Abstract
Objective
Early reports suggest that patients with novel coronavirus disease-2019 (COVID-19) infection carry a significant risk of altered coagulation with an increased risk for venous thromboembolic events. This report investigates the relationship of significant COVID-19 infection and deep venous thrombosis (DVT) as reflected in the patient clinical and laboratory characteristics.
Methods
We reviewed the demographics, clinical presentation, laboratory and radiologic evaluations, results of venous duplex imaging and mortality of COVID-19-positive patients (18-89 years) admitted to the Indiana University Academic Health Center. Using oxygen saturation, radiologic findings, and need for advanced respiratory therapies, patients were classified into mild, moderate, or severe categories of COVID-19 infection. A descriptive analysis was performed using univariate and bivariate Fisher's exact and Wilcoxon rank-sum tests to examine the distribution of patient characteristics and compare the DVT outcomes. A multivariable logistic regression model was used to estimate the adjusted odds ratio of experiencing DVT and a receiver operating curve analysis to identify the optimal cutoff for d-dimer to predict DVT in this COVID-19 cohort. Time to the diagnosis of DVT from admission was analyzed using log-rank test and Kaplan-Meier plots.
Results
Our study included 71 unique COVID-19-positive patients (mean age, 61 years) categorized as having 3% mild, 14% moderate, and 83% severe infection and evaluated with 107 venous duplex studies. DVT was identified in 47.8% of patients (37% of examinations) at an average of 5.9 days after admission. Patients with DVT were predominantly male (67%; P = .032) with proximal venous involvement (29% upper and 39% in the lower extremities with 55% of the latter demonstrating bilateral involvement). Patients with DVT had a significantly higher mean d-dimer of 5447 ± 7032 ng/mL (P = .0101), and alkaline phosphatase of 110 IU/L (P = .0095) than those without DVT. On multivariable analysis, elevated d-dimer (P = .038) and alkaline phosphatase (P = .021) were associated with risk for DVT, whereas age, sex, elevated C-reactive protein, and ferritin levels were not. A receiver operating curve analysis suggests an optimal d-dimer value of 2450 ng/mL cutoff with 70% sensitivity, 59.5% specificity, and 61% positive predictive value, and 68.8% negative predictive value.
Conclusions
This study suggests that males with severe COVID-19 infection requiring hospitalization are at highest risk for developing DVT. Elevated d-dimers and alkaline phosphatase along with our multivariable model can alert the clinician to the increased risk of DVT requiring early evaluation and aggressive treatment
Keywords: COVID-19, Venous disease, Deep venous thrombosis, Hypercoagulable state, Anticoagulation, d-dimer
Article Highlights.
-
•
Type of Research: Single-center, retrospective, nonrandomized cohort study
-
•
Key Findings: Seventy-one patients with novel coronavirus disease-2019 had 107 venous duplex examination studies. Presence of deep venous thrombosis (DVT) was noted in 37% of examinations. The majority of those who experienced DVT were male (67%) with proximal DVT and had a significantly elevated mean d-dimer (5447 ng/mL), alkaline phosphatase (110 IU/L). A d-dimer cutoff 2450 ng/mL provided a 70.0% and 59.5% sensitivity and specificity.
-
•
Take Home Message: A model for calculating the probability of DVT in patients with severe novel coronavirus disease-2019 can be developed that may help identify risk for DVT. Based on our results, patients may need a higher dose of anticoagulation therapy as most of our patients diagnosed with DVT while on anticoagulation.
Novel coronavirus disease-2019 (COVID-19), which is caused by the severe acute respiratory syndrome novel coronavirus-2, can present in mild, moderate, or severe forms.1 Patients who are admitted to either an inpatient facility or to an intensive care unit tend to have moderate to severe symptoms with shortness of breath progressing to pneumonia requiring supportive respiratory therapy with or without the need for multiorgan supportive therapy.2 In a small number of patients, COVID-19 infection leads to cytokine surge, endothelial dysfunction with an increase in acute phase reactants and inflammatory markers resulting in coagulopathy. Increases in d-dimer, fibrinogen, C-reactive protein, and ferritin levels indicate the combination of a prothrombotic and hyperinflammatory state that may contribute to COVID-19-associated severity of illness, morbidity, and mortality.3, 4, 5, 6
The objective of our report is to examine the select group of patients who were admitted to our hospital with COVID-19 infection and underwent venous duplex ultrasound imaging. We report their characteristics in the context of clinical severity and laboratory results. This report examines mortality outcome and comparisons between the two cohorts of patients who were positive for COVID-19 but differed in the presence or absence of deep venous thrombosis (DVT).
Methods
The study was conducted in accordance with the Declaration of Helsinki. The study was granted expedited review status by Indiana University School of Medicine -Institutional Review Board (IRB Protocol 2004249979).
Study cohort
All COVID-19-positive patients between 18 and 89 years of age admitted to the Academic Health Center, Indiana University Health, and who had a duplex examination of their venous system between March 15 and April 14, 2020, were included in this study. These patients were admitted to either an inpatient or intensive care unit of the hospital depending on the level of the care required. Study patients were identified from In Record Time (In Record Time, LLC. Fenton, Mich), our vascular laboratory database that records and maintains every noninvasive vascular imaging and interpretation in the facility. Appropriate annotation of COVID-19 status was made in the vascular database.
Patient and laboratory characteristics
Patient demographics consisted of age, sex, race, insurance status, body mass index, smoking status, renal function with need for renal replacement therapy, history of active or remote cancer, use of immunosuppressive medications, and history of any organ or hematopoietic transplantation were identified from patients records. Patients were classified as mild or moderate severity of infection depending on <94% or >94% oxygen saturation, respectively. Severe category patients had in addition respiratory rate of >30, PaO2/FiO2 of ≤300 mm Hg or need for mechanical ventilation. Using medications charted in Cerner, the hospital electronic medical records, documentation was made of the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, use and type of anticoagulation, hydroxychloroquine, antiviral medications per hospital protocol (remdesivir, lopinavir, ritonavir) used in treatment. Laboratory variables in terms of serum hemoglobin, hematocrit, C-reactive protein (CRP), erythrocyte sedimentation rate, platelets, serum fibrinogen levels (fibrinogen), d-dimer, renal function test with blood urea nitrogen, serum creatinine, liver function test results with aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, serum ferritin, serum procalcitonin, and presence or absence of any abnormality on electrocardiography were recorded. For the purposes of reporting the severity of COVID-19 infection and medication use, they were recorded as ≤48 hours from the day of the venous duplex examination.
Venous duplex examinations
All patients underwent either upper and/or lower extremity venous duplex ultrasound examination at the request of the treating physicians. Patients had imaging of either one or all four extremities. Patients were then categorized into two groups depending on the status of the venous duplex examination as either positive or negative for DVT. Extent (proximal versus distal) as well as the location (superficial venous thrombosis [SVT] and/or DVT) of the venous thrombus was considered for reporting. Time to diagnosis of the venous thrombosis from the time of admission was recorded using the admission date. All examinations were carried out by registered vascular technologists and interpreted by attending physicians in accordance with the protocols suggested by the Intersocietal Accreditation Commission.
Statistical analysis
All these variables, including the venous duplex examination results, were entered into the REDCap database for analysis. REDCap (Research Electronic Data Capture) is a Health Insurance Portability and Accountability Act-compliant, highly secure and intuitive tool designed by Vanderbilt University used by the participating institutions in developing databases to capture data for clinical and translational research.7 Descriptive analysis was performed to examine the distribution of patient characteristics and DVT outcomes in the COVID-19 positive patients using frequency distribution for categorical variables and mean (standard deviation) and median (interquartile range) for continuous variables. Bivariate analyses were conducted to investigate the socio-demographic, clinical and laboratory characteristics of patients with COVID-19 infection and the incidence of DVT using Fisher's exact test and Wilcoxon rank-sum test for categorical and continuous variables, respectively. Multivariable logistic regression was used to examine the adjusted odds ratio of DVT with 95% confidence intervals (CI) of the adjusted odds ratio among COVID patients. The coefficients from the multivariable logistic model were used to define an equation to obtain the probability of developing DVT in male and female patients separately. Mathematically, the logistic regression model can be presented as , which can be used to obtain probability or risk that an outcome = 1 by using the formula , where exp is the natural exponential function, is the logistic regression coefficient and x is the covariate in the model. All variables with a P value of <.20 in the bivariate analysis and those with a P value of >.20 (age, serum ferritin) otherwise considered clinically important were included in multivariable analysis. Multicollinearity was assessed and any potential variables with variance inflation of >10 were excluded from the multivariable modeling. The multivariable model included sex, age, d-dimer, CRP, ferritin, and alkaline phosphatase. Time to diagnosis of DVT from admission was analyzed using log-rank test to determine the time-to-event differences between different severity levels of COVID-19 and was displayed using Kaplan-Meier plots. Receiver operating curve (ROC) analysis for d-dimer as a predictor of DVT was done to report the area under the curve. The Youden index was used to identify the optimal cutoff for the d-dimer that would distinguish between DVT positive and DVT negative cases. Sensitivity, specificity, positive predictive value and negative predictive values were reported for different levels of the cutoff based on an increment of 500 ng/mL. All analyses were performed at 0.05 level of significance using Stata SE/14.2 (StataCorp, L.P., and College Station, Tex).
Results
This study includes 71 unique COVID-19-positive patients who underwent 107 venous duplex examinations between March 15 and April 14, 2020, at the Academic Health Center, Indiana University School of Medicine. The mean age of the cohort was 61.00 ± 14.56 years with a majority male (54%) and African American patients (61%). Forty-two percent of our patients were either former smokers or active smokers at the time of admission. Only 10% of the patients were uninsured. The majority of our patients were categorized into severe COVID-19 infections (83%); 17% were moderate (14%) to mild (2.8%) infections. Patient demographics, comorbidities, and use of medications was compared between the two patient groups consisting of those with or without any form of thrombotic event in the venous system. Among the 34 patients (48%) who had a positive venous duplex examination, we observed 23 patients were males (68%) (P = .032) and race was not found to be significant (P = .329) for venous thrombotic events. Additional results are shown in Table I .
Table I.
Patient characteristics and bivariate relationship with deep venous thrombosis (DVT)
| Characteristics | In sample (n = 71) | DVT status |
P value | |
|---|---|---|---|---|
| Negative (n = 37) | Positive (n = 34) | |||
| Age (n = 71) | .7478 | |||
| Mean ± SD | 61.06 ± 14.56 | 61.11 ± 13.6 | 61 ± 15.74 | |
| Median (IQR) | 63 (20) | 63 (14) | 65 (20) | |
| Sex | .032 | |||
| Male | 38 (54) | 15 (41) | 23 (68) | |
| Female | 33 (46) | 22 (59) | 11 (32) | |
| Race | .329 | |||
| Whites | 22 (31) | 13 (35) | 9 (27) | |
| African Americans | 43 (61) | 23 (62) | 20 (61) | |
| Others | 5 (7) | 1 (3) | 4 (12) | |
| Missing | 1 (1) | – | – | |
| Smoking status | .934 | |||
| Current | 6 (8) | 3 (8) | 3 (10) | |
| Former | 24 (34) | 14 (38) | 10 ((32) | |
| Never | 38 (54) | 20 (54) | 18 (58) | |
| Missing | 3 (4) | – | – | |
| Active cancer | >.99 | |||
| No | 65 (92) | 35 (95) | 30 (94) | |
| Yes | 4 (6) | 2 (5) | 2 (6) | |
| Missing | 2 (2) | – | – | |
| Remote cancer | >.99 | |||
| No | 64 (90) | 34 (92) | 30 (94) | |
| Yes | 5 (7) | 3 (8) | 2 (6) | |
| Missing | 2 (3) | – | – | |
| Insurance | .442 | |||
| Medicare | 33 (46) | 19 (51) | 14 (41) | |
| Medicaid | 7 (10) | 5 (14) | 2 (6) | |
| Private | 22 (31) | 10 (27) | 12 (35) | |
| Other | 2 (3) | 0 (0) | 2 (6) | |
| Uninsured | 7 (10) | 3 (8) | 4 (12) | |
| CKD | .088 | |||
| No | 55 (78) | 26 (70) | 29 (88) | |
| Yes | 15 (21) | 11 (30) | 4 (12) | |
| Missing | 1 (1) | – | – | |
| RRT | .479 | |||
| No | 70 (99) | 37 (100) | 33 (97) | |
| Yes | 1 (1) | 0 (0) | 1 (3) | |
| Immune sup med | >.99 | |||
| No | 63 | 33 (89) | 30 (91) | |
| Yes | 7 | 4 (11) | 3 (9) | |
| Missing | 1 | – | – | |
| HTN | .795 | |||
| No | 21 (30) | 10 (27) | 11 (32) | |
| Yes | 50 (70) | 27 (73) | 23 (68) | |
| CAD | >.99 | |||
| No | 54 (76) | 28 (76) | 26 (76) | |
| Yes | 17 (24) | 9 (24) | 8 (24) | |
| Diabetes | .628 | |||
| No | 43 (61) | 21 (57) | 22 (65) | |
| Yes | 28 (39) | 16 (43) | 12 (35) | |
| Hyperlipidemia | .232 | |||
| No | 35 (49) | 21 (58) | 14 (42) | |
| Yes | 34 (48) | 15 (42) | 19 (58) | |
| Missing | 2 (3) | – | – | |
| COPD | >.99 | |||
| No | 64 (90) | 33 (89) | 31 (91) | |
| Yes | 7 (10) | 4 (11) | 3 (9) | |
| BMI (n = 70) | .4727 | |||
| Mean ± SD | 33.62 ± 8.35 | 34.61 ± 9.12 | 32.54 ± 7.4 | |
| Median (IQR) | 33 (10.2) | 33 (12.9) | 31 (8.9) | |
| COVID severity | .867 | |||
| Mild | 2 (3) | 1 (3) | 1 (3) | |
| Moderate | 10 (14) | 6 (16) | 4 (12) | |
| Severe | 59 (83) | 30 (81) | 29 (85) | |
BMI, Body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, novel coronavirus disease 2019; IQR, interquartile range; RRT, renal replacement therapy; SD, standard deviation.
Values are number (%) unless otherwise indicated.
Bivariate analysis (Supplementary Table I, online only) evaluating the use of angiotensin-converting enzyme (P = .665), angiotensin receptor blockers (P = .599), hydroxychloroquine (P > .99), hypoglycemic agents (P = .315), statins (P > .99), and antiviral medications (P = .479) demonstrated no difference between groups. The majority of patients (99%) were on anticoagulation (84% on prophylactic and 16% on therapeutic dose) with either unfractionated heparin or low-molecular-weight heparin at the time of DVT diagnosis. In the cohort of patients with DVT, laboratory levels of d-dimers (P = .010) and alkaline phosphatase (P = .009) were found to be abnormally elevated and statistically significant, whereas CRP (P = .077) and aspartate aminotransferase (P = .060) trended toward significance on bivariate analysis between those with and without DVT. Table II provides additional information on laboratory parameters in patients with and without DVT.
Table II.
Bivariate analysis of laboratory parameters comparing patients with and without deep venous thrombosis (DVT)
| Laboratory parameters (in sample) | Results | DVT negative | DVT positive | P value |
|---|---|---|---|---|
| Hgb, gm% (n = 71) | 10.72 ± 1.99 | 10.47 ± 1.94 | 11 ± 2.05 | .4038 |
| HCT (n = 71) | 32.48 ± 5.99 | 31.78 ± 5.8 | 33.25 ± 6.18 | .4878 |
| d-Dimer, ng/mL (n = 67) | 3941.21 ± 5240.36 | 2644.03 ± 2378.77 | 5447.61 ± 7032.01 | .0101 |
| Fibrinogen, mg/dL (n = 41) | 664.66 ± 198.69 | 655.57 ± 220.84 | 676.28 ± 171.8 | .8422 |
| Platelets/μL (n = 71) | 282.76 ± 98.91 | 290.73 ± 107.67 | 274.09 ± 89.2 | .5313 |
| CRP mg/L (n = 67) | 18.26 ± 17.35 | 17.54 ± 21.83 | 19.1 ± 10.26 | .0772 |
| Erythrocyte sedimentation rate (n = 11) | 80.82 ± 25.33 | 83.00 ± 31.84 | 79.00 ± 21.51 | .9361 |
| Ferritin, ng/mL (n = 65) | 1038.73 ± 1191.61 | 820.83 ± 1001.94 | 1277.70 ± 1346.15 | .1295 |
| Procalcitonin, ng/mL (n = 29) | 2.18 ± 5.92 | 2.38 ± 7.70 | 1.94 ± 2.72 | .1873 |
| BUN, mg/dL (n = 70) | 32.86 ± 25.95 | 35.94 ± 28.67 | 29.59 ± 22.70 | .2841 |
| Serum creatinine, mg/dL (n = 70) | 1.41 ± 1.65 | 1.57 ± 1.91 | 1.25 ± 1.32 | .3222 |
| Albumin mg/dL (n = 68) | 2.91 ± 0.58 | 2.90 ± 0.44 | 2.91 ± 0.71 | .4015 |
| AST, U/L (n = 68) | 59.16 ± 47.49 | 47.72 ± 36.17 | 72.03 ± 55.44 | .0604 |
| ALT, U/L (n = 68) | 50.49 ± 40.31 | 45.25 ± 34.69 | 56.38 ± 45.67 | .3828 |
| Alkaline phosphatase, IU/L (n = 68) | 89.56 ± 59.67 | 71.39 ± 24.20 | 110.00 ± 78.86 | .0095 |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C-reactive protein; Hgb, hemoglobin; HCT, hematocrit.
Values are mean ± standard deviation.
There were no significant differences identified in abnormal chest radiographs (P > .99) in those patients (n = 68) where the information was available. Electrocardiographic changes with QT prolongation was see in six patients with no differences between the groups with and without DVT (P > .99; three patients in each group).
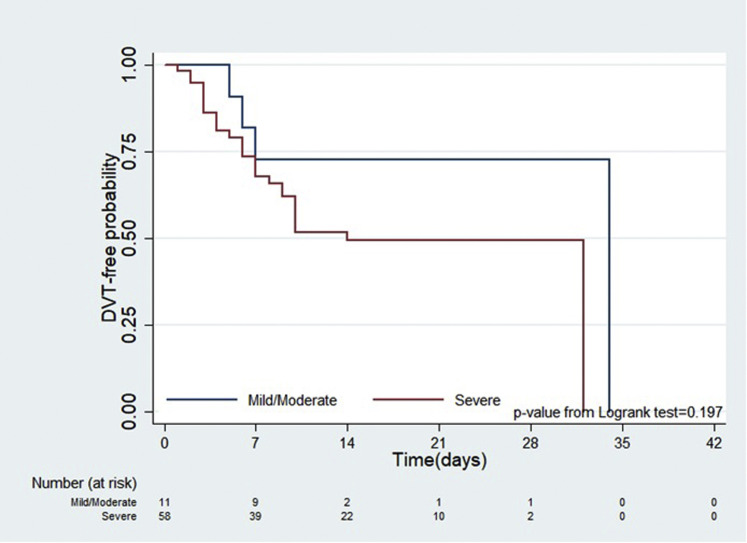
Positive findings on the venous duplex examination for DVT were found in 37% of examinations (n = 40). Patients had venous thrombotic events, either in isolation or in combination with a proximal or distal venous system in the upper and/or lower extremities. The majority of the venous duplex examinations included an evaluation of the lower extremities (70/107 examinations). Fifty-five percent of all the positive lower extremity examinations had a positive finding for venous thrombotic event in both lower extremities, whereas 29% of the upper extremity venous examinations had a positive finding in both upper extremities. Proximal venous thrombosis was found in 39% of lower extremity examinations in the femoral and popliteal veins with 29% of upper extremity examinations having venous thrombosis in the proximal venous system including one or more of the brachial, axillary, and subclavian veins. Isolated SVT was found in 17.8% (n = 19) examinations. SVT was most frequently found in the upper extremities (54% of patients) and in association with proximal DVT. Details of venous thrombotic events are shown in Supplementary Table II (online only). There was no statistical difference in the probability of being diagnosed with DVT among moderate and severe COVID-19 cases (P = .197); however, the time to event analysis by severity of COVID-19 symptoms using Kaplan-Meier plot shows (Fig 1 ) that the likelihood of diagnosis of DVT for severe category of patients with COVID-19 was higher and trending toward significance as was seen in the univariate Cox regression (hazard ratio, 2.13; 95% CI, 0.64-7.08) than that for patients with mild to moderate disease. On an average, the days from admission to the diagnosis of DVT was 10.4 days for mild or moderate disease and 6.83 days for severe disease. This difference was not statistically significant as analyzed by Wilcoxon rank sum test (P = .9416).
Fig 1.
Time to event analysis for determining deep venous thrombosis (DVT)-free probability using log rank test and Kaplan-Meier plot.
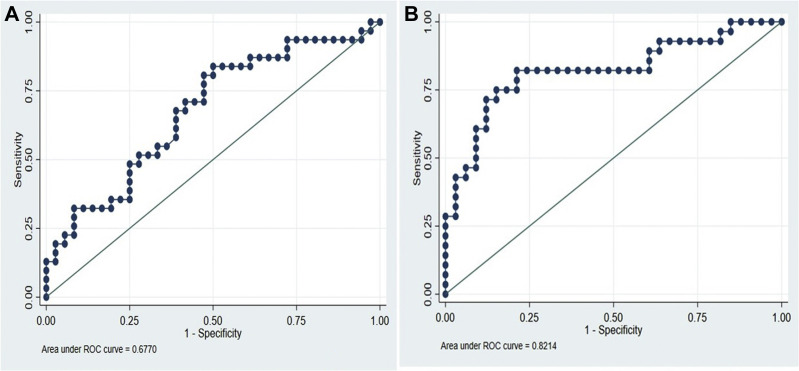
Based on the ROC analysis (Fig 2 , A), the sensitivity, specificity, and predictive values of d-dimers at 500 ng/mL units' intervals was analyzed and obtained an optimal cutoff of 2450 ng/mL. This cutoff was also validated using Youden Index after ROC analysis. Table III shows d-dimer levels in increments of 500 ng/mL along with their specificity, sensitivity, positive predictive value, and negative predictive value. At 2450 ng/mL, these values are 70.6%, 59.5%, 61.5%, and 68.8%, respectively
Fig 2.
A, Receiver operating curve (ROC) for the model predicting deep venous thrombosis (DVT) using d-dimer. B, ROC for the multivariable model predicting DVT.
Table III.
Optimal cutoff of d-dimer values to predict deep venous thrombosis (DVT) in patients with novel coronavirus disease 2019 (COVID-19) infection
| d-Dimer cutoff, ng/mL | Prevalence, % | AUC | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|
| 1450 | 47.9 | 0.63 | 85.3 | 40.5 | 56.9 | 75 |
| 1950 | 47.9 | 0.64 | 76.5 | 51.4 | 59.1 | 70.4 |
| 2450a | 47.9 | 0.65 | 70.6 | 59.5 | 61.5 | 68.8 |
| 2950 | 47.9 | 0.6 | 58.8 | 62.2 | 58.8 | 62.2 |
| 3450 | 47.9 | 0.63 | 55.9 | 70.3 | 63.3 | 63.4 |
| 3950 | 47.9 | 0.59 | 44.1 | 73 | 60 | 58.7 |
AUC, Area under the curve; NPV, negative predictive value; PPV, positive predictive value.
Optimal cutoff based on the Youden Index for d-dimer to predict DVT.
Our multivariable logistic regression model (Fig 2, B) for predicting the odds of DVT gave us an area under the curve of 0.8214, which indicates that the model has a good predictive ability11 and potential of clinical usefulness to discriminate DVT cases from non-DVT cases among patients with COVID-19. Table IV shows patient and laboratory characteristics with the adjusted odds ratio predicting their association with DVT amongst patients with COVID-19. Elevated d-dimers (P = .038) and alkaline phosphatase (P = .021) were significantly associated with the risk of diagnosing DVT. Based on this model, we propose equations for the probability of DVT occurrences in COVID-19 positive patients among male and female cases is as shown elsewhere in this article.
Table IV.
Multivariable analysis of deep venous thrombosis (DVT) among novel coronavirus disease 2019 (COVID-19) positive patients
| Characteristics | Adjusted odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| d-Dimer | 1.000243 | 1.000014 | 1.000472 | .038 |
| CRP | 0.9987068 | 0.9600632 | 1.038906 | .949 |
| ferritin | 1.000353 | 0.999747 | 1.000959 | .254 |
| Alkaline phosphatase | 1.027308 | 1.003987 | 1.05117 | .021 |
| Age | 0.9958539 | 0.9517951 | 1.041952 | .857 |
| Female | 0.2739775 | 0.0701369 | 1.070246 | .063 |
Probability for DVT among female COVID-19-positive patients can be predicted by using
Similarly, for male COVID-19-positive patients the probability for DVT can be predicted by using
Here, e represents the natural exponential function, –2.672 is a constant in the logistic regression, and 0.0002, –0.0013, 0.0004, 0.0269, –0.0042, and –1.2947 are the coefficients respectively for the variables d-dimer, CRP, ferritin, alkaline phosphatase, age, and female sex.
Mechanical ventilation were required in 77.5% patients in the entire cohort with three patients requiring extracorporeal membrane oxygenation. There were no differences in the use of either mechanical ventilation or extracorporeal membrane oxygenation in patients with and without DVT.
In this entire cohort, 10 deaths (14%) occurred during the follow-up period of 13.4 ±7.1 days. All deaths were related to progressive sepsis with multiorgan dysfunction. The analysis of survival comparing the mild/moderate disease and severe disease patients did not reach statistical significance.
Discussion
At the time of writing this article, the United States accounted for both the highest number of patients as well as fatalities due to COVID-19 infections in the world We had a mortality of 14% in our cohort, which is similar to those reported for all COVID-19-related admissions to Intensive care units requiring advanced respiratory therapies and supportive care.8, 9, 10, 11, 12, 13 Our results demonstrate similar observations with significant number of male COVID-19-positive patients with others reporting a high body mass index as an additional risk.14 , 15 Similar to published reports our cohort is composed of approximately 60% African American patients.16
Similar to our results, venous thromboembolism (VTE) in critically ill patients with COVID-19 reportedly ranges from 25% to 31%.17, 18, 19 Patients with severe and fatal COVID-19 are in a prothrombotic and hyperinflammatory state with reportedly higher d-dimer levels.3, 4, 5 , 17 , 20, 21, 22 Given the varying degrees of sensitivity (80%-100%), specificity (23%-63%) d-dimer levels are not advised as a single definitive test for diagnosis18 , 19 for VTE. Tang et al16 defined the high-risk population as having a d-dimer elevation more than six times the upper limit of normal and Sepsis Induced Coagulopathy score of ≥4. In our study, the sensitivity of d-dimer of 2450 g/mL was approximately 70% and specificity was approximately 60% and reflect the limitations of using d-dimer as a stand-alone trigger for treatment. It also must be noted that 99% of our patients were on anticoagulation (84% on prophylactic and 16% on therapeutic dose) at the time of diagnosis. Given the high percentage of patients who were diagnosed with DVT while on prophylactic anticoagulation, one might postulate that these patients need full anticoagulation to be reliably protected against experiencing DVT.
Mechanisms related to such high levels of d-dimer as well as the risk of DVT may be related to a cytokine surge, the upregulation of hypoxia induced transcription pathways15 or potentially the use of continuous positive airway pressure ventilator, thought to compress superficial or deep vessels of the upper limbs, which might lead to thrombosis.23 Our study provides no insights into the underlying pathophysiology.
The presence of liver abnormalities have been observed in the COVID population potentially due to various pathophysiological pathways.24 Worsening transaminases such as alkaline phosphatase, a sign of significant liver disease, is associated with a heightened risk of VTE based on our analysis. Cui et al16 have suggested worsening transaminases is related to worse patient outcomes in COVID-19. Chen et al25 similarly suggest an aspartate aminotransferase >40 U/L (hazard ratio, 2.2; 95% CI, 1.1-6.73) was an independent risk factor associated with a fatal outcome.
Based on our multivariable analysis, d-dimer along with C-reactive protein, alkaline phosphatase, ferritin levels, and sex would be helpful to assess the probability of underlying DVT. An absolute level of d-dimer cannot be used to initiate high-dose anticoagulation in COVID-19-positive patients, we recommend calculating the probability of VTE in these patients and then make decisions based on clinical needs of the patient. This model should help to guide further treatment decisions. A larger cohort is needed to validate our observations.
Similar to sepsis-induced coagulopathy, there are reports that suggest survival benefit in COIVD-19 patients with pneumonia and DVT when treated with anticoagulation.15 , 25, 26 Available algorithms take into consideration Well's pretest probability score27 , 28 advising either prophylaxis, thrombostabilizing protocol, or therapeutic anticoagulation for DVT and PE. However, given the limitations29 , 30 with varying degrees of sensitivity and specificity, as well as age-related challenges and ability to predict only the proximal venous thrombosis, it remains to be seen if the Well's score can be used to advise anticoagulation strategies for patients with COVID-19. To overcome some of these limitations, age-adjusted d-dimer along with Well's probability score31 has been advised.
Based on initial observations, which forms the basis for this report, a high-dose anticoagulant regimen was advised for DVT prophylaxis (Supplementary Table III, online only) in our health facility for patients in intensive care with COVID-19. Because both the upper and lower extremities are often involved with DVT in our cohort, there seems to be little role for inferior vena cava filters in this patient population. The relatively low sensitivity of d-dimer amid a high DVT prevalence warrants consideration of empiric anticoagulation on admission. If the probability score is high based on our multivariate model, we postulate that patients will benefit a therapeutic dose to offer protection against DVT. Our observations are similar to Obi et al,27 indicating that patients with H1N1-associated acute respiratory distress syndrome had a 23.3-fold higher risk for pulmonary embolism and a 17.9-fold increased risk for VTE. This finding led to the authors concluding that empirical systemic heparin anticoagulation in this cohort of patients significantly reduced VTE incidence without increased hemorrhagic complications.32
Given the concerns for acquiring COVID-19 infection and to protect our health care providers, prudent and judicious use of the vascular lab resources is critical. Because anticoagulation will be provided no matter where the DVT is found, termination of extensive testing seems warranted when a major proximal DVT is found. In addition to the concerns expressed by Obi et al,27 we believe that an algorithm of who is at most risk based on sex and other factors from our multivariate model might eventually provide an answer on whom to study.
This report focuses on patients in the moderate to severe categories of COVID-19 infection requiring hospital admission. This study adds to the existing body of literature in that male patients are at highest risk for complications. The study has strengths in the fact that all patients included in the study had a venous duplex ultrasound examination to provide confirmation of DVT. We have identified variables beyond those described to predict the probability of DVT in patients with COVID-19 infection. Besides a small sample size, weakness of the study is very similar to those inherent to any single-center retrospective series and limited by inherent biases related to patient selection and investigation, as well as treatments provided. The multivariable model proposed in this article needs further validation and research. In addition, the study has also its weakness; we did not perform evaluations for the pulmonary embolism or investigating for the caval or iliac venous thrombosis.
Conclusions
Male sex and patients admitted with severe category of COVID-19 infections are at high risk for DVT. Elevated d-dimer and alkaline phosphatase levels have the ability to predict DVT in our model. Our novel multivariate predictive model should provide guidance, as we consider high-dose empiric anticoagulation in this high risk patients with COVID-19. To limit the risk of exposure to healthcare workers considerations should be given in judicious ordering of vascular laboratory imaging.
Author contributions
Conception and design: RM, AD
Analysis and interpretation: RM, LT, SU
Data collection: RK, LT, AG, MI, SR, OR, DR
Writing the article: RM, AD
Critical revision of the article: RM, RK, LT, AG, MI, SU, SR, OR, DR, AD
Final approval of the article: RM, RK, LT, AG, MI, SU, SR, OR, DR, AD
Statistical analysis: LT
Obtained funding: Not applicable
Overall responsibility: RM
Acknowledgments
The authors acknowledge Ms Janet Klein, Research Coordinator for the Division of Vascular Surgery, for all the administrative support. In addition, we acknowledge and dedicate this work to the frontline health care workers of the Indiana University Health and School of Medicine.
Appendix.
Additional material for this article may be found online at www.jvsvenous.org.
From the Society for Vascular Surgery
Appendix (online only).
Supplementary Table I (online only).
Bivariate analysis of patient's medications and status of deep venous thrombosis (DVT)
| Medications | In sample (n = 71) | DVT Negative (n = 37) | DVT Positive (n = 34) | P value |
|---|---|---|---|---|
| Aspirin | .315 | |||
| No | 47 (66.2) | 22 (59.46) | 25 (73.53) | |
| Yes | 24 (33.8) | 15 (40.54) | 9 (26.47) | |
| Angiotensin-converting enzyme inhibitor | .665 | |||
| No | 66 (92.96) | 35 (94.59) | 31 (91.18) | |
| Yes | 5 (7.04) | 2 (5.41) | 3 (8.82) | |
| Angiotensin receptor blockers | .599 | |||
| No | 67 (94.37) | 36 (97.3) | 31 (93.94) | |
| Yes | 3 (4.23) | 1 (2.7) | 2 (6.06) | |
| Missing | 1 (1.41) | |||
| Hydroxychloroquine | >.99 | |||
| No | 28 (39.44) | 15 (40.54) | 13 (38.24) | |
| Yes | 43 (60.56) | 22 (59.46) | 21 (61.76) | |
| Hypoglycemics | .315 | |||
| No | 46 (64.79) | 22 (59.46) | 24 (72.73) | |
| Yes | 24 (33.8) | 15 (40.54) | 9 (27.27) | |
| Missing | 1 (1.41) | |||
| Statins | >.99 | |||
| No | 50 (70.42) | 26 (70.27) | 24 (70.59) | |
| Yes | 21 (29.58) | 11 (29.73) | 10 (29.41) | |
| Antiviral medications | .479 | |||
| No | 61 (85.92) | 30 (83.33) | 31 (91.18) | |
| Yes | 9 (12.68) | 6 (16.67) | 3 (8.82) | |
| Missing | 1 (1.41) | |||
| Anticoagulation status at the time of diagnosis | >.99 | |||
| No | 1 (1.41) | 1 (2.7) | 0 (0) | |
| Yes | 70 (98.59) | 36 (97.3) | 34 (100) | |
| If yes, types | .515 | |||
| Therapeutic | 11 (15.71) | 7 (19.44) | 4 (11.76) | |
| Prophylactic | 59 (84.29) | 29 (80.56) | 30 (88.24) | |
Supplementary Table II (online only).
Description of location and extent of venous thrombotic events
| Location and extent of venous thrombosis (n = 107 examinations: 70 lower extremity+37 upper extremity) | % Positive studies |
|---|---|
| Total number of venous thrombotic events | 55 (n = 59) |
| Total number of DVT | 37.38 (n = 40) |
| Total number of isolated SVT | 17.75 (n = 19) |
| Bilateral lower extremity DVT (of lower extremity examinations) | 55 |
| Bilateral upper extremity DVT of upper extremity examinations) | 29 |
| % Positive proximal DVT in lower extremity examinations (femoral, popliteal veins) | 39 |
| % Positive proximal DVT in upper extremity examinations (axillary, subclavian, jugular veins) | 29 |
DVT, Deep venous thrombosis; SVT, superficial venous thrombosis.
Supplementary Table III (online only).
Indiana University Academic Health Center protocol for prophylactic dosing of anticoagulation in severe novel coronavirus disease 2019 (COVID-19) infections
| Creatinine clearance | Weight <119 kg | Weight 120-150 kg | Weight >150 kg |
|---|---|---|---|
| >30 mL/min | Enoxaparin 30 mg q12h | Enoxaparin 40 mg q12h | Enoxaparin 60 mg q12h |
| <30 mL/min, end-stage renal disease | Heparin 5000 q8h | Heparin 7500 q8h | Heparin 7500 q8h |
q8h, Every 8 hours; q12h, every 12 hours.
References
- 1.Centers for Disease Control and Prevention United States COVID-19 cases and deaths by state. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#anchor_1586782138 Available at:
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan S., Xiang Y., Fang W., Zheng Y., Boqun L., Yanjun H., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Cai Y., Xia J., Zhou X., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Minor B.L., Elliot V., Fernandez M., O’Neal L., et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Indiana COVID-19 Data Report. https://www.coronavirus.in.gov/2393.htm Available at:
- 9.CDC COVID-19 Response Team Severe outcomes among patients with Coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W.J., Ni Z.Y., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J., Tong Z., Guan X., Du B., Qiu H. Clinical characteristics of patients who died of Coronavirus disease 2019 in China. JAMA Netw Open. 2020;3:e205619. doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L.Q., Huang T., Wang Y.Q., wang Z.P., Liang Y., Huang T.B., et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurencin C.T., McClinton A. The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities. J Racial Ethn Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford F., Andras A., Welch K., Sheares K., Keeling D., Chappell F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev. 2016;2016:CD010864. doi: 10.1002/14651858.CD010864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D., Li F., Du X., Zhang X., Zhang Z., Zhao W., et al. Diagnostic accuracy of biomarker D-dimer in patients after stroke suspected from venous thromboembolism: A diagnostic meta-analysis. Clin Biochem. 2019;63:126–134. doi: 10.1016/j.clinbiochem.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maatman T.K., Jalali F., Feizpour C., Douglas A., McGuire S.P., Kinnaman G., et al. Routine Venous Thromboembolism Prophylaxis May Be Inadequate in the Hypercoagulable State of Severe Coronavirus Disease 2019. Crit Care Med. 2020;48:e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trigonis R.A., Holt D.B., Yuan R., Siddiqui A., Craft M.K., Khan B.A., et al. Incidence of venous thromboembolism in critically ill Coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med. 2020;48:e805–e808. doi: 10.1097/CCM.0000000000004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8:694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 Coronavirus disease. J Clin Transl Hepatol. 2020;8:13–17. doi: 10.14218/JCTH.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3 doi: 10.1007/s11239-020-02105-8. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obi A.T., Barnes G.D., Wakefield T.W., Brown S., Eliason J., Arndt E., et al. Practical diagnosis and treatment of suspected venous thromboembolism during COVID-19. J Vasc Surg Venous Lymphat Disord. 2020;8:526–534. doi: 10.1016/j.jvsv.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells P.S., Anderson D.R., Bormanis J., Guy F., Mitchell M., Gray L., et al. Value of assessment of pretest probability of deep-vein thrombosis in 16 clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 29.Shen J.H., Chen H.L., Chen J.R., Xing J.L., Gu P., Zhu B.F. Comparison of the Wells score with the revised Geneva score for assessing suspected pulmonary embolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2016;41:482–492. doi: 10.1007/s11239-015-1250-2. [DOI] [PubMed] [Google Scholar]
- 30.Goodacre S., Sutton A.J., Sampson F.C. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143:129–139. doi: 10.7326/0003-4819-143-2-200507190-00012. [DOI] [PubMed] [Google Scholar]
- 31.van Es N., van der Hulle T., van Es J., Den Exter P.L., Douma R.A., Goekoop R.J., et al. Wells rule and d-Dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253–261. doi: 10.7326/M16-0031. [DOI] [PubMed] [Google Scholar]
- 32.Obi A.T., Tignanelli C.J., Jacobs B.N., Arya S., Park P.K., Wakefield T.W., et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients [published correction appears in J Vasc Surg Venous Lymphat Disord 2019;7:621. J Vasc Surg Venous Lymphat Disord. 2019;7:317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]