Abstract
Background
Shufeng Jiedu capsules (SFJDC), a patented herbal drug composed of eight medicinal plants, is used for the treatment of different viral respiratory tract infectious diseases. Based on its antiviral, anti-inflammatory and immunoregulatory activity in acute lung injury, SFJDC might be a promising candidate for the treatment of COVID-19.
Purpose
To evaluate the antiviral and anti-inflammatory properties and to discover the mechanism of action of SFJDC as a potential drug for the treatment of COVID-19. Furthermore, the study should determine the clinical effectiveness of SFJDC for the treatment of COVID-19.
Design
We analyzed the antiviral and anti-inflammatory effects of SFJDC in a HCoV-229E mouse model on lung index, virus load in the lung, the release of cytokines, and on T- and B-lymphocytes. The mechanism of action was further investigated by network analysis. Additionally, we investigated data from a clinical pragmatic real-world study for patients with confirmed COVID-19, to evaluate the clinical effect of SFJDC and to determine the best time to start the treatment.
Results
SFJDC significantly reduced the virus load in the lung of HCoV-229E mice (from 1109.29 ± 696.75 to 0 ± 0 copies/ml), decreased inflammatory factors IL-6, IL-10, TNF-α, and IFN-γ in the lung, and increased the amount of CD4+ and CD8+ cells in the blood compared to the model group. Network analysis revealed that SFJDC reduces the activity of NFκB via several signaling pathways. Quercetin, wogonin, and polydatin bind directly to the main protease (Mpro) of SARS-CoV-2.
Clinical data showed that SFJDC, added to standard antiviral therapy (AVD), significantly reduced the clinical recovery time of COVID-19 and fatigue (from 3.55 ± 4.09 to 1.19 ± 2.28 days) as well as cough (from 5.67 ± 5.64 to 3.47 ± 3.75) days compared to AVD alone. SFJDC therapy was significantly more effective when used within the first 8 days after the onset of symptoms.
Conclusion
SFJDC might be a promising drug for the treatment of COVID-19, but large-scale randomized, double-blinded, placebo-controlled clinical trials are needed to complement the real-world evidence. It might be beneficial to start SFJDC treatment as early as possible in suspected cases of COVID-19.
Keywords: SARS-CoV-2, COVID-19, Pneumonia, Shufeng Jiedu, Immunomodulation, Inflammation, Antiviral, Mpro
Abbreviations: SFJDC, Shufeng Jiedu Capsule; TCM, Traditional Chinese Medicine; Mpro, Main protease; AVD, Antiviral drugs; n.s., not significant
Introduction
The rapid spread of COVID-19 around the world has resulted in enormous financial efforts for the research and development of new drugs and vaccines. In spite of increasing understanding of SARS-CoV-2 and COVID-19, up to now, no clinical trial has shown a validated significant effect for the treatment of patients with mild and moderate symptoms (Li et al., 2020, Jean et al., 2020). Traditional Chinese Herbal Medicine (TCM) to combat COVID-19 got international attention because it was regularly used during the pandemic. TCM traditionally uses combinations of herbs that can be seen as a mixture of different active components. Hence, binding of active components to different targets can influence distinct signal pathways, producing synergistic effects, e.g. for the treatment of viral respiratory infections (Li et al., 2017, Tao et al., 2017). TCM was already used successfully for the treatment of SARS in 2003 and influenza A (H1N1) in 2009 (Leung, 2007, Li et al., 2016).
One of the promising herbal drugs for the treatment of COVID-19, SFJDC is composed of eight medicinal plants. This drug showed clinical effectiveness for the treatment of upper respiratory tract infections and infectious diseases, such as influenza A (H1N1) by its anti-inflammatory, immunomodulating and antiviral properties (Li et al., 2017, Ji et al., 2020, Yuan et al., 2018, Xi et al., 2010). Since 2009, SFJDC has been used in China for treating H1N1 upper respiratory tract infection by governmental recommendation (NHC 2010) and it represents a first-line TCM in the prevention and treatment of influenza symptoms.
Previous studies showed that SFJDC can effectively reduce the inflammation and immunoregulatory activity during lipopolysaccharide (LPS)-induced acute lung injury; increase the partial pressure of oxygen in the lung tissue; reduce the lactic acid level; inhibit IL-1β and TNF-α inflammatory factors; inhibit P-selectin, TGF-β, KC, C-Jun/AP-1 and NF-κB mRNA; and regulate proteins and key inflammatory pathways, e.g. MAPK/NF-κB signaling pathway (Yuan et al., 2018, Tao et al., 2014). Accordingly, Basis research data indicate that SFJDC is a promising candidate for the treatment of COVID-19 because lung inflammation injury is a major cumulative response to COVID-19.
HCoV-229E is one of seven coronaviruses that can affect humans. It is associated with a range of respiratory symptoms like common cold, pneumonia, and bronchiolitis. In the present study, we used a mice model to further elucidate the antiviral and anti-inflammatory effects of SFJDC. To enlighten the mechanism of action, the main targets and pathways of bioactive compounds were investigated, to find further explanations of its anti-inflammatory and immunomodulating effect. Subsequently, we investigated single compounds of SFJDC which have potential antiviral effects by direct inhibition of SARS-CoV-2 main protease (Mpro, also called 3CLpro).
Real-world studies acquire data regarding the effects of health interventions, which provide data that represent the real-life drug treatments outside controlled RCTs (Zuidgeest et al., 2017). According to a Cochrane analysis, effect estimation is comparable for both trial designs (Anglemyer et al., 2014), but could give different answers to the same clinical questions.
In the first phase of the COVID-19 pandemic, pragmatic treatment of the infected patients was urgent. Hence, the General Office of the Chinese National Health Commission published guidelines for treatment based on theoretical considerations, as well as the transfer of knowledge about other viral infections, which included the use of western antiviral drugs (AVD) (e.g. Lopinavir/Ritonavir, Umifenovir) as well as TCM (e.g. Lianhua Qingwen Capsules, Shufeng Jiedu Capsules (SFJDC) (National Health Commission and State Administration of Traditional Chinese Medicine 2020). This form of management was the best available evidence for treating COVID-19 in this phase of the pandemic and, representing real-world data, became standard medical practice in China. On the basis of this data, we investigated the effectiveness of SFJDC on mild and moderate COVID-19 patients in a clinical real-world pragmatic study to verify prior clinical studies. These studies suggested that the combination of SFJDC with western AVD might have a significant effect on the improvement of the clinical symptoms of mild to moderate COVID-19 pneumonia (Qu et al., 2020, Chen et al., 2020).
Methods
Animal study
Medication
SFJDC (batch number: 3190923, approved and patented by the Chinese Food and Drug Administration in 2009) clinical drug, was purchased from Jiren Pharmaceutical (Cat. Z20090047; Anhui, China). SFJDCs are composed of Polygonum cuspidatum, Forsythia suspensa, Isatis indigotica, Bupleurum chinense, Patrinia scabiosifolia, Verbena officinalis, Phragmites communis and Glycyrrhiza uralensis (see supplement Table 3 for details) and are manufactured according to the Pharmacopoeia of the People’s Republic of China.
Table 3.
Comparison of symptoms and disease course of AVDs only versus AVDs plus SFJDC.
| Duration of (days) | AVD only (n = 33) | AVD + SFJD (n = 43) | p value |
|---|---|---|---|
| Asymptomatic period before virus-negative in NP swabs | 4.22±5.36 | 8.98±6.11 | <0.005 |
| Asymptomatic period, before virus-negative in NP swabs, blood, urine, stool, | 7.24±5.45 | 10.53±7.42 | < 0.05 |
| Fatigue | 3.55±4.09 | 1.19±2.28 | <0.005 |
| Cough | 5.67±5.64 | 3.47±3.75 | <0.05 |
| Fever | 6.30±3.98 | 6.53±3.58 | n.s. |
| Pharyngalgia | 0.70±2.86 | 1.05±2.57 | n.s. |
Animals
Forty Balb / c mice, SPF grade, weight 14 ± 1 g, 20 males and 20 females were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. Mice were fed normal mouse chow and water ad libitum and were maintained under standard conditions with air filtration. All animal studies were approved by the Ethics Committee of the Institute of Chinese Materia Medica (2020D006).
Mouse infection and treatment
Forty Balb / c mice were randomly divided into normal control group (Normal), model control group (Model), SFJDC high-dose (SFJDC H) and SFJDC low-dose (SFJDC L) group, 10 mice in each group (five male, five female). Except for the normal control group, the mice were nasally infected with 50 µl 100TCID50 HCoV-229E virus solution after mild anesthesia on day 1 and day 3. On the first day of infection, each group was administered SFJDC orally, 0.2 ml/10g body weight according to Table 1 , once a day for 4 days. The daily dose of SFJDC was adjusted in accordance with the dosage for clinical use (human daily dosage: 6.24 g of dried herbal extract (DHE) which corresponds to 32.4 g crude herbal drug (CHD); SFJFC L is equal to the clinical equivalent dose used in humans and SFJDC H is twice higher. On day 5 after the first time of infection, blood was collected from the orbits and the lungs were dissected for further analysis.
Table 1.
Treatment of Balb/c mice.
| Group | Drug | Concentration |
|---|---|---|
| Control | Distilled water | 0 g/kg |
| Model | Distilled water | 0 g/kg |
| SFJDC-high | SFJDC | 11.8 g/kg CHD (2.28 g/kg DHE) |
| SFJDC-low | SFJDC | 5.9 g/kg CHD (1.14 g/kg DHE) |
Lung index
The lung was weighed after lung dissection and the following equations were used to calculate the lung index and inhibition rate:
Lung Index = [Lung Wet Weight (g) / Body Weight (g)] × 100
| Lung Index Inhibition Rate = | average lung index of model control group-average lung index of administration group | × 100% |
| average lung index of model control group-average lung index of normal control group |
HCoV-229E virus detection in lung tissue
After dissection of the mice, the lung tissue was collected and stored at -80 ℃ until further use. Virus RNA was isolated using the QIAamp virus RNA purification kit (Kai Jie Enterprise Management Shanghai Limited, China) according to the manufacturer’s instructions. The isolated RNA was dissolved in 20 μl of diethylpyrocarbonate (DEPC) water and stored at -80 ℃ until further use. Real-time PCR was conducted by using the Human Coronavirus (HCoV-229E) Real-time RT-PCR kit (Shanghai Zhijiang Biotechnology Co., Ltd., China). Quantitative detection of the viral nucleic acid load was performed according to the manufacturer’s instructions, using positive control in the kit to generate a standard curve on the real-time system. PCR products were analyzed using QuantStudioTM Design & Analysis software v1.5.1 (QuantStudioTM 5 Real-time instrument, Thermo Fisher Scientific, USA).
Elisa measurement of inflammatory factors in mouse lung tissue
Lung samples were homogenized in physiological saline using an ultrasonic cell disrupter and centrifuged at 1000 × g for 10 min using a low-temperature high-speed centrifuge at -4 °C. Samples were stored as aliquots at -80 °C until further use.
Inflammatory factors were measured with Mouse IL-6 ValukineTM Elisa Kit, Mouse IL-10 ValukineTM Elisa Kit, Mouse TNF-alpha ValukineTM ELISA Kit, and Mouse IFN-gamma ValukineTM ELISA Kit (Bio-Techne, USA) according to the manufacturer’s instructions using an EnSpire Multimode Plate Reader (PerkinElmer, USA).
Flow cytometric detection of T lymphocyte subsets and B-lymphocytes in peripheral blood
Blood samples were centrifuged in phosphate buffered saline (PBS), and after removing the supernatant RBC, lysate (TONBO biosciences, USA) was added and the samples were further treated according to the manufacturer’s instructions. The final cell suspension was incubated with the following antibodies according to the instruction of the manufacturer: PE-labeled anti-mouse CD19, PerCP-Cy5.5-labeled anti-mouse CD4, and APC-labeled anti-mouse CD8a (TONBO biosciences, USA). Flow cytometric analysis was conducted on an Accuri C6 Plus flow cytometer (BD biosciences, USA).
Network analysis
Prediction of potential targets of bioactive compounds in SFJDC
The chemical structures of potentially bioactive compounds in SFJDC in Mol2 or MDL SDF format were screened against the pharmacophores in the PharmMapper database (Guimarães and Cardozo, 2008) to predict potential targets. After that procedure, the related pathways were annotated and analyzed by Molecule Annotation System (MAS 3.0, http://bioinfo.capitalbio.com/mas3/) and Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/). In identifying potential targets and pathways, which are related to immunomodulation, inflammation and direct antiviral effects, Gene Ontology (GO) analysis was performed by using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics resources 6.8 software and the enriched KEGG pathways of targets (p < 0.5 by Fisher’s exact test, FDR < 0.05). The figure for the network mechanism of SFJDC was created with Cytoscape 2.6.0 (http://cytoscape.org/).
Docking simulation of bioactive compounds in SFJDC on COVID-19 Mpro
The structure of COVID-19 virus Mpro (PDB ID: 6LU7) was used to generate receptor grid for docking simulations. The difference of protein-ligand binding affinities in binding free energy is computed for each molecular species, including complex, ligand, and protein. These complexes were further optimized and re-scored by using the MM-GBSA module of Schrödinger (Guimarães and Cardozo, 2008).
Clinical real-world pragmatic study
Study location and setting
Routinely collected data of hospitalized patients from one regular care inpatient ward, specialized for COVID-19 of the Shanghai Public Health Clinic Center, were extracted from existing electronic patients’ health records (CDCs reporting forms) within 3 weeks from 22 January to 11 February 2020. We obtained epidemiological, demographic, clinical, laboratory, management, and outcome data. Clinical outcomes were followed up to 10 March 2020 (hospital dismission of all recruited patients).
The study was approved by the Shanghai Public Health Clinic Center Ethics Committee (YJ-92 2020-S015–02). All patients gave their written informed consent to participate in the study and were informed that the relevant data would be published.
The treatment was in accordance with the guidelines released by the General Office of Chinese National Health Commission (National Health Commission and State Administration of Traditional Chinese Medicine 2020), which represented the best available evidence for treating COVID-19. This evidence represented real-world data because it was the standard medical care in this phase of the pandemic in China. Only marketed commercial drugs were used. The individual treatment regimen was based on the patient’s condition according to the assessment of the attending clinicians. In this study there were no rules for the physicians how to treat patients, besides the current medical practice; diagnostic methods were performed in accordance with medical practice.
Study medications
Umifenovir tablets (Arbidol, Ouyi Pharmaceutical Co., Ltd. of Shiyao group, 0.2 g, t.i.d.) or Lopinavir/Ritonavir Tablets (Abbott, Germany, two tablets, t.i.d) and SFJDCs (Anhui Jiren Pharmaceutical Co., Ltd., 2.08 g, t.i.d.) were used.
Twelve patients received antiviral therapy with Umifenovir, 19 with Lopinavir/Ritonavir alone and two with Umifenovir plus Lopinavir/Ritonavir. Twenty-five patients received Umifenovir plus SFJD, nine Lopinavir/Ritonavir plus SFJD, and nine Umifenovir plus Lopinavir/Ritonavir plus SFJDC.
Population and outcomes
Seventy-six adults, requiring only regular care, fulfilled the inclusion criteria of confirmed COVID-19 with mild (n ==3) or moderate (n ==73) symptomatology, according to the classifications released by the General Office of Chinese National Health Commission (National Health Commission and State Administration of Traditional Chinese Medicine 2020). Written informed consent was obtained.
Patients with mental disease, history of drug abuse or dependence, allergic reactions to the medication(s), or who participated in a clinical trial within the last 3 months were excluded, as were pregnant or lactating women. Three patients were excluded and had to leave the regular care ward because they needed assisted ventilation treatment in the ICU.
The aim of the evaluation was to determine whether addition of SFJDC to standard AVD improves the symptomatology of COVID-19 patients. Therefore, we compared real-world data from patients who only got AVD (n ==33) with patients who received AVD plus SFJDC (n ==43). The number of participants needed for this study was based on prior clinical trials comparing AVD and AVD ++SFJDC (Qu et al., 2020, Chen et al., 2020). Subsequently, in taking this into account, we decided that for a 3-week evaluation period of real-world data, a sufficient set of data was made available for comparing AVD and AVD ++SFJDC.
Primary outcome was the duration of time from the onset of symptoms to the detection of negative virus tests in oral/nasopharyngeal swabs. Secondary outcomes were the duration of time from onset of symptoms to detection of negative virus tests in all specimens (oral/nasopharyngeal swabs, blood, stool); the duration of clinical symptoms, such as cough, fever, pharyngalgia, and fatigue, as well as the influence of the commencement of SFJDC treatment on the primary outcome.
COVID-19 detection
COVID-19 was confirmed by real-time RT-PCR using the protocol of the National Institute for Viral Disease Control and Prevention (China). The nasopharyngeal, oropharyngeal swabs, urine, stool (once every three days), serum (two samples acute and convalescent possibly 2–4 weeks after acute phase) of all patients were collected for viral nucleic acid analysis by reverse-transcriptase polymerase chain reaction (RT-PCR) until two successive (> 24 h) negative results were obtained. Negative RT-PCR results from at least two consecutive sets of nasopharyngeal/throat swabs were collected at least 24 h apart. Four negative specimens are needed to meet this requirement.
Statistical analysis
Statistical analysis for animal experiments was conducted with Graphpad Prism 7.0 software using one-way ANOVA analyses followed by Tukey’s multiple comparison procedures.
Clinical data were analyzed by OriginPro 9.0 using the Shapiro–Wilk test for normality (p < 0.05) and the Two-Sample test for variance (p < 0.05). When normality was rejected, the Man–Whitney U test was conducted. For normally distributed data, t-test was used in case of equal variance and Welch’s correction was applied if data showed an unequal variance. The correlation was determined by Pearson's Correlation Coefficient. P < 0.05 was considered statistically significant.
Results
Animal study
Lung index
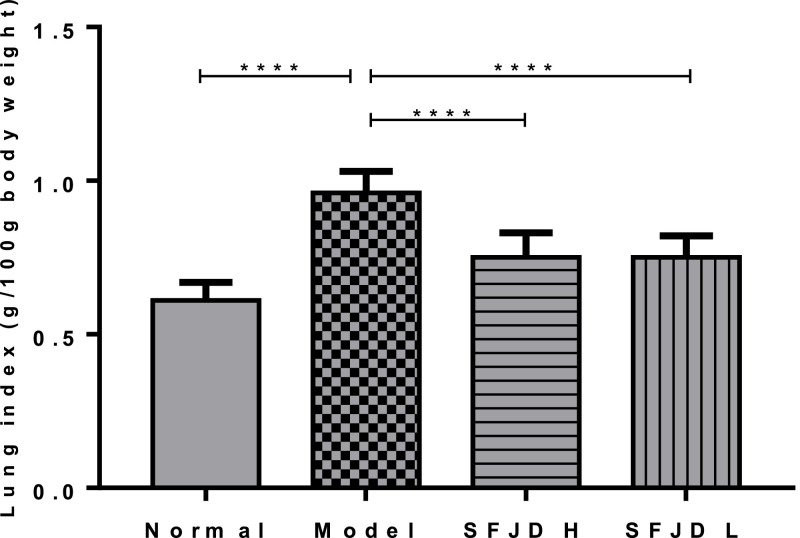
The results in Fig. 1 show that after infecting mice with HCoV-229E, the lung index of the mice increased significantly, and there was a significant difference compared with the normal control group (p < 0.0001). High and low doses of SFJD can inhibit the increase of lung index and their inhibition rates were 58.92% and 60.21%% respectively – rates statistically different from the model control group (p < 0.0001).
Fig. 1.
Inhibition effect of Shufeng Jiedu Capsule on lung index in mice with HCoV-229E infected pneumonia. Results are represented as mean ± SD (n = 10). ⁎⁎⁎⁎p < 0.0001 between groups.
HCoV-229E virus detection in lung tissue
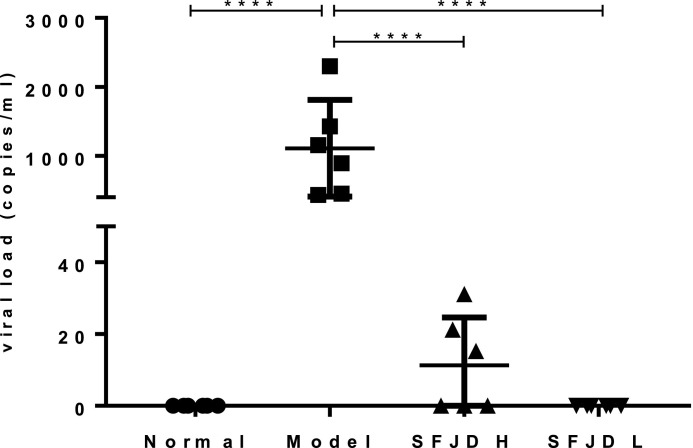
The results in Fig. 2 show that there was no HCoV-229E nucleic acid expression in the lung tissue of normal control animals. After the mice were infected with HCoV-229E, there was a significant viral nucleic acid load of 1109.29 ± 696.75 in the lung tissue of the mice. In both SFJDC high and low dose groups, the viral nucleic acid loads in lung tissue were significantly reduced to 11.27 ± 13.34 and 0 ± 0, respectively.
Fig. 2.
Inhibition effect of Shufeng Jiedu Capsule on viral load in lung tissue of mice. Results are represented as mean ± SD (n = 6). ⁎⁎⁎⁎p < 0.0001 between groups.
Inflammatory factors
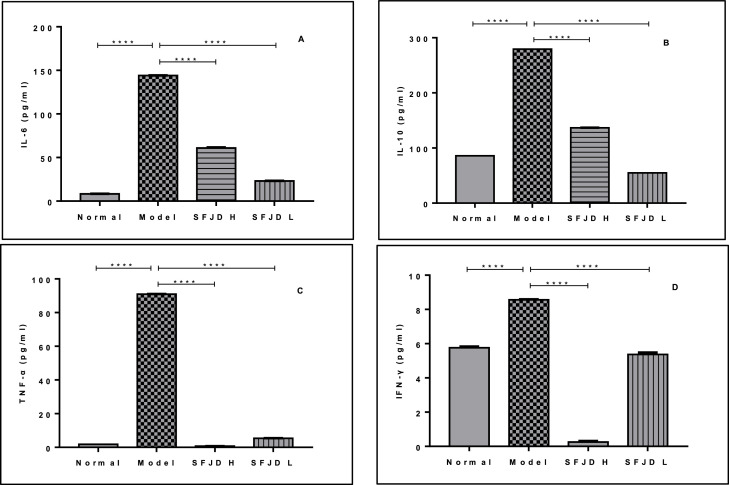
The results in Fig. 3 show that after infecting mice with HCoV-229E, the inflammatory factors IL-6, IL-10, TNF-a and IFN-γ in the lung tissues of the mice were significantly increased to 144.10 ± 0.11, 279.32 ± 0.06, 90.99 ± 0.07, 8.56 ± 0.03, which were significantly different from the normal control group 8.28 ± 0.06, 85.78 ± 0.01, 1.82 ± 0.01, 5.76 ± 0.07 (p < 0.0001), respectively. After 4 days of SFJDC administration, in both high and low dose groups, the levels of IL-6, IL-10, TNF-a and IFN-γ in the lung tissues were significantly reduced to 60.92 ± 0.60, 23.16 ± 0.15, 136.61 ± 0.15, 54.81 ± 0.05; 0.67 ± 0.01, 5.36 ± 0.06; 0.25 ± 0.06, 5.37 ± 0.11, and there was a significant difference compared with the model control group (p < 0.0001).
Fig. 3.
The regulating effect of Shufeng Jiedu Capsule on inflammatory factors in lung tissue of mice. Results are represented as mean ± SD (n = 6). ⁎⁎⁎⁎p < 0.0001 between groups.
Lymphocytes in peripheral blood
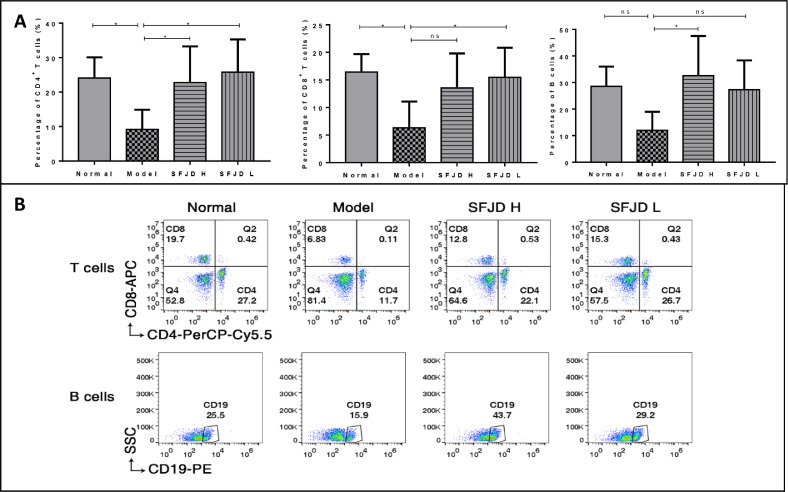
The results in Fig. 4 show that after HCoV-229E infection, the percentages of CD4 + T cells and CD8 + T cells in peripheral blood of the mice were significantly reduced to 9.17 ± 5.21, 6.32 ± 4.35, and there were significant differences between model and normal control groups (24.07 ± 5.48, 16.43 ± 2.97) (p < 0.05). The percentage of B cells showed a decreasing trend, but there was no statistical significance between model and normal control groups (p > 0.05). After SFJDC was administered for 4 days, in both high and low dose groups, the percentage of CD4+ T cells in peripheral blood increased significantly to 22.80 ± 9.55, 25.77 ± 8.67; in the low dose group, the percentage of CD8+ T cells in peripheral blood increased significantly to 15.46 ± 4.92; in the high dose group, the percentage of B cells in peripheral blood increased significantly to 32.57 ± 13.66; and there were significant differences between SFJDC group and model control groups (p < 0.05).
Fig. 4.
The regulating effect of Shufeng Jiedu Capsule on the percentage of CD4+T cells,CD8+T cells and B cells in peripheral blood of mice. Results are represented as mean ± SD (n = 6). * p < 0.05 between groups.
Network analysis
Prediction of potential targets of bioactive compounds in SFJDC
To identify potential bioactive compounds, 94 compounds of SFJDC were included in the screening. The results revealed that 28 compounds might have biological activities, which could be seen as potentially active compounds relating to the effect of SFJDC on SARS-CoV-2 infection (supplement Table 1) (Li et al., 2017, Tao et al., 2017). To further understand the interaction of those bioactive compounds with potential therapeutic targets, an in-silico prediction model was used to predict the target and pathway profiles. A total of 43 targets and 77 pathways were predicted by PharmMapper and KEGG.
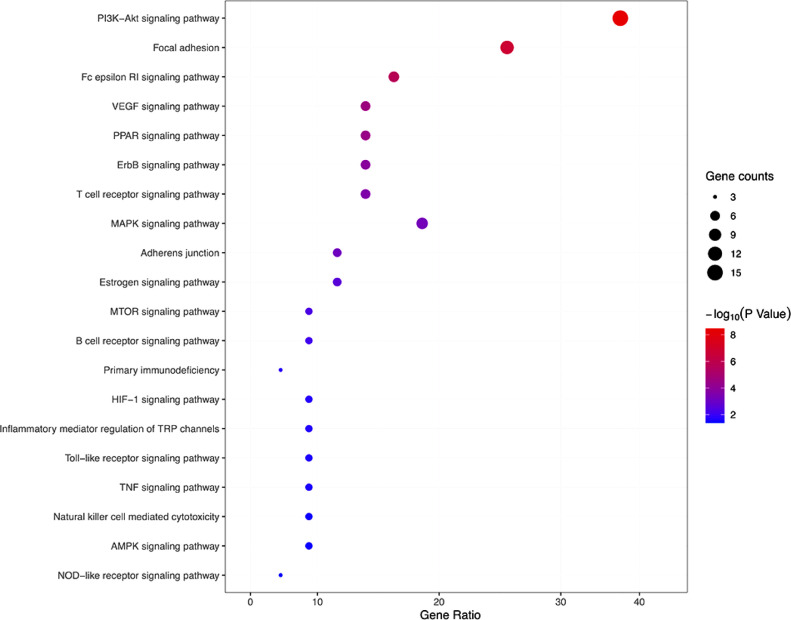
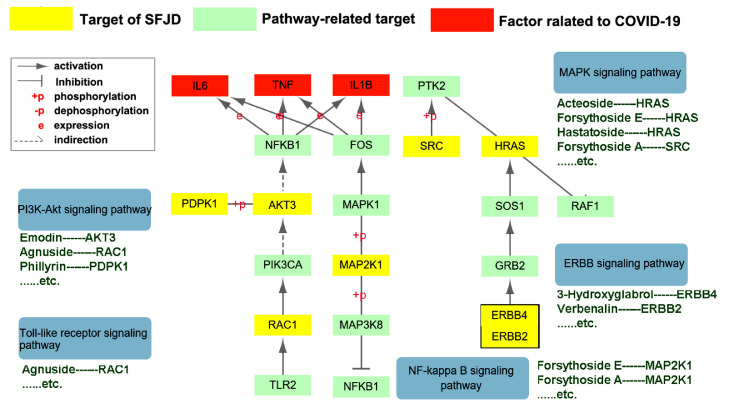
GO-based semantic similarity (p-value ≤ 0.05) and enriched KEGG pathways of targets (FDR < 0.05) analysis showed that 11 pathways, including toll-like receptor, Fc epsilon RI, focal adhesion, MAPK, VEGF, PPAR, ErbB, signaling pathway were related to inflammation; six pathways including the B and T cell receptor pathway, natural killer cell-mediated cytotoxicity, complement and coagulation cascades, primary immunodeficiency signaling pathway and toll-like receptor pathway were related to immunomodulation (supplement Table 2 ). The major pathways are regulated through multiple SFJDC target proteins and many of them have been reported as appropriate target pathways for regulation of immune response and inflammation and could be related to the treatment of viral diseases (Figs. 5 , 6 ) (Kumar et al., 1998, Zhong et al., 2013, Sahpaz et al., 2002, Wang et al., 2018, Tao et al., 2020).
Table 2.
Basic characteristics of the study participants.
| AVD only (n = 33) | AVD+ SFJD (n = 43) | Difference (p-value) | |
|---|---|---|---|
| Sex | 14 females 19 males | 18 females, 25 males, | n.s |
| Age | 45.9 ± 13.3 | 46.95 ± 14.9 | n.s |
| Preexisting chronic disease | N = 9 *1 | N ==10 ⁎2 | n.s. |
| Onset of symptoms before hospitalization | 5.70 ± 3.86 | 4.8 ± 2.61 | n.s. |
*1 chronic bronchitis (1), diabetes mellitus (2), renal insufficiency (1), pulmonal nodules (1), coronary heart disease (1), tuberculosis (1), arterial hypertension (2).
*2 diabetes mellitus (4), breast tumor (1), chronic nephrosis (1), arterial hypertension (4).
Fig. 5.
KEGG enrichment analysis from the potential targets related to immunomodulatory and anti-inflammatory pathways.
Fig. 6.
Network pharmacological action of SFJDC in COVID-19. The main targets and pathways of bioactive compounds predicted by PharmMapper (Wang et al., 2017) and KEGG, respectively.
Potential inhibitors of COVID-19 main protease (Mpro)
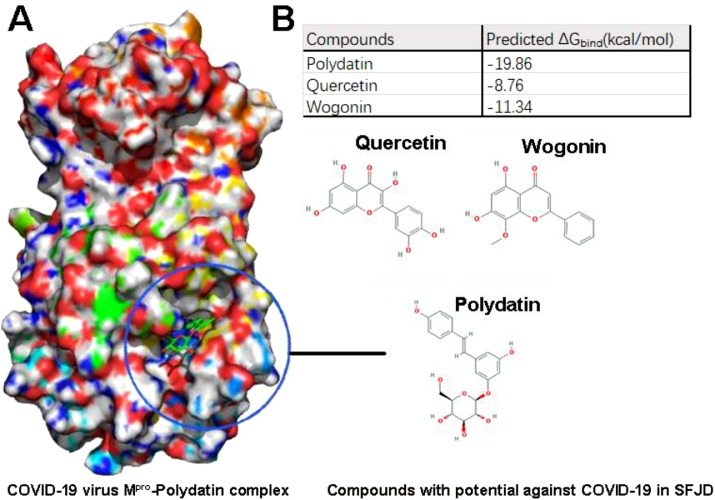
Docking experiments showed that three compounds of SFJDC had a high affinity to the active site pocket of 6LU7 (Fig. 7 ). Polydatin forms H-bonds with the 6LU7 amino acids Asn 142 and His164 and had predicted binding energy of -19.86 kcal/mol. Quercetin forms H-bonds with the 6LU7 amino acids Glu166, Gln189 and, Gln192 and had predicted binding energy of -8.76 kcal/mol. Wogonin forms H-bonds with the 6LU7 amino acids Phe140 and His163 and had predicted binding energy of -11.34 kcal/mol.
Fig. 7.
Molecular docking of SFJDC active compounds with COVID-19 virus Mpro; A) Docking Poses of COVID-19 virus Mpro- Polydatin complex binding mode. B) Affinities for the potential compounds to COVID-19 virus Mpro by using MM-GBSA module integrated in Schrödinger.
Clinical study
Patients basic characteristics
In the AVD group 19 patients were male, 14 were females (mean age 45.9 ± 13.3) years); in the AVD ++SFJD-treated group 25 were males, 18 were females (mean age 46.95 ± 14.88 years).
In the AVD group there were 9/33 patients with preexisting chronic diseases (chronic bronchitis one, diabetes two, renal insufficiency one, pulmonal nodules one, coronary heart disease one, tuberculosis one, arterial hypertension two). In the AVD ++SFJD-treated group 10/43 patients suffered from preexisting chronic diseases (diabetes four, breast tumor one, chronic nephrosis one, arterial hypertension four). Symptoms started 5.70 ± 3.86 days before hospitalization in the AVD group and 4.8 ± 2.61 days in the AVD ++SFJD group (n.s.).
Patients’ basic characteristics are summarized in Table 2.
Outcomes
All recruited patients of the AVD (n ==33) and of the AVD ++SFJD (n ==43) groups were included in each analysis, for each group as originally assigned.
Duration of symptoms
Primary outcome:
Patients in the AVD group were asymptomatic for 4.22 ± 5.36 days when their NP swabs became virus-negative, while the AVD ++SFJD group was already asymptomatic for 8.98 ± 6.11 days (p < 0.005; 95%, CI: -6.64 ~ 0.06; Effect size d 0.83).
Secondary outcomes:
Similarly, patients in the AVD group were asymptomatic for 7.24 ± 5.45 days when their NP swabs, blood, urine, stool specimens were virus-negative, while the AVD+SFJD group was already asymptomatic for 10.5 ± 7.42 days (95%, CI: -8.02 ~ -1.49; Effect size d 0.51).
Fatigue was present in the AVD group for 3.55 ± 4.09 days and 1.19 ± 2.28 days in the AVD ++SFJD group (p < 0.005; 95%CI: -3.8 ~ -0.89; Effect size d 0.71).
Cough lasted for 5.67 ± 5.64 days in the AVD group and 3.47 ± 3.75 days in the AVD ++SFJD group (p < 0.05; 95%, CI: -4.35 ~ -0.05; Effect size d 0.46). The fever duration was 6.30 ± 3.98 days in the AVD group and 6.53 ± 3.58 days in the AVD ++SFJD group (n.s.; 95%, CI: -1.52 ~ 1.96; Effect size d 0.06). Pharyngalgia lasted for 0.70 ± 2.86 days in the AVD group and 1.05 ± 2.57 days in the AVD ++SFJD group (n.s.; 95%, CI: -0.89 ~ 1.59; Effect size d 0.13).
Relevance of start time of SFJDC treatment
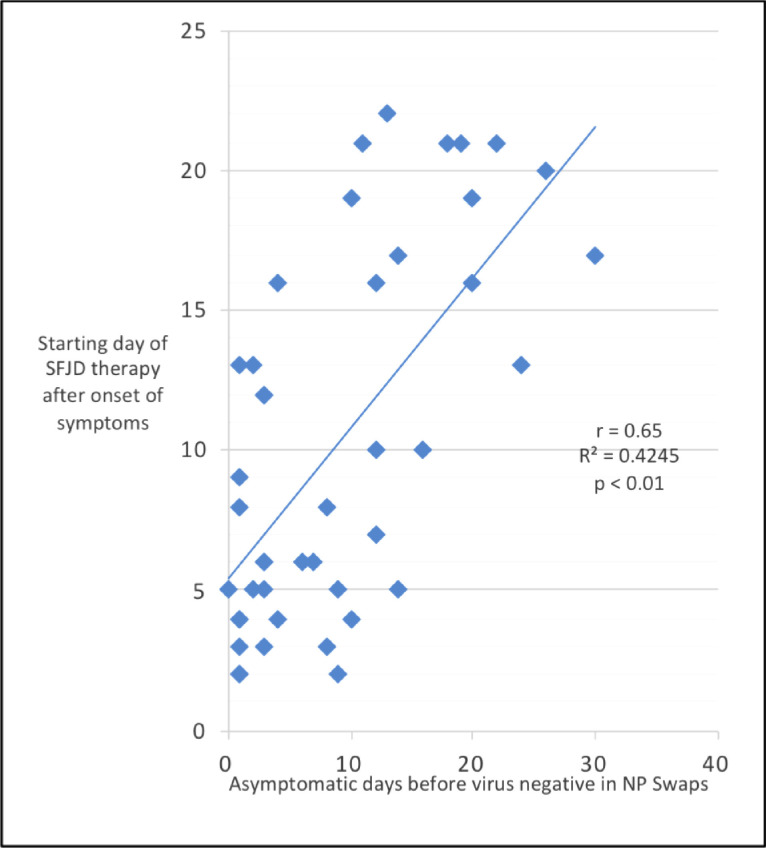
Depending on the patient’s condition, SFJDC was implemented in order to escalate therapy. Hence, the start time of SFJD differed. In 22 cases, SFJDC treatment was initiated early within the first 8 days after onset of symptoms (mean 1.59 ± 1.33, 1–8); in 21 cases 9 days or later (mean 16 ± 4.19, 9–22). Patients treated early had symptoms for 6.8 ± 2.8 days, later treated patients for 9.5 ± 3.2 days (p < 0.005; 95%, CI: 0.97 ~ 4.62; Effect size d 0.9). It took 5.1 ± 4.1 days after the disappearance of symptoms before early treated patients became virus-negative in NP swabs, while it took 13.4 ± 7.9 days for later treated patients (p < 0.005; 95%, CI: 4.03 ~ 12.54; Effect size d 1.31). Similarly, it took 6.9 ± 5.9 days after the disappearance of symptoms before early treated patients became virus-negative in all specimens (NP swabs, blood, urine, stool), while it took 14.3 ± 9.1 days for later treated patients (p < 0.005; 95%, CI: 2.73 ~ 12.11; Effect size d 0.96). Time of application correlated strongly with the asymptomatic period before NP swabs became virus-negative (r ==0.65, p < 0.01), which is demonstrated in Fig 8 .
Fig. 8.
The influence of start time of SFJD treatment on symptom control.
Furthermore, the subgroup of early treated SFJDC patients (n ==22) was superior regarding the whole symptomatic course of COVID-19. Patients were symptomatic for 6.8 ± 2.5 days, while the patients from the control group (n ==33) were symptomatic for 9.10 ± 4.10 days (p < 0.005; 95%, CI: 0.97 ~ 4.63; Effect size d 0.68).
Harms
During the treatment period 8/76 (10.5%) patients, 1/12 of the Umifenovir-alone-treated patients, 2/19 of the Lopinavir/Ritonavir-alone-treated patients, 1/2 of the Umifenovir plus Lopinavir/Ritonavir treated patients, 2/25 of the Umifenovir plus SFJDC treated patients, 1/9 of Lopinavir/Ritonavir plus SFJDC treated patients, and 1/9 Umifenovir plus Lopinavir/Ritonavir plus SFJDC treated patients demonstrated digestive symptoms, which included diarrhea and nausea. No patients discontinued treatment because of adverse effects. All events were classified as mild.
Discussion
The animal experiments of this study indicate that SFJDC demonstrates an antiviral effect. HCoV-229E-infected mice showed a significant increase of the lung index, HCoV-229E nucleic acid expression in the lung, increased inflammatory factors IL-6, IL-10, TNF-α, and IFN-γ in lung tissue, as well as a decreased percentage of CD4+ and CD8+ T cells in peripheral blood. Treatment of mice with SFJDC significantly decreased the lung index and HCoV-229E nucleic acid expression in the lung compared to the control group.
Furthermore, the study showed that SFJDC decreased inflammatory factors Il-6, IL-10, TNF-α, and IFN-γ in lung tissue. Those results proved that the anti-inflammatory effect of SFJDC, which was previously reported for influenza and upper respiratory tract infections, could be found in HCoV-229E-infected mice, too. The reduction of cytokines in coronavirus-infected mice by SFJDC leads to the hypothesis that this herbal drug could reduce the stormy increase of cytokines, which could lead to viral sepsis seen in many COVID-19 patients (Li et al., 2020).
Additionally, the increased percentage of CD4+ and CD8+ T cells in peripheral blood compared to the model group indicates that SFJDC could effectively be attenuated or might prevent lymphopenia caused by COVID-19 (Wang et al., 2020). The importance of this result is supported by a study of Wang et al. (2020) who reported that CD8+ T cells could be an independent predictor for COVID-19 severity as well as for treatment efficacy, because a decrease in CD8+ T cells was associated with severe virus infection and poor treatment efficacy (Wang et al., 2020).
To further evaluate the underlying mechanism of action of the antiviral and anti-inflammatory effect of SFJDC, we conducted a system-level network analysis that can provide useful information for understanding and insight into the Drug-Target-Pathway interactions. Results revealed that 11 inflammation and immunomodulation-related pathways, including PI3K-Akt-, toll-like receptor-, MAPK-, ErbB- and NF-κB signaling pathways were influenced by bioactive compounds of SFJDC (Kumar et al., 1998, Zhong et al., 2013, Sahpaz et al., 2002, Wang et al., 2018, Tao et al., 2020).
PI3-K/Akt and NF-κB signaling pathways regulate inflammatory cytokine production, and promote the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IFN-γ, which could lead to acute lung injury (Tao et al., 2014, Kumar et al., 1998, Yum et al., 2001). Emodin, an anthraquinone found in Polygonum cuspidatum as well as Phragmites communis and Phillyrin, a natural lignan found in Forsythia suspensa and Verbena officinalis attenuate PI3K-Akt signaling and inhibit the NF-κB-mediated transcription of pro-inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, and IFN-γ) (Zhong et al., 2013, Zheng et al., 2019). Furthermore, it was shown that Emodin inhibits TNF-induced-NF-κB activation by inhibition of IκB degradation (Kumar et al., 1998). The attenuation of pro-inflammatory pathways by bioactive compounds of SFJDC, as suggested by our system-level network analysis, could explain the decreased inflammatory factors Il-6, IL-10, TNF-α, and IFN-γ in lung tissue of coronavirus-infected mice treated with SFJDC.
Our molecular docking experiments suggested that the antiviral effect of SFJDC could be partly explained by direct inhibition of Mpro by three bioactive compounds (polydatin, quercetin, and wogonin) found in SFJDC. However, those results need to be confirmed by in-vivo experiments.
Our clinical pragmatic real-live study gives evidence that the addition of SFJDC to conventional AVD significantly reduced the symptomatic period of COVID-19 patients. This is even more relevant in the light of recent studies, which showed the AVD Lopinavir/Ritonavir and Umifenovir used in this study could not show clinical effectiveness against COVID-19 (Cao et al., 2020, Chen et al., 2020).
Symptomatic effectiveness was shown to be significant for patients with cough and fatigue, but not with fever and pharyngalgia. However, both groups received best medical care, including antipyretic drugs during the hospitalization period; pharyngalgia was present in only 10 of all 76 patients.
Furthermore, real-live evidence does not replace RCTs but can be an important complement. Hence, an additional large-scale randomized, double-blinded, placebo-controlled clinical trial is still necessary and pending. This might clarify the question: whether the effect of SFJDC is mainly symptomatic or, as indicated by the animal study, caused by an antiviral property of SFJDC.
Early use of AVD alone or combined with TCM is considered to be essential for virus control (Kai-Wang To et al., 2020), and we additionally compared the early to late initiation of SFJDC treatment. Early application of SFJDC within the first 8 days from the onset of the first symptom(s) of COVID-19 showed a noticeable higher effect, which has to be taken into account in future studies.
Conclusion
The antiviral and anti-inflammatory properties of SFJDC were confirmed by the mouse model. The decreased inflammatory factors in the lung tissue of coronavirus-infected mice can be explained by attenuation of pro-inflammatory pathways by bioactive compounds of SFJDC. Network analysis showed that 11 inflammation and immunomodulation-related pathways were influenced by bioactive compounds of SFJDC. The main ingredients of SFJDC: quercetin, wogonin, and polydatin bind directly to the main protease (Mpro) of SARS-CoV-2. Clinical data provided some promising evidence that SFJDC might shorten the symptomatic course of COVID-19 in patients with mild and moderate symptoms. The results indicated that it is beneficial to administer SFJD immediately from the onset of the first symptoms. Hence, a large-scale randomized, double-blinded, placebo-controlled clinical trial is necessary to confirm the effect of this real-world study of SFJDC for the treatment of COVID-19 patients.
Author contributions
Lu Xia, Yunfei Lu, Yun Ling and Ying Lv had full access to all of the clinical data in the study and take responsibility for the integrity and accuracy of the data analysis. Sven Schröder, Hongzhou Lu, Xiaolan Cui are co–senior authors, Lu Xia, Yujing Shi, Jie Su and Thomas Friedemann are co–first authors. Concept and design: Sven Schröder, Hongzhou Lu, Xiaolan Cui. Acquisition, analysis, or interpretation of data: Lu Xia, Yujing Shi, Jie Su, Thomas Friedemann, Zhenggang Tao, Yunfei Lu, Yun Ling, Ying Lv, Ronghua Zhao, Zihan Geng, Xiaolan Cui, Hongzhou Lu, Sven Schröder. Drafting of the manuscript: Sven Schröder, Thomas Friedemann, Lu Xia, Yujing Shi and
Jie Su. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Thomas Friedemann. Obtained funding: Hongzhou Lu, Xiolan Cui, Sven Schröder. Administrative, technical, or material support: Hongzhou Lu, Sven Schröder, Xiolan Cui. Supervision: Sven Schröder, Xiaolan Cui, Hongzhou
Lu.
All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Trial registration
Chinese Trial Registry (ChiMCTR2000002977)
Declaration of Competing Interest
None.
Footnotes
This work was supported by:
1. The National Key Research and Development Project (grant no. 2018YFE0102300)
2. The National Natural Science Foundation of China (81803805)
3. Research of Early Clinical Treatment System and Pre-strategy of Traditional Chinese Medicine for Acute infectious outbreak disease (No. 2017ZX10305501)
4. HanseMerkur Insurance group, Hamburg, Germany (No 2-07-2020)
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2020.153390.
Appendix. Supplementary materials
References
- Anglemyer A., Horvath H.T., Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.MR000034.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lin S., Niu C., Xiao Q. Clinical evaluation of Shufeng Jiedu Capsules combined with umifenovir (Arbidol) in the treatment of common-type COVID-19: a retrospective study. Expert Rev. Respir. Med. 2020:1–9. doi: 10.1080/17476348.2020.1822741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chinese J. Infect. Dis. 2020;38 E008–E008. [Google Scholar]
- Guimarães C.R.W., Cardozo M. MM-GB/SA rescoring of docking poses in structure-based lead optimization. J. Chem. Inf. Model. 2008;48:958–970. doi: 10.1021/ci800004w. [DOI] [PubMed] [Google Scholar]
- Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: The reality and challenges. Journal of Microbiology, Immunology and Infection. 2020;53 doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., et al. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- Kai-Wang To K., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Dhawair S., Aggarwal B.B. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-κB activation, IκB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- Leung P.C. The efficacy of Chinese medicine for SARS: A review of Chinese publications after the crisis. American Journal of Chinese Medicine. 2007;35:575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- Li J.H., Wang R.Q., Guo W.J., Li J.S. Efficacy and safety of traditional Chinese medicine for the treatment of influenza A (H1N1): A meta-analysis. J. Chinese Med. Assoc. 2016;79:281–291. doi: 10.1016/j.jcma.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. Anti-inflammatory effects of Shufengjiedu capsule for upper respiratory infection via the ERK pathway. Biomed. Pharmacother. 2017;94:758–766. doi: 10.1016/j.biopha.2017.07.118. [DOI] [PubMed] [Google Scholar]
- Li H., et al. Updated approaches against SARS-CoV-2. Antimicrobial Agents and Chemotherapy. 2020;64:1–7. doi: 10.1128/AAC.00483-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission & State Administration of Traditional Chinese Medicine Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. Trial Vers. 2020 [Google Scholar]
- NHC . H1N1 influenza diagnosis and treatment plan; 2010. National Health Commission of the People’s Republic of China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., et al. Observation on the clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsules in the treatment of COVID-19.pdf. Chinese Tradit. Herb. Drugs CN. 2020:12–1108. [Google Scholar]
- Sahpaz S., Garbacki N., Tits M., Bailleul F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002;79:389–392. doi: 10.1016/s0378-8741(01)00415-9. [DOI] [PubMed] [Google Scholar]
- Tao Z., et al. Shufeng Jiedu Capsule protect against acute lung injury by suppressing the MAPK/NF-κB pathway. BioScience Trends. 2014;8:45–51. doi: 10.5582/bst.8.45. [DOI] [PubMed] [Google Scholar]
- Tao Z., et al. Therapeutic Mechanistic Studies of ShuFengJieDu Capsule in an Acute Lung Injury Animal Model Using Quantitative Proteomics Technology. J. Proteome Res. 2017;16:4009–4019. doi: 10.1021/acs.jproteome.7b00409. [DOI] [PubMed] [Google Scholar]
- Tao, Z. et al. Quantitative Proteomics Analysis of Systemic Responses and Biological Mechanisms of ShuFengJieDu Capsule Using H1N1-Infected RAW264.7 Cells. (2020). doi:10.1021/acsomega.0c01545. [DOI] [PMC free article] [PubMed]
- Wang X., et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45 doi: 10.1093/nar/gkx374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: A review. Journal of Ethnopharmacology. 2018;210:318–339. doi: 10.1016/j.jep.2017.08.040. [DOI] [PubMed] [Google Scholar]
- Wang, F. et al. 2020 Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. doi:10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed]
- Xi Z., et al. Effect of Shufengjiedu capsule on the fever caused by viral upper respiratory tract infection: A report of 130 cases. J Tradit Chin Med. 2010;51:426–427. [Google Scholar]
- Yuan Y., et al. Shufeng Jiedu Capsules Alleviate Lipopolysaccharide-Induced Acute Lung Inflammatory Injury via Activation of GPR18 by Verbenalin. Cell. Physiol. Biochem. 2018;50:552–568. doi: 10.1159/000494184. [DOI] [PubMed] [Google Scholar]
- Yum H.-K., et al. Involvement of Phosphoinositide 3-Kinases in Neutrophil Activation and the Development of Acute Lung Injury. J. Immunol. 2001;167:6601–6608. doi: 10.4049/jimmunol.167.11.6601. [DOI] [PubMed] [Google Scholar]
- Zheng, X.-Y. et al. Emodin-induced autophagy against cell apoptosis through the PI3K/AKT/mTOR pathway in human hepatocytes. (2019). doi:10.2147/DDDT.S204958. [DOI] [PMC free article] [PubMed]
- Zhong W.T., et al. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. Fitoterapia. 2013;90:132–139. doi: 10.1016/j.fitote.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Zuidgeest M.G.P., et al. Series: Pragmatic trials and real world evidence: Paper 1. Introduction. J. Clin. Epidemiol. 2017;88:7–13. doi: 10.1016/j.jclinepi.2016.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.