Abstract
Background
Shortages in personal protective equipment, especially respiratory protective devices (respirators), occurred during the initial phase of the coronavirus disease 2019 pandemic. Sterilization of used respirators can reduce these shortages. In this study, respirator testing was carried out after a single cycle of sterilization.
Aim
To determine if steam sterilization and re-use can be applied safely for used respirators.
Methods
An aqueous solution of NaCl (0.02% w/v) was nebulized and passed through a sample of respirator material in a cabinet. Passing particle concentrations were measured directly from the cabinet and via the filter material of the respirator for particles ≥0.3 μm, ≥0.5 μm and ≥1.0 μm in diameter.
Findings
Only three of 10 steam sterilized respirators met the requirement of 94% filtration efficiency.
Conclusion
Heat sterilization cannot be applied generically for safe re-use of respirators.
Keywords: Respirators, FFP2, KN95, Filter efficiency, Disinfection, Heat sterilization
Introduction
The coronavirus disease 2019 (COVID-19) pandemic quickly resulted in enormous demand for personal protective equipment, especially respiratory protective devices (respirators). Initially, China faced a huge demand for respirators [1]. The resulting shortage in respirators spread to other countries as national guidelines made recommendations for the use of respirators by healthcare workers and the public [2,3]. Possible solutions for the shortage in respirators included investigating the most promising decontamination procedures to enable safe re-use. Rubio-Romero et al. [4] reviewed the options for re-use. They concluded that autoclaving/high-temperature steam treatment (121 °C) is not fully recommended. Despite this information, several healthcare institutions, including many hospitals, have continued to explore the effects of heat sterilization on the filter efficiency of respirators. Insight into these effects may allow strained healthcare institutions to re-use respirators if their filter efficiency is not negatively affected by the sterilization process.
Given the enormous demand for respirators, particularly FFP2 and comparable non- Conformité Européenne (CE)-marked KN95, KP95, N95, P95 and R95 respirators, in intensive care units with patients with COVID-19, The Netherlands Organisation for Applied Scientific Research decided to modify their system to test respirators. Note that CE marking should not be confused with ‘China Exported’ marking. The system was developed previously to test the protective properties of packaging material to protect medical equipment after heat sterilization against penetration of contaminants, known as the ‘final pack test’ [5]. This test determines the ability to keep particles of 1-μm diameter (average size of bacteria) out of the packaging after cooling down after sterilization. In the modified system set up to test respirator materials, a range of particles were used ranging in size between 0.3 μm and 1 μm, thus including smaller particles.
The system offers the possibility to determine the penetration of 0.3-, 0.5- and 1.0-mm particles through a respirator at a flow of 28 L/min. These settings differ from the NEN EN 149:2001+A1:2009 [6] European standard, where penetration of material is evaluated at a flow rate of 95 L/min with a polydispersed aerosol of 60–100 nm with a geometric standard deviation of 2–3. Although the set-up is not in agreement with the European standard, the modified system offers the possibility to compare the protective properties of respirators. The current testing protocol also allows for the evaluation of respirators certified according to the Chinese GB2626-2019 standard or the American NIOSH 42 CFR 84 standard.
This paper describes the modification of the test system and implementation of this system to determine the effect of sterilization on the filtering efficiency of respirators, to provide certainty about possible re-use of FPP2 and KN95 respirators based on confirmation of retained filter properties of the respirator material.
Methods
Filter function test unit
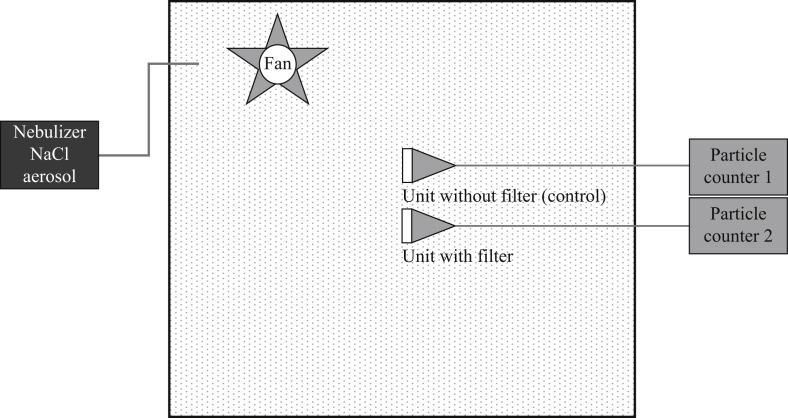
Two test units with a circular dimension of 50-mm diameter were placed in a conditioned cabinet of approximately 1 m3. One of these units was covered by respirator material with a clamp to prevent leakage, and the other remained completely exposed. An aqueous solution of NaCl (0.02% w/v) was nebulized in the cabinet. The resulting particles were sampled with a flow of 28 L/min (1 ft3/min) via both units, with and without respirator filter material, through equal-length Tygon tubing in the cabinet connected to polyurethane tubing outside the cabinet, and subsequently through a Lighthouse Solair particle counter 3100 with respirator material and a Lighthouse Solair particle counter 1100 without respirator material in each channel. These particle counters detected and quantified particles of 0.3-, 0.5- and 1.0-μm diameter for a period of 10 min. The set-up of the test system is presented schematically in Figure 1 .
Figure 1.
Test system for analysing the filter efficiency of respirator material. Nebulized particles were distributed evenly in the space within the cabinet by a fan. The units with and without a filter were connected by equal-length plastic tubing (Tygon and polyurethane, respectively) to validated particle counters that sampled air with a yield of 28 L/min while counting the particles. Sampling and counting of particles were performed for 10 min per test.
Comparative testing of filter efficiency of respirator material
The higher the filter efficiency of the respirator material, the more particles are retained. From the number of particles detected in the surrounding air which went through the unit in the channel without respirator material and the number of particles that penetrated through the respirator, the filter efficiency of the sample of a sterilized respirator was calculated and compared with the filter efficiency of a new reference respirator (non-sterilized) of the same type and brand. Analyses of the respirators were performed in triplicate from different respirators. For each particle size, filter efficiency was calculated using the equation below:
Based on the NEN EN 149:2001+A1:2009 criteria for FFP2 respirators, the following minimum filter efficiency of respirator materials was used in this study: ≥94% for 0.3 μm; ≥99% for 0.5 μm; and ≥99% for 1.0 μm.
New respirators that met the above-mentioned criteria set for this study were included for subsequent heat sterilization for 15 min at 121°C in an autoclave. The respirators included in the test are listed in Table I . Testing of the filter material was performed at least in triplicate. According to the European standard for performance criteria for respiratory protection, FFP respirators should be judged on filter efficiency and other aspects including face seal leakage, leakage of the exhalation valve leakage if fitted, breathing resistance, field of vision, and compatibility with skin. As these tests are performed on the faces of users that are not uniform, these tests require a relatively large number of replicates. In the filter efficiency experiments, the unit with a mounted respirator was uniform and did not allow any leakage because of the use of a tightly closed clamp around the respirator material. As the passage of particles through the respirator material was guaranteed, triplicate measurements of filter efficiency were assumed to be sufficient.
Table I.
Respirators included in the filter efficiency test
| Respirator brand name | Production location | Sample code |
|---|---|---|
| Medicon ref: 2092S-WH | France | FFP2-1 |
| 3M maskers Aura 1862 + | UK | FFP2-2 |
| Meixin MX-2005 CE 2797 FFP2 NR | China | FFP2-3 |
| Prot. Mask (KN95) Folding T 0004-20200317 Blue | China | KN95-1 |
| WEIHUI KN95 9801 | China | KN95-2 |
| YC KN95 | China | KN95-3 |
| Honeywell 2211 FFP2 | Tunisia | FFP2-4 |
| 3M 9320 FFP2 | UK | FFP2-5 |
| Fosan Nanhai Plus Medical KN95 | China | KN95-4 |
| KN95 – FFP2 LMX-520 | China | KN95-5 |
If the disinfected respirators performed comparably with the new reference respirators (non-disinfected) and met the criteria set above, the specific disinfection method applied (in this case, steam sterilization for 15 min at 121°C) was considered to be acceptable. If disinfected respirators showed reduced filter efficiency, they were judged to be unsafe for use by healthcare professionals caring for patients with COVID-19.
Results
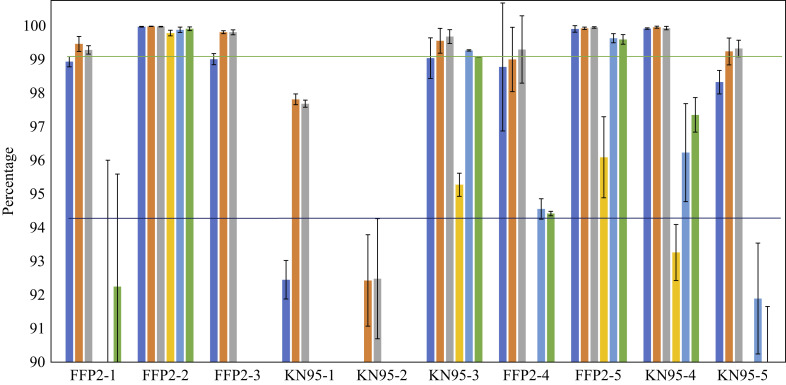
Determination of the filter efficiency of respirator materials showed large differences based on baseline analyses of the unused respirators. The best performing respirators were FFP2-2, FFP2-5 and KN95-4, as can be seen in Figure 2 . This figure also shows the results for respirators after one sterilization cycle, except for those respirators that did not comply with the pre-set criteria described above. As such, KN95-1 and KN95-2 respirators were not included in sterilization testing. The results shown in Figure 2 indicate the robustness of FFP2-2 respirators. These respirators only showed marginal reduction in filter efficiency after sterilization, while the other respirators showed a large decrease in filter efficiency. However, in addition to FFP2-2 respirators, KN95-3 and FFP2-5 respirators also met the minimum requirements for filter efficiency described above.
Figure 2.
Filter performance of respirator material before and after heat sterilization at 121°C for 15 min. The absence of coloured bars means that the efficiency score was below 90%. KN95-1 and KN95-2 respirators were not tested following sterilization due to underperformance before sterilization based on pre-set criteria; as such, post-sterilization filter performance of these respirators is not included in the figure. Dark blue bars, 0.3 μm before sterilization; orange bars, 0.5 μm before sterilization; grey bars, 1 μm before sterilization; yellow bars, 0.3 μm after sterilization; light blue bars, 0.5 μm after sterilization; green bars, 1 μm after sterilization.
Discussion
While the FFP2 respirators (CE-marked) met the pre-set minimal criteria for filter efficiency, non-CE-marked respirators may also be effective for filtering particles based on the observations in this study with the KN95-3, KN95-4 and KN95-5 respirators. A similar observation was described by Wezel et al. [7]. They concluded that a shortage of CE-approved respirators can be resolved by using non-CE-marked KN95 respirators after applying simple in-house testing. The testing of non-CE-marked respirators is advised by the authors based on their test experiences, as many of the supplied certificates appear to be forged. Apart from using these alternative non-CE-marked respirators, heat sterilization has been suggested as an alternative in the fight against respirator shortages [8]. This appears to be a valid option for some brands of respirators, but certainly not for the majority. Although this study only included a limited number of FFP2 and KN95 respirators, it demonstrated that many brands cannot be re-used safely after heat sterilization as they no longer meet the minimum required filter efficiency. However, simple in-house testing may provide essential insight into whether heat sterilization can be used for a particular respirator brand.
In conclusion, heat sterilization cannot be applied generically for safe re-use of respirators. Of the 10 types of respirators tested in this study, only FFP2-2, KN95-3 and FFP2-5 respirators were able to withstand the conditions of heat sterilization. All other FFP2 or KN95 respirators showed a decrease in filter efficiency of the respirator material below the threshold value after sterilization, or did not even meet the pre-set criteria before sterilization.
The FFP2 and KN95 respirators currently on the market are primarily developed for single use. In line with this, re-use after heat sterilization should not become common practice without thorough testing.
Acknowledgements
The authors wish to thank Daniel van Rijswijk and Volmar Hatt for their technical assistance and modification of the test rig.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Wu H., Huang J., Zhang C.J.P., He Z., Ming W.-K. Facemask shortage and the coronavirus disease (COVID-19) outbreak: reflection on public health measures. EClinicalMedicine. 2020;21:100329. doi: 10.1016/j.eclinm.2020.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8:434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2020. Advice on the use of masks in the context of COVID-19: interim guidance, 5 June 2020.https://apps.who.int/iris/handle/10665/332293 Available at: [last accessed November 2020] [Google Scholar]
- 4.Rubio-Romero C.J., Del Carmen Pardo-Ferreira M., Antonio Torrecilla García J., Calero-Castro S. Disposable masks: disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf Sci. 2020;129:104830. doi: 10.1016/j.ssci.2020.104830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastelein J., van der Vossen J.M.B.M. Einfluss des Prionen-Sterilisationszyklus auf die bakterielle Barrierewirkung von Sterilisationsvlies im Endverpackungstest [Influence of prion sterilisation cycle on the bacterial barrier performance of sterilisation wrap according to the final pack test] Zentralsterilisation. 2013;21:277–284. [Google Scholar]
- 6.NEN-EN 149:2001+A1:2009 . marking; 2009. Respiratory protective devices – filtering half masks to protect against particles – requirements, testing. [Google Scholar]
- 7.Van Wezel R.A.C., Vrancken A.C.T., Ernest M., Laurensse J., van Doornmalen Gomez Hoyos J.P.C.M. In-hospital verification of non-CE-marked respiratory protective devices to ensure safety of healthcare staff during the COVID-19 outbreak. J Hosp Infect. 2020;105:447–453. doi: 10.1016/j.jhin.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Man P., van Straten B., van den Dobbelsteen J., van der Eijk A., Horeman T., Koeleman H. Sterilization of disposable face masks by means of standardized dry and steam sterilization processes; an alternative in the fight against mask shortages due to COVID-19. J Hosp Infect. 2020;105:356–357. doi: 10.1016/j.jhin.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]