Abstract
Introduction
The periosteum has a bilayered structure that surrounds cortical bone. The outer layer is rich in connective tissue and fibroblasts, while the inner layer in contact with the cortical surface of the bone predominantly consists of osteoblasts and osteoblast progenitors. The identification of cell-specific surface markers of the bilayered structure of the periosteum is important for the purpose of tissue regeneration.
Materials and methods
We investigated the expression of the discoidin domain tyrosine kinase receptor DDR2, fibroblast specific protein-1 (FSP-1) and alkaline phosphatase (ALP) in the periosteum of cortical bone by immunohistochemistry. Osteogenic differentiation was compared between DDR2- and FSP-1-expressing cells flow-sorted from the periosteum.
Results
We showed that DDR2 predominantly labeled osteogenic cells residing in the inner layer of the periosteum and that Pearson’s coefficient of colocalization indicated a significant correlation with the expression of ALP. The mineralization of DDR2-expressing osteogenic cells isolated from the periosteum was significantly induced. In contrast, FSP-1 predominantly labeled the outer layer of periosteal fibroblasts, and Pearson’s coefficient of colocalization indicated that FSP-1 was poorly correlated with the expression of DDR2 and ALP. FSP-1-expressing periosteal fibroblasts did not exhibit osteogenic differentiation for the induction of bone mineralization.
Conclusion
DDR2 is a novel potential cell surface marker for identifying and isolating osteoblasts and osteoblast progenitors within the periosteum that can be used for musculoskeletal regenerative therapies.
Keywords: Osteoblasts, DDR2, FSP-1, Periosteum, Alkaline phosphatase
Introduction
The periosteum is a dense bilayered connective tissue that surrounds cortical bone [1]. The inner layer of the periosteum, also known as the cambial layer, is rich in osteoblasts and osteoblast progenitors, while the outer layer of the periosteum is rich in fibroblasts [2]. The periosteum plays a critical role in de novo bone formation and fracture repair [2]. Following a bone fracture, the periosteum overlying the injured site expands, and mesenchymal progenitors and osteoblasts are activated to invade the fracture site and form a callus that later ossifies to form new bone [3–5]. The periosteum is also widely recognized as a target for anabolic agents such as sex steroids and intermittently administered parathyroid hormone [6]. The impressive ability of the periosteum to regenerate new bone coupled with its reservoir of progenitor cells and osteoblasts has generated immense enthusiasm regarding the periosteum as a cellular source for tissue engineering and musculoskeletal regeneration [7–9]. There are a number of advantages of using periosteal cells for the purposes of tissue engineering and regeneration. First, the periosteum covers the surface of long bones except for where tendons and muscles attach and can be easily excised using minimal surgical exploration from a readily accessible anatomic site [10]. Second, periosteal osteogenic cells exhibit higher proliferation rates than those of osteoblasts or progenitor cells harvested from cortical or trabecular bone and thus are readily amenable for ex vivo expansion prior to cell therapy or tissue engineering [11]. Finally, the multipotent characteristics of isolated periosteal osteoprogenitors are equivalent to those of bone marrow-derived stromal cells [12]. Despite the scientific rationale and enthusiasm for using periosteal osteoblasts for tissue engineering and regeneration [2, 10], no specific markers exist for identifying and isolating periosteal osteogenic cells.
Discoidin domain tyrosine kinase receptor 2 (DDR2), a membrane-bound tyrosine kinase receptor, is activated by fibrillar collagens and stimulates osteoblast differentiation and bone formation through both ERK1/2 and p38 MAP kinase signaling [13–16]. Human DDR2 mutations cause spondylo-meta-epiphyseal dysplasia (SMED) [17], and mice harboring deletions in the DDR2 locus have a SMED-like phenotype characterized by dwarfism and a reduction in total bone mineral density [18]. DDR2slie/slie cells exhibit defective osteoblast differentiation and accelerated adipogenesis[19]. Furthermore, it was shown that DDR2 controls the expression of bone markers and osteogenic differentiation and chondrocyte maturation via modulation of Runx2, master transcription factor activation [20]. Recently, research showed that DDR2 functions as an inhibitor of osteoclastogenesis and is a potential therapeutic target in osteoporosis [21].However, whether DDR2 itself can be served as a marker for periosteal osteoblasts and osteoblast progenitor has not been reported.
Here, we report that unexpectedly, the bilayered structure of the bone periosteum can be predominately labeled by two cell surface markers, namely, discoidin domain tyrosine kinase receptor 2 (DDR2) and fibroblast specific protein-1 (FSP-1). These newly identified cell surface markers can facilitate the isolation of different cellular components of the periosteum for tissue engineering or regenerative purposes.
Materials and methods
Reagents
Goat anti-DDR2 antibody (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA), rabbit anti-DDR2 antibody (Genetex, Inc. CA, USA), ALP (alkaline phosphatase) antibody (R&D systems, Inc., Minneapolis, MN) and FSP-1 (R&D systems, Inc., Minneapolis, MN) were purchased from respective vendors. ALP activity staining kit was purchased from Sigma.
Immunohistochemistry and immunofluorescence staining
All animal studies were approved by the Institutional Animal Care and Use Committee of the Guangzhou Medical University (the acceptance number: 2016-025) and all animal procedures conform to the National Institutes of Health (NIH) guidelines. Ribs and femurs were harvested from C57bl/6 mice and frozen in OCT. Cryosections (7-μm) were fixed in acetone for 10 min at − 20 °C followed by staining for DDR2, FSP-1 and ALP according to the manufacturer’s instructions (Vector Labs ABC kit, Burlingame, CA, USA) or standard immunofluorescence staining. A Leica SP2 AOBS upright laser scanning confocal microscope was used to obtain images. Then, co-localization analysis of confocal images was performed by the plugin called Just Another Colocalization Plugin or JACoP of the Image J software (NIH) according to the instructions of the software.
Isolation of periosteal osteoblasts from the periosteum
We chose ribs as an example of cortical bone because ribs can be easily cut for cryosections. Periosteal osteoblasts and osteogenic cells were isolated adapting techniques described by Arnsdorf et al. [10]. Ribs were removed from the chest wall and the overlying muscle gently removed by blunt dissection. The ribs were cut into 1 mm3 pieces, gently treated with Collagenase II and washed to remove bone marrow and subsequently plated in T25 tissue culture flasks with DMEM supplemented by 10% fetal bovine serum [22]. Following 7 days of culture, sub-confluent cells were observed to migrate from the leading edges of the cut bone pieces. The bone pieces were discarded and the cells were subsequently used for further experiments without further passaging.
Flow cytometry
Indirect flow cytometry was done to sorting DDR2- and FSP-1-expressing cells isolated from the periosteum. Cells were incubated with the primary antibody DDR2 and FSP-1 for 30 min at 4 °C, followed by staining with fluorescent conjugated secondary antibodies Anti-Rabbit IgG Alexa Fluor 647 (Jackson, Philadelphia, PA, USA) and anti-Rat IgG DyLight 488 (Invitrogen, USA). The cells subjected to flow cytometry without the addition of a primary antibody or secondary antibody served as controls.
Bone mineralization differentiation and alizarin red staining
After flow cytometry sorting, DDR2-expressing cells and FSP-1-expressing cells were plated at a density of 2.5 × 104 cells/cm2 and osteogenesis was induced using differentiation medium (a-MEM supplemented with 10% FBS, 0.1 μM dexamethasone, 10 mM β-glycerol phosphate and 50 μM l-ascorbic acid) [23]. Differentiation was visualized after 21 days by staining with alizarin red staining. In brief, 2% paraformaldehyde-fixed cells were incubated with alizarin red solutions for 15 min at room temperature. After staining, the cells were washed thrice with 1× wash buffer and the images were taken in a Leica microscope. The percentage of alizarin red staining area to high power field (HPF) were analyzed with Image pro plus 6.0 software (Media Cybernetics, USA).
Statistical analysis
Statistical analysis was performed using the GraphPad 6.0 software (GraphPad software, Inc, La Jolla, CA, USA) according to the instructions of software. For paired comparison, we used the Student’s t test (two-tailed). For multiple comparison, one-way analysis of variance (ANOVA) with Bonferroni post-hoc test analysis was applied A probability value of p = 0.05 was considered statistically significant. All results in the text are shown as mean ± SEM (standard error of the mean).
Results
Expression of DDR2 and FSP‑1 in the bilayered structure of the periosteum
The periosteum covers the external surface of cortical bones and consists of two histologically distinct layers. The outer fibrous layer comprises fibroblasts and the extracellular matrix, while the inner layer (in contact with the cortical bone surface), also known as the cambial layer, is highly cellular and predominantly consists of osteoblasts and osteoblast progenitors [2]. DDR2 is a receptor tyrosine kinase that is activated following collagen binding and plays an important role in the remodeling of extracellular matrix [13]. FSP-1 is expressed in fibroblasts in several organs and has been implicated in cell mobility and migration [24]. Periosteal osteoblasts, such as osteoblasts located elsewhere in the skeleton, express ALP (alkaline phosphatase). By performing immunohistochemistry on 7-μm parallel frozen sections of bone, we demonstrated that the inner cambial layer of the periosteum abundantly expressed ALP (Fig. 1a) and was labeled by DDR2 (Fig. 1b, c). In contrast, the outer fibrous layer of the periosteum was labeled by FSP-1 (Fig. 1d). By performing double immunofluorescence staining on frozen sections of bone, we demonstrated that 62.35 ± 1.81% (mean ± SEM) of periosteal osteoblasts that expressed DDR2 also expressed ALP (Fig. 1e, h) and that only 9.30 ± 0.60% of the FSP-1-positive cells located in the outer layer of the periosteum expressed ALP (Fig. 1f,h). Coexpression analysis of both FSP-1- and DDR2-expressing periosteal cells showed that 10.96 ± 1.96% of DDR2-expressing cells also expressed FSP-1 and that 13.59 ± 1.57% of FSP-1-expressing cells also expressed DDR2 (Fig. 1g, i). The Pearson’s coefficient indicated that DDR2 was significantly correlated with ALP (0.74 ± 0.02), while FSP-1 was poorly correlated with DDR2 (0.20 ± 0.20) and ALP (0.31 ± 0.05) (Fig. 1e–g, j).
Fig. 1.

Expression of ALP (alkaline phosphatase), DDR2 and FSP-1 in periosteum surrounding cortical bone (ribs). Outer layer of periosteum (white and black arrowhead); the inner layer of periosteum (arrow). a ALP expression determined by ALP activity in periosteum surrounding rib. Immunohistochemistry for b DDR2 (goat anti-DDR2 antibody), c DDR2 (rabbit anti-DDR2 antibody) and d FSP-1. e–g Immunofluorescence staining of the square area highlighted in a. e DDR2 and ALP staining with merged image demonstrating colocalization of fluorophores (red arrowhead). f Immunofluorescence staining of the outer layer of periosteum for FSP-1 and ALP demonstrating lack of colocalization. g Immunofluorescence staining of the junction of outer and inner layers of periosteum for FSP-1 and DDR2 demonstrating lack of colocalization of fluorophores. h The percentage of DDR2-or FSP-1-expressing cells expressed ALP in e, f. i The colocalization between DDR2- and FSP-1-positive cells in g. j Pearson’s coefficient of colocalization of ALP, DDR2 and FSP-1 in e–g. (All graphs were analyzed by image J and shown as mean ± SEM, n = 3 mice, **p < 0.01, ns not significant, using ANOVA with post hoc tests. Scale bar: 25 μm)
It is well recognized that periosteal anatomy as well as its regulation differ throughout the skeleton in a site-specific manner and that the periosteum around the femoral diaphysis is particularly thin [25]. To determine whether DDR2 and FSP-1 also label osteoblasts and fibroblasts in the periosteum over the femoral diaphysis, we analyzed the periosteum over the diaphysis region at the upper end of the femur. Immunohistochemistry demonstrated that the inner osteoblastic layer of the periosteum over the femoral diaphysis expressed ALP (Fig. 2a, b) and was intensely labeled by DDR2 (Fig. 2c, d), while the outer layer of periosteal fibroblasts expressed FSP-1 and was ALP-negative (Fig. 2a, b, e, f). These findings suggest that DDR2 and FSP-1 are specific markers of periosteal osteoblasts and fibroblasts covering cortical bone.
Fig. 2.

Similar distribution of ALP, DDR2 and FSP-1 expression in the periosteum surrounding femoral diaphysis. a Immunohistochemistry staining for ALP and bALP activity staining on bilayered-periosteum of femoral diaphysis. c–f Immunohistochemistry of DDR2 (c–d) and FSP-1 (e–f) in the similar region indicating no overlap staining between DDR2 and FSP-1. (Arrows point to the inner layer of periosteum, arrowheads point to the outer layer of periosteum, Scale bar: 100 μm). The magnification in figures a, c and e is ×100, magnification in figures b, d and f is ×400
Mineralization of DDR2‑expressing periosteal osteogenic cells can be significantly induced
To investigate the difference in osteogenic differentiation between DDR2- expressing and FSP-1-expressing periosteal osteogenic cells in vitro, we isolated periosteal osteogenic cells from the cortical bone (ribs), taking care not to expose the endosteum so that the isolation of periosteal osteogenic cells was enriched [10]. We first performed immunofluorescence staining of isolated periosteal osteogenic cells. Consistent with the immunohistochemistry results, we observed that 61.56 ± 4.88% of isolated osteogenic cells that expressed DDR2 also expressed ALP, while only 33.38 ± 4.18% of FSP-1-positive cells expressed ALP (Fig. 3a, b). Pearson’s coefficient of colocalization demonstrated a high correlation between DDR2 and ALP (0.62 ± 0.02) but a poor correlation between FSP-1 and ALP (0.19 ± 0.02) (Fig. 3c).
Fig. 3.
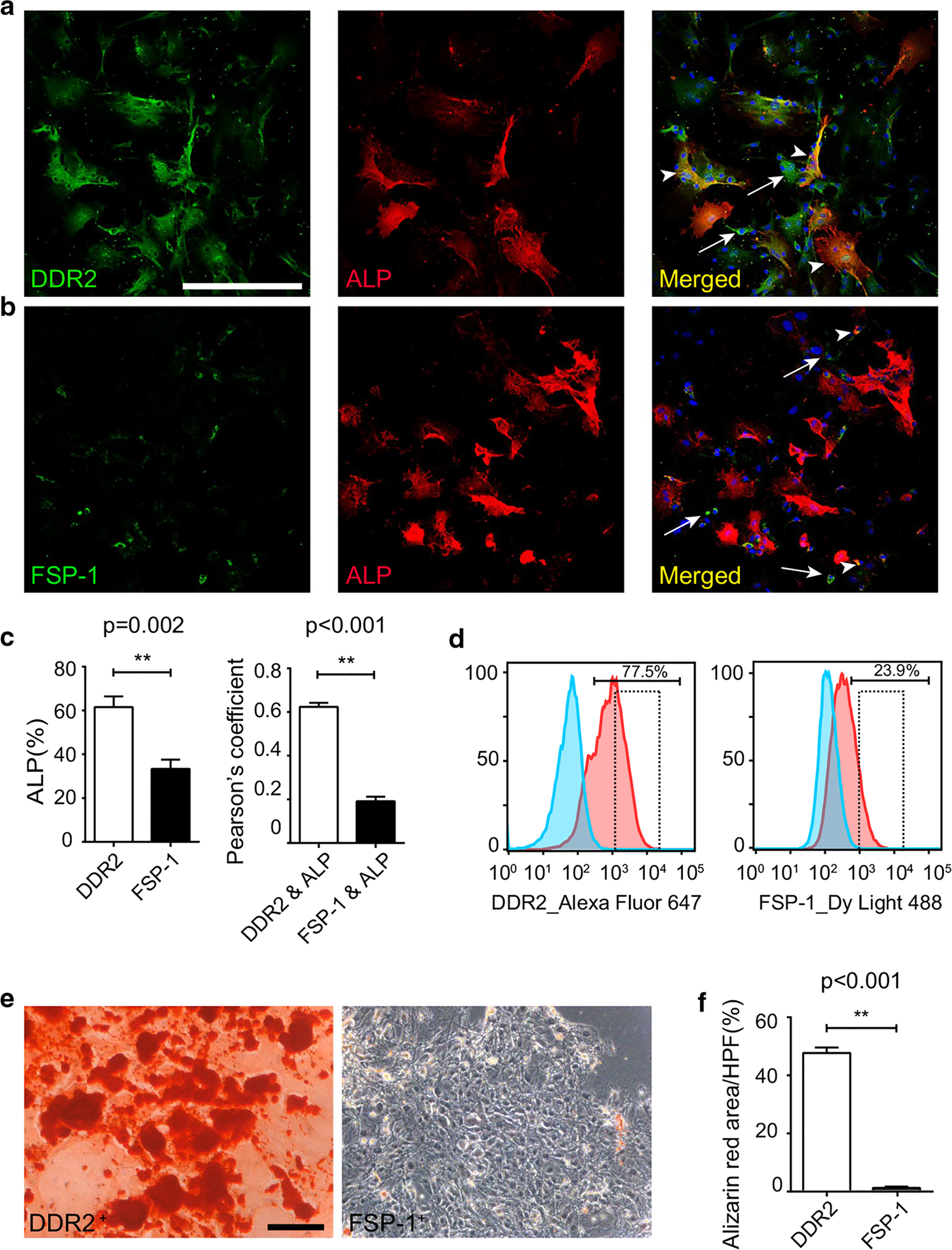
Bone mineralization differentiation of DDR2- and FSP-1-expressing Periosteal osteogenic cells. a–c double immunofluorescence staining of osteogenic cells isolated from the periosteum of ribs a DDR2 and ALP b FSP-1 and ALP c Quantitation of the colocalization in a, b (colocalization of fluorophores indicated by arrowhead; arrow points to the DDR2- or FSP-1-positive alone cells). d Flow sorting of DDR2-expressing cells and FSP-1-expressing cells from isolated periosteal osteogenic cells (dotted box showed the collected cells). e Alizarin red staining after 21 days bone differentiation of DDR2-expressing and FSP-1-expressing periosteal osteogenic cells from d, and f The percentage of alizarin red-positive area relative to the HPF (high power field) in e. (results shown as mean ± SEM; n = 7, **p < 0.01, using an unpaired t test. Scale bar: 200 μm)
We then isolated the periosteal osteogenic cells and used flow cytometry to sort DDR2- and FSP-1-expressing periosteal osteogenic cells for bone mineralization differentiation (Fig. 3d). After 21 days, we observed a significant difference in bone mineralization differentiation between DDR2-expressing cells and FSP-1-expressing cells (Fig. 3e). Clearly, almost all DDR2-expressing cells were differentiated into mineralized cells; however, few FSP-1-expressing cells were alizarin red-positive (Fig. 3e). Based on the percentage of alizarin red-positive area relative to the HPF (high power field), we analyzed and compared the degree of osteogenic differentiation ability between DDR2- and FSP-1-expressing cells. The results showed that the mineralization of 47.3 ± 1.90% of DDR2-expressing cells per HPF was induced, while the mineralization of only 0.82 ± 0.10% of FSP-1-expressing cells per HPF was induced (Fig. 3e, f). These results suggest that compared to FSP-1-expressing cells, DDR2-expressing cells have a significantly greater potential to differentiate into bone and may play a more important role in bone repair. DDR2 is a potential cell marker of periosteal osteogenic progenitors.
Discussion
The periosteum is recognized to have a population of osteoblasts and osteoblast progenitors that have regenerative potential and play a critical role in bone repair. In fact, lineage-tracing studies with bone grafts have suggested that cells of periosteal origin, rather than endosteal osteoblasts, which are in contact with the bone marrow, contribute to callus formation and fracture healing [3–5]. Despite the obvious potential of the periosteum for the purposes of cell therapy in musculoskeletal regenerative medicine, there is a lack of cell surface markers that can be used for the isolation of osteogenic cells, including periosteal osteoblasts or osteoblast progenitors, and to prevent contamination with periosteal fibroblasts. In this report, we observed in situ that the expression of ALP is significantly correlated with the expression of DDR2, but not FSP-1. The mineralization of almost 100% of isolated DDR2-expressing cells from the periosteum can be induced, while few FSP-1-expressing cells exhibit osteogenic differentiation in vitro. More Interestingly, the fluorescence signal intensity of ALP increase from the outer layer to the inner layer, and DDR2-positive cells that express ALP are also increased from the outer layer to the inner layer (Fig. 1e), which indicates that DDR2-positive cells may migrate to the cortical bone surface and the expression of ALP may represent different maturation stages of DDR2-expressing cells.
DDR2 has been reported widely regarding the function and mechanism of DDR2 in osteogenic and chondrogenic differentiation [20]. In recent years, great attention has been paid to the function and signaling of DDR2 in bone-related disease. However, DDR2 itself has not been recognized as a specific marker of periosteal osteoblasts and osteoblast progenitors. Considering our observation were firstly got by using directly frozen ribs, the possible reason may be due to both the antibodies of DDR2 and FSP-1 currently available are more sensitive to fixation and decalcification, which may cause failed Immunohistochemistry staining for the bilayer structure of bone tissue. The phenotype that DDR2 is primarily expressed in periosteal osteoblasts and osteoblast progenitor is consistent with the functional importance of DDR2 in osteogenic and chondrogenic differentiation.
Intriguingly, DDR2 is enriched in cardiac fibroblasts and serves as one of the useful markers for identifying cardiac fibroblasts [26]. Our report raises an interesting question whether DDR2+ cardiac fibroblast cells mainly contribute to the heart and valve calcification.
As this report demonstrates, periosteal fibroblasts rarely express DDR2; however, those in close proximity to the cortical bone express DDR2. Our work suggests that, in addition to its key function in bone-related diseases, DDR2 is also a marker of periosteal osteoblasts and progenitors, which can be used for the isolation and/or targeting of accurate regulation during bone formation and healing. Revealing the exact regulatory mechanism of DDR2 signaling in DDR2-expressing periosteal osteoblasts may be beneficial for effective musculoskeletal regenerative therapies.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC#81670262), Guangzhou lingnan yingjie project and the start-up funding from Guangzhou Women and Children’s Medical Center and grants HL129178, HL137241, AR075867 from the National Institues of Health, and PR190268 and PR161247 from the Department of Defense, United states of America.
Footnotes
Conflict of interest The authors report no conflict of interest.
Ethical approval All animal studies were approved by the Institutional Animal Care and Use Committee of the Guangzhou Medical University (the acceptance number:2016-025) and all animal procedures conform to the National Institutes of Health (NIH) guidelines. This article does not contain any studies with human participants performed by any of the authors.
Informed consent No informed consent needed.
References
- 1.Allen MR, Hock JM, Burr DB (2004) Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 35:1003–1012. 10.1016/j.bone.2004.07.014 [DOI] [PubMed] [Google Scholar]
- 2.Colnot C, Zhang X, Knothe Tate ML (2012) Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res 30:1869–1878. 10.1002/jor.22181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colnot C (2009) Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282. 10.1359/jbmr.081003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O’Keefe RJ, Guldberg RE (2005) Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 20:2124–2137. 10.1359/JBMR.050806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozaki A, Tsunoda M, Kinoshita S, Saura R (2000) Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci 5:64–70 [DOI] [PubMed] [Google Scholar]
- 6.Ogita M, Rached MT, Dworakowski E, Bilezikian JP, Kousteni S (2008) Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology 149:5713–5723. 10.1210/en.2008-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gastel N, Torrekens S, Roberts SJ, Moermans K, Schrooten J, Carmeliet P, Luttun A, Luyten FP, Carmeliet G (2012) Engineering vascularized bone: osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 30:2460–2471. 10.1002/stem.1210 [DOI] [PubMed] [Google Scholar]
- 8.Ferretti C, Borsari V, Falconi M, Gigante A, Lazzarini R, Fini M, Di Primio R, Mattioli-Belmonte M (2012) Human periosteum-derived stem cells for tissue engineering applications: the role of VEGF. Stem Cell Rev Rep 8:882–890. 10.1007/s12015-012-9374-7 [DOI] [PubMed] [Google Scholar]
- 9.Chang H, Knothe Tate ML (2012) Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med 1:480–491. 10.5966/sctm.2011-0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsdorf EJ, Jones LM, Carter DR, Jacobs CR (2009) The periosteum as a cellular source for functional tissue engineering. Tissue Eng Part A 15:2637–2642. 10.1089/ten.TEA.2008.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng AM, Saim AB, Tan K, Tan GH, Mokhtar SA, Rose IM, Othman F, Idrus RBH (2005) Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci 10:192–199. 10.1007/s0077-6-004-0884-2 [DOI] [PubMed] [Google Scholar]
- 12.Park J, Gelse K, Frank S, von der Mark K, Aigner T, Schneider H (2006) Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med 8:112–125. 10.1002/jgm.826 [DOI] [PubMed] [Google Scholar]
- 13.Vogel W (1999) Discoidin domain receptors: structural relations and functional implications. FASEB J 13(Suppl):S77–82. 10.1096/fasebj.13.9001.s77 [DOI] [PubMed] [Google Scholar]
- 14.Vogel W, Gish GD, Alves F, Pawson T (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1:13–23. 10.1016/s1097-2765(00)80003-9 [DOI] [PubMed] [Google Scholar]
- 15.Ruiz PA, Jarai G (2011) Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts. J Biol Chem 286:12912–12923. 10.1074/jbc.M110.143693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD (2013) The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol 15:677–687. 10.1038/ncb2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen DH, Smithson SF, Borochowitz Z, Belostotsky R, Raas-Rothschild A (2009) Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet 84:80–84. 10.1016/j.ajhg.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Mañes S, Brückner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martínez AC, Klein R (2001) The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep 2:446–452. 10.1093/embo-reports/kve094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge C, Wang Z, Zhao G, Li B, Liao J, Sun H, Franceschi RT (2016) Discoidin receptor 2 controls bone formation and marrow adipogenesis. J Bone Miner Res 31:2193–2203. 10.1002/jbmr.2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An essential role of discoidin domain receptor 2 (DDR2) in osteoblast differentiation and chondrocyte maturation via modulation of Runx2 activation—Zhang—2011. —Journal of Bone and Mineral Research—Wiley Online Library; https://asbmr.onlinelibrary.wiley.com/doi/full/10.1002/jbmr.225. Accessed 17 Mar 2020 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Su J, Wu S, Teng Y, Yin Z, Guo Y, Li J, Li K, Yao L, Li X (2015) DDR2 (discoidin domain receptor 2) suppresses osteoclastogenesis and is a potential therapeutic target in osteoporosis. Sci Signal 8:ra31 10.1126/scisignal.2005835 [DOI] [PubMed] [Google Scholar]
- 22.Bakker A, Klein-Nulend J (2003) Osteoblast isolation from murine calvariae and long bones. Methods Mol Med 80:19–28. 10.1385/1-59259-366-6:19 [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64:295–312 [PubMed] [Google Scholar]
- 24.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG (1995) Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130:393–405. 10.1083/jcb.130.2.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck TJ, Ruff CB, Scott WW, Plato CC, Tobin JD, Quan CA (1992) Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int 50:24–29. 10.1007/bf00297293 [DOI] [PubMed] [Google Scholar]
- 26.Baudino TA, Carver W, Giles W, Borg TK (2006) Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol 291:H1015–1026. 10.1152/ajpheart.00023.2006 [DOI] [PubMed] [Google Scholar]


