Graphical abstract
Keywords: COVID-19 pandemic, Vaccine safety, Autoimmunity, Molecular mimicry, Cross-Reactivity, Immune interference
Highlights
-
•
A future COVID-19 vaccine is being developed at unprecedented speed to augment immune system defenses.
-
•
This paper presents myriad infection/vaccine-related mechanisms that could adversely impact vaccine effectiveness and safety.
-
•
It is unclear how the present accelerated vaccine development program can address these longer-term impact vaccine safety issues.
Abstract
A degraded/dysfunctional immune system appears to be the main determinant of serious/fatal reaction to viral infection (for COVID-19, SARS, and influenza alike). There are four major approaches being employed or considered presently to augment or strengthen the immune system, in order to reduce adverse effects of viral exposure. The three approaches that are focused mainly on augmenting the immune system are based on the concept that pandemics/outbreaks can be controlled/prevented while maintaining the immune-degrading lifestyles followed by much of the global population. The fourth approach is based on identifying and introducing measures aimed at strengthening the immune system intrinsically in order to minimize future pandemics/outbreaks.
Specifically, the four measures are: 1) restricting exposure to virus; 2) providing reactive/tactical treatments to reduce viral load; 3) developing vaccines to prevent, or at least attenuate, the infection; 4) strengthening the immune system intrinsically, by a) identifying those factors that contribute to degrading the immune system, then eliminating/reducing them as comprehensively, thoroughly, and rapidly as possible, and b) replacing the eliminated factors with immune-strengthening factors.
This paper focuses on vaccine safety. A future COVID-19 vaccine appears to be the treatment of choice at the national/international level. Vaccine development has been accelerated to achieve this goal in the relatively near-term, and questions have arisen whether vaccine safety has been/is being/will be compromised in pursuit of a shortened vaccine development time. There are myriad mechanisms related to vaccine-induced, and natural infection-induced, infections that could adversely impact vaccine effectiveness and safety. This paper summarizes many of those mechanisms.
1. Introduction
1.1. Background
Over the past two decades, there have been at least three major coronavirus-based infectious disease outbreaks/epidemics/pandemics: Severe Acute Respiratory Syndrome (SARS), 2002–2003; Middle East Respiratory Syndrome (MERS), starting in 2012; COVID-19, starting in December 2019 [1]. There are a number of similarities among these three infectious diseases, including abnormal values of selected biomarkers (e.g., neutrophils, lymphocytes, albumin, CRP, TNF-alpha, etc.), pulmonary inflammation, pulmonary damage, etc. The most important similarity among these infectious diseases is the demographic affected most severely [2]. This demographic tends to be the elderly, with comorbidities and degraded/dysfunctional immune systems, and others with degraded/dysfunctional immune systems [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. While there is some decline in the immune system with age, comorbidity is a stronger predictor of impaired immunity than chronological age in older adults [14,15].
There are also similarities between COVID-19 and influenza: “Both (COVID-19 and influenza) cause fever, cough, body aches and fatigue; sometimes vomiting and diarrhea; can be mild or severe, even fatal in rare cases; can result in pneumonia” [16]. Additionally, “Neither virus is treatable with antibiotics, which only work on bacterial infections; both are treated by addressing symptoms, such as reducing fever; severe cases may require hospitalization and support such as mechanical ventilation” [16,17]. Both COVID-19 and influenza share the demographic affected most severely, as well.
The main measures being taken to control the spread of the SARS-CoV-2 coronavirus (the virus mainly associated with COVID-19) are conceptually those that were taken to control the spread of the SARS-CoV coronavirus in 2002−2003: good hygiene and quarantine (lockdown). The difference is the scale of these measures. Currently, many countries are on lockdown (at different levels of severity), restricting many activities and businesses that involve gatherings of large numbers of people in close proximity. As of early October 2020, it is unknown how long these restrictions will be in place.
In addition to identifying short-term adverse vaccine effects related to the mechanisms, this paper identifies potential mid-and long-term adverse vaccine effects that cannot be identified in short-term human clinical trials characteristic of vaccine efficacy testing. To ensure vaccine safety, long-term human testing under real-life conditions (exposures to multiple toxic stimuli) is required. There is an incompatibility between the accelerated vaccine development times being pursued by government and industry and the long times required for validation of vaccine safety.
In summary, it is difficult to see how safe COVID-19 vaccines can be developed and fully tested for safety on development time scales of one or two years, as proposed presently. The only real protection against a future COVID-19 pandemic or any other viral pandemic/outbreak is the one that was demonstrated to work in the SARS, MERS, and COVID-19 pandemics/outbreaks, and in the annual influenza pandemics/outbreaks: a healthy immune system capable of neutralizing incoming viruses as nature intended.
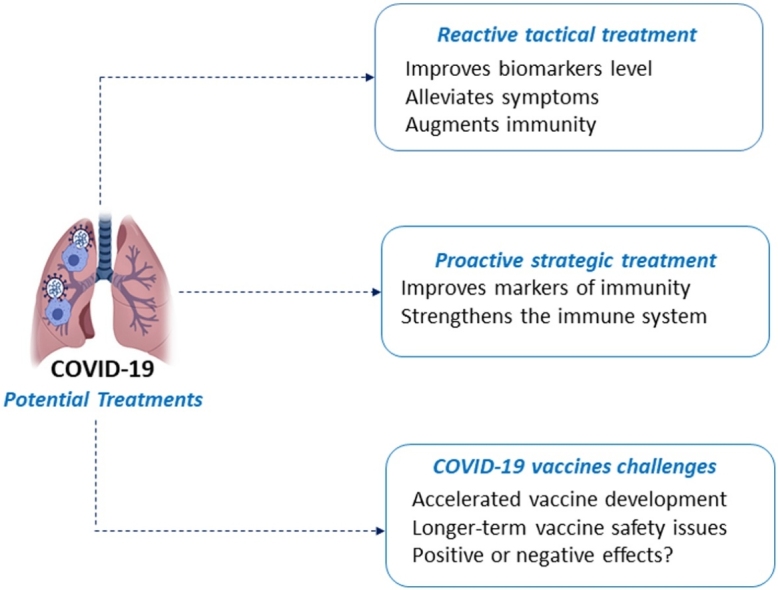
1.2. Potential treatments
There are myriad efforts being pursued to develop treatments and preventative measures for COVID-19. Some of these will now be outlined.
If treatments are defined as a set of actions that improve health, then (at least) two types of treatments are possible.
The first type can be defined as positive treatments. They can be sub-divided into high-tech treatments and low-tech treatments.
The high-tech are the classical treatments where drugs (or supplements) and/or radiation and/or surgery are implemented, and symptoms are alleviated. These high-tech positive treatments are basically a reactive tactical response to abnormal markers of health. They can be applied for the short-term (e.g., antibiotics for bacterial infections, antivirals for viral infections, etc.) [18,19], or for the long-term (e.g., statins, blood thinners, antihypertensives, etc.) [20]. The low-tech treatments involve dietary supplements or natural bioactive compounds [[21], [22], [23]], sleep, and other behavioral changes shown to impact the immune system positively (see section A4-C of our previous COVID-19 monograph [13] for a bibliography of low-tech immune system strengthening factors). For long-term benefit, these low-tech treatments need to be maintained indefinitely [24]. On average, the high-tech treatments have greater risk than the low-tech treatments.
The second type can be defined as negative-negative treatments, where those factors that contribute to disease are first identified and then removed. The name derives from the mathematics world, where a negative of a negative is a positive [25]. These negative-negative treatments are basically a proactive strategic response to abnormal markers of health, and typically involve long-term changes in lifestyle and harmful exposures for improved health [[26], [27], [28], [29]].
1.3. Tactical treatments
Much of the effort to help the most vulnerable COVID-19 demographic at this time has been searching for, and experimenting with, treatments that were/are used to combat other (mainly) viral diseases (aka repurposed treatments). These treatments include, but are not limited to: Actemra/Tocilizumab; Avigan/Favipiravir; Azithromycin; Baricitinib/Olumiant;
Bevacizumab/Avastin; Calquence/Acalabrutinib; Chloroquine; Colcrys/Colchicine; Convalescent Plasma; EIDD-2801; Fingolimod/Gilenya; Galidesivir; Hydroxychloroquine; Ilaris/Canakinumab; Ivermectin; Jakafi/Ruxolitinib; Kaletra/Lopinavir/Ritonavir; Kevzara/Sarilumab;
Kineret/Anakinra; Leronlimab; Mavrilimumab; Methylprednisolone; Olumiant/Baricitinib; Otezla/Apremilast; Remdesivir; Tamiflu/Oseltamivir; Umifenovir/Arbidol; Xeljanz/Tofacitinib [[30], [31], [32], [33], [34]].
Other novel tactical treatments could be identified using our Literature-Related Discovery and Innovation (LRDI)-based treatment repurposing methodology [35,36].
1.4. Strategic treatments
Strategic treatments were the focus of our previous COVID-19 monograph [13]. Their identification is a two-step process. First, markers of immune system health (ranging from specific biomarkers to more general descriptors) are selected. Second, those substances (e.g., smoking, excess alcohol, pesticides, etc.) behaviors (e.g., sedentary lifestyle, substance abuse, etc.), and other toxic stimuli that degrade the levels of these markers (i.e., lead to immune dysfunction, immunotoxicity, immunosuppression, etc.) are then identified and recommended for elimination [37]. The strategic treatments identified in the previous monograph are those contained within the immune system core literature. Additional novel strategic treatments could also be identified using our LRDI-based treatment repurposing methodology [35,36].
1.5. Reactive tactical vs proactive strategic treatments
The reactive tactical treatment approach for countering infections from viral exposure improves biomarker levels and reduces symptoms (if successful), but ordinarily does little to improve the body’s resistance to disease. For viral infections, the tactical treatments will do little to strengthen the degraded/dysfunctional immune (and other) system. After tactical treatments for one viral infection, people with degraded/dysfunctional immune systems will again be vulnerable to serious infectious consequences from exposure to the next harmful virus they encounter.
The proactive strategic treatment approach will strengthen the immune (and other) system by removing those critical factors that contribute to disease and a degraded/dysfunctional immune system (unless irreversible damage has been done to the immune system, or individuals possess congenital or other hereditary damage to their immune system) [[38], [39], [40]]. These strategic treatments tend to require long-term adherence by their recipients. In turn, these recipients of strategic treatments will be less vulnerable to infection from exposure to the next pathogenic virus they encounter (SARS-CoV-2 or otherwise). Like many healthy people who were exposed to SARS-CoV and SARS-CoV-2, these people who follow the (typically) long-term proactive strategic treatment regimen successfully may not even be aware they have been exposed to, or infected by, the coronavirus. The only indication of their infection will be coronavirus antibodies in their serum.
2. Methodology
A hybrid methodology was used to identify references showing potential long-term adverse effects of vaccines and vaccine/infection-induced mechanisms that could contribute to these adverse effects. Based on reading myriad vaccine adverse effects review articles, terms showing mechanisms were extracted (e.g., antibody-dependent enhancement, viral interference, route of infection, original antigenic sin, etc), and used as a Medline query to retrieve potentially relevant articles. All these retrieved articles were read, and the most relevant ones extracted. Their titles were entered into the Web of Science, and the citation network was explored (citing papers, cited papers, papers that shared common references, etc). Those records were read, and the most relevant ones extracted for this monograph.
3. Results
3.1. Overview
The main body of our previous COVID-19 monograph [12] addressed the first type of strategic treatment: 1) identification and removal of factors contributing to weakening the immune system (see section A4-A of the previous monograph [12] for a table of these contributing factors), and 2) identification and addition of factors contributing to strengthening the immune system (see section A4-C of the previous monograph [12] for a bibliography of low-tech immune system strengthening factors). The present section addresses the second type of strategic treatment: development and implementation of a COVID-19 vaccine. The prospects for such a vaccine will be addressed from three criteria perspectives: development time, efficacy, and safety.
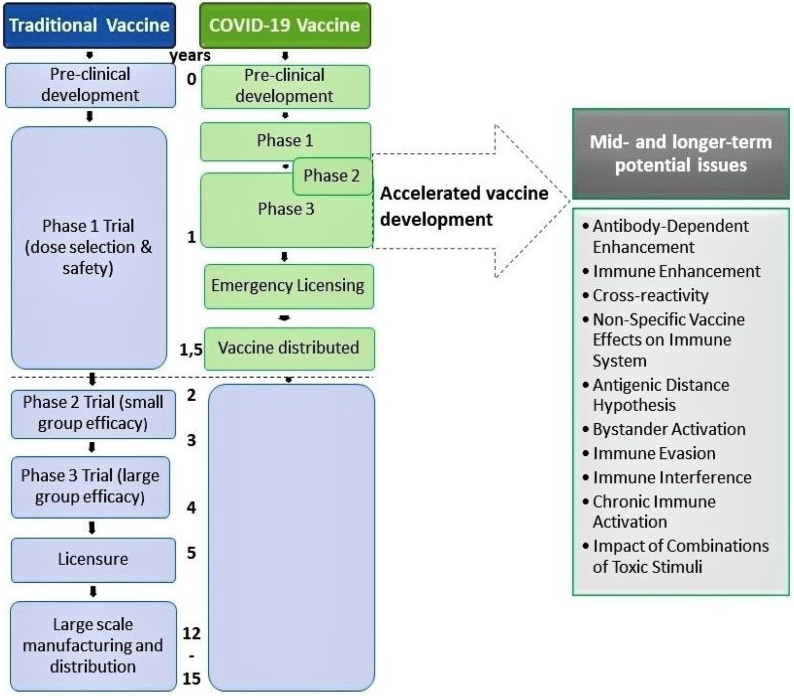
Calina et al. evaluated the ongoing approaches to COVID-19 vaccine development, and stated: “Normally, the period of development of a vaccine is 12‑15 years” [41]. Against this backdrop, SARS-CoV-2 vaccines are targeted for accelerated development, safety testing, manufacturing, and distribution by an order of magnitude [42]. Each of the accelerated steps [41] has drastically reduced the time required from normal development. Some of the potential adverse vaccine effects shown on the right of Fig. 1 may take years to emerge, well after the initial abbreviated vaccine safety testing period.
Fig. 1.
Compares the traditional vaccine development schedule with the accelerated COVID-19 vaccine development schedule.
3.2. Past coronavirus vaccine development history
There have been two prior coronavirus outbreaks in the 21 st century: SARS in 2002–2003, and MERS starting in 2012. Vaccine development for each started/accelerated during the height of each outbreak. What have been the results of these prior coronavirus vaccine development efforts?
According to a comprehensive 2019 article on MERS vaccine development [43], “To date, there is no specific treatment proven effective against this viral disease. In addition, no vaccine has been licensed to prevent MERS-CoV infection thus far … In general, the potential vaccine candidates can be classified into six types: viral vector-based vaccine, DNA vaccine, subunit vaccine, nanoparticle-based vaccine, inactivated-whole virus vaccine and live-attenuated vaccine”
According to a comprehensive 2020 article on SARS and MERS vaccine development [44], “As of April 2020, no vaccine is commercially available for these coronavirus strains”. The rationale for lack of a vaccine is given by the following: “Reasons for the lack of commercial and effective vaccines for SARS and MERS are varied. In the case of MERS, it is likely that the vaccine development was delayed because of the scarcity of suitable and cost-effective small animal models during pre-clinical experimentation. In addition, it is probable that a vaccine has not been delivered because of the low interest in investing in a vaccine for a disease that has produced relatively low and geographically centralized cases (compared with other more global and persistent infectious diseases such as influenza, HIV and tuberculosis). This last factor might have also contributed to the lack of a vaccine for SARS, in the sense that it was considered pointless to continue investing in a vaccine for a disease whose cases ceased to be reported in 2004.”
While interest in a vaccine may have waned after the SARS pandemic/outbreak seemed to have terminated, research on such a vaccine persisted. References in the above article showed SARS vaccine research continued for a decade or more after the pandemic had ended [45,46].
Based on the experiences with SARS and MERS, successful vaccine development was not achieved after about a decade of research, or even more. That does not bode well for COVID-19 coronavirus vaccine development/safety testing/distribution for the one-year timescales being projected.
3.3. Challenges for successful vaccine development - overview
The main challenges facing successful coronavirus vaccine development can be summarized as time to development, efficacy of the vaccine and, most importantly, safety of the vaccine. A complementary perspective on some of the problems listed in [41] can be stated as follows:
First, although the virus’s spike protein is a promising immunogen for protection, optimizing antigen design is critical to ensure optimal immune response. Debate continues over the best approach — for example, targeting the full-length protein or only the receptor-binding domain.
Second, preclinical experience with vaccine candidates for SARS and the Middle East respiratory syndrome (MERS) have raised concerns about exacerbating lung disease, either directly or as a result of antibody-dependent enhancement. Such an adverse effect may be associated with a type 2 helper T-cell (Th2) response. Hence, testing in a suitable animal model and rigorous safety monitoring in clinical trials will be critical” [47].
3.4. Vaccine mechanisms with uncertain consequences
Numerous mid- and longer-term potential issues concerning vaccines have been identified. Their themes are summarized initially, followed by excerpts from specific cited references.
- 1)
-
2)
Vaccine-associated Virus Interference (where vaccinated individuals may be at increased risk for other respiratory viruses because they do not receive the non-specific immunity associated with natural infection) [[70], [71], [72], [73], [74], [75]];
-
3)
Vaccine-Associated Imprinting Reduction (where vaccinations could also reduce the benefits of ‘imprinting’, a protection conferred upon children who experienced infection at an early age) [76,77];
-
4)
Non-Specific Vaccine Effects on Immune System (where previous infections can alter an individual's susceptibility to unrelated diseases) [78,79];
-
5)
Impact of Infection Route on Immune System (where immune protection can be influenced by the route of exposure/delivery) [[80], [81], [82]];
-
6)
Impact of Combinations of Toxic Stimuli (where people are exposed over their lifetime to myriad toxic stimuli that may impact the influence of any vaccine) [78; 83, 84];
-
7)
Antigenic Distance Hypothesis (negative interference from prior season’s influenza vaccine (v1) on the current season’s vaccine (v2) protection may occur when the antigenic distance is small between v1 and v2 (v1 ≈ v2) but large between v1 and the current epidemic (e) strain (v1 ≠ e).) [[85], [86], [87]];
-
8)
Bystander Activation (activation of T cells specific for an antigen X during an immune response against antigen Y) [[88], [89], [90]];
-
9)
Gut Microbiota (Impact of gut microbial composition on vaccine response) [[91], [92], [93], [94], [95]];
-
10)
Homologous Challenge Infection Enhancement (the strain of challenge virus used in the testing assay is very closely related to the seed virus strain used to produce the vaccine that a subject received) [[96], [97], [98]];
-
11)
Immune Evasion (evasion of host response to viral infection) [[99], [100], [101], [102]];
-
12)
Immune Interference (interference from circulating antibody to the vaccine virus) [103,104];
-
12a) Original antigenic sin (propensity of the body's immune system to preferentially utilize immunological memory based on a previous infection when a second slightly different version of that foreign entity (e.g. a virus or bacterium) is encountered.) [[105], [106], [107], [108]];
-
13)
Prior Influenza Infection/Vaccination (effects of prior influenza infection/vaccination on severity of future disease symptoms) [[109], [110], [111], [112], [113], [114], [115], [116]];
-
14)
Timing between Viral Exposures (elapsed time between viral exposures) [[117], [118], [119], [120]];
-
15)
Vaccine-Associated Enhanced Respiratory Disease (where vaccination enhances respiratory disease) [[121], [122], [123]];
-
16)
Chronic Immune Activation (continuous innate immune responses) [[124], [125], [126]].
3.5. Vaccine effectiveness
The previous section addressed mechanisms that could potentially enhance infections, rather than attenuate or prevent them. The present section addresses empirical findings of low vaccine effectiveness, where mechanistic explanations may or may not have been offered. Because of similarities between influenza and COVID-19, and space limitations, the focus will be on the influenza vaccination experience. Significant influenza VE could not be demonstrated for any season, age, or setting after adjusting for county, sex, insurance, chronic conditions recommended for influenza vaccination, and timing of influenza vaccination. [127]. We found a threefold increased risk of hospitalization in subjects who did get the TIV vaccine [128].
Using data from traditional control subjects, VE for those seasons was estimated to be 5 % (95 % CI, −52 % to 40 %), 11 % (95 % CI, −96 % to 59 %), and 37 % (95 % CI, −10 % to 64 %), respectively; confidence intervals included 0 [129]. Participants reporting pH1N1-related ILI during the period 1 April through 5 June 2009 were more than twice as likely to report having previously received seasonal influenza vaccine [130]. Unvaccinated children had more flu-specific CTLs than vaccinated children with CF [131].
Following 2009 H1N1 vaccination, subjects previously given a seasonal influenza virus vaccination exhibited significantly lower antibody responses, as determined by hemagglutination inhibition assay, than subjects who had not received the seasonal influenza virus vaccination [132]. Our study confirms the results from our previous interim report, and other studies, that failed to demonstrate benefit or harm from receipt of seasonal influenza vaccine in patients with confirmed infection with pandemic influenza H1N1 2009. [133]. Influenza vaccination seemed to be associated with an increased risk of non-influenza respiratory virus infections, which is consistent with temporary nonspecific immunity. [134]. A potential doubling of pandemic infection risk among prior seasonal vaccine recipients could be disastrous in the event of a more severe pandemic involving a higher per-case fatality risk” [135].
3.6. Potential short- and long-term diseases resulting from vaccines
-
Tracking Deficiencies for Vaccine Adverse Effects
While the efficacy issues for a COVID-19 vaccine have been enumerated extensively in recent reviews [49,54], more emphasis needs to be placed on ensuring mid- and long-term safety are achieved. Vaccines do not appear to have the same safety requirements as many drugs. For example, consider the following excerpts from selected vaccine inserts relative to safety [136]:
-
MMR Vaccine: M-M-R II has not been evaluated for carcinogenic or mutagenic potential, or potential to impair fertility. Animal reproduction studies have not been conducted with M-M-R II.;
-
Influenza Vaccine FLUARIX QUADRIVALENT has not been evaluated for carcinogenic or mutagenic potential or male infertility in animals.
-
DTAP Vaccine INFANRIX has not been evaluated for carcinogenic or mutagenic potential or for impairment of fertility.
-
HPV Vaccine [137] GARDASIL 9 has not been evaluated for the potential to cause carcinogenicity, genotoxicity or impairment of male fertility.
Long-term safety studies of vaccines are rare. The typical vaccine study is aimed at efficacy. Such studies tend to be a few months long, and the main evaluation criterion is titers of antibody in the serum.
Vaccines, especially childhood vaccines, are administered according to a schedule, which now comprises about seventy + doses covering about sixteen vaccines. The schedule-based combination effects of these seventy + vaccine doses have not been tested, and, therefore, adverse effects due to real-life vaccine synergies are unknown. Such vaccine combination experiments cannot be limited to the pristine environment of the laboratory, but require testing in humans who are exposed to myriad toxic stimuli that could impact vaccine combination synergies.
Much of the published data for vaccine adverse events (at least in the USA) originates from the Vaccine Adverse Event Reporting System (VAERS) database. VAERS is a passive monitoring system, and, like all similar systems, suffers from substantial under-reporting of adverse events [138]. A groundbreaking study [139], performed by Harvard Pilgrim Healthcare, Inc, reported that fewer than 1% of vaccine adverse events are reported. In other words, the actual numbers of adverse reactions to vaccines are one to two orders of magnitude higher than those reported in VAERS!
The methodology used by Harvard Pilgrim Healthcare. Inc, for obtaining this result was as follows: Every patient receiving a vaccine was automatically identified, and for the next 30 days, their health care diagnostic codes, laboratory tests, and medication prescriptions are evaluated for values suggestive of an adverse vaccine event. When a possible adverse event was detected, it was recorded, and the appropriate clinician was to be notified electronically.
Thus, these adverse events that were identified are single-visit short-term adverse events (within thirty days of the vaccination). They do not reflect the results of vaccination combinations administered over a longer period than thirty days, and they do not reflect results of vaccinations of any type in the mid-or long-term [139].
If fewer than 1% of vaccine adverse events are reported, how well does this sample reflect the total number of adverse events actually experienced? This is not a randomly-selected sample, as would be required for a statistically-valid result. Thus, even analyses of short-term adverse effects based on VAERS data are severely flawed. And, if fewer than 1% of these short-term adverse events are reported, what fraction of longer-term adverse events (where the connection between the adverse event and the vaccination becomes more tenuous as time proceeds) would be reported? One can only conclude that a negligible fraction of long-term adverse events is reported in a passive monitoring system like VAERS.
3.7. Diseases triggered by vaccines
A brief analysis was performed of the vaccine biomedical literature to identify diseases potentially triggered by vaccination, especially in the long-term. It should be noted the biomedical literature is very sparse with studies on long-term vaccine effects, especially long-term adverse effects. Large numbers of people and long periods of time are required to identify such adverse events, and draw statistically-valid connections between vaccinations and disease. These efforts would be very resource-intensive, and there appears to be little motivation among the vaccine producers and regulators to make these resources available for such studies. Thus, the following examples reflect the extremely small tip of an extremely large iceberg of long-term adverse vaccine effects.
The two main categories of diseases reported in the biomedical literature triggered by vaccinations are Autoimmune (e.g., Systemic Lupus Erythematosus, Psoriasis, Arthritis, Multiple Sclerosis, Hepatitis, Uveitis, Pseudolymphoma, Guillain-Barre Syndrome, Thrombocytopenic Purpura, etc.) and Neurological (e.g., Central Demyelinating Diseases, Developmental Disability, Febrile seizures, Narcolepsy, Encephalomyelitis, Autonomic Dysfunction, etc.). Others include Diabetes, Gastrointestinal, Joint-related, Necrobiotic Granuloma, Neutropenia, Pulmonary Fibrosis, etc.
Main syndromes associated with systemic toxicity of adjuvanted vaccine: acute phase response (APR), hypersensitivity reactions, induction or worsening of autoimmune diseases, modification of drug hepatic metabolism, vascular leak syndrome (VLS), oral immunosuppression or tolerance post vaccination [140].
Vaccinations may also contribute to the mosaic of autoimmunity. Evidence for the association of vaccinations and the development of these diseases is presented in this review. Infrequently reported post-vaccination autoimmune diseases include systemic lupus erythematosus, rheumatoid arthritis, inflammatory myopathies, multiple sclerosis, Guillain-Barre syndrome, and vasculitis [141].
Toplak et al. reported the production of autoantibodies (such as antinuclear and antiphospholipid antibodies) in 92 healthy medical workers up to 6 months after influenza vaccination. Other studies have demonstrated a latency period of years between HiB vaccination and diabetes mellitus, and between HBV vaccination and demyelinating events. In conclusion, latency periods can range from days to years for postinfection and postvaccination autoimmunity [142].
Adults receiving HBV had significantly increased odds ratios (OR) for multiple sclerosis (OR = 5.2, p < 0.0003, 95 % Confidence Interval (CI) = 1.9–20), optic neuritis (OR = 14, p < 0.0002, 95 % CI = 2.3–560), vasculitis (OR = 2.6, p < 0.04, 95 % CI = 1.03–8.7), arthritis (OR = 2.01, p < 0.0003, 95 % CI = 1.3–3.1), alopecia (OR = 7.2, p < 0.0001, 95 % CI = 3.2–20), lupus erythematosus (OR = 9.1, p < 0.0001, 95 % CI = 2.3–76), rheumatoid arthritis (OR = 18, p < 0.0001, 95 % CI = 3.1–740), and thrombocytopenia (OR = 2.3, p < 0.04, 95 % CI = 1.02–6.2) in comparison to the TCV group. Minimal confounding or systematic error was observed [143].
The difference in cumulative incidence between those receiving 4 doses and those receiving 0 doses is 54 cases of IDDM/100,000 (P = 0.026) at 7 years, (relative risk = 1.26). Most of the extra cases of IDDM appeared in statistically significant clusters that occurred in periods starting approximately 38 months after immunization and lasting approximately 6–8 months. Immunization with pediatric vaccines increased the risk of insulin diabetes in NOD mice…..Exposure to HiB immunization is associated with an increased risk of IDDM. NOD mice can be used as an animal model of vaccine induced diabetes [144].
3.8. Time required for credible COVID-19 vaccine safety studies
As the above results have shown, vaccines can have long-term impacts on the immune system (positive and negative), and short and long-term effects on other diseases. The effects of vaccines can vary according to route of infection, prior history of vaccinations, and, as stated by Benn et al. above, administration “with other vaccines, drugs, or micronutrients and in different sequences [78]. To accelerate the time required to demonstrate long-term safety, laboratory experiments are usually done using animals with relatively short lifespans whose responses to myriad toxic stimuli are similar to that of human beings.
One major difference between these animal experiments and the human model is that the laboratory experiments are usually performed with the administration of a single toxic stimulant, or maybe two, while the human model lives in a sea of toxic stimuli. Also, it is not always clear which animal model simulates the human model best for response to vaccination.
There are many examples in the biomedical literature where combined exposures to toxic stimuli showed adverse effects whereas exposures to the same stimuli in isolation (at the same dosages) showed no adverse effects [83,84]. Thus, unless these laboratory experiments are performed with a range of combinations of associated immunomodulators, they would not be credible for safety assessment purposes. Such experiments would require enormous amounts of financial and time resources.
The other alternative is to perform these safety studies with human beings. For long-term safety studies (e.g., potential vaccine effects on initiating cancer or Alzheimer’s Disease), decades could be required for credible results. Thus, there is a major disconnect between the time required for credible safety studies of a COVID-19 vaccine and the one-year or less vaccine commercialization being propounded by decision-makers and the media today.
3.9. Molecular mimicry and the invalid genetic basis of vaccine pre-clinical tests: the new vaccinology scenario for designing safe and effective vaccines
The above analyzed COVID-19 vaccine safety considerations become even more cogent in light of the fact that cross-reactivity might represent the mechanism underlying the immunopathology and the disease multitude associated with the coronavirus infection [145]. The rationale is that the sharing of peptides between SARS-CoV-2 and human proteins might trigger immune responses hitting not only the virus but also the human proteins, with consequent autoimmune pathologies in the human host [146]. Hence, the massive viral vs. human peptide commonalities described since 2000 [147,148] clearly explain how the protective anti-viral antibody immune response can become a pathogenic autoimmune attack against the human organism, thereby addressing the issue of why SARS-CoV-2 attacks the respiratory system so heavily [149]. The scientific cross-reactivity context and the clinical data showing that immunization with SARS-CoV antigens causes severe pneumonia [150] suggest a prominent pathogenic role of anti-SARS-CoV antibodies in COVID-19. In fact, emerging reports show that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection precedes the appearance of various autoimmune and autoinflammatory diseases, including pediatric inflammatory multisystemic syndrome or multisystem inflammatory syndrome in children [151,152]. Simply put, the current race for obtaining a highly immunogenic anti-SARS-CoV-2 vaccine might actually equate to a race for producing a highly lethal vaccine also in light of the fact that adjuvanted anti-SARS-CoV-2 would have a higher immunogenicity and autoimmune pathogenicity when compared to SARS-CoV-2 infection.
This risk of cross-reactivity further increases when considering that it cannot be estimated with the current vaccine pre-clinical tests [153,154]. Indeed, the level of peptide sharing is highest between pathogens and human, murine, and rat proteomes, and is lowest (or absent) with proteomes from nonhuman primates such as gorilla, chimpanzee, and rhesus macaque. That is, from the genetic point of view, primates are unreliable animal models for revealing potential autoimmune cross-reactions in preclinical testing of immunotherapies since, obviously, no cross-reactions can occur in primates in absentia of shared sequences.
On the whole, the data exposed above open new scenarios in vaccinology by confirming the basic concept first stated in 2000 [147] and then illustrated repeatedly [[155], [156], [157], [158]], according to which only peptide sequences derived from pathogens and absent in the human proteome, i.e., ‘non-self’ peptides’, can lead to safe and efficacious immunotherapies.
3.10. Macro-level considerations
The issues discussed previously can be viewed as micro-level issues. The focus is at the cellular-virus-antibody level. These micro-level issues need to be understood within the larger context of macro-level issues. Two of these macro issues will be discussed in the present section.
The first issue examines the role of vaccines from a larger systemic perspective, especially whether the vaccine target is based on local or global optimization. The second issue relates to the objectivity of the published vaccine effectiveness studies, and how the results are open to bias due to conflicts of interest with research sponsoring agencies.
3.11. Local vs global optimization
In the evolution of COVID-19, the impact of real-life exposures to multiple toxic stressors degrading the immune system is followed by the SARS-CoV-2 virus exploiting the degraded immune system to trigger a chain of events ultimately leading to the disease. A person with such a degraded immune system is more vulnerable to infections and other health assaults. Assume that person is given a vaccine to ‘protect’ against a specific virus. How does it perturb the immune system?
The vaccination is designed to provide a local optimization; it is not designed to provide a global optimization. A successful vaccine may offer some increased protection against the specific viral strain in the vaccine, ranging from one season to a lifetime. What protection does it offer against other strains of the same virus, or other viruses? Does it enhance or decrease protection against other challenges, such as the rapid cell increases characteristic of cancer?
One of the mechanisms examined previously is vaccine-Associated Virus Enhancement (where vaccinated individuals may be at increased risk for other respiratory viruses because they do not receive the non-specific immunity associated with natural infection). This phenomenon doesn't always happen, but it can happen. Its occurrence would strengthen the argument for local optimization, where protection is increased against one viral strain at the expense of reduced protection against another viral strain. Given that (on average) measures are not being taken to reverse the immune system degradation in parallel with administering a vaccine, adding a vaccine may improve one type of protection but degrade another type. The immune system remains degraded, and it will express its limitations against other challenges. Considering the military logistics analogy of the immune system, forces/supplies are being withdrawn from one Front to increase defensive capabilities on another Front. Since overall forces or supplies have not been increased, net overall capabilities have not increased, and, to first order, the vacated Front becomes more vulnerable.
As mentioned previously, many (if not all) vaccine inserts state that the vaccine has not been tested for carcinogenicity (and mutagenicity and fertility) impacts. Such carcinogenicity testing would require long-term tracking and establishing a credible link between the vaccine administered long ago and the onset of cancer. There are few incentives for the developers and regulatory agencies (who tend to be vaccine promoters, especially for a COVID-19 vaccine) to conduct such safety tests; finding e.g. a vaccine-cancer or vaccine-fertility or vaccine-AD link would present major problems. But, if the local/global optimization concept is correct, such a link may be possible, or even greater than possible. Unless the immune system is intrinsically strengthened, it is difficult to see how its operations can be improved in one sector without reducing performance in another sector. Otherwise, vaccines could be developed against every conceivable challenge, and compensate for the degraded immune system.
There is a more fundamental problem with vaccines and other members of the vast armamentarium employed by most of modern-day medicine to treat chronic and infectious diseases. Assume that one of the main operational functions of the body (not subjected to hereditary-based dysfunctions or autoimmune dysfunctionality) is to heal itself continuously. One of the healing mechanisms is signaling when the body is being exposed to harmful substances or harmful behaviors, in order to motivate the elimination of these harmful inputs. Since the body can’t speak vocally, it communicates through the language of symptoms. Rather than listen to the symptoms and take steps to eliminate the offending substances/behaviors, modern medicine uses the approaches of drugs/radiation/surgery and other therapies to attenuate the symptoms. Since the fundamental problem has not been eliminated by the symptom-suppression treatment(s), the body is forced to increase signaling through stronger symptoms, which may emerge short-term or long-term, and could range from modest to lethal. It is inconceivable how such an approach can lead to true healing/disease reversal.
As an example, the first author’s group did a study to develop a protocol that would prevent and reverse Alzheimer’s disease (AD) [159]. As part of the study, Dr. Dale Bredesen, a neurologist who had developed an AD reversal approach, was referenced and quoted as follows: "In the case of Alzheimer’s disease, there is not a single therapeutic that exerts anything beyond a marginal, un-sustained symptomatic effect, with little or no effect on disease progression. Furthermore, in the past decade alone, hundreds of clinical trials have been conducted for AD, at an aggregate cost of billions of dollars, without success. This has led some to question whether the approach taken to drug development for AD is an optimal one [160]." That statement could apply in different degrees to myriad chronic diseases. In fact, it is challenging to identify a chronic disease to which that statement does not apply!
The situation may even be worse with vaccines. Most drugs and other therapies are required to undergo modest short-, mid-, and long-term testing for myriad adverse health effects. One motivator for this range of testing is that the manufacturers/vendors of these therapies can be held liable for damage suffered as a result of their products. Vaccine manufacturers today have waivers from these liabilities (at least in the USA) because of the National Childhood Vaccine Injury Act (NCVIA) of 1986 passed by Congress. So, the financial motivation for thorough testing does not exist for vaccines. Additionally, vaccines are not tested for enhancement or stimulation of serious diseases, as stated previously.
Literature surveys show that vaccines are rarely tested for mid-term adverse effects, and certainly not tested for long-term adverse effects. They are not tested for combinations as administered over time on a prescribed schedule, and they are not laboratory-tested in combination with other toxic substances. There appears to be little interest from the manufacturers or researchers in discovering/identifying these adverse effects. This disinterest is most pronounced in the present efforts to have a COVID-19 vaccine on the market, perhaps made mandatory, within a year after starting development. There cannot be any credible safety testing under such a schedule [161]. There are many potential adverse health effects that can result from vaccine-induced mechanisms, as our present study has shown, and these effects could emerge in the near-term or the long-term. To require the young people (who are not at risk from the most serious consequences of COVID-19) to take such vaccines with potential serious long-term consequences is unjustifiable.
3.12. Objectivity of vaccine effectiveness studies
The following is focused on the USA experience, and probably is relevant to most other countries. Most of the vaccine research and development studies published in the biomedical literature (especially the journals with reasonable Impact Factor threshold values) are sponsored by the pharmaceutical industry and the Federal government, with some funds coming from Foundations. In the USA, the government promotes vaccinations for myriad diseases. It provides funds to the CDC for distributing vaccines, while at the time gives the CDC responsibility for safety monitoring of vaccines. In essence, the Federal government (through different branches) that promotes vaccines also sponsors vaccine research, approves vaccines, distributes vaccines, and monitors the safety of vaccines. These intertwining responsibilities open the door for conflicts-of-interest.
It is in the interests of the Federal government that approved vaccines have high Vaccine Effectiveness (VE), and a reading of the VE literature for the VE section contained in this paper shows clearly the emphasis by the sponsored research community (at least in the High Impact Factor journals) to emphasize high VE for the vaccines examined. For the COVID-19 vaccines under development, and the COVID-19 emergency measures being taken by Federal, State, and Local governments, dissenting voices have to make themselves heard through venues other than peer-reviewed publications in mainline journals. This is a perversion of the scientific process, which requires that all knowledgeable voices be heard, and results in a published literature of questionable credibility.
4. Conclusions
Four types of treatments are being used in different degrees to help counter the COVID-19 pandemic: reducing viral transmission (quarantine, face masks, social distancing, use of sanitizers, etc); treatments (mainly repurposed anti-viral drugs); vaccines (under development); immune system strengthening (eliminating immune degrading toxic stimuli; adding immune enhancing behaviors/substances). The first three types can be viewed as immune-augmenting; the last type is immune-strengthening.
Reducing viral transmission may offer some benefit, but has proven to be damaging psychologically and economically. Anti-viral treatments have had mixed results, and none have achieved consensus within the medical community. Vaccines are under accelerated development. Lifestyle and regulatory changes to strengthen the immune system have been minimal
Vaccines are being promoted by the healthcare industry, politicians, decision-makers, and the mainstream media as the best hope for containing the COVID-19 pandemic, and this is reflected in the funding their accelerated development is receiving (Moderna, a leading COVID-19 vaccine contender, has “scored $2.48 billion in R&D and supply funding from the U.S. government for its program” [161]). The goal appears to be initial vaccine distribution by around end of 2020, although it is questionable whether such an accelerated vaccine development program would include adequate mid-term and long-term safety testing [161].
This optimistic outlook for early vaccine dissemination to the public contradicts vaccine development history, especially for coronavirus vaccines. Vaccine development, including limited safety testing, has taken an average of 12–15 years. Vaccines for the coronaviruses most closely associated with the SARS pandemic/outbreak of 2002 and the MERS pandemic/outbreak of 2012 have yet to be developed successfully, even after one-two decades of research.
The present study examined many viral mechanisms that could lead to vaccines exacerbating rather than attenuating viral infection, based on findings from [162]. Generically, the main problem is that prior viral exposure (vaccine-induced or wild/natural) could impact future viral exposure (vaccine-induced or wild/natural) positively or negatively. Years could be required to determine which outcome would result, both in the short-term and in the long-term. Additionally, many chronic diseases have been shown to result from viral exposure, and years of tracking in human trials could be required to determine which, if any, of these diseases would result from a COVID-19 vaccine. Possibly safer (non-autoimmunity-inducing) vaccines could result using peptide sequences derived from pathogens and absent in the human proteome, although the degree of safety enhancement might require years of tracking to determine.
Authors' contributions
All authors contributed equally, read and approved the final manuscript.
Funding
No funding was received.
Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
Conceptualization: RNK; Data curation: DK, ALP, YS, MBB; Validation, Writing - all authors, Supervision, writing - review & editing: RNK, YS, DC, DAS, AT. All authors contributed equally, read and approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Docea A.O., Tsatsakis A., Albulescu D., Cristea O., Zlatian O., Vinceti M. A new threat from an old enemy: Re‑emergence of coronavirus (Review) Int. J. Mol. Med. 2020;45(6):1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea A.O., Petrakis D., Tsoukalas D., Kostoff R., Rakitskii V., Spandidos D.A., Aschner M., Calina D. COVID‑19 in Northern Italy: An integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020;22(1):20–32. doi: 10.3892/mmr.2020.11079. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima M., Siokas V., Aloizou A.M., Liampas I., Mentis A.A., Tsouris Z., Papadimitriou A., Mitsias P.D., Tsatsakis A., Bogdanos D.P., Baloyannis S.J., Dardiotis E. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr. Treat. Options Neurol. 2020;22(11):37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious. Diseases Soc. Am. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiknakis N., Trivizakis E., Vassalou E.E., Papadakis G.Z., Spandidos D.A., Tsatsakis A., Sánchez-García J., López-González R., Papanikolaou N., Karantanas A.H., Marias K. Interpretable artificial intelligence framework for COVID-19 screening on chest X-rays. Exp. Ther. Med. 2020;20(2):727–735. doi: 10.3892/etm.2020.8797. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos D.A., Tsatsakis A. Obesity ‑ a risk factor for increased COVID‑19 prevalence, severity and lethality (Review) Mol. Med. Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T., Shen Q., Guo W., He W., Li J., Zhang Y. Clinical characteristics of elderly patients with COVID-19 in Hunan Province, China: a multicenter, retrospective study. Gerontology. 2020:1–9. doi: 10.1159/000508734. [DOI] [PubMed] [Google Scholar]
- 13.Kostoff R.N., Briggs M.B., Porter A.L. 2020. COVID-19: Preventing Future Pandemics. Georgia Institute of Technology.https://smartech.gatech.edu/handle/1853/62907 PDF. [Google Scholar]
- 14.Tsatsakis A., Petrakis Demetrious, Nikolouzakis Taxiarchis Konstantinos, Docea Anca Oana, Calina Daniela, Vinceti Marco, Goumenou Marina, Kostoff Ronald N., Mamoulakis Charalampos, Aschner Michael, Hernández Antonio F. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141 doi: 10.1016/j.fct.2020.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castle S.C., Uyemura K., Fulop T., Makinodan T. Host resistance and immune responses in advanced age. Clin. Geriatr. Med. 2007;23(3):463–479. doi: 10.1016/j.cger.2007.03.005. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsatsakis A., Calina D., Falzone L., Petrakis D., Mitrut R., Siokas V., Pennisi M., Lanza G., Libra M., Doukas S.G., Doukas P.G., Kavali L., Bukhari A., Gadiparthi C., Vageli D.P., Kofteridis D.P., Spandidos D.A., Paoliello M.M.B., Aschner M., Docea A.O. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stancioiu F., Papadakis G.Z., Kteniadakis S., Izotov B.N., Coleman M.D., Spandidos D.A., Tsatsakis A. A dissection of SARS‑CoV2 with clinical implications (Review) Int. J. Mol. Med. 2020;46(2):489–508. doi: 10.3892/ijmm.2020.4636. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungureanu A., Zlatian O., Mitroi G., Drocaş A., Ţîrcă T., Călina D., Dehelean C., Docea A.O., Izotov B.N., Rakitskii V.N., Cioboată R., Spandidos D.A., Tsatsakis A.M., Găman A. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol. Med. Rep. 2017;16(6):8771–8780. doi: 10.3892/mmr.2017.7746. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlatian O., Balasoiu A.T., Balasoiu M., Cristea O., Docea A.O., Mitrut R., Spandidos D.A., Tsatsakis A.M., Bancescu G., Calina D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp. Ther. Med. 2018;16(6):4499–4510. doi: 10.3892/etm.2018.6737. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cioboată R., Găman A., Traşcă D., Ungureanu A., Docea A.O., Tomescu P., Gherghina F., Arsene A.L., Badiu C., Tsatsakis A.M., Spandidos D.A., Drakoulis N., Călina D. Pharmacological management of non-alcoholic fatty liver disease: atorvastatin versus pentoxifylline. Exp. Ther. Med. 2017;13(5):2375–2381. doi: 10.3892/etm.2017.4256. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehi B., Rescigno A., Dettori T., Calina D., Docea A.O., Singh L., Cebeci F., Özçelik B., Bhia M., Dowlati Beirami A., Sharifi-Rad J., Sharopov F., Cho W.C., Martins N. Avocado-Soybean Unsaponifiables: A Panoply of Potentialities to Be Exploited. Biomolecules. 2020;10(1):130. doi: 10.3390/biom10010130. Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi B., Sharifi-Rad J., Cappellini F., Reiner Ž, Zorzan D., Imran M., Sener B., Kilic M., El-Shazly M., Fahmy N.M., Al-Sayed E., Martorell M., Tonelli C., Petroni K., Docea A.O., Calina D., Maroyi A. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front. Pharmacol. 2020;11(1300) doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: Perspectives for COVID‑19 (Review) Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostoff R.N., Briggs M.B., Porter A.L., Aschner M., Spandidos D.A., Tsatsakis A. [Editorial] COVID 19: post lockdown guidelines. Int. J. Mol. Med. 2020;46(2):463–466. doi: 10.3892/ijmm.2020.4640. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsatsakis A., Docea A.O., Calina D., Tsarouhas K., Zamfira L.M., Mitrut R., Sharifi-Rad J., Kovatsi L., Siokas V., Dardiotis E., Drakoulis N., Lazopoulos G., Tsitsimpikou C., Mitsias P., Neagu M. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019;8(9):1295. doi: 10.3390/jcm8091295. Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharifi-Rad J., Rodrigues C.F., Sharopov F., Docea A.O., Can Karaca A., Sharifi-Rad M. Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health. 2020;17(7):2326. doi: 10.3390/ijerph17072326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharifi-Rad M., Lankatillake C., Dias D.A., Docea A.O., Mahomoodally M.F., Lobine D. Impact of natural compounds on neurodegenerative disorders: from preclinical to Pharmacotherapeutics. J. Clin. Med. 2020;9(4):1061. doi: 10.3390/jcm9041061. Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrakis D., Vassilopoulou L., Mamoulakis C., Psycharakis C., Anifantaki A., Sifakis S., Docea A.O., Tsiaoussis J., Makrigiannakis A., Tsatsakis A.M. Endocrine disruptors leading to obesity and related diseases. Int. J. Environ. Res. Public Health. 2017;14(10):1282. doi: 10.3390/ijerph14101282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharifi-Rad M., Anil Kumar N.V., Zucca P. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covid-19 Treatment and Vaccine Tracker. Milken Institute. https://milkeninstitute.org/covid-19-tracker.
- 31.Torequl Islam M., Nasiruddin, Khan I.N., Mishra S.K., Kudrat-E-Zahan M., Alam Riaz T., Ali E.S., Rahman M.S., Mubarak M.S., Martorell M., Cho W.C., Calina D., Docea A.O., Sharifi-Rad J. A perspective on emerging therapeutic interventions for COVID-19. Front. Public Health. 2020;8:281. doi: 10.3389/fpubh.2020.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar C., Mondal M., Torequl Islam M., Martorell M., Docea A.O., Maroyi A., Sharifi-Rad J., Calina D. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front. Pharmacol. 2020;11(572870) doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dehelean C.A., Lazureanu V., Coricovac D., Mioc M., Oancea R., Marcovici I., Pinzaru I., Soica C., Tsatsakis A.M., Cretu O. SARS-CoV-2: Repurposed Drugs and Novel Therapeutic Approaches-Insights into Chemical Structure-Biological Activity and Toxicological Screening. J. Clin. Med. 2020;9(7):2084. doi: 10.3390/jcm9072084. Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitulescu G.M., Paunescu H., Moschos S.A., Petrakis D., Nitulescu G.M., Ion G.N.D., Spandidos D.A., Nikolouzakis T.K., Drakoulis N., Tsatsakis A. Comprehensive analysis of drugs to treat SARS‑CoV‑2 infection: Mechanistic insights into current COVID‑19 therapies (Review) Int. J. Mol. Med. 2020;46(2):467–488. doi: 10.3892/ijmm.2020.4608. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostoff R.N. Treatment repurposing using literature-related discovery. J. Scientometr. Res. 2019;8(2):S74–S84. [Google Scholar]
- 36.Kostoff R.N., Briggs M.B., Shores D.R. Treatment repurposing for inflammatory bowel disease using literature-related discovery and innovation. World J. Gastroenterol. 2020;26(33):4889–4899. doi: 10.3748/wjg.v26.i33.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostoff R.N., Briggs M.B., Porter A.L., Hernández A.F., Abdollahi M., Aschner M., Tsatsakis A. The under-reported role of toxic substance exposures in the COVID-19 pandemic. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharifi-Rad J., Rodrigues C.F., Stojanović-Radić Z., Dimitrijević M., Aleksić A., Neffe-Skocińska K., Zielińska D., Kołożyn-Krajewska D., Salehi B., Milton Prabu S., Schutz F., Docea A.O., Martins N., Calina D. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina. 2020;56(9):433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsoukalas D., Fragkiadaki P., Docea A.O., Alegakis A.K., Sarandi E., Vakonaki E., Salataj E., Kouvidi E., Nikitovic D., Kovatsi L., Spandidos D.A., Tsatsakis A., Calina D. Association of nutraceutical supplements with longer telomere length. Int. J. Mol. Med. 2019;44(1):218–226. doi: 10.3892/ijmm.2019.4191. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsoukalas D., Fragkiadaki P., Docea A.O., Alegakis A.K., Sarandi E., Thanasoula M., Spandidos D.A., Tsatsakis A., Razgonova M.P., Calina D. Discovery of potent telomerase activators: unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019;20(4):3701–3708. doi: 10.3892/mmr.2019.10614. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calina D., Docea A.O., Petrakis D., Egorov A.M., Ishmukhametov A.A., Gabibov A.G. Towards effective COVID‑19 vaccines: Updates, perspectives and challenges (Review) Int. J. Mol. Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calina D., Hartung T., Docea A.O. COVID-19 vaccines: ethical framework concerning human challenge studies. DARU J. Pharm. Sci. 2020 doi: 10.1007/s40199-020-00371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front. Microbiol. 2019;10:1781. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect. Dis. Ther. 2020:1–20. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fett C., DeDiego M.L., Regla-Nava J.A., Enjuanes L., Perlman S. Complete protection against severe acute respiratory syndrome coronavirus-mediated lethal respiratory disease in aged mice by immunization with a mouse-adapted virus lacking E protein. J. Virol. 2013;87(12):6551–6559. doi: 10.1128/JVI.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regla-Nava J.A., Nieto-Torres J.L., Jimenez-Guardeno J.M., Fernandez-Delgado R., Fett C., Castano-Rodriguez C. Severe acute respiratory syndrome coronaviruses with mutations in the E protein are attenuated and promising vaccine candidates. J. Virol. 2015;89(7):3870–3887. doi: 10.1128/JVI.03566-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calina D., Sarkar C., Arsene A.L. Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development. Immunol. Res. 2020 doi: 10.1007/s12026-020-09154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huisman W., Martina B.E.E., Rimmelzwaan G.F., Gruters R.A. Osterhaus ADME. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27(4):505–512. doi: 10.1016/j.vaccine.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor A., Foo S.-S., Bruzzone R., Dinh L.V., King N.J.C., Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015;268(1):340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020;20(6):339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tirado S.M.C., Yoon K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16(1):69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 52.Shmelkov E., Nadas A., Cardozo T. Could vaccination with AIDSVAX immunogens have resulted in antibody-dependent enhancement of HIV infection in human subjects? Hum. Vaccin. Immunother. 2014;10(10):3013–3016. doi: 10.4161/21645515.2014.972148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu W., Guo L., Yu H., Niu J., Huang M., Luo X. Involvement of CD16 in antibody-dependent enhancement of porcine reproductive and respiratory syndrome virus infection. J. Gen. Virol. 2015;96(Pt 7):1712–1722. doi: 10.1099/vir.0.000118. [DOI] [PubMed] [Google Scholar]
- 54.Smatti M.K., Al Thani A.A., Yassine H.M. Viral-induced enhanced disease illness. Front. Microbiol. 2018;9:2991. doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ubol S., Halstead S.B. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin. Vaccine Immunol. CVI. 2010;17(12):1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halstead S.B. Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol. Spectr. 2014;2(6) doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 58.Sprokholt J., Helgers L.C., Geijtenbeek T.B. Innate immune receptors drive dengue virus immune activation and disease. Future Virol. 2017;13(4):287–305. doi: 10.2217/fvl-2017-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guzman M.G., Alvarez M., Halstead S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013;158(7):1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 60.Costa V.V., Fagundes C.T., Valadao D.F., Avila T.V., Cisalpino D., Rocha R.F. Subversion of early innate antiviral responses during antibody-dependent enhancement of Dengue virus infection induces severe disease in immunocompetent mice. Med. Microbiol. Immunol. 2014;203(4):231–250. doi: 10.1007/s00430-014-0334-5. [DOI] [PubMed] [Google Scholar]
- 61.Jaume M., Yip M.S., Kam Y.W., Cheung C.Y., Kien F., Roberts A. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med. J. 2012;18(Suppl 2):31–36. [PubMed] [Google Scholar]
- 62.Klonjkowski B., Klein D., Galea S., Gavard F., Monteil M., Duarte L. Gag-specific immune enhancement of lentiviral infection after vaccination with an adenoviral vector in an animal model of AIDS. Vaccine. 2009;27(6):928–939. doi: 10.1016/j.vaccine.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 63.Mier-y-Teran-Romero L., Schwartz I.B., Cummings D.A.T. Breaking the symmetry: immune enhancement increases persistence of dengue viruses in the presence of asymmetric transmission rates. J. Theor. Biol. 2013;332:203–210. doi: 10.1016/j.jtbi.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotez P.J., Bottazzi M.E., Corry D.B. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 2020;22(4–5):165–167. doi: 10.1016/j.micinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clay C., Donart N., Fomukong N., Knight J.B., Lei W., Price L. Primary severe acute respiratory syndrome coronavirus infection limits replication but not lung inflammation upon homologous rechallenge. J. Virol. 2012;86(8):4234–4244. doi: 10.1128/JVI.06791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crunkhorn S. Eliminating vaccine cross-reactivity. Nat. Rev. Drug Discov. 2019;18:826–827. doi: 10.1038/d41573-019-00171-z. [DOI] [PubMed] [Google Scholar]
- 67.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paul L.M., Carlin E.R., Jenkins M.M., Tan A.L., Barcellona C.M., Nicholson C.O. Dengue virus antibodies enhance Zika virus infection. Clin. Transl. Immunology. 2016;5(12):e117. doi: 10.1038/cti.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castanha P.M.S., Nascimento E.J.M., Braga C., Cordeiro M.T., de Carvalho O.V., de Mendonca L.R. Dengue virus-specific antibodies enhance brazilian zika virus infection. J. Infect. Dis. 2017;215(5):781–785. doi: 10.1093/infdis/jiw638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolff G.G. Influenza vaccination and respiratory virus interference among Department of Defense personnel during the 2017-2018 influenza season. Vaccine. 2020;38(2):350–354. doi: 10.1016/j.vaccine.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cowling B.J., Fang V.J., Nishiura H., Chan K.-H., Ng S., Ip D.K.M. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin. Infect. Dis. 2012;54(12):1778–1783. doi: 10.1093/cid/cis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skowronski D.M., De Serres G., Crowcroft N.S., Janjua N.Z., Boulianne N., Hottes T.S. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010;7(4) doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rikin S., Jia H., Vargas C.Y., Castellanos de Belliard Y., Reed C., LaRussa P. Assessment of temporally-related acute respiratory illness following influenza vaccination. Vaccine. 2018;36(15):1958–1964. doi: 10.1016/j.vaccine.2018.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Asten L., Bijkerk P., Fanoy E., van Ginkel A., Suijkerbuijk A., van der Hoek W. Early occurrence of influenza A epidemics coincided with changes in occurrence of other respiratory virus infections. Influenza Other Respir. Viruses. 2016;10(1):14–26. doi: 10.1111/irv.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lisewski A.M. 2020. Association between Influenza Vaccination Rates and SARS-CoV-2 Outbreak Infection Rates in OECD Countries. ( https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3558270) [Google Scholar]
- 76.Skowronski D.M., Sabaiduc S., Leir S., Rose C., Zou M., Murti M. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV) Euro Surveill. 2019;24(46) doi: 10.2807/1560-7917.ES.2019.24.46.1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelvin A.A., Zambon M. Influenza imprinting in childhood and the influence on vaccine response later in life. Euro Surveill. 2019;24(48) doi: 10.2807/1560-7917.ES.2019.24.48.1900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Rakebrandt N., Joller N. Infection history determines susceptibility to unrelated diseases. BioEssays: news and reviews in molecular. Cellular Dev. Biol. 2019;41(6) doi: 10.1002/bies.201800191. [DOI] [PubMed] [Google Scholar]
- 80.Demars A., Lison A., Machelart A., Van Vyve M., Potemberg G., Vanderwinden J.-M. Route of infection strongly impacts the host-pathogen relationship. Front. Immunol. 2019;10:1589. doi: 10.3389/fimmu.2019.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pascual D.W., Yang X., Wang H., Goodwin Z., Hoffman C., Clapp B. Alternative strategies for vaccination to brucellosis. Microbes Infect. 2018;20(9–10):599–605. doi: 10.1016/j.micinf.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguilo N., Alvarez-Arguedas S., Uranga S., Marinova D., Monzon M., Badiola J. Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-Dependent mechanism. J. Infect. Dis. 2016;213(5):831–839. doi: 10.1093/infdis/jiv503. [DOI] [PubMed] [Google Scholar]
- 83.Kostoff R.N., Aschner M., Goumenou M., Tsatsakis A. Setting safer exposure limits for toxic substance combinations. Food Chem. Toxicol. 2020;140 doi: 10.1016/j.fct.2020.111346. [DOI] [PubMed] [Google Scholar]
- 84.Kostoff R.N., Goumenou M., Tsatsakis A. The role of toxic stimuli combinations in determining safe exposure limits. Toxicol. Rep. 2018;5:1169–1172. doi: 10.1016/j.toxrep.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith D.J., Forrest S., Ackley D.H., Perelson A.S. Variable efficacy of repeated annual influenza vaccination. Proc. Natl. Acad. Sci. U.S.A. 1999;96(24):14001–14006. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skowronski D.M., Chambers C., De Serres G., Sabaiduc S., Winter A.-L., Dickinson J.A. Serial Vaccination and the Antigenic Distance Hypothesis: Effects on Influenza Vaccine Effectiveness During A(H3N2) Epidemics in Canada, 2010-2011 to 2014-2015. J. Infect. Dis. 2017;215(7):1059–1099. doi: 10.1093/infdis/jix074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belongia E.A., Skowronski D.M., McLean H.Q., Chambers C., Sundaram M.E., De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev. Vaccines. 2017;16(7):1–14. doi: 10.1080/14760584.2017.1334554. [DOI] [PubMed] [Google Scholar]
- 88.Vadala M., Poddighe D., Laurino C., Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8(3):295–311. doi: 10.1007/s13167-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salemi S., D’Amelio R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 2010;29(3):247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 90.Pacheco Y., Acosta-Ampudia Y., Monsalve D.M., Chang C., Gershwin M.E., Anaya J.-M. Bystander activation and autoimmunity. J. Autoimmun. 2019;103 doi: 10.1016/j.jaut.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y.-N., Yang Y.-C.S.H., Lin I.H., Chen Y.-Y., Lin I.H., Wu C.-Y. Phthalate exposure alters gut microbiota composition and IgM vaccine response in human newborns. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110700. [DOI] [PubMed] [Google Scholar]
- 92.Hagan T., Cortese M., Rouphael N., Boudreau C., Linde C., Maddur M.S. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6) doi: 10.1016/j.cell.2019.08.010. 1313-28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vlasova A.N., Takanashi S., Miyazaki A., Rajashekara G., Saif L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019;37:16–25. doi: 10.1016/j.coviro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki A., Kandasamy S., Michael H., Langel S.N., Paim F.C., Chepngeno J. Protein deficiency reduces efficacy of oral attenuated human rotavirus vaccine in a human infant fecal microbiota transplanted gnotobiotic pig model. Vaccine. 2018;36(42):6270–6281. doi: 10.1016/j.vaccine.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhattacharjee A., Hand T.W. Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin. Sci. (London, England: 1979). 2018;132(11):1169–1177. doi: 10.1042/CS20171106. [DOI] [PubMed] [Google Scholar]
- 96.Karlas J.A., Siebelink K.H., Peer M.A., Huisman W., Cuisinier A.M., Rimmelzwaan G.F. Vaccination with experimental feline immunodeficiency virus vaccines, based on autologous infected cells, elicits enhancement of homologous challenge infection. J. Gen. Virol. 1999;80(Pt 3):761–765. doi: 10.1099/0022-1317-80-3-761. [DOI] [PubMed] [Google Scholar]
- 97.Giannecchini S., Isola P., Sichi O., Matteucci D., Pistello M., Zaccaro L. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts. J. Virol. 2002;76(14):6882–6892. doi: 10.1128/JVI.76.14.6882-6892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller C., Emanuelli M., Fink E., Musselman E., Mackie R., Troyer R. FIV vaccine with receptor epitopes results in neutralizing antibodies but does not confer resistance to challenge. Npj Vaccines. 2018;3:16. doi: 10.1038/s41541-018-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hale B.G., Albrecht R.A., Garcia-Sastre A. Innate immune evasion strategies of influenza viruses. Future Microbiol. 2010;5(1):23–41. doi: 10.2217/fmb.09.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang C., Zhang Q., Feng W.-h. Regulation and evasion of antiviral immune responses by porcine reproductive and respiratory syndrome virus. Virus Res. 2015;202:101–111. doi: 10.1016/j.virusres.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agrawal P., Nawadkar R., Ojha H., Kumar J., Sahu A. Complement evasion strategies of viruses: an overview. Front. Microbiol. 2017;8:1117. doi: 10.3389/fmicb.2017.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shokri S., Mahmoudvand S., Taherkhani R., Farshadpour F. Modulation of the immune response by Middle East respiratory syndrome coronavirus. J. Cell. Physiol. 2019;234(3):2143–2151. doi: 10.1002/jcp.27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toapanta F.R., Craigo J.K., Montelaro R.C., Ross T.M. Reduction of anti-HIV-1 Gag immune responses during co-immunization: immune interference by the HIV-1 envelope. Curr. HIV Res. 2007;5(2):199–209. doi: 10.2174/157016207780077057. [DOI] [PubMed] [Google Scholar]
- 104.Pittman P.R., Liu C.-T., Cannon T.L., Mangiafico J.A., Gibbs P.H. Immune interference after sequential alphavirus vaccine vaccinations. Vaccine. 2009;27(36):4879–4882. doi: 10.1016/j.vaccine.2009.02.090. [DOI] [PubMed] [Google Scholar]
- 105.Bradt V., Malafa S., von Braun A., Jarmer J., Tsouchnikas G., Medits I. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. NPJ Vaccines. 2019;4:38. doi: 10.1038/s41541-019-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larke N., Im E.-J., Wagner R., Wagner R., Williamson C., Williamson A.-L., McMichael A.J. Combined single-clade candidate HIV-1 vaccines induce T cell responses limE.-J.tEd by multiple forms of in vivo immune interference. Eur. J. Immunol. 2007;37(2):566–577. doi: 10.1002/eji.200636711. [DOI] [PubMed] [Google Scholar]
- 107.Zhang A., Stacey H.D., Mullarkey C.E., Miller M.S. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J. Immunol. (Baltimore, Md: 1950) 2019;202(2):335–340. doi: 10.4049/jimmunol.1801149. [DOI] [PubMed] [Google Scholar]
- 108.Vatti A., Monsalve D.M., Pacheco Y., Chang C., Anaya J.-M., Gershwin M.E. Original antigenic sin: a comprehensive review. J. Autoimmun. 2017;83:12–21. doi: 10.1016/j.jaut.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 109.Joshi M., Chandra D., Mittadodla P., Bartter T. The impact of vaccination on influenza-related respiratory failure and mortality in hospitalized elderly patients over the 2013-2014 season. Open Respir. Med. J. 2015;9:9–14. doi: 10.2174/1874306401509010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J.H., Liepkalns J., Reber A.J., Lu X., Music N., Jacob J. Prior infection with influenza virus but not vaccination leaves a long-term immunological imprint that intensifies the protective efficacy of antigenically drifted vaccine strains. Vaccine. 2016;34(4):495–502. doi: 10.1016/j.vaccine.2015.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saito N., Komori K., Suzuki M., Morimoto K., Kishikawa T., Yasaka T. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine. 2017;35(4):687–693. doi: 10.1016/j.vaccine.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 112.Kosikova M., Li L., Radvak P., Ye Z., Wan X.-F., Xie H. Imprinting of repeated influenza A/H3 exposures on antibody quantity and antibody quality: implications for seasonal vaccine strain selection and vaccine performance. Clin. Infect. Dis. 2018;67(10):1523–1532. doi: 10.1093/cid/ciy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLean H.Q., Thompson M.G., Sundaram M.E., Meece J.K., McClure D.L., Friedrich T.C. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin. Infect. Dis. 2014;59(10):1375–1385. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ng T.W.Y., RAPM Perera, Fang V.J., Yau E.M., Peiris J.S.M., Tam Y.H. The Effect of Influenza Vaccination History on Changes in Hemagglutination Inhibition Titers After Receipt of the 2015-2016 Influenza Vaccine in Older Adults in Hong Kong. T.W.Y.he J. Infectious Diseases. 2020;221(1):33–41. doi: 10.1093/infdis/jiz327. [DOI] [PubMed] [Google Scholar]
- 115.Verhees R.A.F., Thijs C., Ambergen T., Dinant G.J., Knottnerus J.A. Influenza vaccination in the elderly: 25 years follow-up of a randomized controlled trial. No impact on long-term mortality. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0216983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohmit S.E., Petrie J.G., Malosh R.E., Cowling B.J., Thompson M.G., Shay D.K. Influenza vaccine effectiveness in the community and the household. Clin. Infect. Dis. 2013;56(10):1363–1369. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Palgen J.-L., Tchitchek N., Rodriguez-Pozo A., Jouhault Q., Abdelhouahab H., Dereuddre-Bosquet N. Innate and secondary humoral responses are improved by increasing the time between MVA vaccine immunizations. NPJ Vaccines. 2020;5:24. doi: 10.1038/s41541-020-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Castilla J., Martinez-Baz I., Martinez-Artola V., Reina G., Pozo F., Garcia Cenoz M. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surv. 2013;18(5) doi: 10.2807/ese.18.05.20388-en. [DOI] [PubMed] [Google Scholar]
- 119.Saito N., Komori K., Suzuki M., Kishikawa T., Yasaka T., Ariyoshi K. Dose-dependent negative effects of prior multiple vaccinations against influenza a and influenza B among schoolchildren: a study of Kamigoto Island in Japan during the 2011-2012, 2012-2013, and 2013-2014 influenza seasons. Clinical infectious diseases: an official publication of the infectious. Diseases Soc. Am. 2018;67(6):897–904. doi: 10.1093/cid/ciy202. [DOI] [PubMed] [Google Scholar]
- 120.Morimoto N., Takeishi K. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: a systematic review and meta-analysis. Vaccine. 2018;36(7):949–957. doi: 10.1016/j.vaccine.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 121.Khurana S., Loving C.L., Manischewitz J., King L.R., Gauger P.C., Henningson J. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci. Transl. Med. 2013;5(200) doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 122.Rajao D.S., Loving C.L., Gauger P.C., Kitikoon P., Vincent A.L. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine. 2014;32(40):5170–5176. doi: 10.1016/j.vaccine.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 123.Rajao D.S., Sandbulte M.R., Gauger P.C., Kitikoon P., Platt R., Roth J.A. Heterologous challenge in the presence of maternally-derived antibodies results in vaccine-associated enhanced respiratory disease in weaned piglets. Virology. 2016;491:79–88. doi: 10.1016/j.virol.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.United States. National Institutes of Health NIHNIoAaID Immune system activation boosts HIV replication in HIV-infected people. TB HIV. 1996;(11):14. [PubMed] [Google Scholar]
- 125.Sharan R., Bucsan A.N., Ganatra S., Paiardini M., Mohan M., Mehra S. Chronic immune activation in TB/HIV Co-infection. Trends Microbiol. 2020;28(8):619–632. doi: 10.1016/j.tim.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paiardini M., Muller-Trutwin M. HIV-associated chronic immune activation. Immunol. Rev. 2013;254(1):78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szilagyi P.G., Fairbrother G., Griffin M.R., Hornung R.W., Donauer S., Morrow A. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch. Pediatr. Adolesc. Med. 2008;162(10):943–951. doi: 10.1001/archpedi.162.10.943. [DOI] [PubMed] [Google Scholar]
- 128.Joshi A.Y., Iyer V.N., Hartz M.F., Patel A.M., Li J.T. Effectiveness of trivalent inactivated influenza vaccine in influenza-related hospitalization in children: a case-control study. Allergy Asthma Proc. 2012;33(2):e23–7. doi: 10.2500/aap.2012.33.3513. [DOI] [PubMed] [Google Scholar]
- 129.Belongia E.A., Kieke B.A., Donahue J.G., Greenlee R.T., Balish A., Foust A. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J. Infect. Dis. 2009;199(2):159–167. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 130.Janjua N.Z., Skowronski D.M., Hottes T.S., Osei W., Adams E., Petric M. Seasonal influenza vaccine and increased risk of pandemic A/H1N1‐related illness: first detection of the association in British Columbia, Canada. Clin. Infect. Dis. 2010;51(9):1017–1027. doi: 10.1086/656586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bodewes R., Fraaij P.L.A., Kreijtz J.H.C.M., Geelhoed-Mieras M.M., Fouchier R.A.M., Osterhaus A.D.M.E. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine. 2012;30(51):7407–7410. doi: 10.1016/j.vaccine.2012.04.086. [DOI] [PubMed] [Google Scholar]
- 132.Choi Y.S., Baek Y.H., Kang W., Nam S.J., Lee J., You S. Reduced antibody responses to the pandemic (H1N1) 2009 vaccine after recent seasonal influenza vaccination. Clin. Vaccine Immunol. CVI. 2011;18(9):1519–1523. doi: 10.1128/CVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kelly H.A., Grant K.A., Fielding J.E., Carville K.S., Looker C.O., Tran T. Pandemic influenza H1N1 2009 infection in Victoria, Australia: no evidence for harm or benefit following receipt of seasonal influenza vaccine in 2009. Vaccine. 2011;29(37):6419–6426. doi: 10.1016/j.vaccine.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 134.Vashishtha V.M., Kumar P. Seasonal influenza vaccination and the heightened risk of coronavirus and other pandemic virus infections: fact or fiction? Indian Pediatr. 2020;57(8):767–768. doi: 10.1007/s13312-020-1936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skowronski D.M., De Serres G. Evidence in a cluster randomized controlled trial of increased 2009 pandemic risk associated with 2008-2009 seasonal influenza vaccine receipt. Clinical infectious diseases : an official publication of the infectious. Diseases Soc. Am. 2019;69(12):2230–2231. doi: 10.1093/cid/ciz351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Package Inserts and Manufacturers for some US Licensed Vaccines and Immunoglobulins. Institute for Vaccine Safety. Johns Hopkins University. https://www.vaccinesafety.edu/package_inserts.htm.
- 137.Boda D., Docea A.O., Calina D., Ilie M.A., Caruntu C., Zurac S., Neagu M., Constantin C., Branisteanu D.E., Voiculescu V., Mamoulakis C., Tzanakakis G., Spandidos D.A., Drakoulis N., Tsatsakis A.M. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (Review) Int. J. Oncol. 2018;52(3):637–655. doi: 10.3892/ijo.2018.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goldman G.S., Miller N.Z. Relative trends in hospitalizations and mortality among infants by the number of vaccine doses and age, based on the Vaccine Adverse Event Reporting System (VAERS), 1990-2010. Human Experimental Toxicol. 2012;31(10):1012–1021. doi: 10.1177/0960327112440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). https://digital.ahrq.gov/ahrq-funded-projects/electronic-support-public-health-vaccine-adverse-event-reporting-system.
- 140.Batista-Duharte A., Portuondo D., Perez O., Carlos I.Z. Systemic immunotoxicity reactions induced by adjuvanted vaccines. Int. Immunopharmacol. 2014;20(1):170–180. doi: 10.1016/j.intimp.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 141.Orbach H., Agmon-Levin N., Zandman-Goddard G. Vaccines and autoimmune diseases of the adult. Discov. Med. 2010;9(45):90–97. [PubMed] [Google Scholar]
- 142.Agmon-Levin N., Paz Z., Israeli E., Shoenfeld Y. Vaccines and autoimmunity. Nat. Rev. Rheumatol. 2009;5(11):648–652. doi: 10.1038/nrrheum.2009.196. [DOI] [PubMed] [Google Scholar]
- 143.Geier D.A., Geier M.R. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38(4):295–301. doi: 10.1080/08916930500144484. [DOI] [PubMed] [Google Scholar]
- 144.Classen J.B., Classen D.C. Clustering of cases of insulin dependent diabetes (IDDM) occurring three years after hemophilus influenza B (HiB) immunization support causal relationship between immunization and IDDM. Autoimmunity. 2002;35(4):247–253. doi: 10.1080/08916930290028175. [DOI] [PubMed] [Google Scholar]
- 145.Kanduc D. From Anti-SARS-CoV-2 immune responses to COVID-19 via molecular mimicry. Antibodies Basel (Basel) 2020;9(3) doi: 10.3390/antib9030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kanduc D. Peptide cross-reactivity: the original sin of vaccines. Front. Biosci. Schol. Ed. (Schol Ed) 2012;4:1393–1401. doi: 10.2741/s341. [DOI] [PubMed] [Google Scholar]
- 147.Natale C., Giannini T., Lucchese A., Kanduc D. Computer assisted analysis of molecular mimicry between human papillomavirus 16 E7 oncoprotein and human protein sequences. Immunol. Cell Biol. 2000;78:580–585. doi: 10.1046/j.1440-1711.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- 148.Kanduc D., Stufano A., Lucchese G., Kusalik A. Massive peptide sharing between viral and human proteomes. Peptides. 2008;29(10):1755–1766. doi: 10.1016/j.peptides.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kanduc D., Shoenfeld Y. On the molecular determinants of the SARS-CoV-2 attack. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.-H. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes. Implications for the vaccine. Immunol. Res. 2020 doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanduc D., Shoenfeld Y. Medical, genomic, and evolutionary aspects of the peptide sharing between pathogens, primates, and humans. Global Med. Genet. 2020;07(02):064–067. doi: 10.1055/s-0040-1716334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kanduc D. Self-nonself" peptides in the design of vaccines. Curr. Pharm. Des. 2009;15(28):3283–3289. doi: 10.2174/138161209789105135. [DOI] [PubMed] [Google Scholar]
- 156.Kanduc D. The self/nonself issue: a confrontation between proteomes. Self Nonself. 2010;1(3):255–258. doi: 10.4161/self.1.3.11897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kanduc D. Immunogenicity, immunopathogenicity, and immunotolerance in one graph. Anticancer Agents Med. Chem. 2015;15(10):1264–1268. doi: 10.2174/1871520615666150716105543. [DOI] [PubMed] [Google Scholar]
- 158.Kanduc D., Shoenfeld Y. From HBV to HPV: designing vaccines for extensive and intensive vaccination campaigns worldwide. Autoimmun. Rev. 2016;15(11):1054–1061. doi: 10.1016/j.autrev.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 159.Kostoff R.N., Porter A.L., Buchtel H.A. Georgia Institute of Technology; 2018. Prevention and Reversal of Alzheimer’s Disease: Treatment Protocol.https://smartech.gatech.edu/handle/1853/59311 PDF. [Google Scholar]
- 160.Bredesen D.E. Reversal of cognitive decline: a novel therapeutic program. Aging. 2014;6(9):707–717. doi: 10.18632/aging.100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kostoff R.N., Briggs M.B., Porter A.L., Spandidos D.A., Tsatsakis A. [Comment] COVID-19 vaccine safety. Int. J. Mol. Med. 2020;(Sept 18) doi: 10.3892/ijmm.2020.4733. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kostoff R.N., Kanduc D., Porter A.L., Shoenfeld Y., Briggs M.B. Georgia Institute of Technology; 2020. COVID-19 Vaccine Safety Considerations.http://hdl.handle.net/1853/63710 PDF. [Google Scholar]