Abstract
Introduction The novel coronavirus disease 2019 pandemic has rapidly spread worldwide, challenging healthcare resources and communities to an unprecedent degree. Simultaneously, the amount of clinical and scientific information released has overwhelmed journal platforms.
Objectives This review aims to summarize the available diagnostic tools and current guidelines to safely assist patients while limiting the exposure of otolaryngologists during this pandemic.
Data Synthesis Key articles were retrieved from the following databases: PubMed, Lancet, Springer Nature, BioMed Central, JAMA network and MEDLINE, as well as updated documents from the Spanish Ministry of Health, World Health Organization, Centers for Disease Control and Prevention, Spanish Association of Surgeons, ENT-UK, American College of Surgeons, and American Academy of Otolaryngology-Head and Neck Surgery. The terms used for the search were: COVID-19 , Test COVID , Surgery in COVID , 2019-nCoV , ‘ coronavirus’ , and SARS-CoV-2 . A total of 10,245 papers were retrieved. The inclusion criteria for the review included: COVID-19 testing ( n = 531), society guidelines for otolaryngology-head and neck surgery patient care in the outpatient clinic ( n = 10) and surgical ( n = 18) settings. Studies not related to COVID-19 diagnosis were excluded.
Conclusion Healthcare institutions around the world are outlining their own protocols regarding laboratory testing and personnel protective equipment usage based upon medical societies recommendations during the COVID-19 pandemic. We have summarized the available laboratory tests and their respective sensitivity and specificity. Moreover, clinical guidelines from different societies were reviewed and summarized to facilitate guidance for otolaryngologists in the operating room and in the clinical settings.
Keywords: COVID-19, otolaryngology, COVID-19 testing, public health, recommendations
Introduction
Since the 16th century, at least 3 pandemics have been documented. Pandemics are characterized primarily for their abrupt onset and rapid spread, leading to great morbidity and mortality in a short period of time, overwhelming the capacity of the existing healthcare system. 1
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has challenged available healthcare resources and has imposed a high risk of exposure to healthcare workers. Otolaryngology-head and neck surgeons are amongst the highest risk specialties since they routinely work in the upper airway, where the virus is known to replicate. Therefore, the coronavirus disease 2019 (COVID-19) pandemic has forced the specialty to address the challenge of maintaining high-quality patient care, while protecting its healthcare workers. 2
During the first days of COVID-19 lockdown, most clinics, inpatient consults, and surgical procedures were halted, and only strict emergencies were seen and treated. In the meantime, medical societies around the world were discussing evidence-based information coming from the recently affected countries, to create management guidelines focused on personnel and patient safety to be implemented in their home countries. 3 This led to the public release of an immense number of guidelines and recommendations; however, the information available is difficult to read or summarize, and some may be challenging to apply in certain countries. Therefore, the present manuscript will summarize the current recommendations regarding COVID-19 diagnosis and how physicians may return to the clinic and the operating room safely. We hope this information can be used as a quick reference tool for otolaryngologists and providers of high-risk specialties.
Review
This is a literature review of the available diagnostic tools and current guidelines to safely assist patients while limiting the exposure of otolaryngologists during the COVID-19 era. Key articles were retrieved from different databases: PubMed, Lancet, Springer Nature, BioMed Central, JAMA network, MEDLINE, combined with updated documents from the Spanish Ministry of Health, World Health Organization (WHO), Centers for Disease Control (CDC), as well as the recommendations of Spanish Association of Surgeons, ENT-UK, American College of Surgeons, and American Academy of Otolaryngology Head and Neck surgery.
The terms used for the literature search were: COVID-19 , Test COVID , Surgery in COVID , 2019-nCoV , coronavirus , and SARS-CoV-2 . A total of 10,245 papers were found. The inclusion criteria for review included: COVID-19 testing ( n = 531), society guidelines for otolaryngology-head and neck surgery patient care in the outpatient clinic ( n = 10) and surgical ( n = 18) settings. Studies not related to COVID-19 diagnosis were excluded.
Discussion
Pathophysiology
The COVID-19 is an ongoing viral pandemic that emerged from East Asia and has quickly spread worldwide. 4 Human-to-human aerosol transmission is undoubtedly the main source of infection. In detail, an infected individual releases contaminated respiratory droplets when coughing, sneezing, or breathing. These droplets can directly infect the mucous membranes of healthy individuals that are in close contact to the infective source or contaminated surfaces where the virus has been shown to remain alive for a variable period of time, depending on the surface (e.g., metal, plastic, or glass). 5 Also, when a healthy individual touches recently contaminated surfaces, the virus will then contaminate his/her hands, followed by contamination of the mucous membranes when subsequently touching his/her eyes or mouth. Virus transmission from asymptomatic patients or those in the incubation period, plays a significant role in the transmissibility of the disease. 6 Therefore, the WHO has continuously promoted correct hand hygiene and social distancing as the steppingstones to control the pandemic. 7
Once the virus reaches the respiratory system, binds to angiotensin-converting enzyme 2 (ACE2) receptors present in pulmonary epithelial cells. Here, the virus replicates and promotes the release of IL-6 and IL-8, which leads to a sequence of cellular and chemical events causing a severe acute respiratory syndrome, hence its name SARS-CoV-2. These molecular events may correlate with the occurrence of silent hypoxemia in association with bilateral pulmonary ground-glass opacities in computed tomography (CT) scan images. 8 The reported incubation period of the virus is between 2 and 12 days (median 5.1 days). 9
Angiotensin-converting enzyme 2 receptors are also found in other cells, including olfactory epithelium, causing anosmia and possible virus direct transmission to the nervous system. 10 Furthermore, it has been reported that the virus binds to other receptors present in antigen-presenting cells and might be responsible for the leukopenia seen in certain patients. 8 More recently, there are reports on patients presenting with strokes, massive pulmonary embolisms, and peripheral thrombosis, suggesting a hematologic component. However, the exact pathophysiologic mechanism remains unknown. 11 At the time of submission, the WHO has reported over 315,100 deaths worldwide. 12
Clinical Presentation
Symptomatology is non-specific, and disease presentation can range from no symptoms (asymptomatic) to severe pneumonia and death. The WHO has confirmed 55,924 cases as of February 20, 2020. Typical symptoms included: fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), shortness of breath (18.6%), sore throat (13.9%), headache (13.6%), myalgia or arthralgia (14.8%), chills(11.4%), nausea or vomiting (5.0%), nasal congestion (4.8%), diarrhea (3.7%), hemoptysis (0.9%), and conjunctival congestion (0.8%). Mild respiratory symptoms and fever, usually present on average 5 to 6 days after the initial infection (range 1–14 days). 13 Involvement of the skin, gastrointestinal tract, and cardiovascular system has been seen with a severe tendency in some patients to have coagulation disorders with generalized thrombosis. 14
A high number of complications were seen in patients with underlying diseases such as diabetes, hypertension, and cardiovascular disease, who had clinical symptoms such as fever (98%), cough (76%) fatigue (44%), sputum production (28%), headache (8%), hemoptysis (5%), and diarrhea (3%). 15
Diagnostic Tests
The SARS-CoV-2 can be detected by taking samples from the upper respiratory tract. The virus can be detected 1 to 2 days before the onset of symptoms and can remain in the mucosa for 7 to 12 days in mild to moderate cases, and up to 2 weeks in severe cases. 12
Three primary test types for laboratory diagnosis of COVID-19 exist: 1) Nucleic acid detection test; 2) Antigen detection tests; and, 3) Antibody detection tests.
Nucleic Acid Detection Test
The main nucleic acid detection test is the polymerase chain reaction (PCR), of which there are multiple variations; however, the real-time PCR (RT-PCR) and its quantified version (qRT-PCR) are the most sensitive of the available tests. The qRT-PCR has become the ‘gold standard’ for diagnosis, as positive results are correlated with infectious capacity (active contagious), even in asymptomatic patients.
The RT-PCR is a diagnostic test utilizing nasal, oro or nasopharyngeal swab, tracheal aspirate or bronchoalveolar lavage (BAL) specimens. Samples collected from the upper respiratory tract, via nasopharyngeal and oropharyngeal swabs are considered the primary and preferred method for diagnosis. The use of bronchoscopy as a diagnostic method for COVID-19 is not recommended because the aerosol sprays generated pose a substantial risk for patients and healthcare staff. Bronchoscopy samples should only be considered when upper respiratory samples are negative in a patient with high pretest probability for the disease, to whom appropriate diagnosis is critical and will change clinical management. 16
Molecular tests that detect viral RNA are not as specific. Although analytical sensitivity is known to be very high, detection depends on several crucial factors, including: time of sampling related to the day of illness, sample types, correct sampling technique, sample quality, transport and storage conditions, detection kits, and gene target sequence mutations. A study in China with PCR detection concluded that BAL samples had the highest positive rates (93%), followed by sputum (72%), nasal swabs (63%), and pharyngeal swabs (32%). 17
This method may not detect the virus in the very early stages of infection (days 0–1) or at the later stages, when viral load is very low. Additionally, false-negative results can be linked to: inadequate sample collection (low quantity), deficient or delayed transportation (the cold chain is not maintained), preanalytical errors (poor labeling of the sample), and low amount of virus related to the stage (presymptomatic, asymptomatic or postsymptomatic) or severity.
False positive results have also been reported, and the primary factors associated to this scenario are: preanalytical errors in the labeling of the sample throughout the process, and cross-contamination between samples. 18
Antigen Detection Tests
Lateral flow immunochromatography (“quick” tests or “quick tests”) tests have a sensitivity of less than 30% and a specificity of less than 50%. These are not considered suitable for diagnosis at present due to insufficient sensitivity and specificity. With a very low reliability early in the disease, there is insufficient evidence to position them as a useful technique despite the speed with which the results are obtained (15–30 minutes). Methodologies based on enzyme-linked immunosorbent assay (ELISA) or chemiluminescent immunoassay (CLIA) are preferred, as they offer greater sensitivity and specificity and can be used in high volume automated systems. 19
Antibody Detection Tests
Serological tests intend to detect the presence of immunoglobulin G (IgG), immunoglobulin M (IgM), or both, on whole blood, serum, or plasma samples obtained from diseased individuals. A positive interpretation has been defined as an IgM-positive, or convalescent serum with an IgG titer that is more than four times higher than in the acute phase.
Antibodies rise late in the course of the disease. Initial detection of IgM antibodies occurs around days 5 to 7, while initial IgG detection occurs ∼ 14 days after the onset of symptoms. 20
Sensitivity for IgM and IgG tests ranges between 72.7 and 100%, while specificity ranges between 98.7 and 100%. 21 There are many antibodies tests in the market. Selection depends on not only specificity and sensitivity rates, but also on the number of tests to run (by the hour, day, week), availability and delivery possibilities by the manufacturer, and the compatibility of the test with the available equipment or technologies. In Table 1 , we provide a summary of the available serologic tests, the manufacturer, date of approval in the US, sensitivities, and specificities.
Table 1. Accuracy for eight selected COVID-19 antibody tests under the United States food and Drug Administration emergency use authorization.
| Company | Test name | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Abbott Laboratories (Abbott Park, Illinois, USA) 40 | Abbott Sars-CoV-2 IgG test | 100.0 | 99.9 |
| Roche Diagnostics (Rotkreuz, Switzerland) 41 | Elecsys anti-Sars-CoV-2 antibody test | 100.0 | 99.8 |
| Bio-Rad Laboratories (Hercules, California, USA) 42 | Platelia Sars-CoV-2 total Ab assay | 98.0 | 99.0 |
| Diasorin (Saluggia, Italy) 43 | Liaison Sars-CoV-2 S1/S2 IgG test | 97.4 | 98.5 |
| Cellex (Research Triangle Park, North Carolina, USA) 44 | Cellex qSars-CoV-2 IgG/IgM cassette rapid test | 93.8 | 95.6 |
| Autobio Diagnostics (Zhengzhou, China) 45 | Anti-Sars-CoV-2 rapid test (IgM and IgG) | 93.0 | 100.0 |
| Ortho-Clinical Diagnostics (Raritan, New Jersey, USA) 46 | Vitros anti-Sars-CoV-2 IgG reagent pack | 87.5 | 100.0 |
| Ortho-Clinical Diagnostics 47 | Vitros anti-Sars-CoV-2 total reagent pack | 100.0 | 100.0 |
Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; IgM, immunoglobulin M; Sars-CoV-2, severe acute respiratory syndrome coronavirus 2.
A useful visual guide ( Fig. 1 ) has been added to illustrate the appropriate test timing during COVID-19. It is necessary to remember that for an asymptomatic patient (responsible for the high and quick spread of the virus), the PCR test is indicated, but antigen test in symptomatic cases could be added as support for the diagnosis. 22
Fig. 1 Severe acute respiratory syndrome coronavirus 2 natural evolution and diagnosis tests.
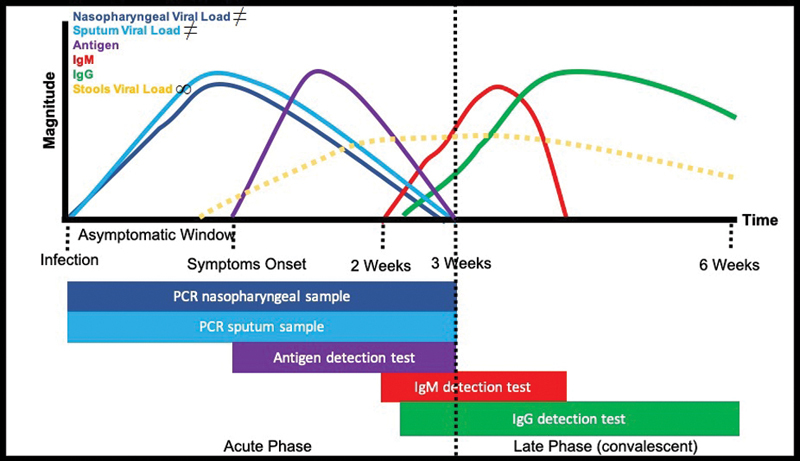
∞Viral load in stools detected by polymerase chain reaction (PCR) has an uncertain clinical significance since it could remain detectable for long time periods (not related to disease severity or evolution). The bar is not showed below to avoid possible confusion. 49 ≠For pedagogic reasons, the sputum & nasopharyngeal viral load curves “stop” at three weeks, following the natural evolution of the disease and its detection by PCR; however, it could be continuing in cases that progress to moderate, severe, or critical coronavirus disease 2019.
In the evaluation of the immune response, and for epidemiological purpose, antibody tests are useful. Currently, there is high demand of PCR testing that keeps continuously increasing as the population reincorporates into the ‘new normal’ life, and limitation in production. Therefore, antibody testing could be of clinical significance in the determination of disease resolution. 23
Recently, the US Food and Drug Administration (FDA) has granted emergency use authorization (EUA) to a new diagnostic kit based on gene-editing technology clustered regularly interspaced short palindromic repeats (CRISPR). This kit programs a CRISPR molecule to detect the specific signature for SARS-CoV-2 in nasal, nasopharyngeal, or oropharyngeal swabs, or in BAL specimens. Once a signature is found, the CRISPR enzyme activates and releases a detectable signal to the tester. According to the company, Sherlock Biosciences, an instrument-free and handheld platform is currently under development, similar to that of at-home pregnancy test. Advantages of this method include rapid one-hour result availability, and the ability to be run on equipment found in most clinical and commercial laboratories. 24
Based on the available diagnostic and follow-up methods, we present on Table 2 a proposal to interpret results in the clinical environment.
Table 2. Proposal for the interpretation of results from different diagnostic and follow-up tests in COVID-19.
| Test results | Clinical significance | ||||||
|---|---|---|---|---|---|---|---|
| PCR Sputum | PCR (NPS) | PCR (Stool) * | CRISPR test ** | Antigen Test | IgM | IgG | |
| - | - | - | - | - | - | - | No infection |
| + | + | + | + | - | - | - | Early infection phase (acute infection) |
| + | + | + | + | + | - | - | Early infection phase (acute infection, symptom onset) |
| + | + | + | + | + | + | - | Acute infection phase |
| + | + | + | + | + | + | + | Acute infection (beginning of transition to late infection or convalescent phase |
| + | + | + | + *** | - | - | + | Late infection phase beginning |
| + | - | + | + *** | - | - | + | Late infection phase |
| - | - | - | + *** | - | + | + | Active disease progression |
| - | - | - | + *** | - | - | + | Late infection (past?) |
| - | - | - | - | - | - | + | Past infection (immunity?) |
| - | - | - | - | - | + | - | False positive **** |
Abbreviations: COVID-19, coronavirus disease 2019; CRISPR, clustered regularly interspaced short palindromic repeats; IgG, immunoglobulin G; IgM, immunoglobulin M; NPS, nasopharyngeal swab; PCR, polymerase chain reaction.
Note: Test for IgM titers, PCR in urine or stool, as well as determination of urinary or fecal antigens, and even virologic culture, are not available in general laboratories (limited only to reference or research).
Table generated based on the Protocolo de Actuación de la Junta de Castilla y León . 48
PCR positivity in stool was observed (57%) infected patients and remained positive in stool beyond nasopharyngeal swab by a median of 4 to 11 days but was unrelated to clinical severity. It may remain positive for over 6 weeks clinical relevance is uncertain (not related to severity of the disease). 49
CRISPR test detects 100 copies severe acute respiratory syndrome coronavirus 2 virus (97% sensitivity/100% specificity). 50
CRISPR test needs to be evaluated in these situations.
Need to retest
The determination of immunity derived from seroconversion of rising IgG levels is still under study. The challenge that we are facing in the next months is to evaluate the real protective activity of the IgG antibodies, as higher titer may not necessarily confer protection. In the worst-case scenario, IgG levels do not provide protection against future infections. 25
Personal Protective Equipment (PPE) for COVID-19
According to the CDC, employers should have adequate PPE to provide care for known or suspected COVID-19 patients while protecting healthcare workers. Employers should also train employees on how to don, use, and doff PPE properly to avoid self-contamination. When interacting with known or suspected COVID-19 patients, the CDC recommends at least:
Respirator or facemask : Use an N95 respirator or higher level [half-facemask with P100 filters, or powered air purifying respirator (PAPR)].
Eye protection : Face shield or goggles. Avoid the use of personal glasses or eye contacts, which are not considered PPE.
Gloves : Clean, non-sterile gloves.
Gown : Clean isolation gown.
For patients that have tested negative or are of low suspicion of COVID-19, a regular surgical facemask and gloves is appropriate. Gown use is optional depending on if a procedure will be done. 26
Mode of Action in Otolaryngology-Head and Neck Clinic Staff
In otolaryngology-head and neck surgery (OHNS), clinical examination and invasive procedures of the respiratory tract, such as paranasal sinuses and middle ear, have the potential for exposure of healthcare providers to SARS-CoV-2. This can occur directly by inhalation or ocular projection of contaminated droplets, and indirectly by contact with contaminated hands, objects, or surfaces. 27
Coronavirus disease 2019 has made these difficult times to practice medicine, balancing physician safety while maintaining high-quality patient care. What is certain is that a systematic review of recommendations focused on the unique needs of OHNS is urgently needed to establish guidelines that can effectively protect both healthcare workers and patients. We, therefore, are summarizing the most updated information at the time of publication regarding physician safety, for performance of routine procedures.
Numerous sources have provided a multitude of proposed recommendations at times contradictory and impractical for daily use. We have attempted to summarize and make practical a set of recommendations from those published by all societies and healthcare organizations ( Table 3 ).
Table 3. General recommendations in the operating room setting during the COVID-19 pandemic.
| Point of interest | Recommendation |
|---|---|
| Aspiration systems | Closed circuit aspiration systems should incorporate antiviral filters |
| OR air flow | OR should incorporate negative pressure system. |
| Staff exposure | Procedures should be performed by the most experienced personnel using the minimum time possible, with the minimal OR staff necessary to perform the procedure safely. |
| Dedicated OR | Institutions are recommended to specify a dedicated OR solely for COVID-19 + patients, and it is required to have specific protection measures (full PPE, proper immediate terminal cleaning). |
| Emergent procedures | In case of emergency procedures, and timing does not allow proper testing, patient should be assumed COVID-19 positive and maximum PPE available should be used. |
| Anesthesia | Avoid orotracheal intubation / general anesthesia as much as possible. |
| Surgical team entry to room | The surgical team (surgeon, assistants, instrumentalist) should not enter the OR until the patient is already intubated. |
| COVID-19 negative patients | Use of protective glasses and FFP2 mask is recommended even in COVID-19 negative patients. 51 |
Abbreviations: COVID-19, coronavirus disease 2019; FFP2, filtering face piece - level 2; OR, operating room; PPE, personal protective equipment.
Mode of Action in the Otolaryngology-Head and Neck Operating Room
Aerosolization in the operating room is a matter of concern when performing intubation maneuvers or procedures in the upper airway. 28 In general, procedures are considered aerosol-generating if they create and disperse aerosols when the patient coughs or sneezes. The CDC outlines several aerosol-generating procedures (AGPs) relevant to otolaryngologists, such as: airways suctioning with an open circuit, endotracheal intubation and extubation, noninvasive ventilation (e.g., bilevel positive airway pressure [BiPAP], continuous positive airway pressure [CPAP]), bronchoscopy, manual ventilation, and nebulizer administration. Furthermore, examination procedures such as nasal endoscopy and flexible nasal laryngoscopy are considered AGPs when associated with suctioning. Even without suctioning, these can also potentially increase the likelihood of cough, gag, and sneeze, with possible subsequent aerosolization; therefore, appropriate precautions should be considered based on individual clinical circumstances. 29
Cases can be labeled as high-risk versus low-risk procedures. High-risk procedures involve the upper airway (oral cavity, oropharynx, middle ear, mastoid), whereas low-risk procedures do not have exposure to the upper airway (thyroid surgery, neck dissection, biopsy excision of lymph nodes). 30
Tables 4 and 5 provide a list of the most common procedures and the suggested management based on the latest recommendations of diverse societies. 31 32 33 34 35 36 37 38 There may be exceptions to each case, and each surgeon should act according to knowledge, experience, and common sense. 30 33 Certainly, availability of COVID-19 varies per institutions and priority needs to be given to patients that are suspected to have high pretest probability of having the disease; therefore, testing recommendations have varied across institutions. While some institutions might have availability to perform 2 COVID-19 tests for high-risk cases, others might not have the same capacity. 39 Therefore, it is imperative that resources are adequately used to avoid scarcity and be able to provide adequate patient care in all circumstances while protecting healthcare workers.
Table 4. Recommendations for different procedures in the otolaryngology clinic during the COVID-19 pandemic.
| Procedure | COVID-19 Testing | Management/PPE recommendations |
|---|---|---|
| Regular physical exam | N/A | COVID-19 known, high suspicion, or patient under investigation follow CDC guidelines. COVID-19 negative or low suspicion, regular surgical mask and gloves. |
| Nasal packing (Epistaxis) | Generally, no time for testing in this situation | Assume COVID-19+ status and follow CDC guidelines Procedure comments: Initially recommend conservative measures (Afrin, Afrin, pinching the nose). If these do not stop the bleeding, packing with absorbable materials (PosiSep vs Gelfoam vs Surgiflo) to avoid a second visit to manipulate the nasal cavity. |
| Nasal endoscopy | If emergent, no testing. If non-emergent, deferred until COVID-19 results | Assume COVID-19 status and follow CDC guidelines. Procedure comments: Do not use nasal sprays. |
| Otologic procedures | Generally enough time to order COVID-19 test | If COVID-19 status or testing not available, assume COVID-19 status and use full PPE as per CDC guidelines. Procedure comments: No ear suctioning if suspicion of tympanic membrane perforation in emergency setting, unless wearing full PPE. |
| Tracheostomy tube change | If emergent, no testing. If non-emergent, deferred until COVID-19 tests is negative | Assume COVID-19 status and use full PPE as per CDC guidelines. |
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; N/A, not applicable; PPE, personal protective equipment.
Table 5. Recommendations for different procedures in the otolaryngology operation room during the COVID-19 pandemic.
| Procedure | COVID-19 Testing | Management/PPE recommendations |
|---|---|---|
| Tracheostomy | Order COVID-19 tests before OR | COVID-19 + with ARDS: No trach COVID-19 + without ARDS: Trach COVID-19 negative: Trach Regardless of COVID status, use full PPE as per CDC guidelines. Procedure comments: Use bipolar instead of monopolar cautery. Use suction regularly to evacuate the smoke. Hold ventilation when accessing the airway and placing the tracheostomy. |
| Nasal endoscopic surgeries, oropharyngeal surgeries and Level 1 Neck Dissections | If emergent , no time for testing. If urgent , order COVID-19 test | If emergent or COVID-19 test not available, assume COVID-19 status and use full PPE as per CDC guidelines |
| Neck dissections, levels 2–4. | Order COVID-19 test | If COVID-19 status, deferred procedure until COVID-19. If COVID-19 negative use PPE (mask face w/shield and standard OR gown, hair cover, gloves) |
| Peritonsillar Abscess | COVID-19 rapid test, if available | If emergent drainage required, assume COVID-19 status and use full PPE as per CDC guideline. Option: Treat medically first and observe while awaiting COVID-19 test results. Procedure comments: Infiltrate with lidocaine (avoid sprays). |
| Neck Abscess |
If
emergent,
no time for testing
Urgent If OR anticipated, order COVID-19 test |
Assume COVID-19 status and use full PPE as per CDC guidelines if emergent drainage is needed Medical treatment and follow clinically, while awaiting test results |
Abbreviations: ARDS, acute respiratory distress syndrome; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; OR, operating room; PPE, personal protective equipment.
Final Comments
Currently, healthcare institutions around the world are outlining their own protocols regarding laboratory testing and PPE usage based upon what medical societies have recommended. We have summarized the available laboratory tests and their respective sensitivity and specificity. Moreover, clinical guidelines from different societies were reviewed and summarized to facilitate guidance to otolaryngologists during procedures in the operating room and in the clinical settings. The accuracy of test results has been controversial, and discussion has been made regarding the false positive/negative results. Therefore, adequate use of PPE is key to decrease exposure of healthcare workers in any setting. However, certain extra precautions need to be considered when performing AGPs or any procedure involving manipulation of mucosal membranes. If these recommendations are applied, high quality patient care can continue with minimal risk of exposure to healthcare workers.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.Madhav N, Oppenheim B, Gallivan M, Mulembakani P, Rubin E, Wolfe N. Washington (DC): 2017. Pandemics: Risks, Impacts, and Mitigation. [PubMed] [Google Scholar]
- 2.Xu K, Lai X, Liu Z.Suggestions on the prevention of COVID-19 for health care workers in department of otorhinolaryngology head and neck surgeryWorld J Otorhinolaryngol Head Neck Surg2020 [DOI] [PMC free article] [PubMed]
- 3.National Institute of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines 2020[Available from:https://www.covid19treatmentguidelines.nih.gov/
- 4.Guan W J, Ni Z Y, Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N, Bushmaker T, Morris D H. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe C, Schunk M, Sothmann P. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization; Coronavirus disease (COVID-19) advice for the public 2020[Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public
- 8.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauer S A, Grantz K H, Bi Q. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172(09):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brann D H, Tsukahara T, Weinreb C.Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia: BioRxiv; 2020 [updated 05/18/ 2020. Available from:https://www.biorxiv.org/content/10.1101/2020.03.25.009084v4.full [DOI] [PMC free article] [PubMed]
- 11.Oudkerk M, Büller H R, Kuijpers D. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020:201629. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Report of the WHO-Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020[Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 13.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020[Available from:https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 14.Giannis D, Ziogas I A, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China Lancet 2020395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascarella G, Strumia A, Piliego C.COVID-19 diagnosis and management: a comprehensive reviewJ Intern Med2020 [DOI] [PMC free article] [PubMed]
- 17.Wang W, Xu Y, Gao R.Detection of SARS-CoV-2 in Different Types of Clinical SpecimensJAMA2020 [DOI] [PMC free article] [PubMed]
- 18.de Sociedad E.Enfermedades Infecciosas y Microbiología Clínica. Recomendaciones Institucionales: Documento de Posicionamiento de la SEIMC sobre el diagnóstico microbiólogo de COVID-19. [Institutional recommendations. SEIMC positioning on the microbiological diagnosis of COVID-19.] 2020[Available from:https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-Posicionamiento_SEIMC_diagnostico_microbiologico_COVID19.pdf
- 19.Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica. Recomendaciones de SEIMC sobre el uso de las pruebas de detección de anticuerpos. [SEIMC Recommendations on the use of Antibody Detection Testing.] 2020[Available from:https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-Recomendaciones_uso_de_las_pruebas_de_deteccion_de_anticuerpos.pdf
- 20.Guo L, Ren L, Yang S. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19) Clin Infect Dis. 2020:ciaa310. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zainol Rashid Z, Othman S N, Abdul Samat M N, Ali U K, Wong K K. Diagnostic performance of COVID-19 serology assays. Malays J Pathol. 2020;42(01):13–21. [PubMed] [Google Scholar]
- 22.Carter L J, Garner L V, Smoot J W. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent Sci. 2020;6(05):591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacofsky D, Jacofsky E M, Jacofsky M. Understanding Antibody Testing for COVID-19. J Arthroplasty. 2020;S0883–5403 20:30442–30443. doi: 10.1016/j.arth.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennings K.FDA Authorizes First-Ever Crispr Application For COVID-19 Coronavirus Test: Forbes; 2020[Available from:https://www.forbes.com/sites/katiejennings/2020/05/07/fda-authorizes-first-ever-crispr-application-for-covid-19-coronavirus-test/#2bdd017a1708
- 25.Chappell B.WHO Says COVID-19 Immunity Is An Unknown; Disease '10 Times Deadlier' Than 2009 Flu: NPR 2020[Available from:https://www.npr.org/sections/coronavirus-live-updates/2020/04/13/833534116/who-says-covid-19-immunity-is-an-unknown-disease-10-times-deadlier-than-2009-flu
- 26.Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019: Infection Control Guidance 2020[Available from:https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
- 27.Couloigner V, Schmerber S, Nicollas R. COVID-19 and ENT Surgery. Eur Ann Otorhinolaryngol Head Neck Dis. 2020;137(03):161–166. doi: 10.1016/j.anorl.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministerio de Sanidad de España. Enfermedad por coronavirus, COVID-19 [Coronavirus disease, COVID-19] 2020[Available from:https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/ITCoronavirus.pdf
- 29.Setzen G, Anne S, Brown E G., IIIGuidance for Return to Practice for Otolaryngology-Head and Neck Surgery: Part One: American Academy of Otolaryngology-Head and Neck Surgery 2020[Available from:https://www.entnet.org/sites/default/files/uploads/guidance_for_return_to_practice_part_one_final_050520.pdf
- 30.Givi B, Schiff B A, Chinn S B.Safety Recommendations for Evaluation and Surgery of the Head and Neck During the COVID-19 PandemicJAMA Otolaryngol Head Neck Surg2020 [DOI] [PubMed]
- 31.American Academy of Otolaryngology-Head and Neck Surgery. Tracheotomy Recommendations During the COVID-19 Pandemic 2020[Available from:https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic
- 32.Boccalatte L A, Larrañaga J J, Perez Raffo G M. Brief guideline for the prevention of COVID-19 infection in head and neck and otolaryngology surgeons. Am J Otolaryngol. 2020;41(03):102484. doi: 10.1016/j.amjoto.2020.102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard B E. High-Risk Aerosol-Generating Procedures in COVID-19: Respiratory Protective Equipment Considerations. Otolaryngol Head Neck Surg. 2020;163(01):98–103. doi: 10.1177/0194599820927335. [DOI] [PubMed] [Google Scholar]
- 34.Patel Z M, Fernandez-Miranda J, Hwang P H. Letter: Precautions for Endoscopic Transnasal Skull Base Surgery During the COVID-19 Pandemic. Neurosurgery. 2020;87(01):E66–E67. doi: 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Public Health England. COVID-19: infection prevention and control guidance 2020[Available from:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/886668/COVID-19_Infection_prevention_and_control_guidance_complete.pdf
- 36.Public Health Scotland. Assessing the evidence base for medical procedures which create a higher risk of respiratory infection transmission from patient to healthcare worker: Public Health Scotland 2020[Available from:https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3055/documents/1_agp-sbar.pdf
- 37.Tran K, Cimon K, Severn M, Pessoa-Silva C L, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(04):e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vukkadala N, Qian Z J, Holsinger F C, Patel Z M, Rosenthal E.COVID-19 and the Otolaryngologist: Preliminary Evidence-Based ReviewLaryngoscope2020 [DOI] [PubMed]
- 39.Al-Muharraqi M A. Testing recommendation for COVID-19 (SARS-CoV-2) in patients planned for surgery - continuing the service and ‘suppressing’ the pandemic. Br J Oral Maxillofac Surg. 2020;S0266–4356(20):30164–30169. doi: 10.1016/j.bjoms.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbott. Abbott Launches COVID-19 Antibody Test 2020[Available from:https://www.abbott.com/corpnewsroom/product-and-innovation/abbott-launches-covid-19-antibody-test.html
- 41.Roche. Roche's COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark 2020[Available from:https://diagnostics.roche.com/global/en/news-listing/2020/roches-covid-19-antibody-test-receives-fda-emergency-use-authorization.html
- 42.Bio-Rad Laboratories Inc. Bio-Rad's SARS-CoV-2 (COVID-19) Serology Test Granted FDA Emergency Use Authorization, the First Total Antibody Test to Receive EUA from the FDA 2020[Available from:https://www.bio-rad.com/en-us/corporate/newsroom/bio-rads-sars-cov-2-covid-19-serology-test-granted-fda-emergency-use-authorization-first-total-antibody-test-receive-eua-span-classforcebreaks-fda?ID=Bio-Rad-s-SARS-CoV-2_1588272430&tlp-link=[COVID-19%20qPCR%20Assay%20Development%20Solutions]%20[SARS-CoV-2%20EUA%20News]
- 43.DiaSorin. The fully automated serology test to detect IgG antibodies against SARS-CoV-2 2020[Available from:https://www.diasorin.com/en/immunodiagnostic-solutions/clinical-areas/infectious-diseases/covid-19
- 44.Cellex. Cellex qSARS-CoV-2 IgG/IgM Rapid Test 2020[Available from:https://www.fda.gov/media/136625/download
- 45.Lassaunière R, Frische A, Harboe Z B.Evaluation of nine commercial SARS-CoV-2 immunoassays: medRxiv 2020[Available from:https://www.medrxiv.org/content/10.1101/2020.04.09.20056325v1.full.pdf
- 46.Ortho-Clinical Diagnostics Inc. VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack 2020[Available from:https://www.fda.gov/media/137363/download
- 47.Ortho-Clinical Diagnostics Inc. VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack 2020[Available from:https://www.fda.gov/media/136967/download
- 48.Junta de Castilla y León. SACYL. Indicaciones y procedimiento de utilización de test diagnósticos de infección COVID-19. [Indications and procedure for utilization of diagnostic tests for COVID-19 infection] 2020[Available from:https://www.saludcastillayleon.es/es/covid-19/informacion-profesionales/atencion-primaria/actuacion-atencion-primaria.ficheros/1599304-Guia%20breve%20para%20la%20utilizacio%C2%BFn%20de%20test%20diagno%C2%BFsticos%20en%20Infeccio%C2%BFn%20por%20COVID%20080520.pdf
- 49.Zheng S, Fan J, Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lall S.SHERLOCK-based one-step test provides rapid and sensitive Covid-19 detection: MIT News 2020[Available from:http://news.mit.edu/2020/sherlock-based-one-step-test-provides-rapid-sensitive-covid-19-detection-0505
- 51.Spanish Association of Surgery (AEC). General Recommendations of Urgent Surgical Care in the Context of The COVID-19 Pandemic (SARS CoV-2) From The Spanish Association Of Surgery (AEC) 2020[Available from:https://www.aecirujanos.es/files/noticias/158/documentos/4_-_Recomendaciones_for_URGENT_Surgical_care_during_the_pandemic_COVID_19__v_2.pdf


