Abstract
Background
Despite numerous advances in the understanding of the pathophysiology, progression, and management of acute respiratory failure (ARF) and ARDS, limited contemporary data are available on the mortality burden of ARF and ARDS in the United States.
Research Question
What are the contemporary trends and geographic variation in ARF and ARDS-related mortality in the United States?
Study Design and Methods
A retrospective analysis of the National Center for Health Statistics’ nationwide mortality data was conducted to assess the ARF and ARDS-related mortality trends from 2014 through 2018 and the geographic distribution of ARF and ARDS-related deaths in 2018 for all American residents. Piecewise linear regression was used to evaluate the trends in age-adjusted mortality rates (AAMRs) in the overall population and various demographic subgroups of age, sex, race, urbanization, and region.
Results
Among 1,434,349 ARF-related deaths and 52,958 ARDS-related deaths during the study period, the AAMR was highest in older individuals (≥ 65 years), non-Hispanic Black people, and those living in the nonmetropolitan region. The AAMR for ARF-related deaths (per 100,000 people) increased from 74.9 (95% CI, 74.6-75.2) in 2014 to 85.6 (95% CI, 85.3-85.9) in 2018 (annual percentage change [APC], 3.4 [95% CI, 2.2-4.6]; Ptrend = .003). The AAMR (per 100,000 people) for ARDS-related deaths was 3.2 (95% CI, 3.2-3.3) in 2014 and 3.0 (95% CI, 3.0-3.1 in 2018; APC, −0.9 [95% CI, −5.4 to 3.8]; Ptrend = .56). The observed increase in rates for ARF mortality was consistent across the subgroups of age, sex, race or ethnicity, urbanization status, and geographical region (Ptrend < .05 for all). The AAMR (per 100,000 people) for ARF (91.3 [95% CI, 90.8-91.8]) and ARDS-related mortality (3.3 [95% CI, 3.2-3.4]) in 2018 were highest in the South.
Interpretation
The ARF-related mortality increased at approximately 3.4% annually, and ARDS-related mortality showed a lack of decline in the last 5 years. These data contextualize important health information to guide priorities for research, clinical care, and policy, especially during the coronavirus disease 2019 pandemic in the United States.
Key Words: ARDS, coronavirus disease, mortality, risk factors
Abbreviations: AAMR, age-adjusted mortality rate; APC, annual percentage change; ARF, acute respiratory failure; COVID-19, coronavirus disease 2019; ICD-10, International Classification of Diseases, Tenth Revision
Acute respiratory failure (ARF) is a life-threatening medical condition characterized by increased preponderance for admission to ICUs and frequently is associated with the need for mechanical ventilation.1, 2, 3 ARDS is a potentially fatal condition characterized by acute-onset, diffuse, inflammatory lung injury that leads to hypoxemic respiratory insufficiency and failure resulting from an increase in pulmonary vascular permeability and loss of ventilated lung parenchyma.4, 5, 6, 7, 8 ARDS is one of the leading causes of ARF. Nearly 3 million patients annually experience ARDS, which contributes to approximately 10% of ICU admissions and approximately 24% of patients receiving mechanical ventilation.3, 4, 5 This potentially fatal respiratory syndrome may be triggered by pulmonary (such as aspiration, coronavirus disease 2019 [COVID-19], pneumonia, and inhalational injury) or nonpulmonary (such as trauma, pancreatitis, sepsis, and drug toxicity) causes and is associated with high mortality of 35% to 46% based on disease severity at the onset.3 , 5 , 7
Despite numerous advances in the understanding of the pathophysiology, progression, and management of ARF and ARDS, limited contemporary data are available on the mortality burden of ARF and ARDS in the United States.3, 4, 5 , 8 Importantly, limited data are available on mortality trends in the United States after the adoption of the Berlin definition of ARDS, which was endorsed by the American Thoracic Society and the Society of Critical Care Medicine and was adopted into routine clinical care.5 , 8 Well-recognized geographic differences in disease management and critical care practices exist that may impact patient outcomes. The contemporary geographic differences in ARF and ARDS mortality in the United States are not known. These geographic differences may be heightened in view of the significant infrastructure strain imposed by the COVID-19 pandemic.
We sought to evaluate the contemporary trends and geographic differences in ARF and ARDS mortality in the United States. We also sought to examine these trends in the demographic subgroups of age, sex, race or ethnicity, and level of urbanization.
Methods
A retrospective analysis of the national mortality from the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research database was performed to evaluate the recent trends and the geographic distribution of mortality secondary to ARF and ARDS.8 , 9 This database incorporates the National Center for Health Statistics’ Multiple Cause of Death data, which includes nationwide mortality data derived from the death certificates of all United States residents.8 , 9 The underlying and contributing causes of death (coded according to the International Classification of Diseases, Tenth Revision [ICD-10]), along with the demographic characteristics of the population, are encompassed within the database.8 , 9 The population estimates incorporated in the Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research are derived from the United States Census Bureau’s intercensal population estimates.10 The Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research database was validated previously and has been used in numerous epidemiologic investigations.11, 12, 13, 14, 15, 16 The study was deemed as nonhuman subjects research and exempt from a full review of the institutional review board.
The crude and age-adjusted mortality rates (AAMRs) for ARF-related deaths and ARDS-related deaths were derived by analyzing the mortality data from January 1, 2014, through December 31, 2018. ARF-related mortality was defined as deaths with relevant ICD-10 codes (J80, J96.0, and J96.9) enlisted as the contributing cause of death. ARDS-related mortality was defined using the ICD-10 code J80 (acute respiratory distress) enlisted as the contributing cause of death.8 In the sensitivity analysis for ARF-related mortality, we included the combination of J80 and J96.0. The AAMRs were age standardized to the 2010 US census population proportions.17 The AAMR estimates were computed and reported as age-adjusted deaths per 100,000 people. The mortality estimates were evaluated by stratifying the population on the basis of sex, race or ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian or Pacific Islander, non-Hispanic American Indian or Alaska Native, and Hispanic), level of urbanization (as per the 2013 Urban-Rural Scheme for Counties), geographic region of residence (defined as per the United States Census Bureau), and state of residence at the time of death. We also evaluated the causes of death associated with ARF-related mortality by identifying common diagnoses enlisted as a contributing cause of death. The previously used ICD-10 codes were used for identifying the causes of ARF-related deaths such as influenza and pneumonia,8 septicemia,8 , 18 trauma and burns,8 pneumonitis caused by food and vomit,8 and acute pancreatitis (e-Table 1). We also explored the seasonal periodicity in the ARF-related and ARDS-related deaths during the study period.
Statistical Analysis
The mortality trends were analyzed using the National Cancer Institute’s Joinpoint Regression Program 4.7.0.0 to assess the ARF-related and ARDS-related mortality during the study period.19 Using a piecewise linear regression approach, the ideal fit for the AAMR trends during the study period was ascertained. The Joinpoint algorithm estimates the best approach to describe the mortality trend over time, either by a straight line or ≥ 1 linear segments, to create a parsimonious model that provides a good weighted-least-squared fit to the data.19 , 20 The annual percentage change (APC) was estimated along with the 95% CIs using previously validated methods.21 , 22 APCs were obtained using Monte Carlo permutation analysis to overcome the uncertainty of the mortality estimates (CIs) by fitting a line through 4,500 different samples of the annual AAMRs.16 The AAPC was calculated for the ARF-related and ARDS-related mortality in the overall population and each predefined subgroups of age, sex, race or ethnicity, and urbanization status. Sensitivity analyses were conducted for ARF-related mortality defined using the restricted ICD-10 codes (J80 and J96.0). The number of deaths attributed to ARF and ARDS was stratified based on the month of death in each study year and cumulatively from 2014 through 2018 to assess the seasonal variation in the mortality. A two-sided type I error of 0.05 was considered statistically significant for all analyses.
Results
During the 5-year study period, 1,434,349 ARF-related deaths and 52,958 ARDS-related deaths were identified nationwide with an overall AAMR (per 100,000 people) of 81.1 (95% CI, 80.9-81.2) and 3.1 (95% CI, 3.0-3.1), respectively, for the 5-year study period (Table 1 ). The ARF-related mortality (per 100,000 people) was highest in men (AAMR, 93.7 [95% CI, 93.5-93.9]), non-Hispanic Black people (AAMR, 92.5 [95% CI, 92.0-92.9]), those ≥ 65 years of age (AAMR, 467.5 [95% CI, 466.7-468.4]), and in those living in nonmetropolitan areas (AAMR, 91.0 [95% CI, 90.6-91.3]) and in South (AAMR, 86.2 [95% CI, 86.0-86.5]) (Table 1). Similarly, ARDS-related mortality was highest in men, non-Hispanic Black people, those ≥ 65 years of age, and those living in nonmetropolitan areas and in the South (Table 1).
Table 1.
Demographic Characteristics for Deaths With ARF and ARDS: 2014-2018
| Variable | ARF |
ARDS |
||
|---|---|---|---|---|
| Deaths | Age-Adjusted Mortality Rate per 100,000 People (95% CI) | Deaths | Age-Adjusted Mortality Rate per 100,000 People (95% CI) | |
| Overall | 1,434,349 | 81.1 (80.9-81.2) | 52,958 | 3.1 (3.0-3.1) |
| Sex | ... | |||
| Male | 713,167 | 93.7 (93.5-93.9) | 27,835 | 3.5 (3.4-3.5) |
| Female | 721,182 | 71.5 (71.3-71.7) | 25,123 | 2.7 (2.7-2.8) |
| Age group, y | ||||
| ≤25 | 12,635 | 2.4 (2.4-2.5) | 1,695 | 0.3 (0.3-0.3) |
| 25-44 | 37,640 | 9.0 (8.9-9.1) | 4,770 | 1.1 (1.1-1.2) |
| 45-64 | 275,222 | 63.0 (62.7-63.2) | 18,778 | 4.4 (4.3-4.4) |
| ≥65 | 1,108,810 | 467.5 (466.7-468.4) | 27,714 | 11.4 (11.3-11.6) |
| Race or ethnicity | ||||
| NH White | 1,114,046 | 82.2 (82.1-82.4) | 37,397 | 3.0 (2.9-3.0) |
| NH Black | 158,197 | 92.5 (92.0-92.9) | 6,747 | 3.6 (3.5-3.7) |
| NH Asian/Pacific Islander | 39,496 | 49.2 (48.7-49.7) | 2,192 | 2.6 (2.4-2.7) |
| NH American Indians/Alaska Native | 9,504 | 88.2 (86.4-90.1) | 706 | 5.9 (5.5-6.3) |
| Hispanic | 108,791 | 68.4 (67.9-68.8) | 5,742 | 3.0 (2.9-3.1) |
| Urbanization | ||||
| Nonmetropolitan | 267,780 | 91.0 (90.6-91.3) | 10,238 | 3.7 (3.7-3.8) |
| Metropolitan | 1,166,569 | 79.1 (79.0-79.3) | 42,720 | 3.0 (2.9-3.0) |
| Region | ||||
| Northeast | 271,190 | 80.3 (80.0-80.6) | 8,387 | 2.6 (2.6-2.7) |
| Midwest | 291,814 | 75.4 (75.1-75.7) | 10,798 | 2.9 (2.9-3.0) |
| South | 564,769 | 86.2 (86.0-86.5) | 21,753 | 3.4 (3.3-3.4) |
| West | 306,576 | 78.1 (77.8-78.4) | 12,020 | 3.1 (3.0-3.1) |
Data are presented as No., unless otherwise indicated. ARF = acute respiratory failure; NH = non-Hispanic.
ARF-Related Mortality Trends
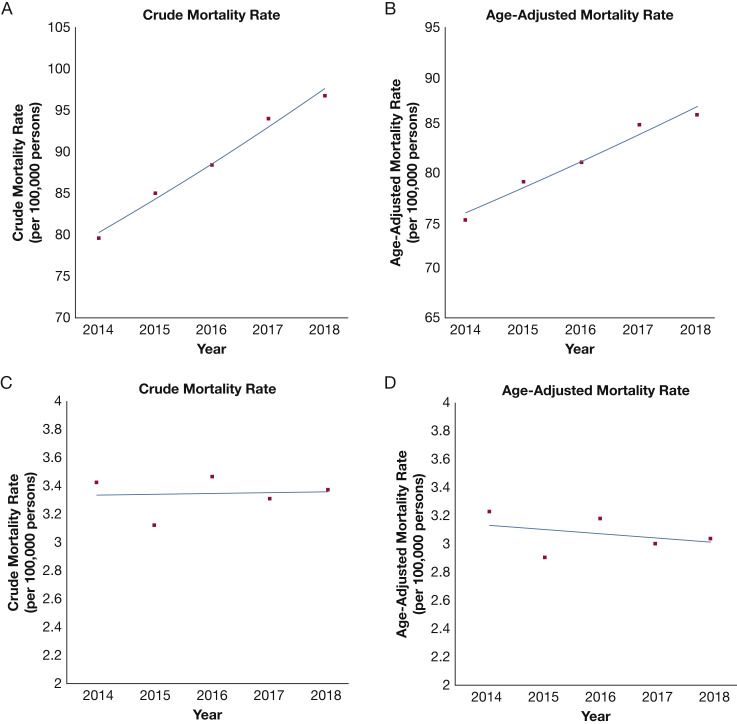
The nationwide crude mortality for ARF-related deaths increased from 79.6 (95% CI, 79.3-79.9) per 100,000 people in 2014 to 96.7 (95% CI, 96.4-97.0) per 100,000 people in 2018 (APC: 5.0 [95% CI, 3.9-6.1]; P = .001). The AAMR showed an increase from 74.9 (95% CI, 74.6-75.2) per 100,000 people in 2014 to 85.6 (95% CI, 85.3-85.9) per 100,000 people in 2018 (APC, 3.4 [95% CI, 2.2-4.6]; P = .003) (Fig 1 ). Among women, the AAMR (per 100,000 people) was 66.0 (95% CI, 65.7-66.4) in 2014 and 75.5 (95% CI, 75.2-75.9) in 2018 (APC, 3.5 [95% CI, 2.2-4.8]; P = .004). The men also showed an increase in AAMR (per 100,000 people) from 86.9 (95% CI, 86.4-87.4) in 2014 to 98.7 (95% CI, 98.3-99.3) in 2018 (APC, 3.2 [95% CI, 2.1-4.3]; P = .002) (Fig 2 ).
Figure 1.
A-D, Line graphs showing trends in acute respiratory failure-related and ARDS-related mortality stratified by crude (A, C) and age-adjusted mortality (B, D) rates from 2014 through 2018.
Figure 2.
A-D, Line graphs showing trends in acute respiratory failure-related and ARDS-related mortality stratified by sex (A, C) and race or ethnicity (B, D) from 2014 through 2018. NH = non-Hispanic.
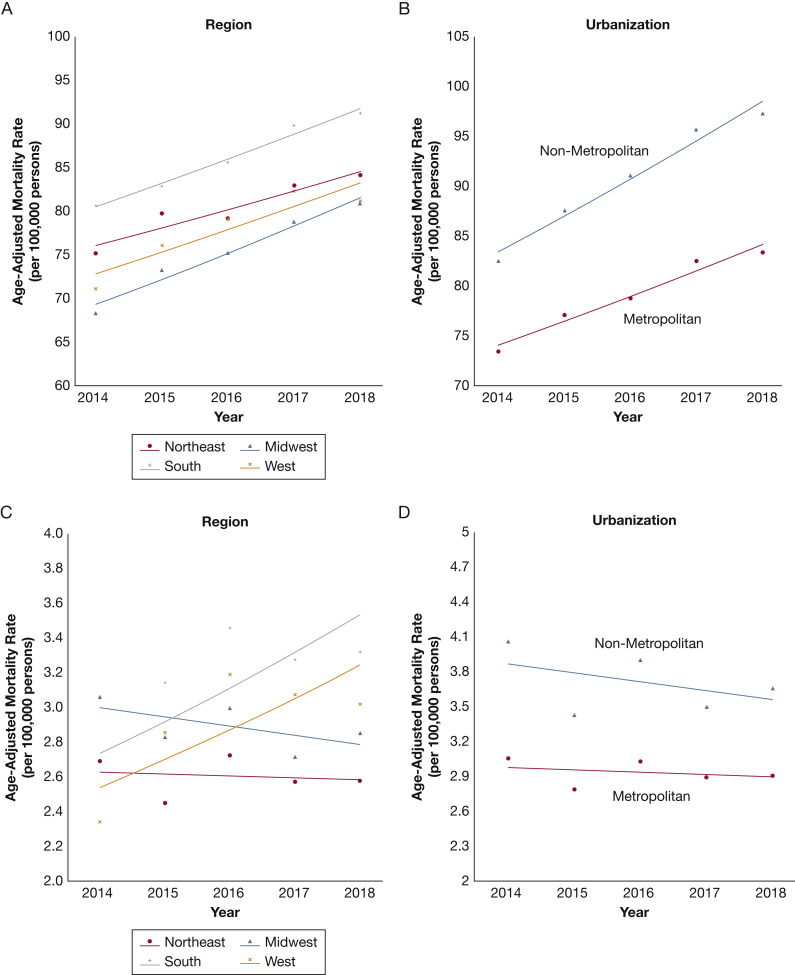
The ARF-related mortality (per 100,000 people) was highest in older people (age ≥ 65 years) from 2014 (431.7 [95% CI, 429.8-433.7]) through 2018 (492.2 [95% CI, 490.2-494.1]; APC, 3.3 [95% CI, 1.7-4.9]; P = .007), with a significant increase in younger (25-44 years of age) and middle-aged (45-64 years of age) adults (P < .05 for both) (Table 2 ). The ARF-related mortality increased in all racial and ethnic subgroups (P < .05 for all) except non-Hispanic American Indians or Alaska Natives (P = .12) (Fig 2, Table 2). This increasing trend was consistent across urbanization status, with both metropolitan (APC, 4.2 [95% CI, 2.8-5.6]; P = .002) and nonmetropolitan (APC, 3.2 [95% CI, 2.1-4.4]; P = .003) areas showing an increase in AAMR (Fig 3 , Table 2).
Table 2.
Age-Adjusted Mortality Rates for ARF-Related and ARDS-Related Mortality Stratified by Demographic Subgroups
| Variable | Age-Adjusted Mortality Rate per 100,000 People (95% CI) |
Annual Percentage Change (95% CI) | P Value | ||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | |||
| Overall | |||||||
| ARF | 74.9 (74.6-75.2) | 78.8 (78.5-79.1) | 80.8 (80.5-81.1) | 84.6 (84.3-84.9) | 85.6 (85.3-85.9) | 3.4 (2.2-4.6) | .003 |
| ARDS | 3.2 (3.2-3.3) | 2.9 (2.8-2.9) | 3.2 (3.1-3.2) | 3.0 (2.9-3.0) | 3.0 (3.0-3.1) | −0.9 (−5.4 to 3.8) | .56 |
| Sex | |||||||
| ARF | |||||||
| Male | 86.9 (86.4-87.4) | 91.3 (90.8-91.7) | 93.4 (92.9-93.8) | 97.4 (96.9-97.9) | 98.7 (98.3-99.3) | 3.2 (2.2-4.3) | .002 |
| Female | 66.0 (65.7-66.4) | 69.5 (69.1-69.8) | 71.2 (70.9-71.6) | 74.9 (74.5-75.3) | 75.5 (75.2-75.9) | 3.5 (2.2-4.8) | .004 |
| ARDS | |||||||
| Male | 2.3 (2.3-2.4) | 3.4 (3.3-3.5) | 3.6 (3.5-3.7) | 3.4 (3.4-3.5) | 3.4 (3.3-3.5) | 5.6 (−8.8 to 22.3) | .32 |
| Female | 2.3 (2.3-2.4) | 2.5 (2.5-2.6) | 2.8 (2.7-2.9) | 2.6 (2.6-2.7) | 2.8 (2.7-2.8) | 3.2 (−2.2 to 8.9) | .16 |
| Age Group, y | |||||||
| ARF | |||||||
| ≤ 24 | 2.3 (2.2-2.4) | 2.3 (2.2-2.4) | 2.6 (2.5-2.7) | 2.4 (2.3-2.5) | 2.5 (2.4-2.6) | 2.5 (−1.2 to 6.2) | .13 |
| 25-44 | 8.2 (8.0-9.4) | 8.3 (8.1-8.5) | 9.2 (9.0-9.4) | 9.3 (9.1-9.5) | 9.8 (9.6-10.0) | 4.6 (2.2-7.1) | .009 |
| 45-64 | 58.3 (57.8-58.9) | 60.1 (59.6-60.6) | 63.7 (63.2-64.3) | 65.5 (64.9-66.0) | 67.1 (66.5-67.6) | 3.7 (2.6-4.9) | .002 |
| ≥ 65 | 431.7 (429.8-433.7) | 457.7 (455.7-459.6) | 463.3 (461.4-465.3) | 489.1 (487.1-491.0) | 492.2 (490.2-494.1) | 3.3 (1.7-4.9) | .007 |
| ARDS | |||||||
| ≤ 24 | 0.3 (0.3-0.4) | 0.3 (0.3-0.3) | 0.4 (0.3-0.4) | 0.3 (0.3-0. .3) | 0.3 (0.3-0.4) | 1.0 (−6.9 to 9.6) | .73 |
| 25-44 | 1.3 (1.2-1.3) | 1.0 (0.9-1.1) | 1.2 (1.1-1.2) | 1.1 (1.0-1.2) | 1.2 (1.1-1.3) | −1.0 (−10.0 to 9.0) | .77 |
| 45-64 | 4.8 (4.7-4.9) | 3.9 (3.8-4.1) | 4.6 (4.5-4.7) | 4.1 (4.0-4.3) | 4.3 (4.2-4.5) | −1.7 (−9.9 to 7.3) | .58 |
| ≥ 65 | 11.5 (11.2-11.8) | 11.3 (11.0-11.7) | 11.7 (11.3-12.0) | 11.5 (11.2-11.8) | 11.1 (10.8-11.4) | −0.60 (−2.6 to 1.4) | .41 |
| Race or ethnicity | |||||||
| ARF | |||||||
| NH White | 75.7 (75.4-76.0) | 80.0 (79.6-80.3) | 81.8 (81.5-82.2) | 86.1 (85.7-86.4) | 87.1 (86.8-87.5) | 3.6 (2.3-4.9) | .003 |
| NH Black | 86.2 (85.1-87.2) | 89.3 (88.2-90.3) | 93.0 (92.0-94.1) | 95.6 (94.6-96.7) | 97.4 (96.4-98.5) | 3.2 (2.3-4.0) | .001 |
| NH Asian/Pacific Islander | 47.1 (46.0-48.2) | 48.6 (47.5-49.7) | 48.7 (47.6-49.8) | 50.7 (49.6-51.7) | 50.6 (49.6-51.7) | 1.9 (0.8-3.0) | .01 |
| NH American Indian/Alaska Native | 82.0 (77.8-86.1) | 89.2 (85.0-93.4) | 88.6 (84.5-92.7) | 90.8 (86.7-94.8) | 90.0 (86.1-93.9) | 1.9 (−1.0 to 4.9) | .12 |
| Hispanic | 63.3 (62.4-64.3) | 64.8. (63.8-65.7) | 68.4 (67.5-69.4) | 71.4 (70.4-72.3) | 72.7 (71.7-73.6) | 3.8 (2.6-5.0) | .002 |
| ARDS | |||||||
| NH White | 3.2 (3.1-3.2) | 2.8 (2.7-2.9) | 3.1 (3.0-3.2) | 2.9 (2.8-3.0) | 3.0 (2.9-3.0) | −1.2 (−6.2 to 4.2) | .53 |
| NH Black | 3.7 (3.5-3.9) | 3.4 (3.3-3.6) | 3.7 (3.5-3.9) | 3.6 (3.4-3.8) | 3.7 (3.5-3.9) | 0.3 (−2.8 to 3.6) | .76 |
| NH Asian/Pacific Islander | 2.6 (2.4-2.9) | 2.6 (2.3-2.8) | 2.5 (2.3-2.8) | 2.6 (2.4-2.8) | 2.5 (2.3-2.7) | −0.9 (−2.7 to 1.0) | .22 |
| NH American Indian/Alaska Native | 6.9 (5.8-8.0) | 6.1 (5.1-7.0) | 5.6 (4.6-6.5) | 5.6 (4.7-6.6) | 5.7 (4.8-6.6) | −4.3 (−9.6 to 1.4) | .09 |
| Hispanic | 3.1 (2.9-3.3) | 2.8 (2.6-2.9) | 3.2 (3.0-3.4) | 3.1 (2.9-3.3) | 2.9 (2.8-3.1) | 0.1 (−6.3 to 7.0) | .96 |
| Urbanization | |||||||
| ARF | |||||||
| Nonmetropolitan | 82.6 (81.8-83.3) | 87.6 (86.8-88.4) | 91.1 (90.3-91.9) | 95.8 (95.0-96.6) | 97.4 (96.6-98.1) | 4.2 (2.8-5.6) | .002 |
| Metropolitan | 73.4 (73.1-73.7) | 77.1 (76.8-77.4) | 78.8 (78.5-79.1) | 82.5 (82.2-82.8) | 83.4 (83.1-83.7) | 3.2 (2.1-4.4) | .003 |
| ARDS | |||||||
| Nonmetropolitan | 4.1 (3.9-4.2) | 3.4 (3.3-3.6) | 3.9 (3.7-4.1) | 3.5 (3.3-3.6) | 3.7 (3.5-3.8) | −2.0 (−9.0 to 5.6) | .45 |
| Metropolitan | 3.1 (3.0-3.1) | 2.8 (2.7-2.9) | 3.0 (3.0-3.1) | 2.9 (2.8-3.0) | 2.9 (2.8-3.0) | −0.7 (−4.6 to 3.5) | .64 |
| Region | |||||||
| ARF | |||||||
| Northeast | 75.2 (74.5-75.9) | 79.7 (79.0-80.4) | 79.2 (78.5-79.9) | 82.9 (82.3-83.6) | 84.2 (83.5-84.9) | 2.7 (1.1-4.3) | .01 |
| Midwest | 68.3 (67.7-68.9) | 73.3 (72.7-73.9) | 75.2 (74.6-75.8) | 78.8 (78.2-79.4) | 80.9 (80.3-81.6) | 4.2 (2.8-5.5) | .002 |
| South | 80.7 (80.2-81.2) | 82.9 (82.4-83.4) | 85.6 (85.1-86.1) | 89.9 (89.4-90.4) | 91.3 (90.8-91.8) | 3.3 (2.4-4.2) | .001 |
| West | 71.2 (70.5-71.8) | 76.1 (75.4-76.7) | 79.1 (78.5-79.7) | 82.3 (81.7-83.0) | 81.2 (80.6-81.8) | 3.4 (0.7-6.2) | .03 |
| ARDS | |||||||
| Northeast | 2.7 (2.6-2.8) | 2.4 (2.3-2.6) | 2.7 (2.6-2.9) | 2.6 (2.4-2.7) | 2.6 (2.5-2.7) | −0.4 (−5.1 to 4.5) | .80 |
| Midwest | 3.1 (2.9-3.2) | 2.8 (2.7-2.9) | 3.0 (2.9-3.1) | 2.7 (2.6-2.8) | 2.8 (2.7-3.0) | −1.8 (−6.0 to 2.6) | .28 |
| South | 2.3 (2.3-2.4) | 3.1 (3.0-3.2) | 3.5 (3.4-3.6) | 3.3 (3.2-3.4) | 3.3 (3.2-3.4) | 6.6 (−5.5 to 20.2) | .19 |
| West | 2.3 (2.3-2.4) | 2.9 (2.7-3.0) | 3.2 (3.1-3.3) | 3.1 (2.9-3.2) | 3.0 (2.9-3.1) | 6.3 (−3.1 to 16.6) | .13 |
ARF = acute respiratory failure; NH = non-Hispanic.
Figure 3.
A-D, Line graphs showing trends in acute respiratory failure-related and ARDS-related mortality stratified by geographical region (A, C) and urbanization (B, D) from 2014 through 2018.
The sensitivity analysis of the ARF-related mortality trends using the ICD-10 codes J80 and J96.0 also demonstrated an increasing mortality trend (e-Fig 1). This was consistent across the subgroups of sex, race and ethnicity, census regions, and urbanization status (e-Figs 2 and 3).
ARDS-Related Mortality Trends
The crude mortality (per 100,000) for ARDS-related deaths was 3.4 (95% CI, 3.3-3.4) in 2014 and 3.3 (95% CI, 3.3-3.4) in 2018 (APC, 0.2 [95% CI, −4.2 to 4.7]; P = .91). The AAMR also was stable at 3.2 (95% CI, 3.2-3.3) per 100,000 people in 2014 and 3.0 (95% CI, 3.0-3.1) per 100,000 people in 2018 (APC, −0.9 [95% CI, −5.4 to 3.8]; P = .56) (Fig 1). Among women, the AAMR (per 100,000 people) was 2.3 (95% CI, 2.3-2.4) in 2014 and 2.8 (95% CI, 2.7-2.8) in 2018 (APC, 3.1 [95% CI, −2.2 to 8.9]; P = .16). The AAMR for ARDS-related mortality in men (per 100,000 people) was 2.3 (95% CI, 2.3-2.4) in 2014 and 3.4 (95% CI, 3.3-3.5) in 2018 (APC, 5.6 [95% CI, −8.8 to 22.3]; P = .32) (Fig 2). The ARDS-related mortality (per 100,000 people) was highest in older (≥ 65 years of age) people from 2014 (11.5 [95% CI, 11.2-11.8]) through 2018 (11.1 [95% CI, 10.8-11.4]; APC, −0.6 [95% CI, −2.6 to 1.4]; P = .41) (Table 2). The ARDS-related mortality (per 100,000 people) was highest in non-Hispanic American Indians or Alaska Natives from 2014 (6.9 [95% CI, 5.8-8.0]) through 2018 (5.7 [95% CI, 4.8-6.6]; APC, −4.3 [95% CI, −9.6 to 1.4]; P = .09) (Fig 2, Table 2). Similar to ARF-related mortality, the ARDS-related mortality (per 100,000 people) was higher in nonmetropolitan areas between 2014 (4.1 [95% CI, 3.9-4.2]) and 2018 (3.7 [95% CI, 3.5-3.8]; APC, −2.0 [95% CI, −9.0 to 5.6]; P = .45) (Fig 3, Table 2).
Geographic Distribution of ARF-Related Mortality and ARDS-Related Mortality
All the geographical regions showed an increase in ARF-related mortality rates (P < .05 for all) (Fig 3). The ARDS-related mortality remained stable across all census regions between 2014 and 2018 (P > .05 for all) (Fig 3). The state-level geographic distribution of AAMR for ARF-related and ARDS-related mortality (per 100,000 people) in 2018 are shown in Figure 4 . The highest AAMR (per 100,000 people) for ARF-related deaths was seen in Arkansas (AAMR, 154.8 [95% CI, 150.7-159.0]), and the lowest was noted in Maryland (AAMR, 45.0 [95% CI, 43.4-46.5]). In 2018, the ARF-related mortality (per 100,000 people) was highest in the South (AAMR, 84.7 [95% CI, 84.2-85.2]) and lowest in the Midwest (AAMR, 75.3 [95% CI, 74.7-75.9]). The ARDS-related mortality (per 100,000 people) was highest in the South (AAMR, 3.3 [95% CI, 3.2-3.4]) and lowest in the Northeast (2.6 [95% CI, 2.5-2.7]).
Figure 4.
A, B, Map of the United States depicting the geographic distribution of (A) acute respiratory failure-related and (B) ARDS-related mortality in 2018, stratified by quartiles. The heatmap represents the age-adjusted mortality (AAMR) in the various states. The age-adjusted prevalence in each state is reported alongside the 95% CIs.
Seasonal Variation in ARF-Related Mortality and ARDS-Related Mortality
The numbers of deaths resulting from ARF-related mortality and ARDS-related mortality are described in e-Figures 4 and 5. Both ARF-related deaths and ARDS-related deaths were higher during the months of December through March in all the study years (2014-2018).
Causes of Death Associated with ARF-Related Mortality
The contribution of associated causes of ARF-related mortality are depicted in e-Tables 2 and 3. Influenza and pneumonia together and septicemia contributed nearly 41.3% and 37.8%, respectively, of ARF-related deaths with cause evaluated through the study period. In the study period, 16.3%, 3.7%, and 0.9% ARF-related deaths with cause evaluated were accounted for by food- and vomit-associated pneumonitis, trauma and burns, and acute pancreatitis, respectively. The AAMR of the various associated causes of ARF-related deaths is depicted in e-Table 3. An increase was found in the ARF-related deaths associated with influenza and pneumonia (APC, 3.1 [95% CI, 1.5-4.6]; P = .008), septicemia (APC, 4.5 [95% CI, 2.0-7.0]; P = .01), pneumonitis resulting from food and vomit (APC, 4.9 [95% CI, 1.3-8.7]; P = .02), and trauma and burns (APC, 3.5 [95% CI, 2.5-4.4]; P = .001) (e-Fig 6).
Discussion
Our investigation highlights an increasing and perturbing trend for ARF-related mortality and a lack of decline in ARDS-related mortality in the United States. This increase in ARF-related mortality was consistent across the subgroups of age, sex, race and ethnicity, and level of urbanization. The increasing mortality trends were seen consistently in all geographic regions. The ARDS-related mortality showed a lack of improvement and stagnation across the subgroups of sex, race and ethnicity, and urbanization. The ARF-related mortality was contributed mostly by influenza and pneumonia together and septicemia. Substantial geographic heterogeneity was found in the ARF and ARDS mortality across the nation, with the states in the southern region having a disproportionately higher mortality rate compared with those in other regions. Collectively, this portends high ARF- and ARDS-related mortality in certain regions in the United States, which is expected to increase as the COVID-19 pandemic burgeons throughout the country. To the best of our knowledge, no nationwide data exist that report the trends of ARDS-related mortality after the release of the Berlin definition in 2012.
Prior nationwide studies in the American population suggested an increasing incidence of ARDS,23 but with declining national mortality between 1999 and 2013, with a plateau from 2010 through 2013.8 We note that the ARDS-related mortality has continued to show a lack of decline after 2013 with an increase in ARF-related deaths after 2013. The observed lack of decline in the ARDS-related mortality and increase in the ARF-related deaths in the United States in our study may be consequent of numerous factors. Underrecognition and undertreatment have been stipulated to contribute to the high in-hospital mortality rate of approximately 40% associated with the development of ARDS.3 , 24 Because of underrecognition, up to one in five cases of severe ARDS may be overlooked, which may result in undertreatment, leading to a greater adverse outcome burden.24 Consequently, the more sensitive definition of ARDS was introduced in 2012.5 , 6 Also, an unaccounted lag may exist between the introduction of the Berlin definition and its dissemination and adoption into routine clinical care. This also may have contributed to the observed lack of decline in AAMR for ARDS-related deaths. The rising ARF mortality and nondeclining ARDS mortality in recent years may be attributed partially to the aging population. Increasing age has been associated with increased mortality secondary to ARDS,25 although the mechanisms remain somewhat unclear.26 The striking racial differences in ARF and ARDS mortality observed in our study also warrant explanation. Variations in race-based susceptibility to ARDS have been documented previously.27 , 28 Higher ARDS mortality in non-Hispanic Black people observed in our study indicate that the racial disparities in ARDS mortality, first recognized more than two decades ago, continue to persist. It has also been postulated that a greater burden of comorbidities among non-Hispanic Black people and Hispanic Americans at the time of admission to the ICU may be associated with greater mortality resulting from ARDS.29 The observed increase also may be a consequence of the increasing incidence of the ARF and ARDS, limited availability of mechanical ventilation devices, and appropriately trained personnel contributing to the burdened of the critical care infrastructure.3, 4, 5 , 30, 31, 32, 33 The inclusion of the wider ICD-10 codes aided in increased recognition of deaths attributable to ARF. Although a nonsignificant change in ARDS-related mortality was found, the demographic patterns remained mostly consistent with ARF-related mortality. We found that influenza and pneumonia together and septicemia are the most common causes associated with ARF-related mortality. We also noted a higher ARF-related and ARDS-related mortality during the winter season, which has a close temporal association with the annual increase in influenza cases in the United States.8 , 34
The observed geographic variation in the ARF and ARDS-related mortality in the United States may be explained by the variability in the health care infrastructure, demographic substrate, and prevalent comorbidity burden with the states.3, 4, 5 , 31, 32, 33 , 35, 36, 37 The greater proportions of non-Hispanic Black people reside in the southern United States, where the ARF and ARDS mortality is concentrated disproportionately. The role of socioeconomic status and its geographic distribution also may have an impact on ARDS-related mortality, and further research is needed to evaluate the impact of the social determinants of health. The limited availability of mechanical ventilation systems and adequately trained personnel, especially in certain regions, may explain the higher mortality rates seen in the nonmetropolitan areas compared with the metropolitan areas. Furthermore, research is needed to evaluate the impact of access to health care, the proximity to well-equipped hospitals, and the type of hospitals (academic or community hospitals) on the prevalent trends of ARDS-related deaths across the United States. Additionally, geographic variations in critical care practices exist that may contribute to the geographic heterogeneity.31, 32, 33 , 35, 36, 37 In the context of COVID-19, nearly 4% to 29% of patients experience ARDS,38, 39, 40 which is the most common cause of death in COVID-19 patients.41 The pandemic may burden health-care systems disproportionately in certain geographic regions and may amplify the existing regional and state-level differences in ARDS-mortality.
Our data have significant public health implications, especially during the COVID-19 pandemic. First, our data regarding ARF and ARDS mortality trends may allow for advanced planning for health-care staffing and scarce inpatient resources, such as ventilators and extracorporeal membrane oxygenation machines, in areas where mortality is traditionally high. Second, our data regarding ARDS mortality could be used further to organize local or regional centers of excellence for the management of severe ARDS-associated COVID-19 in areas where a high prior burden of ARDS mortality exists. This may centralize intensive care resources and expertise for the management of this devastating condition. Finally, our data also should be a call to action to align different health policies and social mechanisms during this pandemic. A vast number of local, regional, and federal government organizations and nongovernmental organizations exist that often are working independently to provide funding, supplies, and urgent administrative support throughout the United States. By demonstrating that differential ARF and ARDS mortality is likely to exist on the basis of historical trends, we hope that all of these organizations may seek collaborative alignment to prioritize resources to areas with the highest proportions of high-risk candidates or higher historic ARDS mortality. In addition to broad national health objectives, addressing risk factors at the local level by the use of focused and tailored health goals and health interventions specific to the population will have positive implications in the improvement of health status nationally in the long term.
We acknowledge that our investigation has several important limitations. The study is limited by the absence of individual-level measures such as comorbidity burden, etiologic agent, the severity of ARDS, global clinical condition, and provision of optimal medical therapy. The subjective ascertainment of the cause of death and the use of administrative ICD-10 codes may result in underreporting of ARDS as the cause of death. Additionally, it is difficult to differentiate if the observed increase is the result of an increase in disease incidence or the case-fatality rate. We used a broader definition of ARF using the ICD-10 codes, which allowed for increased sensitivity for capturing ARF-related mortality and was guided primarily by the previously reported underrecognition of ARDS by physicians and the general issues with the use of administrative diagnosis codes. However, this may have lead to a reduction in precision for estimating ARDS-related mortality. Additionally, we also performed sensitivity analyses using the restricted definition of ARF. Despite these limitations, our nationwide analysis used a validated approach8 to report robust temporal and demographically stratified mortality patterns of ARF-related and ARDS-related mortality in the United States.
Interpretation
An increase in ARF-related mortality has occurred at approximately 3.4% per year, and a lack of decline in ARDS-related mortality, nationally, has occurred in the last 5 years. This worrisome trend is consistent across the subgroups of age, sex, race and ethnicity, urbanization, and geographical regions. Identifying these trends and adapting public health and policy planning efforts to them may help to guide resource and infrastructure organization for the vast morbidity and mortality burden that is expected to accompany the full-scale of ARF and ARDS associated with COVID-19 in the United States.
Acknowledgments
Author contributions: P. A. and V. P. conceived the study. V. P. and R. K. executed the search and extracted data. V. P. and P. A. performed the analysis of data, with inputs from R. K., S. P. B., L. B., and G. A. All authors contributed to the interpretation of data. V. P. and R. K. wrote the initial draft of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version. V. P. and P. A. are guarantors for the study. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. P. B. has received consulting fees from GlaxoSmithKline and Sunovion. None declared (V. P., R. K., L. B., G. A., P. A.).
Role of sponsors: The funding agency had no role in the study design, analysis, interpretation, drafting, or revision of the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work is supported by the National Institutes of Health Mentored-Patient Oriented Research Award [Grant 5K23 HL146887-02 to P. A.].
Supplementary Data
References
- 1.Scala R., Heunks L. Highlights in acute respiratory failure. Eur Respir Rev. 2018;27(147):1–4. doi: 10.1183/16000617.0008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreyro B.L., Angriman F., Munshi L., et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Pham T., Rubenfeld G.D. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 5.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 6.Force A.D.T., Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Pelosi P., D’Onofrio D., Chiumello D., et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 8.Cochi S.E., Kempker J.A., Annangi S., Kramer M.R., Martin G.S. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc. 2016;13(10):1742–1751. doi: 10.1513/AnnalsATS.201512-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United States Department of Health and Human Services Multiple cause of death 1999-2018. Department of Health and Human Services website. https://wonder.cdc.gov/wonder/help/mcd.html
- 10.United States Department of Health and Human Services Multiple cause of death 1999-2018. Department of Health and Human Services website. https://wonder.cdc.gov/wonder/help/mcd.html#Population%20Data
- 11.Bevan G.H., Zidar D.A., Josephson R.A., Al-Kindi S.G. Mortality due to aortic stenosis in the United States, 2008-2017. JAMA. 2019;321(22):2236–2238. doi: 10.1001/jama.2019.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein M.J., Bahiru E., Achenbach C., et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidney S., Go A.S., Jaffe M.G., Solomon M.D., Ambrosy A.P., Rana J.S. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4(12):1280–1286. doi: 10.1001/jamacardio.2019.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah N.S., Lloyd-Jones D.M., O’Flaherty M., et al. Trends in cardiometabolic mortality in the United States, 1999-2017. JAMA. 2019;322(8):780–782. doi: 10.1001/jama.2019.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis F.G., Dolecek T.A., McCarthy B.J., Villano J.L. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson R.N., Rosenberg H.M. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47(3):1–16. 20. [PubMed] [Google Scholar]
- 18.Melamed A., Sorvillo F.J. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care. 2009;13(1):R28. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Division of Cancer Control and Population Sciences, National Cancer Institute Joinpoint regression program, version 4.7.0.0. January 2019. National Cancer Institute website. https://surveillance.cancer.gov/joinpoint/
- 20.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.J., Luo J., Chen H.S., et al. Improved confidence interval for average annual percent change in trend analysis. Stat Med. 2017;36(19):3059–3074. doi: 10.1002/sim.7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clegg L.X., Hankey B.F., Tiwari R., Feuer E.J., Edwards B.K. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28(29):3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eworuke E., Major J.M., Gilbert McClain L.I. National incidence rates for acute respiratory distress syndrome (ARDS) and ARDS cause-specific factors in the United States (2006–2014) J Crit Care. 2018;47:192–197. doi: 10.1016/j.jcrc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Frohlich S., Murphy N., Doolan A., Ryan O., Boylan J. Acute respiratory distress syndrome: underrecognition by clinicians. J Crit Care. 2013;28(5):663–668. doi: 10.1016/j.jcrc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Ely E.W., Wheeler A.P., Thompson B.T., Ancukiewicz M., Steinberg K.P., Bernard G.R. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136(1):25–36. [PubMed] [Google Scholar]
- 26.Schouten L.R.A., Bos L.D.J., Serpa Neto A., et al. Increased mortality in elderly patients with acute respiratory distress syndrome is not explained by host response. Intensive Care Med Exp. 2019;7(1):58. doi: 10.1186/s40635-019-0270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie J.D., Ma S.F., Aplenc R., et al. Variation in the myosin light chain kinase gene is associated with development of acute lung injury after major trauma. Crit Care Med. 2008;36(10):2794–2800. doi: 10.1097/ccm.0b013e318186b843. [DOI] [PubMed] [Google Scholar]
- 28.Gao L., Grant A., Halder I., et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34(4):487–495. doi: 10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erickson S.E., Shlipak M.G., Martin G.S., et al. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37(1):1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azoulay E., Lemiale V., Mourvillier B., et al. Management and outcomes of acute respiratory distress syndrome patients with and without comorbid conditions. Intensive Care Med. 2018;44(7):1050–1060. doi: 10.1007/s00134-018-5209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace D.J., Angus D.C., Seymour C.W., Barnato A.E., Kahn J.M. Critical care bed growth in the United States. A comparison of regional and national trends. Am J Respir Crit Care Med. 2015;191(4):410–416. doi: 10.1164/rccm.201409-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr B.G., Addyson D.K., Kahn J.M. Variation in critical care beds per capita in the United States: implications for pandemic and disaster planning. JAMA. 2010;303(14):1371–1372. doi: 10.1001/jama.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laffey J.G., Madotto F., Bellani G., et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med. 2017;5(8):627–638. doi: 10.1016/S2213-2600(17)30213-8. [DOI] [PubMed] [Google Scholar]
- 34.Tokars J.I., Olsen S.J., Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis. 2018;66(10):1511–1518. doi: 10.1093/cid/cix1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta A.B., Syeda S.N., Bajpayee L., Cooke C.R., Walkey A.J., Wiener R.S. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993-2012. Am J Respir Crit Care Med. 2015;192(4):446–454. doi: 10.1164/rccm.201502-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta A.B., Syeda S.N., Wiener R.S., Walkey A.J. Epidemiological trends in invasive mechanical ventilation in the United States: a population-based study. J Crit Care. 2015;30(6):1217–1221. doi: 10.1016/j.jcrc.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooke C.R. Risk of death influences regional variation in intensive care unit admission rates among the elderly in the United States. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.