Abstract
Nebulized gamma interferon (IFN-γ) protein has been studied for clinical safety and efficacy against pulmonary tuberculosis (TB). The protein is expensive, requires a cold chain, and is difficult to deploy in limited-resource, high-incidence settings. We generated a preclinical proof of concept (PoC) for a dry powder inhalation (DPI) containing DNA constructs to transiently transfect the lung and airway epithelium of mice with murine IFN-γ. Bacterial colony-forming units (CFU) in the lungs of mice infected with Mycobacterium tuberculosis (Mtb) reduced from about 106/g of tissue to ~104 after four doses given once a week. Nodular inflammatory lesions in the lungs reduced significantly in number. Immunohistochemistry of infected lung sections for LC3-1 and LAMP-1 indicated autophagy induction between 18 and 48 h after inhalation. ELISA on bronchoalveolar lavage (BAL) fluid showed differences in kinetics of IFN-γ concentrations in the epithelial lining fluid of healthy versus infected mice. Uninfected mice receiving DNA constructs expressing a fluorescent protein were live-imaged. The fluorescence signals from the intracellular protein peaked at about 36 h after inhalation and declined by 48 h. These results establish preclinical PoC of the efficacy of a DPI and dosing regimen as a host-directed and transient gene therapy of experimental pulmonary TB in mice, justifying preclinical development.
Keywords: gamma interferon, dry powder inhalation, pulmonary tuberculosis, preclinical proof of concept, host-directed therapy, gene delivery, gene therapy
Graphical Abstract

Misra and colleagues present a preclinical proof of concept that transiently transfecting the lung epithelium by means of inhaled DNA encoding gamma interferon is efficacious in treating mice infected with Mycobacterium tuberculosis. They argue that their non-sterile, non-invasive, and potentially storage-stable dry powder inhalation formulation of plasmid DNA has advantages over nebulized protein.
Introduction
Gamma interferon (IFN-γ) has long been in clinical use for cancer therapy1,2 and has been marketed as Actimmune since 1999. In a clinical investigation of IFN-γ as a potential host-directed therapy (HDT) of pulmonary tuberculosis (TB), Jaffe et al.3 demonstrated that delivery of the cytokine to the lungs activated lung macrophages, the immune cells that play a key role in host defense. Condos et al.4,5 reported airway and lung deposition and clinical efficacy of nebulized IFN-γ in patients with multi-drug-resistant (MDR) pulmonary TB.5 A subsequent study by the same group found that nebulized, but not subcutaneously injected, IFN-γ resolved classic symptoms of pulmonary TB faster and cleared the bacteria from the sputum when administered to patients at 200 μg/day for 3 days a week during 4 months, along with the standard anti-TB drug regimen.6 However, 500-μg doses nebulized to severely ill patients of MDR TB may not be capable of “salvage therapy” of patients with advanced pulmonary TB.7
Treatment of drug-sensitive (DS) and MDR/extensively drug-resistant (XDR) TB is prolonged over several months. Anti-TB agents have severe adverse effects,8 and reduction of the duration of treatment is an important objective. Host-directed intervention is an emerging approach to TB therapy.9, 10, 11 Agents aimed at inducing host responses to kill the pathogen, limiting immunopathology by modulating immune homeostasis,12 promoting autophagy,13 and shortening treatment duration can be delivered directly to the lungs. Combining inhaled IFN-γ with existing regimens may hold promise for improving treatment of pulmonary TB.14 However, the cost of nearly 10 mg (0.2 mg × three doses a week × 16 weeks) of IFN-γ per patient and the requirement of a “cold chain” for the use of IFN-γ at the point of care are impediments in developing nebulized IFN-γ. This is especially important in limited-resource settings where the incidence of TB is high.
The target indications of the product we aim to develop are DS and MDR/XDR TB, but the relevance of such a product to the treatment of respiratory viral infections, occlusive pulmonary disease, and fibrosing alveolitis,15 among others, is arguable from first principles.16 It is prudent to mention here that additional engineering of the construct to provide a 5′ untranslated region that could avoid “cap snatching” by viral RNases would be required for deploying cytokine gene therapy against coronavirus respiratory infections.17
We hypothesized that it is possible to transiently transfect the lung and airway epithelium with IFN-γ for an optimal period of gene expression that would induce host response against the pathogen, but not immunopathology in the host. This hypothesis derives from the early and counter-intuitive observation by Ernstoff et al.2 that intermittent intravenous infusion of up to 1,000 mg/m2 of IFN-γ during 2 h was safer than continuous infusion of the same amount spread out over 24 h, as well as from observations by Condos et al.5 and Dawson et al.6 that intermittent inhalation of IFN-γ provided healing effects rather than inflammatory pathology in patients with pulmonary TB. IFN-γ is known, among other host defense functions, to induce autophagy in cells infected with the intracellular pathogen Chlamydia.18 Our group has also observed that the lung architecture of Mycobacterium tuberculosis (Mtb)-infected mice and guinea pigs improves significantly upon pulmonary delivery of drugs that induce autophagy.13,19 We therefore hypothesized that intermittent exposure to IFN-γ would drive a host defense program against the pathogen that would include induction of autophagy, without inducing immunopathology. A dry powder inhalation (DPI) formulation for transient transfection of the airways and deep lungs was prepared and evaluated to test these hypotheses and provide preclinical proof of concept (PoC) for IFN-γ HDT using species-autologous gene delivery.
Results
Bacterial Burden and Lung Pathology
We infected 36 mice by the aerosol route with a low dose of Mtb and started treatment with once-weekly inhalations 28 days later (Figure 1A). The mice were made to inhale about 100 μg of a dry powder containing about 5 ng of plasmid bearing the mouse gene for IFN-γ (Ifng) by nose-only exposure.20 The initial inoculum of bacteria received by the mice was about 100 colony-forming units (CFU) per gram of lung tissue as shown in Figure 1B. The CFU/g grew to about a million in the lungs and 10,000 in the spleen by the time treatment was started. At the end of the fourth week, no culturable bacteria were found in the lungs of one of the two mice receiving once-weekly inhalations. In a two-sample t test of actual power 0.99 at an alpha of 0.05, the mean lung CFU/g in the group after 4 weeks of treatment was significantly different from that in the untreated mice. The CFU counts in the spleen were apparently reduced on treatment but were not significantly different between treated and untreated animals (Figure S1).
Figure 1.
Efficacy of Inhaled Ifng against Experimental TB in Mice
(A) Mice were infected with Mtb by whole-body exposure in a Glas-Col chamber on day 0 and treated once a week starting at day 28. Two untreated control animals were culled on days 0, 28, and 56. Two treated animals were culled on days 28, 35, 42, and 56. Lungs and spleens were harvested as indicated by arrows. (B) Dashed line connecting open circles (○) show average ± standard error of mean (n=2) of log10–transformed values of CFU/g of lung, and open squares (□) show these values in respect of the spleens. Filled symbols connected by solid lines starting from day 28 indicate CFU counts in these tissues in treated animals.
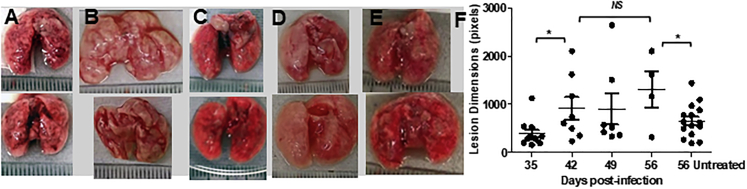
Figure 2 shows the lungs of the mice after they had received one, two, three, or four doses of the DPI. There was progressive improvement in the gross organ pathology and reduction in the number of visible nodular lesions. When the mice received no treatment, severe pathology was evident at 56 days post-infection (Figure 2E).
Figure 2.
Lung Pathology
(A–E) Photographs (scale graduations, mm) of freshly harvested lungs after the first (A), second (B), third (C), and fourth (D) doses show progressive reduction in nodular lesions, while the lungs of mice receiving no treatment showed a large number of lesions (E). Photographs were subjected to image analysis using ImageJ software. (F) The numbers of nodular lesions (scatter points) reduced progressively, and their dimensions were more diffuse in animals receiving inhalations. Scatter points refer to individual lesions. The average number of lesions at each time point/group is shown as a line, with error bars showing standard deviations. Statistically significant differences (t test, α = 0.05, actual power 0.67) are shown by asterisks. NS, not significant.
Autophagy in Lung Tissue
Lung sections of mice infected with Mtb 28 days prior to receiving a single inhaled dose (100 μg of DPI, 5 ng of plasmid) were stained for markers of autophagy. Figure 3 shows that LC3-II and LAMP-1 expression was low in animals that received no treatment and in treated animals examined at 7 h after inhalation. After 18 h, expression of these markers increased discernibly. The two markers co-localized extensively at 48 h post-inhalation.
Figure 3.
Induction of Autophagy
(A) Confocal micrographs of representative lung sections of mice that were infected 21 days prior to the experiment but received no treatment, Untreated control stained with DAPI to reveal nuclei, and with antibodies to LC3-1 and LAMP-1 conjugated with green and red fluorescent dyes, respectively. (B) Six hours after inhalation of the plasmid bearing Ifng. (C) Punctate fluorescence (arrows) was observed to initiate at 18 h after the dose due to sporadic co-localization of the two markers. (D) Extensive co-localization of the two markers indicative of autophagic flux was observed at 48 h after inhalation. Scale bars, 75 μm.
Gene Expression Kinetics In Vitro
Figure 4 shows transient transfection of A549 human alveolar epithelial cells by DPI particles added to culture wells, as well as a pattern of rising and falling concentrations of IFN-γ in the bronchoalveolar lavage (BAL) fluid of infected mice receiving inhalations. Cells were transfected either with a plasmid bearing Ifng or a chimeric construct of green fluorescent protein (-gfp-Ifng) under the cytomegalovirus (CMV) promoter. IFN-γ was secreted into the culture medium in a dose-dependent manner, showing peak concentrations at 12 and 24 h after adding plasmid DNA in DPI particles. Flow cytometry of cells transfected with gfp-Ifng indicated that intracellular concentrations of the foreign protein peaked at 12 h post-transfection. Confocal microscopy at different time intervals revealed that fluorescence could be observed starting 6 h after exposure to particles. At 48 h post-transfection, GFP co-localized with LysoTracker red dye, suggesting that the expressed protein was targeted for degradation at this time.
Figure 4.
Transient Transfection In Vitro
(A) IFN-γ secreted in culture supernatant by 3 × 105 A549 alveolar epithelial cells transfected with about 1 ng (filled symbols) or 1.5 ng (open symbols) of plasmid DNA. Means ± SD of three replicates are plotted. (B) A chimeric GFP-IFN-γ protein was not secreted, but it could be detected by flow cytometry. Means ± SD of three replicates are plotted. (C) The green fluorescence attributable to the chimeric protein co-localized with LysoTracker red at 48 h post-transfection to generate yellow fluorescence. (D) Detail of (C).
Pharmacokinetics of IFN-γ in Uninfected and Infected Mice
We drew blood samples, conducted BAL, and prepared homogenates of the lavaged lung tissue recovered at different time points from uninfected mice (n = 3 per time point) after a single inhalation dose of 5 ng of plasmid DNA (Figure 5). IFN-γ peaked at about 8 h after inhalation in the BAL fluid and was, surprisingly, discernible almost immediately after inhalation in the lung homogenate. The half-life (t½) in the BAL fluid was about 3 h. In blood plasma, we observed fairly high concentrations, approaching those in the BAL fluid at the earliest time point. Because IFN-γ is an endogenous protein, we cannot discriminate between the concentrations produced by expression of plasmid DNA and concentrations resulting from background endogenous expression at any time point. Blood concentrations did not appear to change much with time, and pharmacokinetic modeling calculated the value of t½ in the blood as >458 h (Table S1), which is absurd.
Figure 5.
Pharmacokinetics of IFN-γ in Uninfected and Infected Mice
(A–C) Scatter points show values from individual animals (A–C, uninfected mice, n = 3; D, infected mice, n = 2); points with error bars indicate mean ± SEM, and solid lines depict model-fitted values of concentrations of IFN-γ as determined by ELISA in BAL fluid (A), lung homogenate (B), and blood plasma (C) of uninfected mice. (D) The concentration-time profile in the BAL fluid of infected mice showing drastic shift in Cmax and tmax (note the logarithmic scale of the y axis; see also Table S1).
Concentration-time profiles of IFN-γ recovered from the lung and airway epithelial lining fluid sampled by BAL from mice infected with Mtb 28 days before the experiment were markedly different. The time (Tmax) taken to attain maximal concentrations (Cmax) differed strongly between uninfected (~8 h) and infected mice (~30 h) because of a lag time (Tlag) of nearly 18 h in the latter case. We note a correspondence between the pharmacodynamic outcome of induction of autophagy (Figure 3) starting at about 18 h and the Tlag calculated here (Table S1). The Cmax in BAL fluid was nearly 3-fold higher in infected animals. A one-compartment pharmacokinetic model with a time lag following extravascular administration was fitted to the data using PKSolver 2.0.21 The values of other calculated pharmacokinetic parameters are given in Table S1.
Gene Expression Kinetics of Intracellular Protein
Mice (n = 3) receiving inhalations of ~100 μg of particles containing ~5 ng of plasmid DNA with red fluorescent protein (RFP) under the CMV promoter were imaged at 6, 12, 24, 36, and 48 h post-inhalation (Figure 6). At 12 h after the dose, fluorescence was observed in the nasal/buccal area but not at other time points. It is likely that the mucosal epithelium of the nares and mouth was also transiently transfected during administration of the inhalation. Importantly, note that one of the mice showed fluorescence in only one lung (Figure 6G). Control mice received particles comprising all components except DNA. Background fluorescence in the urogenital, rectal, and peri-anal region, most likely due to dietary components, gut microflora, and other factors, was observed in these mice, as well as in mice that had received plasmid DNA inhalations. The kinetics of foreign gene expression indicate that RFP expression was detectable from 6 h onward, peaking at 24 h and depleting substantially by 48 h after dosing.
Figure 6.
In Vivo Imaging. Mice were imaged at different time intervals after inhalation of a plasmid bearing RFP under the CMV promoter
(A–E) 6, 12, 18, 24, and 48 h after inhalation. (F) The animal that received control particles without DNA at 6 h. (G) One animal showed signal in the nares and buccal regions and only in the left lung at the 12 h time point (corresponding to the animal shown in B). (H) Control animal, the same as shown in (F), at 6 h.
Discussion
As the first step in the process of translational research, we present a preclinical PoC of transient transfection with IFN-γ as a stand-alone intervention in TB. As shown in Figure 1, transient transfection with inhaled Ifng reduces bacterial burden in the lungs by two orders of magnitude. It progressively and significantly improves gross morphology of the lungs. Reduction in bacterial burden (Figure 1B) and differences in gross morphology of the spleens of the same animals (Figure S1) were not significant. Lack of significant efficacy in the spleen is an expected result. Unlike the long-lived insulin molecule, which is amenable to pulmonary delivery for systemic effects, it is unlikely that significant amounts of functional IFN-γ secreted by transfected epithelial cells on the lumen of the lungs and airways would diffuse intact into systemic circulation from the respiratory epithelium. Exclusion from peripheral blood is potentially an advantage for treatment of lung disease because it spares non-target organs from the pleiotropic effects of the cytokine. However, exclusion of IFN-γ from systemic circulation also implies that inhaled gene therapy would not be available for HDT of extra-pulmonary TB, unless it can be demonstrated that macrophages and dendritic cells located in the lungs and stimulated by the cytokine released there are able to drive amplification of systemic IFN-γ.
The effects of IFN-γ as HDT include, among others, induction of the self-cleaning cellular process of autophagy in TB-infected macrophages.19,22 We have earlier reported that inhalation of pharmacological inducers of autophagy improves alveolar architecture in mice and guinea pigs infected with Mtb.23 Figure 2 illustrates that autophagy was indeed induced in experiments reported herein, as early as 18 h after the first inhaled dose, and extensively after 48 h.
It has been demonstrated that most lipopolysaccharide (LPS)-induced proteins (including IFN-γ) are expressed by macrophages within 3 h of the stimulus.24 Similar to other cytokines, IFN-γ acts in a paracrine manner to amplify its own production. The observed time frame of IFN-γ expression in vitro and in vivo (Figures 4 and 5) is consistent with the induction of autophagy in the TB-infected lung. Autophagy was not observed in the lungs of mice that were infected but received no treatment. These observations support the claim that autophagy was induced by secretion of functional IFN-γ in the lungs.
The kinetics of disposition of IFN-γ in the BAL fluid represent single-dose preclinical pharmacokinetics in a tissue compartment that is rarely sampled in clinical studies.25 In a study on plasmid delivery by nebulization to patients of cystic fibrosis, vector-specific DNA (but not RNA) could be quantified in bronchial brushings recovered by bronchoscopy.26 Polyplexes of mRNA administered by nebulization and whole-body exposure to mice also generated a profile of protein expression that was similar to the results shown in Figures 4 and 5 with respect to uninfected mice.27 However, the contrast between IFN-γ secreted in the lungs of infected versus healthy animals is noteworthy. We have earlier demonstrated that type 1 cytokines such as IFN-γ are downregulated in the animal model of TB that we use, and that administration of even inert biodegradable and biocompatible polymeric particles on the lung tends to induce their secretion into the lung lumen.28 Results shown in Figure 5 appear to suggest that the kinetics of upregulation of IFN-γ in the BAL fluid recovered from healthy animals reflect the response of a healthy lung, while the initial delay and later enhancement of IFN-γ secretion show how transfection with Ifng “rescues” the type 1 response of the Mtb-infected lung. Thus, we speculate that the protein produced by transfection does not form the bulk of the amount observed in the BAL fluid. Instead, most of the IFN-γ is made by bystander macrophages, dendritic cells, and T cells that receive the stimulus from transfected cells. This hypothesis can be tested in IFN-γ double-knockout (Ifng−/−) mice that we cannot afford, but we can gratefully and rapidly supply our formulation and inhalation apparatus to any research team interested in joining this open innovation effort.
Finally, results of live animal imaging (Figure 6) corroborated the time course of gene expression following inhalation of plasmids formulated in the same way as plasmids bearing Ifng. The imaging study also points out a limitation of inhalations in general and their use for in vivo transfection in particular. One mouse out of three that received inhalations of a plasmid expressing RFP showed the fluorescence signal in only one lung (Figure 6G). This could have resulted either from deposition of the powder in only one lung, or because one of the lungs was not transfected even though the powder was deposited. It is unlikely that all of the powder that deposited in the lung lobes that did not show fluorescence was devoid of DNA. Although 5 ng of DNA in 100 μg of powder appears to be a very small amount, we prepare the powder by spray-drying a solution of all components (Table S2). Because a solution has uniform composition throughout its bulk, “uniformity of content” in the product is assured. We also routinely establish the DNA content (“assay”) and uniformity of content of each batch of the DPI powder as critical quality attributes (CQAs) of a pharmaceutical product. Non-uniform and idiosyncratic deposition of inhaled powder in the airways and lungs29 is therefore the likely explanation for this observation. If this were to happen in a patient with a tubercular lung, it would amount to missing a dose. This is also why our product is positioned as an adjunct or add-on therapy of pulmonary TB.
There are several limitations of the studies reported herein. The first is sample size in the efficacy study. Within the constraints of resources available, the actual statistical power of the study on bacterial counts in lungs and spleens was 0.83, suggesting a 17% chance that the efficacy reported is an artifact. The situation is also complicated by the observation that one mouse had no culturable bacteria in the lungs at the end of 4 weeks. The presence of bacteria in the same animal’s spleen (Figures 1B and S2) and residual nodular lesions in the lungs (Figure 2D) assures us that it received the initial inoculum, but ambiguity remains whether the animal’s lungs were significantly colonized to begin with. Transfection of only one lung (Figure 6G) points out that it is possible that the mouse that had no bacteria in the lungs received a smaller initial inoculum. Next, IFN-γ is intended as an adjunct HDT, and not as a stand-alone intervention. Although the standard four-drug regimen for TB does not clear bacteria from the lungs and spleens of mice in 4 weeks in the facility where these experiments were done, we have no control group in which standard treatment by the oral route was given, either alone or in addition to inhalations. This shortcoming prevents us from inferring whether the intervention is 'superior' or 'non-inferior' to standard treatment.
The many limitations of the mouse model of pulmonary TB apply to the present study.30 Primary among these is the observation that mice we used do not develop cavitary, necrotizing, caseous granulomas, and the bacteria are present mostly inside cells. In human disease, apart from the difference in pathology, extracellular bacteria and bacterial biofilms cannot be eliminated by host-directed mechanisms, including autophagy. The effect of gene therapy needs to be studied in a model such as the guinea pig or non-human primate to evaluate efficacy against a mix of bacterial populations residing inside or outside host cells. Finally, we used the mouse cytokine gene rather than the human gene in these experiments, so it might be argued that our results would not apply to human IFN-γ. However, it is only logical to use the species-autologous gene in order to express a protein that will be treated as “self” by the host immune system.
With these caveats, we submit that we establish preclinical PoC of non-viral, transient transfection with species-autologous IFN-γ to justify preclinical development of the proposed intervention. We aim to prepare a self-administered, non-invasive, non-sterile, storage-stable DPI providing transient transfection of the lungs and respiratory tract with human IFN-γ. The results justify a full preclinical development program to address the limitations pointed out above, as well as detailed safety/toxicity studies. Efficacy in mice, guinea pigs, and ideally in non-human primates is required to be established. Safety should also be established in infected animals (guinea pigs and non-human primates) with different chronicity of infection. Clinical development (as an adjunct to standard treatment, for initiation as soon after the first confirmed diagnosis of pulmonary TB as possible, and with the objective of reducing the duration of treatment) may be undertaken if warranted by the results of animal studies.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
Materials and Methods
Materials
Plasmids bearing Ifng or gfp in tandem with Ifng were sourced from Origene Technologies (USA). The plasmid carrying the gene for RFP (pCMV-DsRed-Express) was from Clonetech (USA). Plasmids were amplified in sufficient yield using a process that is easily adaptable to good manufacturing practices (GMPs) mandatory for regulatory approvals for human use.31 Plasmid DNA mixed with branched-chain polyethylenimine (bPEI) of 10 kDa molecular weight (Polysciences, USA) yielded stable complexes. Drug master file (DMF)-grade pharmaceutical excipients were obtained from various suppliers. Culture media, fetal calf serum (FCS), and supplements were sourced from Sigma-Aldrich (India). Fluorescent-tagged antibodies to LC3-1 and LAMP-1, LysoTracker red, and DAPI were from Elabscience Biotechnology. Absolute ethanol was procured from Merck (EMSURE), and other reagents and chemicals were of molecular biology grade.
Preparation of DPI
The DNA-PEI polyplex was mixed with various excipients (Table S2) in distilled water/ethanol (85:15 v/v). The solution was spray dried (Buchi 290, Switzerland) using an inert loop, at an inlet temperature of 110°C, feed rate of 5 mL/min, total solids content of ~8.1% w/v, and an aspirator setting of 85%. These conditions resulted in outlet temperatures between 606°C and 5°C.
Characterization
The size and morphology of the particles was determined by laser scattering (Malvern Mastersizer 2000, Malvern Instruments, UK) and scanning electron microscopy (Tecnai, Phillips, USA). Cascade impaction (MOUDI 100NRl, MSP, USA) was used to establish aerosol properties. We used an in-house apparatus for administration of dry powder inhalations to laboratory animals.20 The delivery port of this apparatus was interfaced with a MOUDI 100 cascade impactor, and 10 mg of powder samples (n = 3) were fluidized at a negative airflow rate of 30 L/min. Powder deposited at different stages was weighed using a five-digit balance (Mettler, USA). Agarose gel electrophoresis was used to confirm the quality of DNA after recovering it from the DPI particles by the standard phenol-chloroform-isoamyl alcohol method. DNA in samples collected on different stages was estimated quantitatively using the NanoDrop 2000c apparatus (Thermo Scientific). CQAs of the DPI formulations are illustrated in Figure S2.
Cell Culture, Microscopy, and ELISA
Human alveolar basal epithelial adenocarcinoma A549 cells (ATCC CCL-185) were maintained in RPMI 1640 with 10% FCS. Confluent wells of 24-well plates (with lysine-coated glass coverslips placed inside) were transfected in vitro to ascertain time kinetics of expression, secretion, and localization of foreign proteins within phagolysosomes (LysoTracker red, Invitrogen, USA). Confocal microscopy (Leica, TCS SP8) was carried out on the coverslips bearing A549 cells placed on glass slides. Apart from fluorescent proteins encoded by transfecting plasmids, cells were stained with DAPI and LysoTracker red according to the manufacturer’s instructions. ELISA was carried out on culture supernatants and BAL fluid (see below) using DuoSet ELISA reagents for mouse IFN-γ (R&D Systems, USA).
In Vivo Experiments
Animal experiments were conducted with approval by, and oversight of, the Institutional Animal Ethics Committee (IAEC/2017/283/Renew-0, dated October 31, 2017).
For assessment of whole-organ morphology and bacterial burden, animals were dosed once weekly starting at 28 days after low-dose aerosol infection. All procedures were carried out under animal biosafety level 3 (ABSL3) containment. Two animals were euthanized by cervical dislocation at the end of each week. Organs were harvested and their photographs were taken using a 48-megapixel cell phone camera through an observation port located outside the ABSL3 area. Tissue homogenates were plated in duplicate on 7H11 medium with oleic albumin dextrose catalase (OADC) supplement (Difco, Becton Dickinson, India). Colonies were counted after incubating the plates for 28 days in a biochemical oxygen demand (BOD) incubator maintained at 37°C. For kinetics of IFN-γ expression in Mtb-infected animals, BAL was conducted under ketamine-xylazine anesthesia on three mice each at time points of 6, 12, 18, 24, 36, and 48 h after the dose. After the BAL procedure, mice were perfused with 4% paraformaldehyde, and lung tissue was harvested for transport out of ABSL3 containment. Lung tissue was embedded in paraffin, and microtome sections were deparaffinized and stained for immunohistochemistry as per the manufacturer’s instructions.
For imaging, six male Swiss mice weighing between 15 and 20 g were randomly allocated to control and treatment groups (n = 3/group). The DPI was administered using an in-house inhalation apparatus for a period of 30 s.20 At time intervals of 6, 12, 18, and 31 h, mice were anesthetized with isoflurane, and in vivo imaging was carried out as per the manufacturer’s instructions (IVIS Spectrum, PerkinElmer, USA).
Acknowledgments
This work was supported by Department of Biotechnology (DBT), Government of India grant BT/PR10468/MED/29/815/2013, and Indian Council of Medical Research (ICMR) grant AMR/IN/112/2017-ECD-II. R.B. and D.V.S.R. received Senior Research Fellowships (SRFs) from the Council of Scientific and Industrial Research, India (CSIR), while H.S. is an SRF of the ICMR. T.R. and K.V. received Junior Research Fellowships (JRFs) from the University Grants Commission, India, and S.K.R. from the CSIR. L.R. received a post-doctoral Senior Associateship from the CSIR. S.V. was funded by the ICMR grant listed above. Funders had no role in study design, execution, or reporting. We thank Dr. Kalyan Mitra, Sophisticated Analytical Instruments Facility, CSIR-CDRI, for electron and confocal microscopy. The graphical abstract used some icons from BioRender. This is CSIR-CDRI communication no. 10128.
Author Contributions
Conceptualization, A.M., L.R., and R.B.; Methodology, L.R., J.S., and A.K.S.; Investigation, R.B., T.R., K.V., D.V.S.R., H.S., S.V., S.K.R., A.K.S, and L.R.; Writing – Original Draft, R.B. and A.M.; Writing –Review & Editing, A.M.; Funding Acquisition, A.M., A.K.S., L.R., and J.S.; Resources, A.M., A.K.S., J.S., and L.R.; Supervision, A.M., L.R., A.K.S., and J.S.
Disclosure of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.10.023.
Supplemental Information
References
- 1.Foon K.A., Sherwin S.A., Abrams P.G., Stevenson H.C., Holmes P., Maluish A.E., Oldham R.K., Herberman R.B. A phase I trial of recombinant gamma interferon in patients with cancer. Cancer Immunol. Immunother. 1985;20:193–197. doi: 10.1007/BF00205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernstoff M.S., Trautman T., Davis C.A., Reich S.D., Witman P., Balser J., Rudnick S., Kirkwood J.M. A randomized phase I/II study of continuous versus intermittent intravenous interferon gamma in patients with metastatic melanoma. J. Clin. Oncol. 1987;5:1804–1810. doi: 10.1200/JCO.1987.5.11.1804. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe H.A., Buhl R., Mastrangeli A., Holroyd K.J., Saltini C., Czerski D., Jaffe H.S., Kramer S., Sherwin S., Crystal R.G. Organ specific cytokine therapy. Local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-gamma to the human lung. J. Clin. Invest. 1991;88:297–302. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condos R., Hull F.P., Schluger N.W., Rom W.N., Smaldone G.C. Regional deposition of aerosolized interferon-γ in pulmonary tuberculosis. Chest. 2004;125:2146–2155. doi: 10.1378/chest.125.6.2146. [DOI] [PubMed] [Google Scholar]
- 5.Condos R., Rom W.N., Schluger N.W. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-γ via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 6.Dawson R., Condos R., Tse D., Huie M.L., Ress S., Tseng C.H., Brauns C., Weiden M., Hoshino Y., Bateman E., Rom W.N. Immunomodulation with recombinant interferon-γ1b in pulmonary tuberculosis. PLoS ONE. 2009;4:e6984. doi: 10.1371/journal.pone.0006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis R.S. Lack of a therapeutic role for interferon γ in patients with tuberculosis. J. Infect. Dis. 2014;209:627–628. doi: 10.1093/infdis/jit555. [DOI] [PubMed] [Google Scholar]
- 8.Ormerod L.P., Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber. Lung Dis. 1996;77:37–42. doi: 10.1016/s0962-8479(96)90073-8. [DOI] [PubMed] [Google Scholar]
- 9.Palucci I., Delogu G. Host directed therapies for tuberculosis: futures strategies for an ancient disease. Chemotherapy. 2018;63:172–180. doi: 10.1159/000490478. [DOI] [PubMed] [Google Scholar]
- 10.Tobin D.M. Host-directed therapies for tuberculosis. Cold Spring Harb. Perspect. Med. 2015;5:a021196. doi: 10.1101/cshperspect.a021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zumla A., Ippolito G., Ntoumi F., Seyfert-Margolies V., Nagu T.J., Cirillo D., Chakaya J.M., Marais B., Maeurer M. Host-directed therapies and holistic care for tuberculosis. Lancet Respir. Med. 2020;8:337–340. doi: 10.1016/S2213-2600(20)30078-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann S.H.E., Lange C., Rao M., Balaji K.N., Lotze M., Schito M., Zumla A.I., Maeurer M. Progress in tuberculosis vaccine development and host-directed therapies—a state of the art review. Lancet Respir. Med. 2014;2:301–320. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A., Pant G., Mitra K., Madan J., Chourasia M.K., Misra A. Inhalable particles containing rapamycin for induction of autophagy in macrophages infected with Mycobacterium tuberculosis. Mol. Pharm. 2014;11:1201–1207. doi: 10.1021/mp4006563. [DOI] [PubMed] [Google Scholar]
- 14.Sachan M., Srivastava A., Ranjan R., Gupta A., Pandya S., Misra A. Opportunities and challenges for host-directed therapies in tuberculosis. Curr. Pharm. Des. 2016;22:2599–2604. doi: 10.2174/1381612822666160128150636. [DOI] [PubMed] [Google Scholar]
- 15.Britton J. Interferon gamma-1b therapy for cryptogenic fibrosing alveolitis. Thorax. 2000;55(Suppl 1):S37–S40. doi: 10.1136/thorax.55.suppl_1.s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smaldone G.C. Repurposing of gamma interferon via inhalation delivery. Adv. Drug Deliv. Rev. 2018;133:87–92. doi: 10.1016/j.addr.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa K., Lokugamage K.G., Makino S. Viral and cellular mRNA translation in coronavirus-infected cells. Adv. Virus Res. 2016;96:165–192. doi: 10.1016/bs.aivir.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Zeer M.A., Al-Younes H.M., Braun P.R., Zerrahn J., Meyer T.F. IFN-γ-inducible Irga6 mediates host resistance against Chlamydia trachomatis via autophagy. PLoS ONE. 2009;4:e4588. doi: 10.1371/journal.pone.0004588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A., Misra A., Deretic V. Targeted pulmonary delivery of inducers of host macrophage autophagy as a potential host-directed chemotherapy of tuberculosis. Adv. Drug Deliv. Rev. 2016;102:10–20. doi: 10.1016/j.addr.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur J., Muttil P., Verma R.K., Kumar K., Yadav A.B., Sharma R., Misra A. A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur. J. Pharm. Sci. 2008;34:56–65. doi: 10.1016/j.ejps.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Huo M., Zhou J., Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Rovetta A.I., Peña D., Hernández Del Pino R.E., Recalde G.M., Pellegrini J., Bigi F., Musella R.M., Palmero D.J., Gutierrez M., Colombo M.I., García V.E. IFNG-mediated immune responses enhance autophagy against Mycobacterium tuberculosis antigens in patients with active tuberculosis. Autophagy. 2014;10:2109–2121. doi: 10.4161/15548627.2014.981791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A., Sharma D., Meena J., Pandya S., Sachan M., Kumar S., Singh K., Mitra K., Sharma S., Panda A.K. Preparation and preclinical evaluation of inhalable particles containing rapamycin and anti-tuberculosis agents for induction of autophagy. Pharm. Res. 2016;33:1899–1912. doi: 10.1007/s11095-016-1926-0. [DOI] [PubMed] [Google Scholar]
- 24.Eichelbaum K., Krijgsveld J. Rapid temporal dynamics of transcription, protein synthesis, and secretion during macrophage activation. Mol. Cell. Proteomics. 2014;13:792–810. doi: 10.1074/mcp.M113.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodvold K.A., Yoo L., George J.M. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antifungal, antitubercular and miscellaneous anti-infective agents. Clin. Pharmacokinet. 2011;50:689–704. doi: 10.2165/11592900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Alton E.W.F.W., Armstrong D.K., Ashby D., Bayfield K.J., Bilton D., Bloomfield E.V., Boyd A.C., Brand J., Buchan R., Calcedo R., UK Cystic Fibrosis Gene Therapy Consortium Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015;3:684–691. doi: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., DeRosa F., Langer R., Anderson D.G. Inhaled nanoformulated mrna polyplexes for protein production in lung epithelium. Adv. Mater. 2019;31:e1805116. doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R., Muttil P., Yadav A.B., Rath S.K., Bajpai V.K., Mani U., Misra A. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 2007;59:499–506. doi: 10.1093/jac/dkl533. [DOI] [PubMed] [Google Scholar]
- 29.Li D., Li Y., Li G., Zhang Y., Li J., Chen H. Fluorescent reconstitution on deposition of PM2.5 in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. USA. 2019;116:2488–2493. doi: 10.1073/pnas.1818134116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A.K., Gupta U.D. Animal models of tuberculosis: lesson learnt. Indian J. Med. Res. 2018;147:456–463. doi: 10.4103/ijmr.IJMR_554_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakker N.A.M., de Boer R., Marie C., Scherman D., Haanen J.B.A.G., Beijnen J.H., Nuijen B., van den Berg J.H. Small-scale gmp production of plasmid DNA using a simplified and fully disposable production method. J. Biotechnol. X. 2019;2:100007. doi: 10.1016/j.btecx.2019.100007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.