Abstract
Background and study aims Endoscopic ultrasound guided pancreatic duct drainage (EUS-PDD) is a minimal-invasive therapeutic option to surgery and in patients with failed endoscopic retrograde pancreatography (ERP). The aim of this review was to quantitatively appraise the clinical outcomes of EUS-PDD by meta-analysis methods.
Methods We searched multiple databases from inception through March 2020 to identify studies that reported on EUS-PDD. Pooled rates of technical success, successful drainage of pancreatic duct, clinical success, and adverse events were calculated. Study heterogeneity was assessed using I 2 % and 95 % prediction interval.
Results A total of 22 studies (714 patients) were included. The pooled rate of technical success in EUS-PDD was 84.8 % (95 % CI 79.1–89.2). The pooled rate of successful PD drained by EUS-PDD was 77.5 % (95 % CI 63.1–87.4). The pooled rate of clinical success of EUS-PDD was 89.2 % (95 % CI 82.1–93.7). The pooled rate of all adverse events was 18.1 % (95 % CI 14.2–22.9). On sub-group analysis, the pooled technical success and clinical success of EUS-PDD from Japanese data were considerably superior (91.2 %, 83–95.6 & 92.5 %, 83.9–96.7, respectively). The pooled rate of post EUS-PDD acute pancreatitis was 6.6 % (95 % CI 4.5–9.4), bleeding was 4.1 % (95 % CI 2.7–6.2), perforation and/or pneumoperitoneum was 3.1 % (95 % CI 1.9–5), pancreatic leak and/or pancreatic fluid collection was 2.3 % (95 % CI 1.4–4), and infection was 2.8 % (95 % CI 1.7–4.6).
Conclusion EUS-PDD demonstrates high technical success and clinical success rates with acceptable adverse events. Technical success was especially high for anastomotic strictures.
Introduction
Patients with an obstructed pancreatic duct (PD) can suffer from pain that can be severe. Etiologies of an obstructed pancreatic duct PD include chronic pancreatitis with inflammatory stenosis of the duct and/or papilla, obstructions due to pancreato-lithiasis, compressing pseudocysts, disconnected pancreatic duct syndrome (DPDS), and stenosis of the pancreatico-enteral anastomosis following surgery, typically a pancreaticojejunostomy created during pancreaticoduodenectomy 1 2 3 .
The current mainstay of treatment is to relieve the obstruction with endoscopic retrograde transpapillary (ERP) drainage or, less commonly, surgery 1 2 . Traditionally, surgical intervention included lateral pancreatico-jejunostomy (Peustow procedure) in patients with a dilated main pancreatic duct (MPD) or pancreaticoduodenectomy (Whipple procedure) versus distal pancreatectomy 1 2 . Although surgery is effective in this setting with success rates of 65 % to 85 %, adverse event (AE) rates of up to 30 % and mortality rates up to 2 % have been reported 4 . Patients who underwent prior pancreaticojejunostomy creation can have this anastomosis revised, although often at the cost of further loss of pancreatic parenchyma.
Since the first report of EUS guided pancreaticogastrostomy in 2002, endoscopic ultrasound-guided pancreatic duct drainage (EUS-PDD) has emerged as a therapeutic option in patients who have failed conventional methods of ERP-PDD 5 . EUS-PDD can help avoid invasive surgery. Data on the efficacy and safety of EUS-PDD are limited 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 . The aims of this study were to qualitatively and quantitatively appraise the current available data on EUS-PDD by meta-analysis methods.
Methods
Search strategy
The literature was searched by a medical librarian for the concepts related to EUS-PDD. Search strategies were created using a combination of keywords and standardized index terms. Searches were run in March 2020 in ClinicalTrials.gov, Ovid EBM Reviews, Ovid Embase (1974 +), Ovid Medline (1946 + including epub ahead of print, in-process & other non-indexed citations), Scopus (1970 +) and Web of Science (1975 +). Results were limited to English language manuscripts. All results were exported to Endnote where 1120 obvious duplicates were removed leaving 1744 citations. The full search strategy is available in Appendix 1 . The MOOSE and PRISMA checklists were followed and are provided as Appendixes 2 and 3 28 29 . Reference lists of evaluated studies were examined to identify other studies of interest.
Study selection
In this meta-analysis, we included studies that evaluated the clinical outcomes of EUS-PDD. Studies were included irrespective of inpatient/ outpatient setting, follow-up time, route of access and/or drainage, presence of surgically altered anatomy, and geography as long as they provided the appropriate data needed for the analysis.
Our exclusion criteria were: (1) case reports and case series studies; (2) studies with sample size < 10 patients; (3) studies performed in the pediatric population (age < 18 years); and (4) studies not published in English language. In cases of multiple publications from a single research group reporting on the same patient, same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained. When needed, authors were contacted via email for clarification of possible study-cohort overlap. The retained studies were selected by two authors (BPM, SC) based on publication timing (most recent) and/or sample size of the study (largest). In situations in which a consensus could not be reached, overlapping studies were included in the final analysis and any potential effects were assessed by sensitivity analysis of the pooled outcomes by leaving out one study at a time.
Data abstraction and quality assessment
Data on study-related outcomes from the individual studies were abstracted independently onto a standardized form by at least two authors (BPM, SRK). Authors SC and LLK cross-verified the collected data for possible errors and two authors (BPM, SRK) did the quality scoring independently. The primary study authors were contacted via email as and when needed for further information and/or clarification on data.
The Newcastle-Ottawa scale for cohort studies was used to assess the quality of studies 30 . This quality score consisted of eight questions, the details of which are provided in Supplementary Table 1.
Outcomes assessed
Pooled rates of technical success of pancreatic duct access (defined as successful pancreatography and/or insertion of pancreatic duct wire and/or duodenal wire),
Pooled rate of successful drainage of the pancreatic duct (defined as resolution of MPD obstruction via drainage),
Pooled rate of clinical success (defined as successful clinical resolution of symptoms, such as pain)
Pooled rate of AEs (defined by the American Society for Gastrointestinal Endoscopy [ASGE] lexicon for endoscopic adverse events) and AE subtypes 31 ,
Pooled rate of stent-related complications (defined as stent migration and/or stent occlusion) and the need for EUS-PDD reintervention (defined as the need for repeat procedure in patients who achieved clinical success before irrespective of the presence and/or absence of stents).
Statistical analysis
We used meta-analysis techniques to calculate the pooled estimates in each case following the methods suggested by DerSimonian and Laird using the random-effects model 32 . When the incidence of an outcome was zero in a study, a continuity correction of 0.5 was added to the number of incident cases before statistical analysis 33 .
We assessed heterogeneity between study-specific estimates by using Cochran Q statistical test for heterogeneity, 95 % prediction interval (PI) and the I 2 statistics 33 34 35 . In this, values < 30 %, 30 % to 60 %, 61 % to 75 %, and > 75 % were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively. The PI gives an idea about the range of dispersion of the pooled results in the wider universe, and I 2 tell us what proportion of the dispersion is true vs chance.
Publication bias was ascertained, qualitatively, by visual inspection of funnel plot and quantitatively, by the Egger test 36 . When publication bias was present, further statistics using the fail-Safe N test and Duval and Tweedie’s ‘Trim and Fill’ test was used to ascertain the impact of the bias 37 . Three levels of impact were reported based on the concordance between the reported results and the actual estimate if there were no bias. The impact was reported as minimal if both versions were estimated to be same, modest if effect size changed substantially but the final finding would still remain the same, and severe if basic final conclusion of the analysis is threatened by the bias 38 .
Meta-regression analysis was attempted, when feasible, to study the effects of patient variables on the pooled outcomes. Knapp-Hartung two-tailed P < 0.05 was considered statistically significant and R 2 value was calculated to study the goodness-of-fit. All analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 3 (BioStat, Englewood, New Jersey, United States).
Results
Search results and population characteristics
A total of 22 studies were included in the final analysis 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 . The schematic diagram demonstrating our study selection is illustrated in Supplementary Fig. 1.
A total of 714 patients were analyzed from the included studies. 55 % were males. Mean age ranged from 36 years to 69 years. The pre- EUS-PDD mean main pancreatic duct diameter ranged from 3.5 mm to 8.1 mm. Further details along with the population characteristics are described in Supplementary Table 1 .
EUS-PDD was attempted via the following approaches: (1) ERP-EUS rendezvous procedure if the papilla can be reached endoscopically with successful advancement of a guidewire to the bowel lumen, either through the papilla or through an anastomosis. Endoscopic retrograde cholangiopancreatography (ERCP) using the rendezvous guide-wire can then be performed; (2) primary transgastric or transduodenal drainage with ante- or retrograde outflow if the papilla or the anastomosis cannot be reached endoscopically. PDD in these situations is achieved via transmural fistula creation followed by dilation and stent placement; or (3) internal antegrade drainage if the papilla cannot be reached but the stenotic ductal segment can be passed by the guidewire, in which case the stent is pushed into the small intestine through the ampulla or the anastomosis.
Characteristics and quality of included studies
There were no population-based studies. Six studies were based on multicenter data 7 11 13 22 24 26 . The detailed study quality evaluation is presented in Supplementary Table 3 . Based on the New-Castle Ottawa scoring system, five studies 7 10 22 24 27 were considered to be of high quality and 17 studies 6 8 9 11 12 13 14 15 16 17 18 19 20 21 23 25 26 were considered to be of medium quality. There were no low-quality studies.
Meta-analysis outcomes
Technical success
The pooled rate of technical success in EUS-PDD was 84.8 % (95 % CI 79.1–89.2). Forest plot ( Fig. 1 ). The pooled rate of technical success from multicenter data was 87.5 % (95 % CI 77.7–93.4) and from single-center data was 83.5 % (95 % CI 76.1–88.9). The pooled rate of technical success of studies published as full manuscripts was 84.4 % (95 % CI 77.4–89.5) and of studies in abstract form was 86.5 % (95 % CI 74.3–93.5). The pooled rate of technical success when evaluating follow up time > 12 months was 84.1 % (95 % CI 71.8–91.6) and < 12 months was 92.6 % (95 % CI 84.4–96.6) ( Table 1 ).
Fig. 1 .
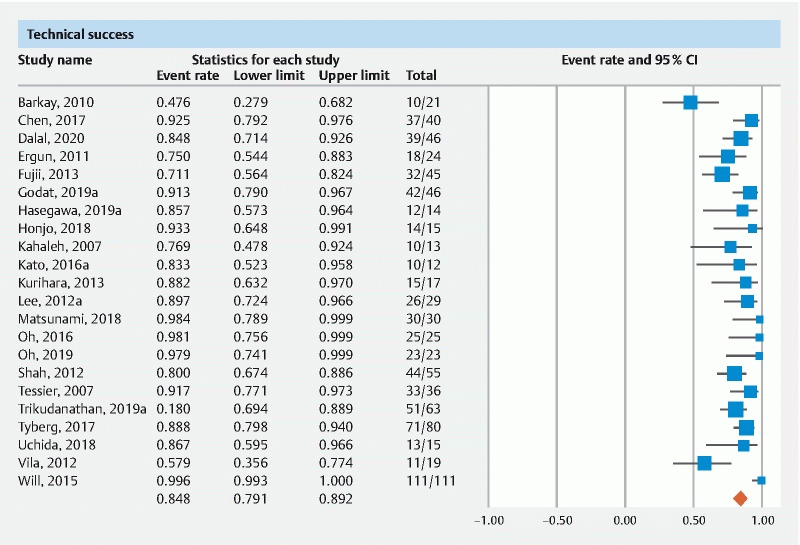
Forest plot of pooled technical success.
Table 1. Summary of pooled rates.
| Outcome | Pooled rate (95 % confidence interval) | I2; 95 % prediction interval |
| Technical success | 84.8 % (79.1–89.2); 22 studies | 61 %; 56 to 96 |
|
77.5 % (61.2–88.3); 4 studies | 0 %; 38 to 95 |
|
85.3 % (72–92.9); 5 studies | 79 %; 11 to 99 |
|
91.2 % (83–95.6); 9 studies | 0 %; 81 to 96 |
|
82 % (67.1–91.1); 4 studies | 84 %; 4 to 99 |
|
87.5 % (77.7–93.4); 6 studies | 66 %; 41 to 98 |
|
83.5 % (76.1–88.9); 16 studies | 58 %; 54 to 95 |
|
84.4 % (77.4–89.5); 17 studies | 68 %; 48 to 97 |
|
86.5 % (74.3–93.5); 5 studies | 0 %; 63 to 96 |
|
84.1 % (71.8–91.6); 5 studies | 64 %; 41 to 94 |
|
92.6 % (84.4–96.6); 6 studies | 30 %; 52 to 94 |
| Successful PD drained | 77.5 % (63.1–87.4); 11 studies | 88 %; 21 to 98 |
|
69.2 % (47–85); 4 studies | 85 %; 2 to 99 |
|
89.4 % (75.7–95.8); 4 studies | 9 %; 46 to 99 |
|
Insufficient data | -NA- |
|
87.2 % (69.5–95.3); 4 studies | 80 %; 4 to 95 |
|
70.2 % (52.1–83.5); 7 studies | 87 %; 43 to 93 |
|
79.5 % (47.7–94.3); 3 studies | 68 %; 3 to 98 |
|
84.2 % (53–96.2); 3 studies | 93 %; 2 to 98 |
| Clinical success | 89.2 % (82.1–93.7); 18 studies | 73 %; 50 to 98 |
|
92.5 % (83.9–96.7); 9 studies | 50 %; 44 to 99 |
|
76.2 % (58.9–87.7); 4 studies | 76 %; 9 to 99 |
|
Insufficient data | -NA- |
|
91 % (80.2–96.2); 4 studies | 0 %; 65 to 98 |
|
90.2 % (77.9–96); 5 studies | 49 %; 47 to 99 |
|
88.3 % (79–93.8); 13 studies | 71 %; 41 to 98 |
|
89.6 % (81.2–94.5); 14 studies | 75 %; 45 to 99 |
|
88.8 % (67.7–96.8); 4 studies | 64 %; 7 to 99 |
|
86.4 % (71.9–94.1); 5 studies | 71 %; 43 to 98 |
|
85.3 % (70.3–93.5); 6 studies | 77 %; 42 to 99 |
| Adverse events (all) | 18.1 % (14.2–22.9); 22 studies | 45 %; 8 to 36 |
|
18.7 % (12.4–27.2); 5 studies | 32 %; 6 to 44 |
|
15.8 % (10.3–23.6); 9 studies | 31 %; 5 to 39 |
|
12.8 % (7.4–21.3); 4 studies | 0 %; 4 to 40 |
|
27.9 % (19.4–38.2); 4 studies | 48 %; 6 to 70 |
|
24.3 % (16.8–33.7), 6 studies | 51 %; 8 to 55 |
|
15.9 % (11.8–21); 16 studies | 30 %; 7 to 31 |
|
17.9 % (13.4–23.5); 17 studies | 50 %; 7 to 39 |
|
18.2 % (10.8–29); 5 studies | 32 %; 5 to 49 |
| Adverse events by ASGE Lexicon | ||
|
13 % (8.3–19.9); 19 studies | 69 %; 2 to 49 |
|
9.9 % (6.5–14.8); 19 studies | 48 %; 3 to 30 |
|
3.9 % (2.5–5.9); 19 studies | 0 %; 2 to 6 |
| Post EUS-PDD individual adverse events | ||
|
6.6 % (4.5–9.4); 21 studies | 5 %; 4 to 11 |
|
4.1 % (2.7–6.2); 21 studies | 0 %; 3 to 6 |
|
3.1 % (1.9–5); 21 studies | 0 %; 2 to 5 |
|
2.3 % (1.4–4); 21 studies | 0 %; 1 to 4 |
|
2.8 % (1.7–4.6); 21 studies | 0 %; 2 to 5 |
|
13.9 % (8.2–22.6); 10 studies | 65 %; 3 to 49 |
|
21.3 % (11.5–36.2); 12 studies | 85 %; 2 to 80 |
|
15.2 % (9.1–24.1); 6 studies | 44 %; 4 to 45 |
| Publication bias | Eggers P = 0.01 | |
PD, pancreatic duct; ASGE, American Society for Gastrointestinal Endoscopy; EUS-PDD, endoscopic ultrasound-guided pancreatic duct drainage.
Successful PD drainage
The pooled rate of successful PD drainage by EUS-PDD was 77.5 % (95 % CI 63.1–87.4) ( Fig. 2 ).
Fig. 2.
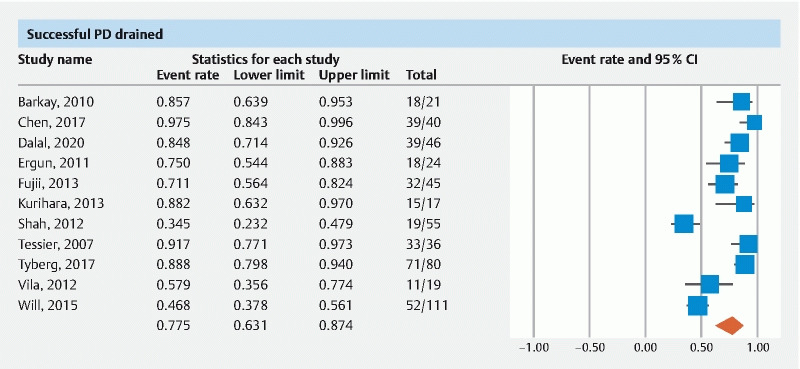
Forest plot of pooled successful PD drainage.
The pooled rate of successful PD drained from multi-center data was 87.2 % (95 % CI 69.5–95.3) and from single center data was 70.2 % (95 % CI 52.1–83.5). The pooled rate of successful PD drained when evaluating follow up time > 12 months was 79.5 % (95 % CI 47.7–94.3) and < 12 months was 84.2 % (95 % CI 53–96.2) ( Table 1 ).
Clinical success
The pooled rate of clinical success of EUS-PDD was 89.2 % (95 % CI 82.1–93.7)( Fig. 3 ).
Fig. 3.
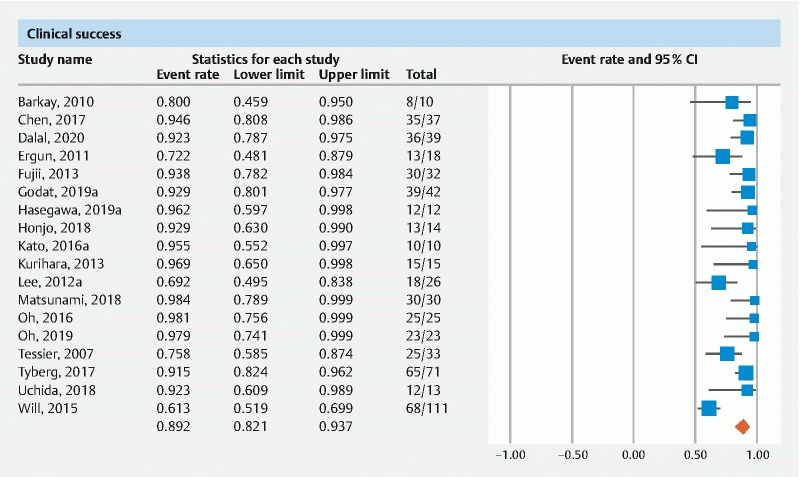
Forest plot of pooled clinical success.
The pooled rate of clinical success from multicenter data was 90.2 % (95 % CI 77.9–96) and from single center data was 88.3 % (95 % CI 79–93.8). The pooled rate of clinical success of studies published as full manuscripts was 89.6 % (95 % CI 81.2–94.5) and of studies in abstract form was 88.8 % (95 % CI 67.7–96.8). The pooled rate of clinical success when evaluating follow up time > 12 months was 86.4 % (95 % CI 71.9–94.1) and < 12 months was 85.3 % (95 % CI 70.3–93.5) ( Table 1 ).
Adverse events
The pooled rate of all AEs was 18.1 % (95 % CI 14.2–22.9)( Fig. 4 ). The pooled rate of AEs from multicenter data was 24.3 % (95 % CI 16.8–33.7) and from single-center data was 15.9 % (95 % CI 11.8–21). The pooled rate of AEs of studies published as manuscripts was 17.9 % (95 % CI 13.4–23.5) and of studies in abstract form was 18.2 % (95 % CI 10.8–29).
Fig. 4 .
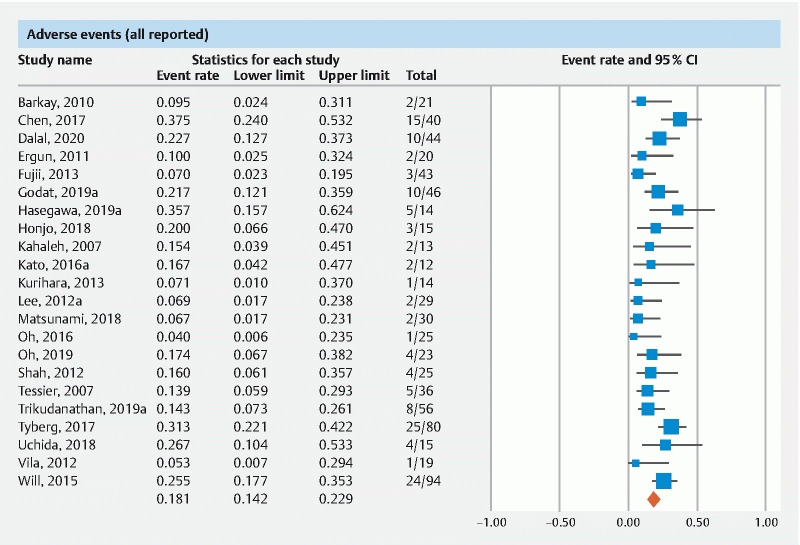
Forest plot of pooled adverse events.
Based on the ASGE lexicon of AEs, the pooled rate of procedure-related mild AEs was 13 % (95 % CI 8.3–19.9), moderate AEs 9.9 % (95 % CI 6.5–14.8), and severe AEs was 3.9 % (95 % CI 2.5–5.9).
In terms of the specific type of AE (defined as post EUS-PDD acute pancreatitis, bleeding, perforation and/or pneumoperitoneum, pancreatic leak and/or pancreatic fluid collection, and infection), the pooled rate of post EUS-PDD acute pancreatitis was 6.6 % (95 % CI 4.5–9.4), bleeding was 4.1 % (95 % CI 2.7–6.2), perforation and/or pneumoperitoneum was 3.1 % (95 % CI 1.9–5), pancreatic leak and/or pancreatic fluid collection formation was 2.3 % (95 % CI 1.4–4), infection was 2.8 % (95 % CI 1.7–4.6) and non-specific post-procedure abdominal pain warranting inpatient monitoring was 13.9 % (95 % CI 8.2–22.6).
Stent related
AEs, defined as stent migration and/or occlusion, occurred in 21.3 % of patients (95 % CI 11.5–36.2). The pooled rate of EUS-PDD reintervention was 15.2 % (95 % CI 9.1–24.1).
Meta-regression analysis
Meta-regression analysis was performed to assess the impact on calculated pooled rates of technical success and clinical success for the following variables: chronic pancreatitis, anastomotic strictures, transmural stenting and rendezvous technique. A statistically significant two-tailed P value by Knapp-Hartung method was noticed with anastomotic strictures as a single variable ( P = 0.03, R 2 = 0.29) on the calculated technical success. Rest of the variables did not demonstrate significant influence on the calculated technical and/or clinical success rates.
Validation of meta-analysis results
Sensitivity analysis
To assess whether any one study had a dominant effect on the meta-analysis, we excluded one study at a time and analyzed its effect on the main summary estimate. In this analysis, no single study significantly affected the outcome or the heterogeneity. The results of subgroup analysis are summarized in Table 1 .
Heterogeneity
We assessed dispersion of the calculated rates using the prediction interval (PI) and I 2 percentage values. The calculated PIs are reported with the pooled rates in Table 1 . Overall, none to considerable heterogeneity was noted across the analysis. Specifically, data from Japan demonstrated minimal heterogeneity with narrow prediction intervals. In addition, EUS-PDD in patients with anastomotic strictures demonstrated statistical significance when assessed as a single variable on meta-regression analysis. Therefore, study geography and clinical indication for EUS-PDD are explainable causes of heterogeneity in this analysis.
Publication bias
Based on visual inspection of the funnel plot as well as quantitative measurement that used the Egger regression test, there was evidence of publication bias (funnel plot, Supplemental Fig. 7 , Eggers two-tailed P = 0.01). Further statistical analysis using the fail-Safe N test and Duval and Tweedie’s ‘Trim and Fill’ test revealed that the reported pooled results would not be significantly affected by the unpublished studies.
Discussion
In this meta-analysis, EUS-PDD demonstrated high technical and clinical success rates, with acceptable AE rates. We report a pooled technical success rate of 84.8 % with EUS-PDD, a pooled successful PD drainage rate of 77.5 %, and a pooled clinical success rate of 89.2 %. To the best of our knowledge, this is the first meta-analysis on this topic.
EUS-PDD continues to be one of the most challenging procedures in interventional EUS and the reasons are as follows: (1) even a dilated PD is smaller than a pancreatic fluid collection, the gallbladder, or even a dilated bile duct; (2) the stomach does not typically create a stable platform for an echoendoscope during EUS-PDD; and (3) currently, there are no dedicated PD stents designed for EUS-PDD.
Technically EUS-PDD can be done by the rendezvous method, wherein drainage can be achieved in either antegrade or retrograde fashion with or without stent placement. On the other hand, drainage can also be achieved in transmural/transluminal fashion by placement of a self-expanding metal stent (SEMS) and/or lumen apposing metal stent (LAMS). The feasibility, difficulty, and safety of EUS-PDD largely depend on the procedure methodology. A previous qualitative review suggested 91 % technical success with transmural stenting and 72 % with rendezvous 39 . Unfortunately, in this study, we were not able to classify the outcomes by the procedure methodology due to the fact that the included studies did not analyze outcomes in subgroups by procedure methodology.
Similarly, the clinical indication can also affect the feasibility and safety of EUS-PDD. EUS-PDD can be difficult to perform in chronic pancreatitis when compared to post-surgical anastomotic causes, due to the accompanying multiple strictures and calcifications in chronic pancreatitis. Studies did not stratify the outcomes based on the clinical indication and we acknowledge this limitation. Nevertheless, we attempted to analyze the effects of clinical indications (chronic pancreatitis and post-surgical anastomosis) and EUS-PDD technique (transmural stenting and rendezvous drainage) by meta-regression analysis. We did not find any statistically significant influence on the outcomes from transmural stenting, rendezvous drainage, and chronic pancreatitis as single variables. However, EUS-PDD in post-surgical anastomosis demonstrated significant effect on the technical success (Knapp-Hartung two-tailed P = 0.03), indicating that the technical success of EUS-PDD seemed to be better in postsurgical anastomosis patients. It is important to note that meta-regression analysis is a weak statistic in terms of assessing the predictability of patient variables and additionally the R 2 goodness-of-fit was only 29 % in our analysis.
Studies defined clinical success as resolution of symptoms, especially pain. In this study we noted the pooled rate of clinical success to 89 %, which was greater than the technical success and/or the rate of successful PD drained. Although the exact explanations are unclear for this finding, concurrent pain management by conservative methods like use of analgesics and alternative procedures like celiac plexus nerve blocks might have helped achieve resolution of pain in addition to EUS-PDD. Therefore, we cannot be certain that the reported clinical success rate is entirely due to EUS-PDD procedure.
To assess the safety of EUS-PDD, we analyzed the pooled rate of AEs in multiple angles. The pooled rate of all AEs together was 18.1 %. The pooled rate of pancreatitis was 6.6 %, bleeding was 4.1 %, perforation and/or pneumo-peritoneum was 3.1 %, pancreatic leak and/or pancreatic fluid collection was 2.3 %, and infection was 2.8 %. Based on the ASGE lexicon for endoscopic AEs, the pooled rate of mild AEs was 13 %, moderate was 9.9 % and severe was 3.9 %. There were no procedure-related deaths. It is important to note that all studies were done at tertiary care centers by advanced therapeutic endoscopists. Stent-related AEs (stent occlusion and/or stent migration) were seen in 21.3 % of patients and reintervention was required in 15.2 % of patients.
The strengths of this review are as follows: systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good quality studies with detailed extraction of data, and rigorous evaluation of study quality. There are limitations to this study, most of which are inherent to any meta-analysis. The included studies were not entirely representative of the general population and community practice, with most studies being performed in tertiary-care referral centers. Our analysis had studies that were retrospective in nature contributing to selection bias.
Our analysis has the limitation of heterogeneity. However, based on our sensitivity analysis and subgroup analyses, we have attempted to provide plausible explanations. Variability in the geography where studies were conducted, clinical indication for EUS-PDD, and differences in procedural techniques seemed to explain the heterogeneity. Other possible causes that we were not able to study were details of stenting (choice, type, and number used), and the differences in procedural tools across centers and countries. Finally, our study did not compare EUS-PDD to other techniques such as enteroscopy-assisted ERCP (e-ERCP). Chen et al performed a multicenter trial comparing the two and reported a technical success rate of 92.5 % and clinical success rate of 87.5 % with EUS-PDD compared to 20 % and 23.1 %, respectively, with e-ERCP. Nevertheless, this study is the best available in the literature thus far quantitatively summarizing the clinical outcomes of EUS-PDD.
Conclusion
In conclusion, based on our meta-analysis, EUS-PDD achieved a technical success rate of 84.8 %, a successful PD drainage rate of 77.5 %, and a clinical success rate of 89.2 %. The rate of moderate AEs was 9.9 % and severe AEs were 3.9 %. Pancreatitis was the most commonly observed AE at 6.6 %. Owing to its technical complexity, EUS-PDD should be performed at advanced tertiary care centers with adequate expertise. Future studies are warranted to stratify the results in terms of clinical indication and EUS-PDD technique and study the efficacy of newer procedural tools including LAMS in EUS-PDD.
Acknowledgement
The authors thank Dana Gerberi, MLIS, Librarian, Mayo Clinic Libraries, for help with the systematic literature search.
Footnotes
Competing interests Dr. Adler is a consultant for Boston Scientific
Supplementary material :
References
- 1.Baars J E, Chen F, Sandroussi C et al. EUS-guided pancreatic duct drainage: Approach to a challenging procedure. Endosc Ultrasound. 2018;7:284–285. doi: 10.4103/eus.eus_104_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannini M. EUS-guided pancreatic duct drainage: ready for prime time? Gastrointestinal endoscopy. 2013;78:865–867. doi: 10.1016/j.gie.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau J-M, Devière J, Le Moine O et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547–555. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 4.Adams D B, Ford M C, Anderson M C. Outcome after lateral pancreaticojejunostomy for chronic pancreatitis. Ann Surg. 1994;219:481–489. doi: 10.1097/00000658-199405000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.François E, Kahaleh M, Giovannini M et al. EUS-guided pancreaticogastrostomy. Gastrointest Endosc. 2002;56:128–133. doi: 10.1067/mge.2002.125547. [DOI] [PubMed] [Google Scholar]
- 6.Barkay O, Sherman S, McHenry L et al. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010;71:1166–1173. doi: 10.1016/j.gie.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y I, Levy M J, Moreels T G et al. An international multicenter study comparing EUS-guided pancreatic duct drainage with enteroscopy-assisted endoscopic retrograde pancreatography after Whipple surgery. Gastrointest Endosc. 2017;85:170–177. doi: 10.1016/j.gie.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Dalal A, Patil G, Maydeo A. Six-year retrospective analysis of endoscopic ultrasonography-guided pancreatic ductal interventions at a tertiary referral center. Digest Endosc. 2020;32:409–416. doi: 10.1111/den.13504. [DOI] [PubMed] [Google Scholar]
- 9.Ergun M, Aouattah T, Gillain C et al. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518–525. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 10.Fujii L L, Topazian M D, Abu Dayyeh B K et al. EUS-guided pancreatic duct intervention: outcomes of a single tertiary-care referral center experience. Gastrointest Endosc. 2013;78:854–8640. doi: 10.1016/j.gie.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Godat S, David G, Maxime R et al. Endoscopic ultra-sonography guided drainage of the main pancreatic duct: A Swiss multi-center experience. Swiss Med Weekly. 2019;149:11–12. [Google Scholar]
- 12.Hasegawa S, Sato T, Kubota K et al. Usefulness of endoscopic ultrasonography-guided pancreatic duct drainage for patients in ERCP failure with pancreatitis due to obstruction of pancreatic duct. Pancreas. 2019;48:1439–1440. [Google Scholar]
- 13.Honjo M, Itoi T, Tsuchiya T et al. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video) Endosc Ultrasound. 2018;7:376–382. doi: 10.4103/eus.eus_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahaleh M, Hernandez A J, Tokar J et al. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–230. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Mizukawa S, Yabe S et al. The feasibility and effectiveness of EUS-guided pancreatic duct drainage. Pancreatology. 2016;16:S16–S17. [Google Scholar]
- 16.Kurihara T, Itoi T, Sofuni A et al. Endoscopic ultrasonography-guided pancreatic duct drainage after failed endoscopic retrograde cholangiopancreatography in patients with malignant and benign pancreatic duct obstructions. Digest Endosc. 2013;25:109–116. doi: 10.1111/den.12100. [DOI] [PubMed] [Google Scholar]
- 17.Lee S S, Jang J W, Park D H. EUS-guided pancreatic duct drainage for symptomatic patients with pancreatic duct obstruction who are unsuitable for transpapillary drainage: Long-term follow-up results. Gastrointest Endosc. 2012;1:845. [Google Scholar]
- 18.Matsunami Y, Itoi T, Sofuni A et al. Evaluation of a new stent for EUS-guided pancreatic duct drainage: long-term follow-up outcome. Endoscopy International Open. 2018;6:E505–E512. doi: 10.1055/s-0044-101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh D, Park D H, Cho M K. Feasibility and safety of a fully covered self-expandable metal stent with antimigration properties for EUS-guided pancreatic duct drainage: early and midterm outcomes (with video) Gastrointest Endosc. 2016;83:366–373. doi: 10.1016/j.gie.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Oh D, Park D H, Song T J. Long-term outcome of endoscopic ultrasound-guided pancreatic duct drainage using a fully covered self-expandable metal stent for pancreaticojejunal anastomosis stricture. J Gastroenterol Hepatol. 2020;35:994–1001. doi: 10.1111/jgh.14897. [DOI] [PubMed] [Google Scholar]
- 21.Shah J N, Marson F, Weilert F et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56–64. doi: 10.1016/j.gie.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Tessier G, Bories E, Arvanitakis M et al. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Trikudanathan G, Dirweesh A, Attam R et al. EUS-guided pancreatic duct intervention: Technical outcomes at a single tertiary care center. Pancreas. 2019;48:1537. [Google Scholar]
- 24.Tyberg A, Sharaiha R Z, Kedia P et al. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85:164–169. doi: 10.1016/j.gie.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Uchida D, Kato H, Saragai Y et al. Indications for endoscopic ultrasound-guided pancreatic drainage: for benign or malignant cases? Can J Gastroenterol Hepatol. 2018;2018:8.216109E6. doi: 10.1155/2018/8216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vila J J, Perez-Miranda M, Vazquez-Sequeiros E et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76:1133–1141. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Will U, Reichel A, Fueldner F et al. Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct. World J Gastroenterol. 2015;21:13140–13151. doi: 10.3748/wjg.v21.i46.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroup D F, Berlin J A, Morton S C et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 31.Cotton P B, Eisen G M, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Sutton A J, Abrams K R, Jones D R et al. Methods for meta-analysis in medical research. John Wiley & Sons Ltd., New York. 2000:205–228. [Google Scholar]
- 34.Higgins J, Thompson S G, Spiegelhalter D J. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan B P, Adler D G. Heterogeneity in systematic review and meta-analysis: how to read between the numbers. Gastrointest Endosc. 2019;89:902–903. doi: 10.1016/j.gie.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J P, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duval S, Tweedie R. Trim and Fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 38.Rothstein H R, Sutton A J, Borenstein M. New York: John Wiley & Sons Ltd; 2006. Publication bias in meta-analysis: Prevention, assessment and adjustments. [Google Scholar]
- 39.Itoi T, Kasuya K, Sofuni A et al. Endoscopic ultrasonography-guided pancreatic duct access: Techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Digest Endosc. 2013;25:241–252. doi: 10.1111/den.12048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


