survival time; NA, not available.
Discussion
The AKT pathway (Fig. 4) has been reported to contribute to cell proliferation, cell survival, cell size, and response to nutrient availability (3-5). Its aberrant expression has also been known to promote tumorigenesis in many cancers (5). By evaluating expression of multiple components of the AKT pathway in a large number of NSCLC using a TMA, this study was able to show for the first time the interaction between various members of the AKT pathway, including elF4E, S6, and their significance in the clinical outcome of NSCLC patients. We found that overexpression of p-eIF4E is an independent prognostic factor in NSCLC. Although it was not independent of stage, very few biological markers are found to be stronger predictors of survival than stage. It also shows the status of Erk1/2 expression in NSCLC and its relationship with the downstream mediators of the AKT pathway.
Fig. 4.
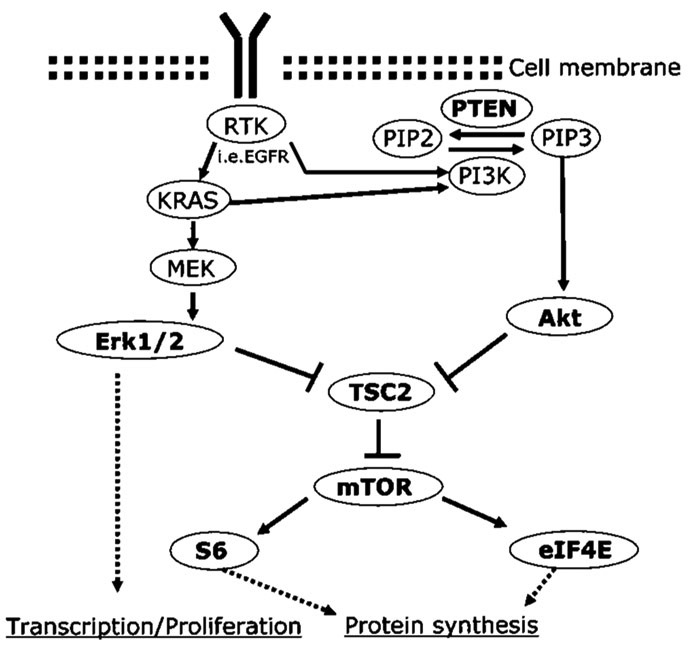
Conventional PI3K-AKT-mTOR signaling pathway and RAS/MEK/ERK signaling pathway diagram derived from in vitro studies on NSCLC. AKT activation is most directly brought about by inhibition of TSC2, which results in the activation of S6 and e-IF4E signaling and enhanced protein synthesis through the activation of mTOR. mTOR may be affected by other genetic or metabolic changes. RTK, receptor tyrosine kinase; EGFR, epidermal growth factor receptor; PIP2/3, phosphatidylinositol 4,5-/3,4,5-phosphate; PI3K, phosphatidylinositol 3-kinase.
AKT expression and the association with clinical variabilities or other proteins in the AKT pathway.
This study shows a p-AKT–positive phenotype in 78.8% of NSCLC. Tsurutani et al. (39) summarized that the reported frequency of p-AKT overexpression in NSCLC ranges between 20% and 89%. This study also found that AKT activation correlates with poor prognosis in NSCLC, confirming data from several previous studies (34, 42, 43), including a previous analysis of this same cohort of patients (39). Balsara et al. (16) reported that prevalence of p-AKT expression was similar in low stage and high stage. However, our data showed no correlation between AKT activation and tumor stage. On the other hand, p-mTOR expression was observed in 46.7% (129 of 277) of total NSCLC. Interestingly, a positive phenotype was observed in squamous cell carcinoma when compared with adenocarcinoma. These results are the first that suggest that mTOR activation may contribute to tumor progression in lung squamous cell carcinoma.
The protein coexpression section of this study found that the expression of p-AKT correlated significantly with expression of p-TSC2, p-S6, and p-eIF4E, but the expression of p-AKT did not correlate with expression of p-mTOR. The p-TSC2 phenotype strongly corresponded with expression of p-eIF4E.
In cluster analysis, groups 9 and 10, with phenotypes characterized by showing positive for p-AKT, p-mTOR, p-S6, and p-eIF4E, had significantly lower survival rates than the overall survival rates of all cases. Conversely, group 3, which showed negative for p-AKT, p-TSC2, p-eIF4E, and p-S6 but positive for p-mTOR, had significantly higher survival rates than the overall survival rates of all cases. These findings indicate that there could be a pathway in which AKT regulates TSC2 and eIF4E, dependent or independent of mTOR, and that an AKT-mTOR-S6-eIF4E–positive phenotype correlates with poor prognosis. Much literature supports the premise that mTOR is activated, either directly p-AKT or indirectly through tuberous sclerosis complex, in many types of tumors (5, 13, 14).
About lung cancer, specifically, Balsara et al. (16) described that positive staining for p-mTOR was associated with significant activation of AKT based on immunohistochemical experiments. Han et al. (17) reported that AKT small interfering RNA and rapamycin, which inhibit AKT and mTOR functions, respectively, inhibit fibronectininduced phosphorylation of p70S6K and 4E-BP1 and lung carcinoma cell proliferation. These reports indicate that AKT might contribute to proliferation of NSCLC through mTOR activation in NSCLC. In contrast, some in vitro studies have questioned the central role of mTOR as the main downstream mediator of AKT signaling, suggesting that the tuberous sclerosis complex might mediate S6K activation independent of mTOR or that S6K might directly phosphorylate mTOR (44, 45). Sun et al. (18) interestingly reported that rapamycin induces phosphorylation of AKT and eIF4E, and they seem to attenuate the growth-inhibitory effects of rapamycin in NSCLC. In the immunohistochemical observations of this study, correlations were lacking between p-mTOR and p-TSC2 and between p-mTOR and p-AKT. Taken together, these results indicate that there could be an unknown pathway through which AKT activates S6 and eIF4E independent of mTOR function or that mTOR may be influenced by additional signaling pathways in response to genetic changes or metabolic changes independently of AKT in NSCLC (14). Anagnostou et al. (46) recently reported high expression of mTOR was associated with better outcome in early-stage lung adenocarcinoma; however, we did not observe this in our data set.
eIF4E expression and the association with clinical variables.
Regulation of translation is important for the accurate expression of a broad variety of genes that function in critical processes, such as cell cycle progression and differentiation (21, 24). Deregulation at protein synthesis is associated with aberrant gene expression, leading to altered cell growth and possibly cancer. Translational initiation is controlled by multiple eukaryotic initiation factors (21, 24). Eukaryotic initiation factor 4E (eIF4E) plays a central role in the regulation of translation with a strong correlation between phosphorylation of eIF4E, eIF4F complex formation, rate of protein synthesis, and cell growth (21, 24). eIF4E exists in both phosphorylated (active) and nonphosphorylated (inactive) forms. Phosphorylation of eIF-4E is controlled by 4E-BP1, which is preferentially phosphorylated by the mTOR. mTOR thus controls protein translation, which, in turn, is crucial for proper control of cell growth and cell cycle progression (21, 24). In human and experimental cancers, function of eIF4E is enhanced either as a result of signaling through the PI3 kinase/AKT/ mTOR pathway and or the RAS/MEK/ERK pathway (26, 29). The mechanism of eIF4E overexpression, however, is still under investigation.
Recently, many studies have found overexpression of eIF4E in a variety of human cancers, such as breast, bladder, colorectum, uterine cervix, non-Hodgkin lymphoma, and head and neck cancers, showing that overexpression of eIF4E is well correlated with clinical outcomes and histologic malignancy (24). Rosenwald et al. (28) have observed overexpression of p-eIF4E in bronchioloalveolar carcinoma but not in squamous cell carcinoma. Seki et al. (27) also showed that eIF4E seems increased in peripheral lung adenocarcinomas and suggests a correlation between the magnitude of the eIF4E increase and the invasiveness of the tumors. On the other hand, Yang et al. (47) reported that there was no significant difference of eIF4E expression level between adenocarcinoma and squamous cell carcinoma of lung. In this study, p-eIF4E was overexpressed in 39.9% (111 of 278) of NSCLC, but it did not find a difference between overexpression in adenocarcinoma compared with squamous cell carcinoma. Yang et al. (47) also observed the moderate correlation of eIF4E with VEGF and cyclin D1 in NSCLC, which supports a role for eIF4E in translational regulation of proteins related to angiogenesis and growth. This study found that p-eIF4E is overexpressed predominantly in stage III or IV NSCLC cases compared with stage I or II. In addition, overexpression of eIF4E was strongly associated with worse prognoses in NSCLC patients. These results provide the first clinical documentation supporting previous molecular studies that eIF4E may contribute to tumor progression in NSCLC.
Erk expression and the relationship between Erk and the downstream mediators of the AKT pathway.
Erk1/2, which is one of the members of the RAS/MEK/ERK pathway, is known to contribute to tumorigenesis in many cancers (32, 33). Erk1/2 activation in 22% to 63% of NSCLC is reported in a few studies (25, 34-37). However, there are no reports in NSCLC correlating Erk1/2 activation with the status of the downstream mediators of the AKT pathway. This study found overexpression of p-Erk1/2 in 16.6% (47 of 281) of NSCLCs. Expression of p-Erk1/2 strongly correlated with expression of p-TSC2 and p-S6 (P < 0.001 and P < 0.001 respectively), but it did not correlate with expression of p-eIF4E (P = 0.382). Recent studies suggest that phosphorylation by Erk suppresses TSC2 function toward S6 kinase and cellular proliferation and transformation independently of AKT function (48, 49). Taken together, the findings of this study indicate that S6 may be regulated by Erk through TSC2 in NSCLC. Conversely, p-S6 correlated strongly not only with p-Erk1/2 (P < 0.001) but also with p-mTOR (P = 0.010), whereas p-Erk1/2 expression correlated weakly with p-mTOR (P = 0.014), suggesting that Erk1/2 seems to regulate S6 through mTOR.
It is striking that an association between p-AKT expression and p-Erk1/2 expression was not observed, although p-AKT and p-Erk1/2 strongly correlated with p-TSC2 and p-S6, which was shown to be an independent predictive factor in our study. These results suggest that AKT and Erk1/2 may independently contribute to tumorigenesis and survival through TSC2 in NSCLC. In cluster analysis, group 3, which included cases with loss of expression of p-AKT and p-Erk1/2, showed a more favorable prognosis (median survival time, 9.4 years; 5-year overall survival rate, 75.0%) than the other groups, suggesting that AKT and Erk1/2 are very important factors for survival in NSCLC.
In summary, these results show for the first time that TSC2, eIF4E, and S6, which are proposed downstream mediators in the AKT signaling pathway, are more likely to be phosphorylated in tumors with AKT phosphorylation in NSCLC, whereas no correlation of mTOR phosphorylation with AKT and TSC2 phosphorylation was seen. In addition, we showed that eIF4E overexpression is an independent prognostic factor in NSCLC. These findings indicate that TSC2, eIF4E, and S6 are likely regulated by AKT independent of mTOR in NSCLC. Taken together, these data lend further support that dysregulation of the AKT pathway plays an important role in the development of NSCLC but that activation of the AKT signaling pathway in NSCLC may not be an appropriate selection criterion for including patients in mTOR inhibitor treatment because Erk1/2 phosphorylation could also contribute to expression of the downstream factors in the AKT pathway, especially p-TSC2 and p-S6.
Supplementary Material
Translational Relevance.
Overexpression of eukaryotic translation initiation factor complex 4E (eIF4E) is important in many cancers in playing a fundamental role in carcinogenesis by promoting the posttranscriptional expression of cancer-related genes and as a poor prognostic factor. In our study on non–small cell lung carcinoma (NSCLC), we showed that eIF4E is a poor prognostic factor but also that the combined overexpression of eIF4E with AKT results in a worse prognosis than other AKT and eIF4E combinations. We also showed for the first time that tuberin (TSC2), eIF4E, and S6, which are proposed downstream mediators in the AKT signaling pathway, are more likely to be phosphorylated in tumors with AKT phosphorylation in NSCLC, but there was no correlation of mammalian target of rapamycin phosphorylation with AKT and TSC2 phosphorylation. These results indicate that TSC2, eIF4E, and S6 are likely regulated by AKT independent of mammalian target of rapamycin. These data may help in defining more aggressive NSCLC and in developing targeted therapies involving eIF4E or other members of the AKT pathway.
Acknowledgments
We thank Stacy Johnson for editing the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology & genetics of tumours of the lung, thymus and heart. IARC/World Health Organization of Tumours; 2004. [Google Scholar]
- 3.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol 2004;22:4217–26. [DOI] [PubMed] [Google Scholar]
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005;4:988–1004. [DOI] [PubMed] [Google Scholar]
- 5.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455–64. [DOI] [PubMed] [Google Scholar]
- 6.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol 2006;19:238–45. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Ittmann MM, Ayala G, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis 2005;8:108–18. [DOI] [PubMed] [Google Scholar]
- 8.Riemenschneider MJ, Betensky RA, Pasedag SM, Louis DN. AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res 2006;66:5618–23. [DOI] [PubMed] [Google Scholar]
- 9.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3’-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res 2003;63:2742–46. [PubMed] [Google Scholar]
- 10.Pallares J, Bussaglia E, Martinez-Guitarte JL, et al. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod Pathol 2005;18:719–27. [DOI] [PubMed] [Google Scholar]
- 11.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304:1497–500. [DOI] [PubMed] [Google Scholar]
- 12.Adjei AA. Novel combinations based on epidermal growth factor receptor inhibition. Clin Cancer Res 2006;12:4446–50. [DOI] [PubMed] [Google Scholar]
- 13.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 2005;37:19–24. [DOI] [PubMed] [Google Scholar]
- 14.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–48. [DOI] [PubMed] [Google Scholar]
- 15.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene 2006;25:6423–35. [DOI] [PubMed] [Google Scholar]
- 16.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis 2004;25:2053–9. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Khuri FR, Roman J. Fibronectin stimulates non-small cell lung carcinomacell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res 2006;66:315–23. [DOI] [PubMed] [Google Scholar]
- 18.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 2005;65:7052–8. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K, Ogura T, Yokose T, et al. Loss of heterozygosity in the tuberous sclerosis gene associated regions in adenocarcinoma of the lung accompanied by multiple atypical adenomatous hyperplasia. Int J Cancer 1998;79:384–9. [DOI] [PubMed] [Google Scholar]
- 20.Takamochi K, Ogura T, Suzuki K, et al. Loss of heterozygosity on chromosomes 9q and 16p in atypical adenomatous hyperplasia concomitant with adenocarcinoma of the lung. Am J Pathol 2001; 159:1941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggero D, Montanaro L, Ma L, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med 2004;10:484–6. [DOI] [PubMed] [Google Scholar]
- 22.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene 2006;25:6416–22. [DOI] [PubMed] [Google Scholar]
- 23.Miyagi Y, Sugiyama A, Asai A, Okazaki T, Kuchino Y, Kerr SJ. Elevated levels of eukaryotic translation initiation factor eIF-4E, mRNA in a broad spectrum of transformed cell lines. Cancer Lett 1995;91:247–52. [DOI] [PubMed] [Google Scholar]
- 24.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004;23:3189–99. [DOI] [PubMed] [Google Scholar]
- 25.Conde E, Angulo B, Tang M, et al. Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res 2006;12:710–7. [DOI] [PubMed] [Google Scholar]
- 26.Wislez M, Spencer ML, Izzo JG, et al. Inhibition of mammalian target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res 2005;65:3226–35. [DOI] [PubMed] [Google Scholar]
- 27.Seki N, Takasu T, Mandai K, et al. Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin CancerRes 2002;8:3046–53. [PubMed] [Google Scholar]
- 28.Rosenwald IB, Hutzler MJ, Wang S, Savas L, Fraire AE. Expression of eukaryotic translation initiation factors 4E and 2α is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 2001;92:2164–71. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Geng J, Wang JH, Chu XY, Geng HC, Chen LB. Overexpression of eukaryotic initiation factor 4E (eIF4E) and its clinical significance in lung adenocarcinoma. Lung Cancer 2009. [DOI] [PubMed] [Google Scholar]
- 30.Khoury T, Alrawi S, Ramnath N, et al. Eukaryotic initiation factor-4E and cyclin D1 expression associated with patient survival in lung cancer. Clin Lung Cancer 2009;10:58–66. [DOI] [PubMed] [Google Scholar]
- 31.McDonald JM, Pelloski CE, Ledoux A, et al. Elevated phospho-S6 expression is associated with metastasis in adenocarcinoma of the lung. Clin Cancer Res 2008;14:7832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279–90. [DOI] [PubMed] [Google Scholar]
- 33.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26:3291–310. [DOI] [PubMed] [Google Scholar]
- 34.Han SW, Kim TY, Hwang PG, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 2005;23: 2493–501. [DOI] [PubMed] [Google Scholar]
- 35.Han SW, Kim TY, Jeon YK, et al. Optimization of patient selection for gefitinib in non-small cell lung cancer by combined analysis of epidermal growth factor receptor mutation, K-ras mutation, and Akt phosphorylation. Clin Cancer Res 2006;12:2538–44. [DOI] [PubMed] [Google Scholar]
- 36.Vicent S, Lopez-Picazo JM, Toledo G, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer 2004;90:1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukohara T, Kudoh S, Yamauchi S, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer 2003;41:123–30. [DOI] [PubMed] [Google Scholar]
- 38.Fukuoka J, Fujii T, Shih JH, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indications in non-small cell lung cancer. Clin Cancer Res 2004;10:4314–24. [DOI] [PubMed] [Google Scholar]
- 39.Tsurutani J, Fukuoka J, Tsurutani H, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol 2006;24: 306–14. [DOI] [PubMed] [Google Scholar]
- 40.UICC, International Union Against Cancer. TNM classification of malignant tumours. 6th ed. New York: Wiley and Sons; 2002. [Google Scholar]
- 41.Ma L, Teruya-Feldstein J, Bonner P, et al. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res 2007;67:7106–12. [DOI] [PubMed] [Google Scholar]
- 42.David O, Jett J, LeBeau H, et al. Phospho-Akt overexpression in non small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res 2004;10:6865–71. [DOI] [PubMed] [Google Scholar]
- 43.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt over expression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer 2006;51:181–91. [DOI] [PubMed] [Google Scholar]
- 44.Jaeschke A, Hartkamp J, Saitoh M, et al. Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J Cell Biol 2002;159: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 2005;280:26089–93. [DOI] [PubMed] [Google Scholar]
- 46.Anagnostou VK, Bepler G, Syrigos KN, et al. High expression of mammalian target of rapamycin is associated with better outcome for patients with early stage lung adenocarcinoma. Clin Cancer Res 2009;15:4157–64. [DOI] [PubMed] [Google Scholar]
- 47.Yang SX, Hewitt SM, Steinberg SM, et al. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol Rep 2007; 17:281–7. [PubMed] [Google Scholar]
- 48.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and −2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem 2003;278:37288–96. [DOI] [PubMed] [Google Scholar]
- 49.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 2005;121: 179–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


