Abstract
The pandemic coronavirus disease 2019 (COVID-19), caused by the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly spreading globally. Clinical observations found that systemic symptoms caused by SARS-CoV-2 infection are attenuated when using the anticoagulant agent heparin, indicating that heparin may play other roles in managing COVID-19, in addition to prevention of pulmonary thrombosis. Several biochemical studies show strong binding of heparin and heparin-like molecules to the Spike protein, which resulted in inhibition of viral infection to cells. The clinical observations and in vitro studies argue for a potential multiple-targeting effects of heparin. However, adverse effects of heparin administration and some of the challenges using heparin therapy for SARS-CoV-2 infection need to be considered. This review discusses the pharmacological mechanisms of heparin regarding its anticoagulant, anti-inflammatory and direct antiviral activities, providing current evidence concerning the effectiveness and safety of heparin therapy for this major public health emergency.
Abbreviations: ACE2, angiotensin-converting enzyme 2; aPTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; AT-III, antithrombin-III; COVID-19, coronavirus disease 2019; DVT, deep vein thrombosis; FDP, fibrin(ogen) degradation product; GAG, glycosaminoglycan; gC, glycoprotein C; GlcA, Glucuronic Acid; GlcN, Glucosamine; HIT, heparin-induced thrombocytopenia; HIV, human immunodeficiency virus; HPMECs, human pulmonary microvascular endothelial cells; HS, heparan sulfate; IdoA, Iduronic Acid; IL-1, interleukin-1; LMWH, low molecular weight heparin; LPS, lipopolysaccharide; MERS-CoV, middle east respiratory syndrome-coronavirus; MW, molecular weight; NF-κB, nuclear factor kappa-B; PF4, platelet factor 4; PrV, pseudorabies virus; PT, prothrombin time; RBD, receptor binding domain; SARS-CoV, severe acute respiratory syndrome coronavirus; SGP, spike glycoprotein; SIC, sepsis-induced coagulopathy; TCMs, traditional Chinese medicines; TNF-α, tumor necrosis factor-α; UFH, unfractionated heparin; VTE, venous thromboembolism
Keywords: Heparin, COVID-19, SARS-CoV-2, Anticoagulation, Anti-inflammation, Antivirus
1. Introduction
Since December 2019, a highly infectious coronavirus, causing coronavirus disease 2019 (COVID-19), is rapidly spreading throughout the globe (Zhu et al., 2020). COVID-19 can induce a severe respiratory illness similar to that caused by severe acute respiratory syndrome coronavirus (SARS-CoV) infection. On February 11, 2020, the International Committee on Taxonomy of Viruses officially named the new type of coronavirus that caused COVID-19 epidemic as SARS-CoV-2 (Walls et al., 2020). The clinical manifestations of COVID-19 include fever, dry cough and fatigue while a few patients are accompanied by nasal congestion, runny nose, sore throat, myalgia and diarrhea. Severe patients often develop dyspnea and/or hypoxemia one week after the disease’s onset. For these more severe cases, symptoms can quickly progress to acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, coagulopathy, and multiple organ failures (Guan et al., 2020). As of September 30, 2020, the number of SARS-CoV-2 infected patients had already risen to 33 million, with an increase of more than 280,000cases in last 24 hours. By this date the cumulative number of deaths exceeded 800,000, with the estimated mortality of COVID-19 being approximately 3.0%. This rate is much higher than influenza caused fatality (<1%) (Mehta et al., 2020). A large number of studies have been performed to investigate the structural characteristics, pathophysiology as well as thein vivo processes of SARS-CoV-2, which would facilitate the discovery of effective treatments to control the spreading of COVID-19.
The ultimate approaches to manage COVID-19 lie in the discovery of effective vaccines and antivirals. However, the process of developing novel therapeutics is complicated and often time consuming. In this regard, the use of existing, approved therapies with demonstrated safety profile is a promising strategy to meet the urgent requirement of reducing the rising mortality. Heparin, a natural polysaccharide, is widely used as an anticoagulant agent with well-characterized bioavailability, safety, stability and pharmacokinetic profiles (Lindahl & Li, 2020; Thachil, 2020). In addition, heparin possesses other biological activities including increasing the release of hepatic lipase and lipoprotein lipase (Persson & Nilssonehle, 1990), suppressing complement activation (Boackle, Caughman, Vesely, Medgyesi, & Fudenberg, 1983), inhibiting angiogenesis (Folkman, Langer, Linhardt, Haudenschild, & Taylor, 1983), and modulating tumor progress and metastasis (Atallah, Khachfe, Berro, & Assi, 2020). Crucially, heparin also shows a broad-spectrum activity against a multitude of distinct viruses (Baba, Pauwels, Balzarini, Arnout, & De Clercq, 1988; Howell et al., 1996; Zhang et al., 2010). Given current insight on the pharmacological activity and clinical efficacy of heparin, it could be an under-exploited effective therapy for COVID-19 and warrants investigation.
Here we discuss the pharmacological mechanisms of the anticoagulant, anti-inflammatory and direct antiviral activities of heparin. On the basis of these critical functions of heparin, we illustrate both the preclinical and clinical evidence that heparin therapy has multiple effects for COVID-19. The therapeutic approach of using heparin to manage COVID-19 may provide a strategy to decrease SARS-CoV-2 related morbidity and mortality. Meanwhile, we also bring up the innate adverse effects of heparin and the underlying challenges of implementing heparin therapy for SARS-CoV-2 infection.
2. Structure and classification of heparin
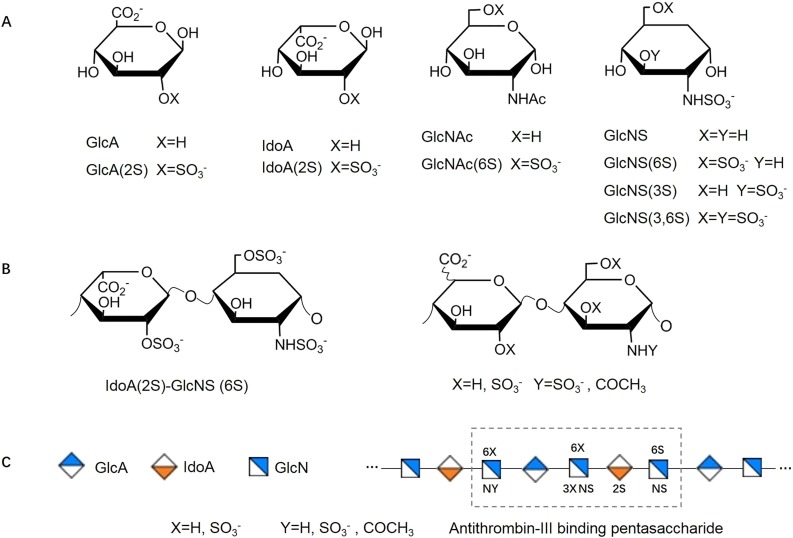
Heparin is structurally one of the most complex members in the family of polysaccharides, which is constructed from the monosaccharide building blocks of Glucosamine (GlcN), Iduronic Acid (IdoA) and Glucuronic Acid (GlcA) (Casu & Lindahl, 2001). GlcN can be N-acetylated (GlcNAc) or N-sulfated (GlcNS), both of which can be further 6-O-sulfated (GlcNAc(6S) and GlcNS(6S)).GlcNS and GlcNS(6S) can also be sulfated at carbon-3(GlcNS(3S) and GlcNS(3,6S)). GlcA and IdoA can be 2-O-sulfated (GlcA(2S) and IdoA(2S)) (Fig. 1 A) (Baytas & Linhardt, 2020). Disaccharide subunits composed of the above monosaccharides form the basic sequence units of the long, linear and highly-sulfated chain of heparin. Of these, a disaccharide composed of IdoA(2S)-GlcNS(6S) accounts for the highest proportion in heparin while other combinations of monosaccharides make up the remainder (Rabenstein, 2002; Zhang et al., 2019) (Fig. 1B). With the highly-sulfated nature of the glycosaminoglycan (GAG) chain, heparin shows the highest negative charge density (∼3.3 negative charges per disaccharide) among the known biomolecules (Weiss, Esko, & Tor, 2017). Moreover, the special arrangement sequence of this negative GAG chain can create binding sites that allow heparin to selectively and strongly interact with various proteins (Esko & Lindahl, 2001). The most well-characterized interaction is the highly specific interaction of a pentasaccharide with serine protease inhibitor antithrombin-III (AT-III), which confers heparin with excellent anticoagulant activity (Fig. 1C).
Fig. 1.
Structure of Heparin. (A) Monosaccharide units of heparin. (B) The dominant repeating disaccharides of heparin. (C) Schematic illustration of pentasaccharide sequence of antithrombin-III binding site.
Generally, the molecular weight (MW) of heparin is between 3 to 30 kDa with an average MW of 15 kDa. According to the length of polysaccharide chain, heparin can be classified into unfractionated heparin (UFH) and low molecular weight heparin (LMWH). Because of the heterogeneous mixture of linear chains, UFH has variable biological activities (Bounameaux, 1998). LMWH is generated from UFH via different methods including chemical, physical or enzymatic depolymerization. The resulting products have a mean molecular weights of 4-5 kDa, a mix of polysaccharide chain lengths and different pharmacological properties (Merli & Groce, 2010). If there is no specific designation, heparin refers to both UFH and LMWH through the text.
3. Anticoagulant therapy of heparin in COVID-19
3.1. Coagulopathy in COVID-19
Clinical observations and laboratory evidence confirm that coagulopathy is a common phenomenon in COVID-19. Numerous SARS-CoV-2 infected patients who had no other risk factors for thrombosis, suffered from various thrombotic events, such as microvascular thrombosis, venous thromboembolism, pulmonary embolism, and acute arterial thrombosis (Klok et al., 2020). While the pathogenesis of coagulopathy in COVID-19 has not been fully elucidated, several mechanisms may be involved here. For many severe infections, including pulmonary infection, the main causes for coagulation disorder include excessive inflammatory cytokine production, increased levels of damage-associated molecular patterns, the stimulation of cell-death and vascular endothelial damage (Helms et al., 2020). Similarly, SARS-CoV-2 infection could induce a cytokine storm with the activation of leukocytes, endothelium and platelets, which would promote the upregulation of tissue factor, coagulation activation, thrombin generation, and fibrin formation (Engelmann & Massberg, 2013; Sara, 2011). Moreover, as SARS-CoV-2 shows high affinity to angiotensin-converting enzyme 2 (ACE2) expressed on endothelial cells, endothelial cell activation may be a specific mechanism for COVID-19-inducedthrombosis (Zhang, Penninger, Li, Zhong, & Slutsky, 2020). In addition, profound hypoxemia has been observed in COVID-19, which may result in vasoconstriction, reducing blood flow and promoting vascular occlusion (Grimmer & Kuebler, 2017). Hypoxia also activates hypoxia-inducible factors and decreases hydroxylation, resulting in the induction or inhibition of many genes including plasminogen-activator inhibitor-1 (PAI-1) and tissue factor (Gupta, Zhao, & Evans, 2019).
Among the coagulation parameters found related to COVID-19,the most common coagulation abnormality is the remarkably elevated D-dimer concentration (up to 45% of patients) (Wu et al., 2020; Zhou et al., 2020). This increased D-dimer level is correlated with severity of illness. Compared to patients with normal D-dimer levels, the patients with a concentration of 4-fold above the normal value showed approximately 5-fold higher odds of critical illness (Petrilli et al., 2020). Elevated D-dimer levels at the time of admission and during hospital stay are also associated with the mortality of patients. The concentration of D-dimer on admission of non-survivors (2.12 (0.77-5.27) μg/mL) is significantly higher than that of survivors (0.61 (0.35-1.29) μg/mL) (Tang, Li, Wang, & Sun, 2020). Another typical change of coagulation parameters in COVID-19 is the dramatic increase of fibrin(ogen) degradation product (FDP), suggesting a secondary hyper-fibrinolysis following coagulation activation (Iba, Levy, Levi, & Thachil, 2020). The prothrombin time (PT) and activated partial thromboplastin time (aPTT) are normal or slightly prolonged, which has not been shown to be related with severity of COVID-19 (Lee, Fralick, & Sholzberg, 2020). However, a prolongation of PT > 3 or aPTT> 5 seconds are independent predictors for thrombosis (Klok et al., 2020). It was found that fibrinogen levels are elevated in the initial phase but drop late in the course of non-survivors, which may be a signal for impending death (Tang, Li, et al., 2020). In addition, a declining level of AT-III is found in COVID-19, which appears to be an indicator of progressive disease and is associated with death (Iba et al., 2020).Thrombocytopenia is also observed in COVID-19 patients with a low platelet count being related to the elevating risk of disease severity and mortality (Lippi, Plebani, & Henry, 2020). As coagulopathy plays an essential role in the progress and prognosis of patients, it is urgent to effectively manage the hypercoagulable conditions of COVID-19 patients.
3.2. Anticoagulant activity of heparin in COVID-19
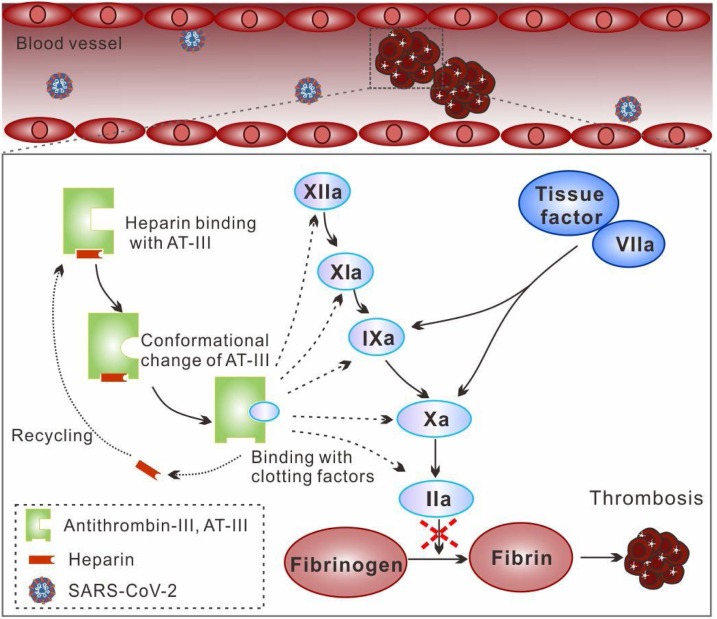
Discovered in 1916, heparin was introduced clinically in 1935, and became firmly established as an excellent agent to treat or prevent thromboembolism since 1960 (Conrad, 1997). It can be used in various settings to prevent thrombotic complications, including systemic administration, catheter instillation, using an extracorporeal circuit, or by the addition of an artificial surface coating to medical devices (Lau, Barnes, & Streiff, 2018). Mechanically, heparin cannot lyse existing thrombi as it has no intrinsic fibrinolytic activity. The major mechanism of its anticoagulant effect dependents on the presence of the active pentasaccharide sequence for binding to AT-III (Lindahl, Backstrom, Thunberg, & Leder, 1980). Once heparin binds with AT-III, it induces a conformational change of AT-III. It has been shown that this might involve a two-step binding mechanism (Olson, Srinivasan, Bjork, & Shore, 1981). Firstly, the trisasaccharide at the non-reducing end of the pentasaccharide sequence forms a low-affinity complex with AT-III, which then induces the conformational change of AT-III, thereby forming a high-affinity complex. The remaining disaccharide at the reducing end is not responsible for the activation process but can stabilize the activated conformation of AT-III (Maurice, Térésa, Jean-Pascal, & Glycobiology, 1997). The activated AT-III can increase its affinity to and inactivate coagulation factors (XIIa, XIa, IXa, Xa and IIa) (Mackman, 2008). Interestingly, heparin can readily dissociate from the heparin/AT-III complex and bind to additional AT-III, with a recycling effect that provides a continuous anticoagulant effect (Fig. 2 ). Among the coagulation factors, factor Xa and IIa are the most sensitive to the AT-heparin complex. Heparin’s inhibition of factor Xa only requires the pentasaccharide sequence, while inhibition of factor IIa requires not only the pentasaccharide sequence but additional sugar residues adjacent to the pentasaccharide sequence (Griffith, 1982). Therefore, heparin with a degree of polymerization of less than 18 (MW around 5.4 kDa) has very low efficacy in inactivating factor IIa but a similar affinity to factor Xa (Carter, Kelton, Hirsh, & Gent, 1981). Apart from its anticoagulant effects, heparin has been found to increase vessel wall permeability and suppress the proliferation of vascular smooth muscle cells (Hirsh & Raschke, 2004).
Fig. 2.
Schematic illustration of anticoagulant activity of heparin in COVID-19. Heparin binds with AT-III and induces a conformational change of AT-III for activation. The activated AT-III further inactivates coagulation factors (XIIa, XIa, IXa, Xa and IIa) and inhibits the formation of thrombosis.
3.3. The clinical application of anticoagulant therapy in COVID-19
The clinical application of heparin for anticoagulant therapy in COVID-19 has shown promising outcomes. It has been reported that anticoagulant therapy reduced mortality of severe COVID-19 patients. Tang et al. (Tang, Bai, et al., 2020) conducted a retrospective study that enrolled 449 patients with severe COVID-19 in Tongji hospital, Wuhan, China. In the study, 94 of the patients received LMWH (40-60 mg enoxaparin/d) while 5 of patients received UFH (10,000-15,000 U/d) for 7 days or longer. No significant difference was observed in the overall mortality between patients with or without heparin treatment (30.3% vs 29.7%, P = 0.910). However, there were significant differences in 28-day mortality in the subgroup of patients with a concentration of D-dimer (>3 μg/mL) higher than 6-fold of the normal upper limit (32.8% vs 52.4%, P = 0.017), or who had a sepsis-induced coagulopathy (SIC) score ≥4 (40.0% vs 64.2%, P = 0.029). The results indicated that anticoagulant therapy with heparin was related with a better prognosis in severe COVID-19 patients with remarkably elevated D-dimer levels or patients who met the criteria of SIC. In another report, the use of LMWH for anticoagulation therapy was recommended for COVID-19 patients with D-dimer concentrations 4-fold higher than the upper limit of what is considered normal (Lin, Lu, Cao, & Li, 2020). Autopsy findings from COVID-19 patients have detected microthrombi in pulmonary microvasculature, indicating refractory hypoxemia (Tian et al., 2020; Yao et al., 2020). In a study of 27 consecutive COVID-19 patients in SirioLibanes Hospital, São Paulo-Brazil, the researchers found that heparin therapy for COVID-19 improved oxygenation. The PaO2/FiO2 ratio, a marker for respiratory distress, was significantly increased from 254(±90) to 325(±80) (P = 0.013) after anticoagulation therapy for 72 hours (Negri et al., 2020).
Heparin also plays a critical role in venous thromboembolism prophylaxis for COVID-19 patients. Zhang et al. (Zhang et al., 2020) performed a single institutional study to investigate deep vein thrombosis (DVT) in hospitalized COVID-19 patients. For the subgroup with a Padua prediction score ≥ 4 and whose ultrasound scans were performed <72 hours after admission, DVT was found in 18 (34.0%) patients of the subgroup receiving venous thromboembolism (VTE) prophylaxis therapy with LMWH, compared with 35 (63.3%) in the non-prophylaxis group (P = 0.010). Thus, using heparin for VTE prophylaxis has been included in some practice guidelines in its efficacy regarding COVID-19 management (Bikdeli et al., 2020; Popoola et al., 2017; Qiu, Wang, Zhang, & Qian, 2020; Thachil et al., 2020). According to expert recommendations from the American College of Cardiology (ACC), hospitalized patients with respiratory failure or comorbidities, bedridden patients and those requiring intensive care, should receive pharmacological VTE prophylaxis in the absence of any contraindications (Bikdeli et al., 2020). Mechanical prophylaxis should be considered in immobilized patients with a contraindication to pharmacological prophylaxis (Ho & Tan, 2013). The International Society on Thrombosis and Haemostasis (ISTH) recommended that aprophylactic dose of LMWH should be considered for all patients (without contraindications) who require hospital admission. The optimal dosages need to be adjusted according to the specific conditions of individual patient (Thachil et al., 2020). For the choice of agents, the World Health Organization interim guidance statement recommends once daily dosing regimen of LMWH, or twice daily of subcutaneous UFH (Organization, 2020). Receiving all scheduled doses of pharmacological VTE prophylaxis has been shown to be important, with missed doses likely resulting in the worse outcomes (Popoola et al., 2017). In this regard, once daily dose of LMWH may be advantageous over the twice daily UFH. However, UFH is recommended for patients with severe renal failure (creatinine clearance CrCl< 30 mL/min) (Qiu et al., 2020).
4. Anti-inflammatory activity of heparin in COVID-19
4.1. Cytokine storm in COVID-19
ARDS is extremely life-threatening to COVID-19 patients with a high mortality rate of 40% to 50% (Wu et al., 2020). Although the exact mechanism of ARDS in COVID-19 is not fully understood, initiation of a cytokine storm is considered to be one of the major contributing factors (Chen, Zhou, et al., 2020; Chousterman, Swirski, & Weber, 2017; Huang et al., 2020). Broadly speaking, a cytokine storm is usually characterized by high release of interleukins, tumor-necrosis factors, interferons, chemokines, and other mediators (Sinha, Matthay, & Calfee, 2020). Under ideal circumstances, the coordinated secretion of sustained inflammatory cytokines is a well-conserved consequence of appropriate innate and adaptive immune responses for efficient clearance of microorganisms (Pedersen & Ho, 2020). However, in several viral infections including SARS-CoV and middle east respiratory syndrome-coronavirus (MERS-CoV), an overwhelming systematic response is triggered, leading to the excessive activation and proliferation of immune cells, with the release of immense amounts of cytokines (Lau et al., 2013). Not surprising given the phylogenetic relationship between these two coronaviruses, a similar phenomenon is also observed in patients in response to SARS-CoV-2 infection (Ragab, Salah Eldin, Taeimah, Khattab, & Salem, 2020). However, while SARS-CoV infection mainly produces pro-inflammatory cytokines, SARS-CoV-2 infection produces both pro-inflammatory cytokines (e.g., interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α)) and anti-inflammatory cytokines(e.g., IL-4 and IL-10) (Zhang, Wu, Li, Zhao, & Wang, 2020). According to the clinical findings, the pro-inflammatory IL-6 is the most frequently reported cytokine elevated and serves as a main contributor to the acute inflammatory responses in COVID-19 (Sinha et al., 2020). Gao et al. (Gao et al., 2020) reported that IL-6 levels were remarkably increased in severe patients (n = 15) compared to the milder cases (n = 28). Similarly, Chen et al. (Chen, Liu, et al., 2020) divided 29 COVID-19 patients into three groups based on relevant diagnostic criteria. Among the three groups, critical cases (n = 5) had the highest IL-6 levels, while severe cases (n = 9) had higher IL-6 levels than mild cases (n = 15). In addition to the severity of illness, IL-6 is also correlated with the mortality of COVID-19 patients. In a multicenter retrospective study of 150 patients, a significantly higher concentration of IL-6 was found in fatal cases compared with discharged cases (Ruan, Yang, Wang, Jiang, & Song, 2020). Based on this, anti-inflammatory therapies that alleviate the cytokine responses, especially IL-6, may ease severe symptoms and decrease the mortality of COVID-19 patients.
4.2. Anti-inflammatory activity of heparin in COVID-19
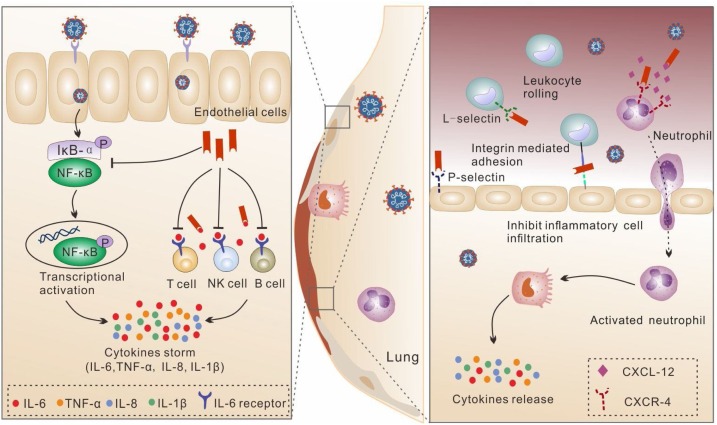
Heparan sulfate (HS) is a type of GAG expressed by virtually all mammalian cells (Bishop, Schuksz, & Esko, 2007). The common form of HS found in the cell surface is as HS proteoglycans (HSPG), formed via the covalently linking of one or more linear HS molecules with a core protein (Weiss et al., 2017). HS is composed of the same saccharide building blocks as heparin. However, the primary structure of HS shows remarkable difference from heparin. The disaccharide subunits in HS present higher GlcA/IdoA and GlcNAc/GlcNS and lower SO4 content (Rabenstein, 2002), with unsulfated GlcA-GlcNAc being the most common disaccharide subunit (Malcolm Lyon, 1998).The biological activities of HS are mainly through interaction with a wide range of proteins, including cytokines, chemokines, enzymes, enzyme inhibitors, selectins, growth factors, and extracellular matrix molecules (Sarrazin, Lamanna, & Esko, 2011). With a highly similar structure to HS, heparin also interacts with the HS-binding proteins. By competing with HS for protein binding, heparin will replace the original HS-mediated protein anchoring, thereby disrupting the corresponding functional action (Fig. 3 ).
Fig. 3.
Schematic illustration of anti-inflammatory activity of heparin. The anti-inflammatory effects include two general mechanisms: (1) Preventing inflammatory cell infiltration by inhibiting the recruitment and adhesion of neutrophils and leukocytes. (2) Inhibiting the function of IL-6 by indirectly suppressing NF-κB, and directly interacting with IL-6 and preventing the formation of the cell surface receptor complex of IL-6.
Based on the interactions of chemokines and selectins with HS on endothelial cells, heparin can inhibit chemotaxis of neutrophils and migration of leukocytes during inflammation (Farrugia, Lord, Melrose, & Whitelock, 2018).Heparin is able to interact with the chemokines (e.g. CXCL-12) responsible for driving neutrophils to inflammatory area (Ma et al., 2012). Meanwhile, heparin binds to P/L-selectin which is involved in the early adhesion between leukocytes and vessel walls during the process of cell rolling. Heparin also interacts with integrin adhesion molecules(e.g. macrophage-1 antigen), inhibiting the activation and tight adhesion of leukocytes to the endothelium (Yan et al., 2017).The suppressed recruitment of immune cells by heparin can suppress the subsequent immune activation and cytokine release.
Excessive IL-6 signaling can mature naïve T cells into eff ;ector T cells, promote vascular endothelial growth factor expression and increase the permeability of blood vessels, contributing to severe organ damage (Tanaka, Narazaki, & Kishimoto, 2016). An animal study of SARS-CoV demonstrated that inhibiting nuclear factor kappa-B (NF-κB), a key transcription factor of IL-6, increased animal survival with decreased IL-6 levels (DeDiego et al., 2014). In lipopolysaccharide (LPS)-stimulated human pulmonary microvascular endothelial cells (HPMECs), heparin was shown to inhibit the activation of NF-κB through degradation of IκB-α and nuclear translocation of p65, reducing the release of IL-6 by stimulated HPMECs (Li, Li, Shi, Yu, & Ma, 2020). In another study, heparin reduced the expression of LPS-stimulated chemokines and cytokines through inactivation of the NF-κB signaling pathway in human endothelial cells (Li, Ma, Chen, Tang, & Ma, 2016). These findings indicated that heparin could be a promising candidate to reduceIL-6 release by inhibiting the expression of NF-κB. Further, HS on the cell surface can bind with IL-6, which can protect IL-6 from proteolysis and yield a sufficient local concentration to activate downstream signaling receptors (Bernfield et al., 1999). Heparin can disrupt this process by competing with HS for IL-6 interaction. With higher levels of sulfate than HS, heparin has a much higher affinity for IL-6 (Hasan, Najjam, Gordon, Gibbs, & Rider, 1999). Also, heparin may prevent the formation of the cell surface receptor complex of IL-6 by strongly competing with the binding of the rIL-6/srIL-6Ra dimer to soluble glycoprotein 130 (Mummery & Rider, 2000). Therefore, heparin shows a dual effect in IL-6 inhibition by reducing both the release and biological activity.
Apart from the typical inflammatory factors released, some of the clinical features of cytokine storm in COVID-19 are characterized by disseminated intravascular coagulation, capillary leak syndrome and loss of blood pressure, indicating the crosstalk between hemostasis and cytokines (Mangalmurti & Hunter, 2020). As discussed above, heparin has high anticoagulant activity, which could help attenuate the cytokine storm-induced systematic syndrome and organ damage.
4.3. The clinical application of heparin for anti-inflammatory therapy
Clinical investigations on the use of heparin as an anti-inflammatory therapy have been reported (Mousavi, Moradi, Khorshidahmad, & Motamedi, 2015). In a randomized, double-blind, crossover clinical trial for asthma, five doses of UFH (1,000 U/kg/dose) or placebo were inhaled by the patients. Compared with the placebo, heparin significantly reduced the late asthmatic response after allergen administration (P = 0.005) (Diamant et al., 1996). In a quasi-experimental (non-randomized clinical trial) study, nadroparin, a type of LMWH, remarkably improved the endoscopic and histological signs of inflammation in steroid refractory ulcerative colitis (Vrij et al., 2001). Despite the clinical evidence, there are very limited applications of heparin as an anti-inflammatory therapy in COVID-19. In a retrospective cohort study, we found that patients who were not treated with LMWH had a slight increase in IL-6 level after conventional treatment. However, in patients treated with LMWH, IL-6 level was significantly reduced (14.96 ± 151.09, -32.46 ± 65.97, p = 0.031) while other inflammatory factors did not have significant changes (Shi et al., 2020). The possible mechanism could be as follows: on one hand, LMWH could reduce the release of IL-6 by inhibiting the expression of NF-κB, which is consistent with the protective effect of LMWH; on the other hand, IL-6 can bind to HS on the cell surface to produce a high local concentration to activate signal receptors, protect them from proteolysis, and promote paracrine effects (Sarrazin et al., 2011). This study provides direct evidence for the favorable anti-inflammatory effect of heparin therapy in COVID-19.
5. Antiviral effect of heparin
5.1. Preclinical evidence for an antiviral effect of heparin
In addition to its anticoagulant and anti-inflammatory activity, heparin may possess a direct antiviral effect to SARS-CoV-2, based on the preclinical studies for other viral infections. Ito et al. (Ito et al., 1987) investigated the antiviral effect of heparin on human immunodeficiency virus (HIV) infection in vitro. At a concentration of 7.5 μg/mL, heparin showed a 50% reduction in HIV-induced cytopathogenicity to MT-4 cells. To further clarify the underlying mechanism, the same group elucidated that heparin cannot directly neutralize HIV but can prevent the adhesion of HIV to MT-4 cells. The inhibitory effect of fragmented heparin with various MWs ranging from 1400 to 11,000 were further examined. Compared with the original non-fragmented heparin, all of the fragmented heparins were significantly less active in inhibiting the replication of HIV in MT-4 cells, demonstrating a reduced antiviral activity with the smaller fragments of heparin (Baba et al., 1988). Excessive studies have indicated that many viruses show HS dependence of viral infection, providing evidence for antiviral effect by heparin. Milewska et al. (Milewska et al., 2014) illustrated that HS was attachment receptors for human coronavirus NL63 to infect the target cells. Modhiran et al. (Modhiran et al., 2019) showed that PG545, a clinical-stage heparan sulfate mimetic, served as an inhibitor for virus-cell attachment and potently inhibited the infectivity of dengue virus. Sasaki et al. (Sasaki et al., 2018) reported that cellular HS facilitated the adhesion and subsequent entry of Rabies virus (RABV) into target cells. They further demonstrated that heparin blocked the viral adhesion and infection by competitive inhibition for the binding of cellular HS to RABV. Tanaka et al. (Tanaka et al., 2017) revealed that N-sulfation of HS played a critical role in the infectivity of Chikungunya virus to host cells using genome-wide screening. Tamhankar et al. (Tamhankar et al., 2018) found that interrupting the interaction of cellular HS with virus by iota-carrageenan or heparin lyase, lead to significant inhibition of basolateral infection of Ebola virus in the polarized Caco-2 cells. Differently, Gao et al. (Gao et al., 2019) demonstrated that HS is not required for the cell attachment of Zika virus but involved in the viral replication, and mediated the virus infection-induced cell apoptosis. In addition to these findings, the most relevant evidence is that heparin could inhibit the cell entry of SARS-CoV as HSPG provides a binding site for SARS-CoV invasion at the initial attachment phase (Lang et al., 2011). Accumulated evidence indicates that heparin may be a promising candidate for antiviral therapy in COVID-19.
5.2. The potential antiviral activity of heparin against SARS-CoV-2
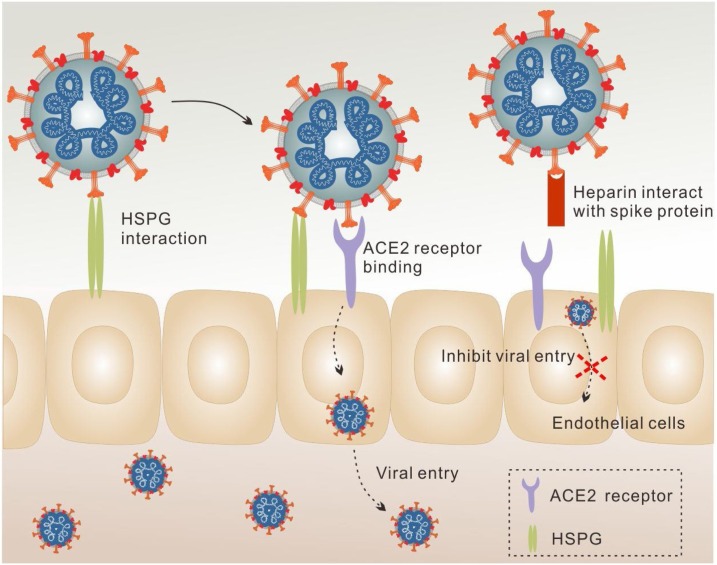
ACE2 is well-characterized as the major receptor for viral entry of SARS-CoV-2 (Hoffmann et al., 2020). However, many viruses including coronaviruses, also use cellular GAG, notably HS, as co-receptors to allow the attachment of viruses to the cell surface, increasing the local concentration of viral particles for invasive infection (Tandon et al., 2020). According to the sequence analysis of Spike glycoprotein (SGP), potential GAG binding domains and GAG binding-like motifs have been shown in SARS-CoV-2. With deeper investigation, these binding domains have been found to be distributed at site 1 (within the receptor binding domain (RBD), Y453-S459), site 2 (proteolytic cleavage site at S1/S2 junction, P681-S686) and site 3 (the S2’ proteolytic cleavage site, S810-S816) (Kim et al., 2020). Strikingly, the S1-S2 proteolytic cleavage motif is novel for SARS-CoV-2, which is not presented in the SGPs of SARS-CoV or MERS-CoV. This specific viral binding to cell surface GAG provides the possibility for the interaction of heparin with SARS-CoV-2 by competing with HS (Fig. 4 ). In the direct binding assay, heparin was found to bind more tightly with both the monomeric (equilibrium dissociation constant, KD = 40 pM) and trimeric (KD = 73 pM) SARS-CoV-2 spike, compared to that of SARS-CoV (KD = 500 nM) and MERS-CoV SGPs (KD = 1 nM) (Kim et al., 2020). It has also been reported that a conformational change of SARS-CoV-2 S1 RBD is induced upon heparin binding. The basic amino acids constituting heparin binding domains are solvent accessible on the protein surface and can form a continuous patch for heparin binding (Mycroft-West, Su, Li, et al., 2020). Guimond et al. (Guimond et al., 2020) observed similar results in HS mimetic PG545, which also bind directly to SARS-CoV-2 S1 RBD and altered its conformation. It has been demonstrated that heparanase can promote viral infection and spread (Hadigal et al., 2015). Heparin, LMWH and non-anticoagulant heparin can inhibit the enzyme activity of heparinase, thereby inhibiting viral export and spread (Nasser et al., 2006).
Fig. 4.
Schematic illustration of antiviral effect of heparin. Under normal conditions, SARS-CoV-2 utilizes ACE-2 as the major receptor and HSPG as a co-receptor to infect the cells. Heparin competes with HSPG for virus binding, inhibiting the attachment of SARS-CoV-2 to the cell surface and thereby decreasing viral entry.
Underlying the high binding affinity between heparin and SARS-CoV-2 SGP, the properties of heparin itself plays an important role. It was found that the degree and position of sulfate in heparin influence its binding to SARS-CoV-2. In a competitive SGP binding study, the IC50 values of immobilized heparin, trisulfated HS (NS-2S-6S) and LWMH were 0.056 μM, 0.12 μM and 26.4 μM, respectively. Both heparin and trisulfated HS are at N-, 2-O-, and 6-O-sulfation. However, heparin has a higher inhibitory activity to bind with SARS-CoV-2 SGP than trisulfated HS, which could be attributed to the additional 3-O-sulfationin heparin (O’Donnell, Kovacs, Akhtar, Valyi-Nagy, & Shukla, 2010; Shukla et al., 1999). In comparison, LMWH showed the lowest affinity for SGP binding, indicating that the chain length could be critical in this binding process (Kim et al., 2020). Tandon et al. (Tandon et al., 2020) observed a similar result. They pseudotyped SARS-CoV-2 SGP on a third-generation lentiviral (pLV) vector and investigated the effect of heparin on transduction efficiency in HEK293 T cells. The concentration-response curves displayed that the IC50 value in neutralizing pLV-S particles in UFH (599 ng/L) was significantly lower than enoxaparin, a type of LMWH (108 μg/L). Moreover, heparin’s inhibition of SARS-CoV-2 infection displays a concentration-dependent manner. When at a concentration of 100 μg/mL, heparin was capable of inhibiting 70% of invasion to Vero cells by SARS-CoV-2 (Mycroft-West, Su, Pagani, et al., 2020).
6. Challenges in heparin therapy
Several adverse effects of heparin therapy are related with the wide biological activities of heparin, posing considerable challenges (Alban, 2012). Bleeding is the major safety concern of heparin use (Schulman, Beyth, Kearon, & Levine, 2008). Despite the fact that the bleeding incidence is hard to define, various factors could increase the risk. The elderly and patients with renal insufficiency are typical independent baseline indicators of bleeding in all patients receiving antithrombotic therapy (Bounameaux & Perrier, 2008). In addition, recent trauma or bleeding, surgery, long hospital stay, anemia, cancer, pulmonary embolism and elevated cardiac biomarkers are also associated with heparin induced bleeding (Alban, 2012). Apart from bleeding, heparin-induced thrombocytopenia (HIT) has been a recurrent concern. HIT is an immunological side effect and usually occurs in the second week of heparin therapy (Warkentin, 2015). The pathogenesis of HIT is caused by the binding of heparin to platelet factor 4 (PF4) and the induction of platelet-activating IgG antibodies (Chong, Pitney, & Castaldi, 1982). As a result of the antibody-mediated platelet activation, massive thrombin generation is induced, thereby enhancing the risk of thrombosis (Theodore E Warkentin, 2003). HIT also induces skin lesions, which typically, occurs at heparin injection sites, while some skin necrosis also occurs at remote sites (Hirsh et al., 2001). Systemic allergic and anaphylactic immune responses to heparin have been described more than 60 years ago (Bernstein, 1956). However, these immediate-type reactions are rare under current practice, with the improved pharmaceutical quality of heparin and application of LMWH. Osteoporosis is one of the most common severe side effects of long-term UFH use, with a 2.2–5% incidence of heparin-induced osteoporotic fracture (Dahlman, 1993). The risk of LWMH for osteoporosis is lower than that of UFH, but should be noticed in pregnant women, elderly and children (Rajgopal, Bear, Butcher, & Shaughnessy, 2008; Templier & Rodger, 2008).
In contrast to the beneficial effects of heparin in COVID-19 patients, unfortunately, HIT has been reported. Riker et al. (Riker et al., 2020) reported three cases of thrombocytopenia with anti-PF4 antibodies among the 16 intubated COVID-19 patients with respiratory distress syndrome, where one case was confirmed to be HIT according to the results of a serotonin release assay. The authors recommended to monitor platelet counts during heparin therapy, facilitating the early recognition of HIT. Bleeding was also observed in COVID-19 patients who received heparin as an anticoagulation therapy. Musoke et al. (Musoke et al., 2020) reported that 20 of 80 patients had developed bleeding events and ICU patients accounted for 45% of these events. Thirty percent of these bleeding events were from gastrointestinal sources while 30% of the events were in other sites including pulmonary, intraabdominal and retroperitoneal. According to the ISTH guidelines (Thachil et al., 2020), if bleeding is developed, similar principles to septic coagulopathy with respect to blood transfusions may be followed (Wada et al., 2013). Antifibrinolytic agents could be used for patients who present a hyperfibrinolytic state such as trauma (moderate quality) or leukemia (low quality). It should be noted that the bleeding patients should keep a platelet count >50 × 109/L, fibrinogen > 1.5 g/L and PT ratio <1.5 (not the same as INR). Heparin resistance, defined as the requirement of extremely high doses of heparin to achieve the target aPTT ratio, was found in COVID‑19 patients under intensive care. In a cohort of 69 patients in an intensive care unit, 15 of them received either UFH or LMWH for anticoagulant therapy. 80% (8/10) of UFH-treated patients were heparin resistant while 100% (5/5) of LMWH-treated patients displayed a sub-optimal peak anti-Xa (White et al., 2020). Robert et al. (Beun1, Kusadasi, Sikma1, Westerink, & Huisman, 2020) observed a similar phenomenon. All of the patients (4/75) receiving therapeutic anticoagulation necessitated a very high dose of UFH to realize the perceived adequate coagulation. Factor VIII was found to be remarkably elevated in these SARS-CoV-2 patients. As high level of factor VIII is a common reason for heparin resistance, this may be the critical cause of heparin resistance in COVID-19 patients.
7. Summary and perspectives
The rapid spread of COVID-19 poses a great threat to global health authorities as no effective antiviral drugs or vaccines are currently available. Heparin, a well-tolerated anticoagulant drug, has been used clinically for over 80 years with excellent bioactivity, stability and pharmacokinetic profiles. In this review, we comprehensively discussed the potential of heparin therapy for COVID-19. With multi-functions including its anticoagulant, anti-inflammatory and antiviral activities, heparin may contribute to the management of COVID-19 severity. There are both preclinical evidence and clinical data to demonstrate the benefits of heparin therapy for SARS-CoV-2 infection. With anticoagulant and anti-inflammatory effects, heparin can offer supportive treatment and alleviate the systematic symptoms of COVID-19. Notably, under the current status of lacking effective antiviral agents and vaccines, heparin shows direct antiviral efficiency by inhibiting viral entry. However, considerable safety concerns including bleeding, HIT, skin lesions and osteoporosis set a critical concern to heparin therapy. Heparin resistance was also found in COVID-19 patients. Moreover, except the anticoagulant index, heparin has not been approved as a direct anti-inflammatory or antiviral agent.
As the clinical benefits and safety concerns exist side by side, efforts should be concentrated on maximizing the therapeutic effects while minimizing the adverse effects of heparin. More detailed preclinical research should be conducted to clarify the mechanisms of heparin’s function, which would provide theoretical support to develop effective solutions for the safety and effectivity of heparin in COVID-19 therapy. Meanwhile, more clinical trials could be performed to give greater clinical evidence and an improved understanding on the anti-inflammatory and antiviral effects of heparin. With deliberate attempts and comprehensive investigation, heparin therapy can be optimized for various medical applications beyond its traditional role as an anticoagulation agent. Under the current status of the COVID-19 pandemic, the multifunctional heparin could be a promising candidate in the treatment of COVID-19.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [81901884, 81603037]; Natural Science Foundation of Hubei Province [2019CFB490]; Swedish Research Council [2020-05759]. We appreciate editorial suggestions from Professor Robert G Gilbert (The University of Queensland and Yangzhou University).
References
- Alban S. In: Heparin - a century of progress. Lever R., Mulloy B., Page C.P., editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. Adverse effects of heparin; pp. 211–263. [Google Scholar]
- Atallah J., Khachfe H.H., Berro J., Assi H.I. The use of heparin and heparin-like molecules in cancer treatment: a review. Cancer Treatment and Research Communications. 2020;24 doi: 10.1016/j.ctarc.2020.100192. [DOI] [PubMed] [Google Scholar]
- Baba M., Pauwels R., Balzarini J., Arnout J.D., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(16):6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baytas S.N., Linhardt R.J. Advances in the preparation and synthesis of heparin and related products. Drug Discovery Today. 2020 doi: 10.1016/j.drudis.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Gotte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., et al. Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry. 1999;68(1):729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bernstein I.L. Anaphylaxis to heparin sodium; report of a case, with immunologic studies. JAMA. 1956;161(14):1379–1381. doi: 10.1001/jama.1956.62970140005009b. [DOI] [PubMed] [Google Scholar]
- Beun1 R., Kusadasi N., Sikma1 M., Westerink J., Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. International Journal of Laboratory Hematology. 2020;42(Suppl. 1):19–20. doi: 10.1111/ijlh.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. Journal of the American College of Cardiology. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J.R., Schuksz M., Esko J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Boackle R.J., Caughman G.B., Vesely J., Medgyesi G., Fudenberg H.H. Potentiation of factor H by heparin: A rate-limiting mechanism for inhibition of the alternative complement pathway. Molecular Immunology. 1983;20(11):1157–1164. doi: 10.1016/0161-5890(83)90139-6. [DOI] [PubMed] [Google Scholar]
- Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vascular Medicine. 1998;3:41–46. doi: 10.1177/1358836X9800300109. [DOI] [PubMed] [Google Scholar]
- Bounameaux H., Perrier A. Duration of anticoagulation therapy for venous thromboembolism. Hematology. 2008;2008(1):252–258. doi: 10.1182/asheducation-2008.1.252. [DOI] [PubMed] [Google Scholar]
- Carter C.J., Kelton J.G., Hirsh J., Gent M. Relationship between the antithrombotic and anticoagulant effects of low molecular weight heparin. Thrombosis Research. 1981;21:169–174. doi: 10.1016/0049-3848(84)90045-8. [DOI] [PubMed] [Google Scholar]
- Casu B., Lindahl U. Advances in Carbohydrate Chemistry and Biochemistry. Academic Press; 2001. Structure and biological interactions of heparin and heparan sulfate; pp. 159–206. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu H., Liu W., Liu J., Liu K., Shang J., et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong B.H., Pitney W.R., Castaldi P.A. Heparin-induced thrombocytopenia: association of thrombotic complications with heparin-dependent IgG antibody that induces thromboxane synthesis and platelet aggregation. Lancet. 1982;320(8310):1246–1249. doi: 10.1016/s0140-6736(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Conrad H.E. Academic; San Diego: 1997. Heparin-Binding Proteins. [Google Scholar]
- Dahlman T.C. Osteoporotic fractures and the recurrence of thromboembolism during pregnancy and the puerperium in 184 women undergoing thromboprophylaxis with heparin. American Journal of Obstetrics and Gynecology. 1993;168(4):1265–1270. doi: 10.1016/0002-9378(93)90378-v. [DOI] [PubMed] [Google Scholar]
- DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeno J.M., Fernandez-Delgado R., Fett C., et al. Inhibition of NF-kappaB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. Journal of virology. 2014;88(2):913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant Z., Timmers M.C., Veen H. v. d., Page C.P., Meer F. J. v. d., Sterk P.J. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. American Journal of Respiratory and Critical Care Medicine. 1996;153(6):1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nature Reviews Immunology. 2013;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. Journal of Clinical Investigation. 2001;108(2):169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia B.L., Lord M.S., Melrose J., Whitelock J.M. The role of heparan sulfate in inflammation, and the development of biomimetics as anti-inflammatory strategies. Journal of Histochemistry Cytochemistry. 2018;66(4):321–336. doi: 10.1369/0022155417740881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R.J., Haudenschild C.C., Taylor S. Angiogenesis Inhibition and Tumor Regression Caused by Heparin or a Heparin Fragment in the Presence of Cortisone. Science. 1983;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Gao H., Lin Y., He J., Zhou S., Liang M., Huang C., et al. Role of heparan sulfate in the Zika virus entry, replication, and cell death. Virology. 2019;529:91–100. doi: 10.1016/j.virol.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li T., Han M., Li X., Wu D., Xu Y., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. Journal of Medical Virology. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M.J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. Journal of Biological Chemistry. 1982;257(13):7360–7365. [PubMed] [Google Scholar]
- Grimmer B., Kuebler W. The endothelium in hypoxic pulmonary vasoconstriction. Journal of Applied Physiology. 2017;123:1635–1646. doi: 10.1152/japplphysiol.00120.2017. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond S.E., Mycroft-West C.J., Gandhi N.S., Tree J.A., Buttigieg K.R., Coombes N., et al. Pixatimod (PG545), a clinical-stage heparan sulfate mimetic, is a potent inhibitor of the SARS-CoV-2 virus. bioRxiv. 2020 2020.2006.2024.169334. [Google Scholar]
- Gupta N., Zhao Y.-Y., Evans C. The stimulation of thrombosis by hypoxia. Thrombosis Research. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- Hadigal S.R., Agelidis A.M., Karasneh G.A., Antoine T.E., Yakoub A.M., Ramani V.C., et al. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nature Communications. 2015;6(1):6985. doi: 10.1038/ncomms7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Najjam S., Gordon M.Y., Gibbs R.V., Rider C.C. IL-12 Is a heparin-binding cytokine. Journal of Immunology. 1999;162(2):1064–1070. [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Raschke R. Heparin and low-molecular-weight heparin : the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):188–203. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Warkentin T.E., Shaughnessy S.G., Anand S.S., Halperin J.L., Raschke R., et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(Suppl):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- Ho K.M., Tan J.A. Stratified Meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003–1020. doi: 10.1161/CIRCULATIONAHA.113.002690. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleineweber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A.L., Taylor T.H., Miller J.D., Groveman D.S., Eccles E.H., Zacharski L.R. Inhibition of HIV-1 infectivity by low molecular weight heparin. International Journal of Clinical and Laboratory Research. 1996;26(2):124–131. doi: 10.1007/BF02592355. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Pauwels R., De Clercq E., Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Research. 1987;7(6):361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Kim S.-Y., Jin W., Sood A., Montgomery D., Grant O., Fuster M., et al. Glycosaminoglycan binding motif at S1/S2 proteolytic cleavage site on spike glycoprotein may facilitate novel coronavirus (SARS-CoV-2) host cell entry. bioRxiv. 2020 doi: 10.1101/2020.04.14.041459. [DOI] [Google Scholar]
- Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., et al. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.F., Barnes G.D., Streiff M.B. Unfractionated Heparin and Low Molecular-Weight Heparin. Springer International Publishing AG; 2018. Anticoagulation therapy. [Google Scholar]
- Lau S.K.P., Lau C.C.Y., Chan K., Li C.P.Y., Chen H., Jin D., et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. Journal of General Virology. 2013;94(12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- Lee S.G., Fralick M., Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li L., Shi Y., Yu S., Ma X. Different signaling pathways involved in the anti-inflammatory effects of unfractionated heparin on lipopolysaccharide-stimulated human endothelial cells. Journal of Inflammation. 2020;17 doi: 10.1186/s12950-020-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Ma Y., Chen T., Tang J., Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced expression of chemokines in human endothelial cells through nuclear factor-KappaB signaling pathway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016;28(2):117–121. doi: 10.3760/cma.j.issn.2095-4352.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerging Microbes & Infections. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Backstrom G., Thunberg L., Leder I.G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Li J.P. Heparin-an old drug with multiple potential targets in Covid-19 therapy. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.14898(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clinica Chimica Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Qiao H., He C., Yang Q., Cheung C.H.A., Kanwar J.R., et al. Modulating the interaction of CXCR4 and CXCL12 by low-molecular-weight heparin inhibits hepatic metastasis of colon cancer. Investigational New Drugs. 2012;30(2):508–517. doi: 10.1007/s10637-010-9578-0. [DOI] [PubMed] [Google Scholar]
- Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451(7181):914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm Lyon J.T.G. Bio-specific sequencesand domainsin heparan sulphate and the regulation of cell growth and adhesion. Matrix Biology. 1998;17:485–493. doi: 10.1016/s0945-053x(98)90096-8. [DOI] [PubMed] [Google Scholar]
- Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P., Térésa B., Jean-Pascal H., Glycobiology H.J.-M.J. A unique trisaccharide sequence in heparin mediates the early step of antithrombin III activation. Glycobiology. 1997;7(3):323–327. doi: 10.1093/glycob/7.3.323-e. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli G.J., Groce J.B. Pharmacological and clinical differences between low-molecular-weight heparins implications for prescribing practice and therapeutic interchange. Physical Therapy. 2010;35(2):95–105. [PMC free article] [PubMed] [Google Scholar]
- Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. Journal of Virology. 2014;88(22):13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modhiran N., Gandhi N.S., Wimmer N., Cheung S., Stacey K., Young P.R., et al. Dual targeting of dengue virus virions and NS1 protein with the heparan sulfate mimic PG545. Antiviral Research. 2019;168:121–127. doi: 10.1016/j.antiviral.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Advances in Pharmacological Sciences. 2015;2015 doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery R.S., Rider C.C. Characterization of the heparin-binding properties of IL-6. Journal of Immunology. 2000;165(10):5671–5679. doi: 10.4049/jimmunol.165.10.5671. [DOI] [PubMed] [Google Scholar]
- Musoke N., Lo K.B., Albano J., Peterson E., Bhargav R., Gul F., et al. Anticoagulation and bleeding risk in patients with COVID-19. Thrombosis Research. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycroft-West C.J., Su D., Li Y., Guimond S.E., Rudd T.R., Elli S., et al. SARS-CoV-2 Spike S1 Receptor Binding Domain undergoes Conformational Change upon Interaction with Low Molecular Weight Heparins. bioRxiv. 2020 doi: 10.1101/2020.04.29.068486. [DOI] [Google Scholar]
- Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Guimond S.E., et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the surface protein (spike) S1 receptor binding domain with heparin. bioRxiv. 2020 doi: 10.1101/2020.04.28.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser N.J., Sarig G., Brenner B., Nevo E., Goldshmidt O., Zcharia E., et al. Heparanase neutralizes the anticoagulation properties of heparin and low-molecular-weight heparin. Journal of Thrombosis and Haemostasis. 2006;4(3):560–565. doi: 10.1111/j.1538-7836.2006.01792.x. [DOI] [PubMed] [Google Scholar]
- Negri E.M., Piloto B., Morinaga L.K., Jardim C.V.P., Lamy S.A.E.-D., Ferreira M.A., et al. Heparin therapy improving hypoxia in COVID-19 patients - a case series. medRxiv. 2020 doi: 10.1101/2020.04.15.20067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C.D., Kovacs M., Akhtar J., Valyi-Nagy T., Shukla D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology. 2010;397(2):389–398. doi: 10.1016/j.virol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S.T., Srinivasan K.R., Bjork I., J.D.J.J.o.B.C. Shore Binding of high affinity heparin to antithrombin III. Stopped flow kinetic studies of the binding interaction. Journal of Biological Chemistry. 1981;256(21):11073–11079. [PubMed] [Google Scholar]
- Organization W.H. Interim guidance. World Health Organization; 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected.https://www.who.int/docs/defaultsource/coronaviruse/clinical-management-ofnovel-cov.pdf.: 28 January 2020. Available at: [Google Scholar]
- Pedersen S., Ho Y.-C. SARS-CoV-2: a storm is raging. Journal of Clinical Investigation. 2020;130(5):2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson E., Nilssonehle P. Release of lipoprotein lipase and hepatic lipase activities. Effects of heparin and a low molecular weight heparin fragment. Scandinavian Journal of Clinical Laboratory Investigation. 1990;50(1):43–49. doi: 10.1080/00365519009091563. [DOI] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoola V.O., Tavakoli F., Lau B.D., Lankiewicz M., Ross P.A., Kraus P.S., et al. Exploring the impact of route of administration on medication acceptance in hospitalized patients: Implications for venous thromboembolism prevention. Thrombosis Research. 2017;160:109–113. doi: 10.1016/j.thromres.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Qiu S., Wang N., Zhang L.-q., Qian Y. Rational use and pharmaceutical care of anticoagulant drugs in patients with COVID-19. Central South Pharmacy. 2020;18 [Google Scholar]
- Rabenstein D.L. Heparin and heparan sulfate: structure and function. Natural Product Reports. 2002;19(3):312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Frontiers in Immunology. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgopal R., Bear M., Butcher M.K., Shaughnessy S.G. The effects of heparin and low molecular weight heparins on bone. Thrombosis Research. 2008;122(3):293–298. doi: 10.1016/j.thromres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W.R., et al. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Research and Practice in Thrombosis and Haemostasis. 2020;4(5):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J.J.I.C.M. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara C.S. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. %J Current pharmaceutical biotechnology. Current Pharmaceutical Biotechnology. 2011;9(12):1481–1496. doi: 10.2174/138920111798281171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S., Lamanna W.C., Esko J.D. Heparan sulfate proteoglycans. Cold Spring Harbor PerspectivesinBiology. 2011;3(7) doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Anindita P.D., Ito N., Sugiyama M., Carr M., Fukuhara H., et al. The role of heparan sulfate proteoglycans as an attachment factor for rabies virus entry and infection. Journal of Infectious Diseases. 2018;217(11):1740–1749. doi: 10.1093/infdis/jiy081. [DOI] [PubMed] [Google Scholar]
- Schulman S., Beyth R.J., Kearon C., Levine M. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6):2–7. doi: 10.1378/chest.08-0674. [DOI] [PubMed] [Google Scholar]
- Shi C., Wang C., Wang H., Yang C., Cai F., Zeng F., et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective corhort study. Clinical and Translational Science. 2020 doi: 10.1111/cts.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D., Liu J., Blaiklock P., Shworak N.W., Bai X., Esko J.D., et al. A Novel Role for 3-O-Sulfated Heparan Sulfate in Herpes Simplex Virus 1 Entry. Cell. 1999;99(1):13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Sinha P., Matthay M.A., Calfee C.S. Is a "cytokine storm" relevant to COVID-19? JAMA Internal Medicine. 2020 doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- Tamhankar M., Gerhardt D.M., Bennett R.S., Murphy N., Jahrling P.B., Patterson J.L. Heparan sulfate is an important mediator of Ebola virus infection in polarized epithelial cells. Virology Journal. 2018;15(1):135. doi: 10.1186/s12985-018-1045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Tumkosit U., Nakamura S., Motooka D., Kishishita N., Priengprom T., et al. Genome-wide screening uncovers the significance of N-sulfation of heparan sulfate as a host cell factor for Chikungunya virus infection. Journal of Virology. 2017;91(13):1–22. doi: 10.1128/JVI.00432-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T.J.I. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Tandon R., Sharp J.S., Zhang F., Pomin V.H., Ashpole N.M., Mitra D., et al. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. bioRxiv. 2020 doi: 10.1101/2020.06.08.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templier G.L., Rodger M.A. Heparin-induced osteoporosis and pregnancy. Current Opinion in Pulmonary Medicine. 2008;14(5):403–407. doi: 10.1097/MCP.0b013e3283061191. [DOI] [PubMed] [Google Scholar]
- Thachil J. The versatile heparin in COVID-19. Journal of Thrombosis and Haemostasis. 2020;18(5):1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. Journal of Thrombosis and Haemostasis. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology. 2020 doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrij A.A., Janssen J.M., Schoon E.J., De Bruine A.P., Hemker H.C., R.W.J.S.J.o.G. tockbrugger Low molecular weight heparin treatment in steroid refractory ulcerative colitis: clinical outcome and influence on mucosal capillary thrombi. Scandinavian Journal of Gastroenterology. 2001;234(234):41–47. doi: 10.1080/003655201753265091. [DOI] [PubMed] [Google Scholar]
- Wada H., Thachil J., Di Nisio M., Mathew P., Kurosawa S., Gando S., et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. Journal of Thrombosis and Haemostasis. 2013;11(4):761–767. doi: 10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin T.E. Heparin‐induced thrombocytopenia. Annual Review of Medicine. 2003;40(7):31–44. doi: 10.1146/annurev.me.40.020189.000335. [DOI] [PubMed] [Google Scholar]
- Warkentin T.E. Heparin-induced thrombocytopenia. Current Opinion in Critical Care. 2015;21(6) doi: 10.1097/MCC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Weiss R.J., Esko J.D., Tor Y. Targeting heparin and heparan sulfate protein interactions. Organic & Biomolecular Chemistry. 2017;15(27):5656–5668. doi: 10.1039/c7ob01058c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., MacDonald S., Bull T., Hayman M., de Monteverde-Robb R., Sapsford D., et al. Heparin resistance in COVID-19 patients in the intensive care unit. Journal of Thrombosis and Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019pneumonia in Wuhan, China. JAMA Internal Medicine. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Ji Y., Su N., Mei X., Wang Y., Du S., et al. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydrate Polymers. 2017;160:71–81. doi: 10.1016/j.carbpol.2016.12.037. [DOI] [PubMed] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. International Journal of Antimicrobial Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., A.S.J.I.C.M. Slutsky Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine. 2020;46(5) doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Feng X., Zhang D., Jiang C., Mei H., Wang J., et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- Zhang T., Liu X., Li H., Wang Z., Chi L., Li J.P., et al. Characterization of epimerization and composition of heparin and dalteparin using a UHPLC-ESI-MS/MS method. Carbohydrate Polymers. 2019;203:87–94. doi: 10.1016/j.carbpol.2018.08.108. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu X., Chen J., Su H., Luo Q., Ye J., et al. Heparin sulphate d-glucosaminyl 3-O-sulfotransferase 3B1 plays a role in HBV replication. Virology. 2010;406(2):280–285. doi: 10.1016/j.virol.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]