Abstract
Great strides over the past few decades have increased our understanding of the pathophysiology of hypophosphatemic disorders. Phosphate is critically important to a variety of physiologic processes, including skeletal growth, development and mineralisation, as well as DNA, RNA, phospholipids, and signaling pathways. Consequently, hypophosphatemic disorders have effects on multiple systems, and may cause a variety of nonspecific signs and symptoms. The acute effects of hypophosphatemia include neuromuscular symptoms and compromise. However, the dominant effects of chronic hypophosphatemia are the effects on musculoskeletal function including rickets, osteomalacia and impaired growth during childhood. While the most common causes of chronic hypophosphatemia in children are congenital, some acquired conditions also result in hypophosphatemia during childhood through a variety of mechanisms. Improved understanding of the pathophysiology of these congenital conditions has led to novel therapeutic approaches. This article will review the pathophysiologic causes of congenital hypophosphatemia, their clinical consequences and medical therapy.
Keywords: hypophosphatemia, XLH, rickets, fibroblast growth factor 23, FGF23
Introduction:
Phosphate is critically important in childhood for a variety of physiologic processes, including skeletal growth, development and mineralisation, as well as having important intracellular roles in DNA, RNA, phospholipids, energy metabolism and signaling pathways. Consequently, hypophosphatemic disorders have effects on multiple systems, and may cause a variety of nonspecific signs and symptoms [1]. The acute effects of severe hypophosphatemia include neuromuscular symptoms and compromised cardiac or respiratory function [1]. However, these effects are not typical of the congenital hypophosphatemias. Due to the importance of phosphate to hydroxyapatite and hence the growth and mineralization of the skeleton, the dominant clinical features of chronic hypophosphatemia during childhood are the effects on musculoskeletal function including rickets, osteomalacia and impaired growth during childhood [2].
Hypophosphatemia at the growth plate has been proposed as a common unifying feature of nearly all types of rickets (including nutritional rickets and vitamin D metabolism defects as well as hypophosphatemic rickets) [3, 4]. Hypophosphatemia impairs apoptosis of growth plate hypertrophic chondrocytes, resulting in expansion of this layer and impaired mineralisation [3, 4]. This leads to the radiographic features associated with rickets including widening, lucency, fraying and separation at the metaphysis of long bones [5]. Similarly at the osteoblasts, hypophosphatemia inhibits maturation and mineralization of osteoid resulting in osteomalacia[4]. These rachitic changes at the growth plate and the osteomalacia contribute to the skeletal deformities and overall growth impairment common to the congenital hypophosphatemias.
Phosphate metabolism is discussed in greater detail in other sections of this special issue, and is only briefly summarized here. Specifically in regards to the evaluation of hypophosphatemic disorders in children, it is important to remember that the primary tissues involved in regulating serum phosphate are the bone, intestine, kidney and parathyroid glands, and the hormones involved include parathyroid hormone (PTH), Fibroblast growth factor 23 (FGF23) and 1,25dihydroxyvitamin D [1,25(OH)2D] [6]. Important to the pathophysiology of phosphate disorders, PTH stimulates 1-alpha-hydroxylase activity and inhibits 24-hydroxylase activity (increasing 1,25(OH)2D), but FGF23 has the opposite effects on vitamin D metabolism (decreasing 1,25(OH)2D). While 1,25(OH)2D upregulates active phosphate transport in the intestines, PTH and FGF23 both downregulate renal tubular phosphate reabsorption, via modifying the brush border expression of sodium phosphate co-transporters.
Indeed, the primary mode of regulating the serum phosphate concentration is through altering renal tubular phosphate transport to restore homeostasis. This can be assessed clinically using the transport maximum of phosphate adjusted for glomerular filtration rate (TmP/GFR), requiring measurement of fasting serum phosphate and creatinine, simultaneous with a second morning urine void (or ideally a 2-hour morning urine collection) for phosphate and creatinine[7, 8]. While a nomogram can be used to determine the TmP/GFR [7], the nomogram does not incorporate the upper end of the normal range for young children and a more accurate method for determining TmP/GFR in children uses the equation: Serum phosphate – [(Urine phosphate x Serum creatinine)/Urine creatinine] [8]. However, both methods typically will reliably detect low TmP/GFR in children. The normal range for TmP/GFR in children generally is numerically similar to the normal ranges of serum phosphate at each age.
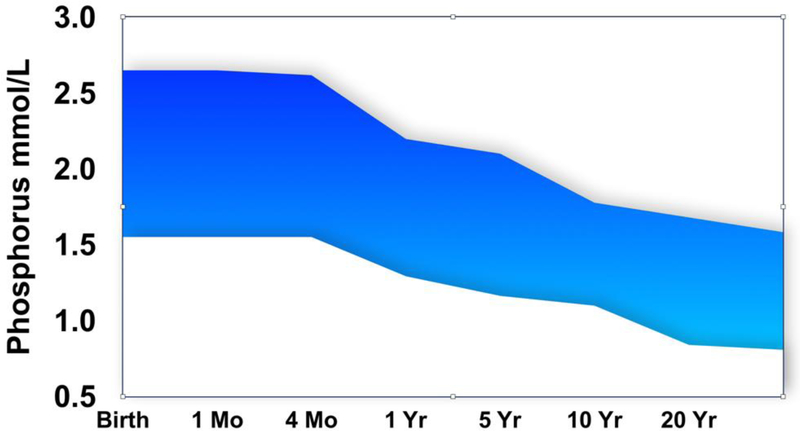
The first step to clinical evaluation and management of pediatric hypophosphatemic disorders involves recognizing the signs and symptoms of chronic hypophosphatemia, including muscle weakness, rickets and impaired growth. Indeed, chronic hypophosphatemia in childhood is usually detected due to presentation with rachitic deformities or occasionally due to fragility or insufficiency fractures. Consequently, serum phosphate should be assessed routinely in rickets and other pediatric musculoskeletal disorders, including assessment of TmP/GFR if hypophosphatemia is present. The next step includes recognition that the normal ranges for serum phosphate (Figure 1) and for TmP/GFR are higher in infants and children than in adults, though the precise mechanism for this is not certain. This higher range is necessary for appropriate mineralization of the growing skeleton. Failure to apply appropriate normal ranges leads to missing or delaying the detection of hypophosphatemia.
Figure 1.
Normal serum phosphate concentrations decrease with age during childhood. Stylized illustration adapted from the range tables provided by the Indiana University Health Pathology Laboratory (Abbreviations: Mo, month; Yr, year)
If TmP/GFR is low during hypophosphatemia, then measurement of FGF23 can be useful. Available assays include intact FGF23 assays that measure only the whole biologically active molecule, and C-terminal FGF23 assays which detect the combination of intact FGF23 and Cterminal fragments. While intact FGF23 levels appear similar when measured in plasma or serum, the C-terminal FGF23 concentrations are much lower in serum compared to plasma[9], and most publications reflect the plasma values of C-terminal FGF23. C-terminal FGF23 ranges are reported to be higher in young children compared to adults [10–17], while values of intact FGF23 are generally more similar across age ranges[12–18]. In addition, some conditions raise C-terminal FGF23 fragments without raising intact FGF23 such as iron deficiency (in which case phosphate is generally normal, except in certain circumstances) [19], or hyperphosphatemic tumoral calcinosis (due to impaired ability to secrete intact FGF23)[20].
General clinical features of congenital hypophosphatemic conditions.
The clinical presentation of chronic hypophosphatemia in childhood varies widely, but can be similar across a range of pathophysiology. Infants with congenital forms may be detected asymptomatically due to screening because of a parent or sibling having the disease. However, outside of such screening, the typical presentation involves features of rickets, especially bowing deformities of the lower limbs, along with frontal bossing, dolichocephaly, widened wrists or ankles, costo-chondral junction enlargement, tibial torsion and impaired growth (Table 1) [2]. However, the upper extremities are not usually bowed. The severity of these deformities is highly variable though, even within a given genetic cause or a given family. Delayed motor milestones or muscle weakness may also sometimes be present, though these may be more pronounced in the most severe hypophosphatemia or in conditions with delayed onset of hypophosphatemia. Patients may complain of limb pain. Fractures are not the typical presentation of hypophosphatemic rickets in childhood (nor in other forms of rickets), however they do sometimes occur and may be the presenting feature, leading to detection of hypophosphatemia. As patients with a variety of causes of hypophosphatemic rickets grow, patients may manifest disproportionate limitations in growth of the lower extremities compared to the trunk or upper extremities, and short stature is common[21–23]. Additional clinical features of the various forms of congenital hypophosphatemia are summarized in Table 2.
Table 1.
General clinical features of congenital hypophosphatemias
| Rachitic features (varus or valgus deformity of lower limbs, torsion of tibia, widening of wrists, widened costochondral junction, frontal bossing, dolicocephaly) |
| Gait abnormalities (waddling) |
| Impaired growth, short stature (disproportionately affecting lower limbs) |
| Delayed motor milestones (variable) |
| Muscle weakness (variable) |
| Fractures (though not a common presentation in children) |
| Tooth abnormalities (abscesses, periodontitis) |
Table 2.
Disease specific features of congenital hypophosphatemias
| Impaired enteral intake | XLH | ADHR (FGF23) | ARHR DMP1 | ARHR ENPP1 | ARHR FAM20 C | FD | LNSS | OD | ADHR (SGK3) | TIO | HHRH | Dent’s disease | Cystinosis | Fanconi syndrome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory | |||||||||||||||
| Phosphate | low | low | low | low | low | low | lowb | low | low | low | low | low | low | low | low |
| TmP/GFR | high | low | low | low | low | low | lowb | low | low | low | low | low | low | low | low |
| Calcium | normala | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal |
| Urine calcium excretion | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | high | high | high | high |
| Alkaline phosphatase | high | high | high | high | high | high | high | high | high | high | high | high | high | high | high |
| PTH | normal to high | normal to high | normal to high | normal to high | normal | normal | normal | normal | normal | normal | normal to high | normal to high | normal to high | normal to high | normal to high |
| 25-hydroxyvitaminD | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal | normal |
| 1,25-dihydroxyvitaminD | normal to high | low to normal | low to normal | low to normal | low to normal | low to normal | low to normal | low to normal | low to normal | low to normal | low to normal | normal to high | normal to high | normal to high | normal to high |
| Intact FGF23 | low | high | high | high | high | high | high b | high | high | normal | high | low | low | low | low |
| iron stores | low | ||||||||||||||
| Urine amino acids/protein, glucose, and/or electrolytes | high | high | high | ||||||||||||
| Clinical features | |||||||||||||||
| Rickets | X | X | X | X | X | X | Sometimes | X | X | X | If present in childhood | X | X | X | X |
| Growth impairment | X | X | X | X | X | X | Sometimes | X | X | X | Sometimes | X | X | X | X |
| Tooth abscesses | X | X | X | ||||||||||||
| Enthesopathy | X | X | X | X | |||||||||||
| Pseudofractures | X | X | X | X | X | X | X | X | X | X | X | ||||
| Nephrolithiasis or nephrocalcinosis | c | c | c | c | c | c | X | X | X | X | |||||
| Osteoarthritis | X | ||||||||||||||
| Delayed onset | X | X | |||||||||||||
| Biochemical waxing/Waning | X | ||||||||||||||
| Focal bone lesions | X | X | X | ||||||||||||
| Skin lesions | Cafe au- lait macules | Epiderma I nevi | |||||||||||||
| Arterial calcifications | X | ||||||||||||||
| Osteosclerosis of long bones | X | ||||||||||||||
| Cerebral calcifications | X | ||||||||||||||
| Facial dysmorphism | X | X | |||||||||||||
| Seizures | X | ||||||||||||||
Abbreviations listed in alphabetical order: ADHR (autosomal dominant hypophosphatemic rickets), ARHR (autosomal recessive hypophosphatemicrickets), DMP1 (dentin matrix protein 1), ENPP1 (ecto-nucleotide pyrophosphatase/phosphodiesterase 1), FAM20C (family with sequence similarity 20, member C), FGF23 (fibroblast growth factor 23), FD (fibrous dysplasia of bone), HHRH (hereditary hypophosphatemic rickets with hypercalciuria), LNSS (linear sebaceous nevus syndrome), OD (Osteoglophonic dysplasia), PTH (parathyroid hormone), SGK3 (serum- and glucocorticoid- inducible kinase 3), TmP/GFR (transport maximum of phosphate adjusted for glomerular filtration rate), TIO (tumor induced osteomalacia), XLH (X-linked hypophosphatemia).
Risk of hypocalcemia during treatment with phosphate.
Patients with fibrous dysplasia do not necessarily get hypophosphatemia. It is related to total body fibrous dysplasia burden. Patients with fibrous dysplasia may manifest other signs of McCune-Albright Syndrome.
In these conditions, nephrocalcinosis or nephrolithiasis occurs only as a complication of medical therapy.
A detailed family history is often useful. The most common genetic hypophosphatemia is Xlinked dominant hypophosphatemia (XLH), however, other inheritance patterns should prompt considerations of autosomal recessive or autosomal dominant forms[24]. XLH is also frequently caused by new sporadic mutations. Parents with milder manifestations of genetic hypophosphatemias may also sometimes be first recognized in adulthood when their child presents for evaluation. The time course and growth patterns of a patient may suggest acquired forms versus missed congenital diagnoses in patients with apparent delayed presentation of hypophosphatemia. However, in particular, autosomal dominant hypophosphatemic rickets can have delayed onset of hypophosphatemia in adulthood[17]. A dietary history should include the intake of calcium, phosphate, and vitamin D, as well as use of amino acid based infant or pediatric formulae [25, 26]. A detailed medication history may reveal other causes of impaired intestinal phosphate absorption or renal phosphate losses. Additional history or exam features may point to specific etiologies.
A complete physical examination should detect features such as frontal bossing, dolichocephaly, valgus or varus deformities, tibial torsion, inward toeing during gait, apparent widening of joints, as well as length/standing height, sitting height and limb length. Additionally, strength and joint range of motion are relevant to chronic hypophosphatemias. A dental evaluation should note timing of eruption of primary and permanent dentition, as well as periodontal disease and tooth abscesses. Skin features such as café au-lait macules or epidermal nevi may point to specific underlying conditions.
Congenital hypophosphatemic conditions can be categorized as those involving low TmP/GFR (indicating renal losses) and those having high TmP/GFR (involving appropriate phosphate conservation), and also categorized according to whether or not the condition is mediated by FGF23. While hypophosphatemia with high TmP/GFR is not due to FGF23 effects, hypophosphatemia with low TmP/GFR may or may not be mediated by FGF23.
Hypophosphatemia with high TmP/GFR
Conditions of chronic hypophosphatemia associated with high TmP/GFR may occur in children, but are not typically congenital. Hypophosphatemia with a high TmP/GFR are due to impaired intake or intestinal absorption, which are usually acquired conditions associated with generalized malnutrition or use of phosphate binding agents[1]. The high TmP/GFR indicates an appropriate response to renally conserve phosphate in the face of enteral deficiency. If measured, FGF23 concentrations should be low, and 1,25(OH)2D concentrations are often elevated. Elimination of the causative factor typically normalizes phosphate metabolism. However, some conditions such as extreme prematurity where the phosphate needs are higher, and use of certain infant or pediatric formulae may also predispose to hypophosphatemia. Specifically “elemental” or amino acid based formulae have been reported to cause chronic hypophosphatemia due to impaired intestinal phosphate absorption [25, 26], attributed to impaired bioavailability of phosphate and possibly influenced by additional factors such as proton pump inhibitors. This hypophosphatemia may be associated with skeletal manifestations including rickets and fragility fractures. It is of note that the brand of formulae most associated with cases of hypophosphatemia (Neocate) did make changes to its formulation in 2018[27], after the initial publication of a large case series [25]. It is not yet clear whether this risk was eliminated by the change in formulation or whether there are also patient specific factors that contributed to the risk. However, in the original reports, switching formula did result in normalization of phosphate metabolism.
Hypophosphatemia with low TmP/GFR
Hypophosphatemia with low TmP/GFR may be further categorized according to whether or not it is mediated by FGF23 [1]. Several FGF23-mediated causes of hypophosphatemia have led to important discoveries about the mechanisms of phosphate metabolism, which include autosomal dominant hypophosphatemic rickets (ADHR), X-linked hypophosphatemic rickets (XLH), autosomal recessive hypophosphatemic rickets (ARHR, due to mutations in multiple genes), fibrous dysplasia (FD), epidermal nevus syndrome and tumor-induced osteomalacia (TIO). Non-FGF23 mediated causes of hypophosphatemia with low TmP/GFR include hereditary hypophosphatemic rickets with hypercalciuria, Dent’s disease, nephropathic cystinosis, and other congenital or acquired (drug induced) generalized renal tubulopathies in various forms of Fanconi syndrome.
The FGF23-mediated hypophosphatemic disorders biochemically share common features which are the consequence of excess FGF23 activity including low serum phosphate, increased urinary phosphate excretion (or decreased TmP/GFR), normal serum and urine calcium, high alkaline phosphatase (ALP), normal 25(OH)D, and decreased or inappropriately normal 1,25(OH)2D [1]. While PTH is often cited as being normal in these conditions, a secondary hyperparathyroidism is common even before treatment, which can be exacerbated by treatment with phosphate [28]. In contrast, in those conditions that are not mediated by FGF23, the 1,25(OH)2D may be elevated, with resultant increases in serum or urine calcium.
X-linked hypophosphatemia (XLH)
XLH is the most common of the congenital hypophosphatemias and the most common genetic form of rickets. The prevalence has been estimated at 1 in 20,000 to 25,000 persons[29, 30]. XLH is caused by a mutation in the PHEX gene. PHEX is expressed in osteocytes and odontoblasts which also express FGF23[31, 32]. PHEX deficiency leads to increased gene expression of FGF23, and elevated FGF23 protein levels [31], which decrease renal tubular phosphate reabsorption, causing hypophosphatemia, as discussed above. Intact FGF23 is generally elevated in XLH, though upper normal range FGF23 may also be seen[33]. While hypophosphatemia mediates most of the important clinical features of XLH during childhood, other consequences of PHEX deficiency, such as increased osteopontin[34], may also contribute to impaired skeletal mineralization and to features such as tooth abscesses.
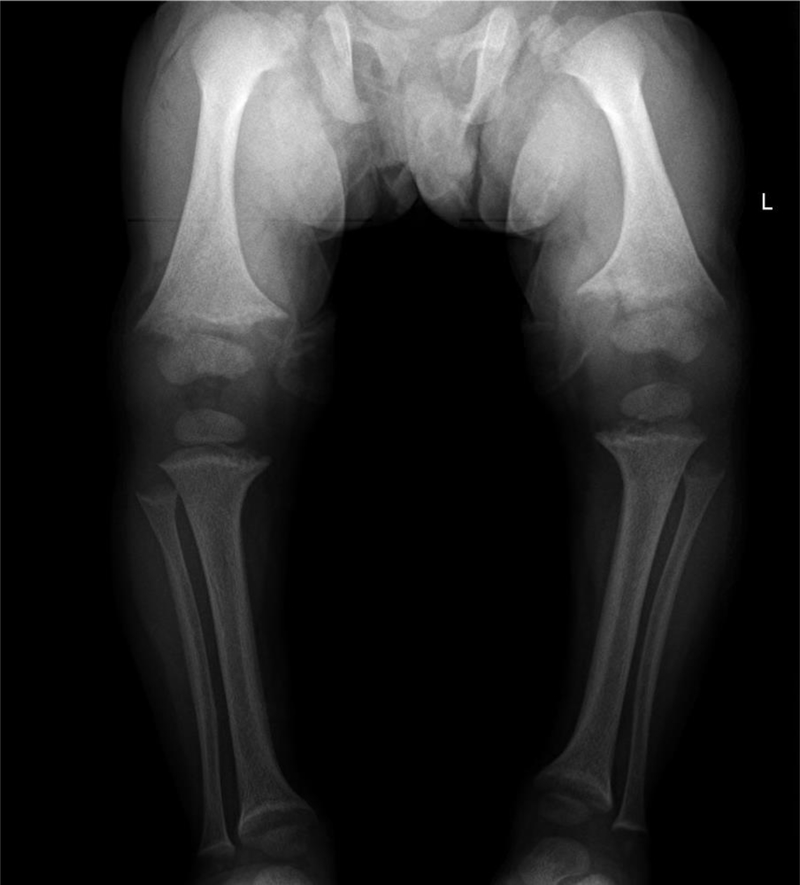
At birth, patients do not typically have bowed legs or impaired growth, but evidence of rickets develops over time and by 1 year of age many patients with XLH are manifesting bowed legs, and concurrently begin to show growth impairments as they begin weight bearing[23, 24, 30, 35]. Figure 2 shows the lower limb radiograph of a 15 month old male with XLH demonstrating typical rachitic features. Despite conventional therapy however, the mean height of adults with XLH remains < −2 SDS compared to country specific norms [23]. The growth of the lower limbs is disproportionately impaired compared to that of the upper limbs[23].
Figure 2.
Radiographs from 15-month-old male with XLH, demonstrating rachitic features in the lower limbs including metaphyseal widening, lucency, fraying, and separation from the epiphysis along with bowing deformities.
While medical treatments can improve the degree of skeletal deformity in growing children, skeletal deformities in the lower limbs still often require surgical intervention to straighten long bones and alignment or to correct torsional abnormalities[30]. Osteotomies with internal or external fixation, or guided growth procedures using plates across the physis of the distal femur or proximal tibia are used to correct these deformities[36]. Challenges exist regarding the optimal timing for such procedures. Guided growth procedures must be completed while there is still time for sufficient growth, while osteotomies may be better performed when growth is near complete because of concerns for recurrence of the deformity. While patients often start with bowing deformities, valgus deformity of the knees can develop over time.
Tooth abscesses and periodontal disease are very common with 62–78% of adults having moderate to severe dental disease, often result in tooth loss[37, 38]. However tooth abscesses are also very common in children with XLH [30]. Teeth of XLH patients demonstrate impaired mineralization of the cementum and dentin [34]. The role of hypophosphatemia itself in the dental phenotype is supported by studies that suggest treatment with phosphate and active vitamin D is beneficial for reducing, though not eliminating, these dental complications [37, 38].
Pseudofractures are a common complication of osteomalacia in adults with XLH, contributing to bone pain. Retrospective studies suggested that fractures may not be increased in XLH patients based on patient self-report[39, 40]. However, when subjected to skeletal radiographs in cross sectional studies, up to half of adults with XLH have active fractures or pseudofractures detected [41, 42]. This suggests that unrecognized pseudofractures likely contribute to the symptomatology of XLH. However, fractures have not been commonly reported among children with XLH
Enthesopathy and osteoarthritis become important clinical complications of XLH in adulthood, and though not generally described in children, affected parents often worry about their children growing up to have similar degrees of debility to themselves. Enthesopathy is common at the spine, shoulders, hips, knees, ankles and feet, approaching 100% in some studies, leading to osteophytes and contributing to limited joint range of motion, pain and quality of life impairments, especially when severe[41, 43, 44]. Orthopedic procedures for joint replacements are also quite common in adults with XLH, generally at a younger age than unaffected adults[45]. Several neurologic issues are important to patients with XLH. Skull shape abnormalities are common and are related at least in part to early suture fusion. Craniosynostosis occurred in one study in 59%, especially of the sagittal suture[46]. Furthermore, the posterior fossa is more shallow, likely contributing to the observed 25% rate of Chiari type 1 malformations[46–48]. Sensorineural hearing loss may be seen in childhood (9%) but becomes more frequent in adulthood (29–82% in various studies)[39, 49, 50]. Enthesopathy of the spine can lead to spinal stenosis, nerve compression and myelopathy in at least 12 % of adults[51].
Autosomal Dominant Hypophosphatemic Rickets (ADHR) due to FGF23 mutations
The gene for FGF23 was discovered during linkage studies searching for the genetic cause of autosomal dominant hypophosphatemic rickets (ADHR)[52]. This mutation changes arginine amino acids at an RXXR cleavage motif and causes impaired cleavage of the FGF23 protein from the active hormone to inactive fragments[53]. The expected biochemical phenotype of hypophosphatemia and impaired activation of vitamin D results from elevated FGF23 in ADHR. However ADHR is unique among the inherited phosphate disorders in that the gene mutation itself is not solely responsible for the phenotype, and there is an interaction with an acquired condition, namely iron deficiency[19, 54]. Although autosomal dominant hypophosphatemic rickets (ADHR) can present as a congenital hypophosphatemia, it does not always[17, 55]. ADHR has a clinical phenotype that can change from normal to hypophosphatemic and back again spontaneously. Thus, ADHR may present in childhood with hypophosphatemic rickets and osteomalacia. However, kindreds with ADHR have also demonstrated clear examples of normophosphatemia during childhood with normal growth and development, and subsequent onset of hypophosphatemia with severe osteomalacia and complications such as pseudofractures, muscle weakness and bone pain[17, 55]. This delayed onset of hypophosphatemia characterizes about half of the adult ADHR patients we have studied. This delayed onset also means that ADHR should always be considered in the differential diagnosis for patients with a clinical presentation suggesting possible tumor induced osteomalacia, as the severity of acquired hypophosphatemic symptoms is often similar. The hypophosphatemic patients also sometimes normalized their serum phosphate concentrations, apparently spontaneously[17, 55]. The periods of hypophosphatemia are driven by excess intact FGF23, while during the quiescent phases, the intact FGF23 and serum phosphate concentrations become normal[17, 19].
Clinical and animal model studies have demonstrated the mechanism of this effect is due to iron stores[19, 54]. Animal models have demonstrated that in the setting of iron deficiency Hif1alpha accumulates leading to increased FGF23 gene expression[54]. The increased FGF23 protein that is produces is normally cleaved to maintain normal intact FGF23 levels and normophosphatemia generally persists, as is demonstrable by an inverse relationship between serum iron or ferritin concentrations and C-terminal FGF23 concentrations in otherwise healthy patients, but absence of a relationship between iron or ferritin with intact FGF23 concentrations[19, 56–59].
However, this adaptation to maintain normal intact FGF23 can be disrupted. If iron deficiency occurs in persons having the ADHR mutation in the FGF23 gene, cleavage of the protein is impaired, leading to elevated intact FGF23 concentrations and resultant hypophosphatemia[19]. Thus, women with ADHR are particularly at risk for developing hypophosphatemia during their reproductive years. The importance of iron to this phenotype was recently demonstrated in both a case report and a prospective clinical trial demonstrating resolution of hypophosphatemia and normalization of FGF23 concentrations in iron deficient patients with ADHR after oral iron supplementation[60, 61]. Thus, testing for iron deficiency and maintaining normal iron stores may restore phosphate homeostasis in ADHR. Unfortunately data from both mouse studies and cross sectional human studies would indicate that iron deficiency effects do not influence intact FGF23 concentrations in the related disorder, XLH[62, 63].
The story of iron and FGF23 is even more complex, however. Importantly, while oral iron supplementation in iron deficient ADHR patients is recommended, intravenous iron administration could be detrimental. This is because, for reasons that are not yet understood, some intravenous iron formulations given to iron deficient patients can trigger an acute and prolonged rise in intact FGF23 and resultant hypophosphatemia[64–69]. Remember that FGF23 gene expression is elevated in the setting of iron deficiency, even among patients without FGF23 mutations. Several case reports and now multiple prospective clinical trials have clearly demonstrated that intravenous iron in this setting can cause an acute rise in intact FGF23, which is thought to be due to some other factor, possibly the carbohydrate moiety of the iron infusion, impairing cleavage of FGF23[64–69]. Indeed, the propensity to develop hypophosphatemia after intravenous iron varies with the formulation, with most reports citing iron carboxymaltose or iron polymaltose. This is clinically important in non-ADHR patients who may manifest severe osteomalacia or other hypophosphatemic signs and symptoms. In a patient with already impaired FGF23 cleavage due to ADHR, the effect might be worse, though we deliberately did not attempt intravenous iron in our prospective iron clinical trial for ADHR, for patient safety[60].
Autosomal Recessive Hypophosphatemic Rickets (ARHR)
Mutations in multiple genes are noted to cause rare autosomal recessive forms of hypophosphatemic rickets mediated by FGF23 excess. These conditions have varied additional phenotypic features as well. Overall, forms of ARHR are far more rare than XLH.
Loss of function mutations in dentin matrix protein 1 (DMP1) lead to severe rickets and osteomalacia in both humans and mouse models[70, 71]. Patients and mice have defective organization of osteocyte lacunae, and elevations of FGF23 gene expression, with resultant increases in serum FGF23 that are not observed in heterozygotes[70]. Patients with DMP1 mutations also develop short stature, deformation of long bones, and enthesopathies, similar to XLH [72]. Dental abscesses and enlarged pulp chambers are also seen, consistent with the importance of DMP1 to dental development[72, 73]. In a mouse model, ablation of FGF23 ameliorated the osteomalacia in the DMP1 null mouse[74].
Mutations in ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) cause generalized arterial calcification of infancy (GACI) resulting in calcification and stenosis of medium to large arteries[75–79]. Multiple deleterious mutations in this gene have been reported, which impair generation of the mineralization inhibitor pyrophosphate[80]. This condition is often lethal in infancy (66% of cases in one series)[77]. However, of those treated with bisphosphonates 65% survived past infancy versus only 31% of those not treated with bisphosphonates [77]. Knockout mice for enpp1 demonstrate increased gene expression for FGF23 and hypophosphatemia [81], and patients with ENPP1 mutations also have high FGF23 levels, which can result in hypophosphatemia[78, 79]. Some patients with ENPP1 mutations are reported having hypophosphatemic rickets without GACI[78]. Interestingly, development of hypophosphatemia has also been associated with improved survival in GACI[77]. Thus, there may be some concerns that treating the hypophosphatemic rickets in this setting might increase risk of vascular calcifications. One case report in a patient with symptomatic rickets suggests that treating the hypophosphatemia with calcitriol and phosphate did not worsen vascular calcifications[82]. While PHEX or DMP1 deficiencies cause lower cementum mineralization, mutations in ENPP1 cause increased cementum thickness and density leading to delayed exfoliation of primary teeth and disruption of tooth movement[83]. Ligament calcifications and enthesopathies also occur[84, 85].
Raine syndrome is an osteosclerotic bone disorder with cerebral calcifications. Siblings were reported with a non-lethal form of Raine syndrome due to recessive FAM20C (family with sequence similarity 20, member C) mutations[86] having tooth decay, osteosclerosis of long bones, cerebral calcifications and facial and acral dysmorphisms, and the unexpected finding of hypophosphatemia due to elevated FGF23 levels. Inactivation of FAM20C in mice and cell lines leads to high FGF23 expression[87]. FAM20C phosphorylates FGF23, which inhibits O-glycosylation by GALNT3, thus promoting FGF23 cleavage to limit intact FGF23 secretion[88]. Thus, loss of FAM20C allows for increased secretion of intact FGF23, and resulting hypophosphatemia.
Fibrous Dysplasia (FD) of bone and similar disorders
Fibrous dysplasia of bone is genetic in nature but does not usually present with congenital features. However, hypophosphatemic bone disease may present during childhood in patients with fibrous dysplasia. Fibrous dysplasia of bone is typically due to post-zygotic mutations in GNAS, and may occur in isolation (as monostotic or polyostotic forms) or as part of the McCune-Albright Syndrome[89]. The somatic mutations result in constitutive activation of adenylyl cyclase in those cells[90]. In cross-sectional studies, half of patients with fibrous dysplasia demonstrate renal phosphate wasting mediated by high FGF23 concentrations, which correlate to the total body burden of fibrous dysplasia though rickets is a rare complication in FD [91, 92], likely because most of the FGF23 produced by the affected cells is cleaved[93].
Post-zygotic somatic activating mutations in HRAS, KRAS, and NRAS cause linear nevus sebaceous syndrome (LNSS),a neurocutaneous disorder affecting the skeletal and central nervous systems with prominent seizures and neurodevelopmental abnormalities[94, 95]. Patients with LNSS develop skeletal lesions consistent with fibrous dysplasia along with hypophosphatemic rickets due to elevated FGF23 levels [96]. Evidence suggests the most likely source for the excess FGF23 production in these patients is the bony fibrous dysplasia lesions[97].
Patients having the autosomal dominant disorder of osteoglophonic dysplasia also have non-ossifying bone lesions similar in radiographic appearance to fibrous dysplasia, along with systemic skeletal dysplastic features of rhizomelic dwarfism, craniofacial defects, and hypodontia. This disorder is caused by dominant activating mutation in the FGFR1 receptor[98, 99]. Hypophosphatemia in this condition has been demonstrated to be due to elevated FGF23 levels hypothesized to be from the non-ossifying bone lesions. Mechanistically it should be noted that in cell culture and animal models the activation of FGFR1 in osteoblasts or osteocytes stimulates FGF23 production[100, 101], while in the hyp (XLH) mouse model, deletion of FGFR1 from the osteocytes partially suppresses the excess FGF23 production [102].
Hypophosphatemia with low TmP/GFR and suppressed FGF23.
Several forms of congenital hypophosphatemia involve renal phosphate losses due to issues endogenous to the renal tubule, in which case FGF23 decreases as the appropriate homeostatic response. Here some notable examples are presented.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH)
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a rare autosomal recessive disorder due to loss of function mutations affecting the sodium phosphate co-transporter type 2c[21]. Affected individuals may present with signs of rickets and osteomalacia (bone pain, lower limb deformities and gait abnormalities) and/or nephrolithiasis or nephrocalcinosis. Since in this setting the primary defect is in renal phosphate transporters, FGF23 is not involved in the pathophysiology and is decreased. As a result, 1,25(OH)2D levels become elevated, resulting in increased intestinal calcium absorption, and hypercalciuria in addition to their renal phosphate losses. Thus, patients with HHRH have a strong propensity to nephrocalcinosis and nephrolithiasis, even without medical therapy. In fact, even heterozygous family members are at risk for hypercalciuria, nephrocalcinosis and nephrolithiasis[103]. Although usually presenting in childhood, a homozygous case with late onset of clinical manifestations with nephrolithiasis and osteoporosis has been described[104].
HHRH is important to distinguish from other causes of congenital hypophosphatemia as the treatment is different in important ways. Patients with HHRH are treated using monotherapy with phosphate salts, without active vitamin D[104], while FGF23 mediated hypophosphatemia is never treated with phosphate alone[24]. Active vitamin D would be contraindicated in HHRH since the 1,25(OH)2D is high, and adding active vitamin D could worsen hypercalciuria or cause hypercalcemia. However, treating HHRH with phosphate monotherapy does improve the rachitic bone disease, while such treatment is not sufficient for XLH.
Autosomal dominant hypophosphatemic rickets due to SGK3 mutations.
Another autosomal dominant form of hypophosphatemic rickets was recently described. Mutations in the gene for serum-and glucocorticoid-inducible kinase 3 (SGK3) were reported in one kindred as a cause of hypophosphatemic rickets due to impaired renal phosphate reabsorption [105]. The mutation, a splice mutation that resulted in exon 13 skipping and in frame deletion of 29 amino acids, affects the protein kinase domain. SGK3 null mice demonstrate mild hyperphosphaturia with normal serum phosphate [106]. The mechanism for phosphaturia is not fully understood. These mice did not have alterations in gene expression or brush border membrane protein for sodium phosphate cotransporters. However, coexpression of SGK3 with sodium-phosphate cotransporter type 2a in Xenopus oocytes, enhanced both sodium-phosphate cotransporter type 2a surface expression and phosphate transport [106], suggesting that lack of SGK3 can alter phosphate transport by impairing function of this transporter.
SGK3 null mice also had lower plasma 1,25(OH)2D and FGF23 concentrations compared to wild type [106]. In the only kindred reported so far with hypophosphatemia due to SGK3 mutations, low or low normal 1,25(OH)2D was seen, but calcium was normal [105]. FGF23 was tested in 3 affected patients. One had high C-terminal FGF23 and intact FGF23 that the authors attributed to iron deficiency, while the other two had normal C-terminal FGF23 and intact FGF23 concentrations. The authors concluded that this was not FGF23 mediated hypophosphatemia, but acknowledged that FGF23 might still also be dysregulated since it was not fully suppressed despite hypophosphatemia.
Other tubulopathies
Several forms of general renal tubulopathy may present in childhood and should be recognized as being FGF23-independent. In general, these share the features of Fanconi syndromes having renal losses of multiple substrates besides phosphate to varying degrees, such as calcium, potassium, glucose, salt, magnesium, and amino acids or frank proteinuria. Thus, it is worth evaluating children presenting with renal hypophosphatemia for other evidence for tubulopathy. Some examples are included below.
Sodium phosphate co-transporters type 2a
Mutations affecting sodium phosphate co-transporters type 2a do not merely cause hypophosphatemic rickets but instead have been described causing an autosomal recessive Fanconi syndrome with proximal tubular renal phosphate losses, amino aciduria and glucosuria[107]. These patients also had absorptive hypercalciuria due to high 1,25(OH)2D. These mutations resulted in a failure to transport the mutant protein to the membrane and the general tubulopathy was hypothesized to be due to resulting intracellular accumulation and tubular damage.
Dent’s disease
Dent’s disease is an X-linked recessive form of hyphophosphatemia which may also present with a variable phenotype including hypophosphatemic rickets and osteomalacia along with high 1,25(OH)2D, hypercalciuria[108]. Proteinuria is a prominent feature. Nephrocalcinosis or nephrolithiasis is common, and this condition leads to chronic kidney disease and often renal failure by adulthood. Dent’s disease is caused by loss of function mutations in the gene for chloride channel 5 or by certain mutations in OCRL1 (a gene in which different mutations cause Lowes syndrome), both of which result in impair lysosomal trafficking in the renal tubule[108].
Cystinosis
Nephropathic cystinosis is an autosomal recessive disease where mutations in the CTNS gene, which encodes the lysosomal cystine transporter cystinosin, causes accumulation of stored cystine in lysosomes and subsequent tissue damage in many organs [22]. In the nephropathic form, infants present with a Fanconi syndrome with urinary losses of electrolytes, minerals including calcium and phosphate, water, glucose, bicarbonate and amino acids. The resulting metabolic bone disease causes rickets and poor linear growth. Repletion of phosphate is necessary to treat the rickets, and active vitamin D is added, but patients must be carefully monitored for hypercalciuria[22]. Disease targeted treatment with cysteamine can delay end organ damage in the kidney and other tissues.
Medical treatment
XLH was originally categorized as a vitamin D resistant rickets due to the inability to cure it by repleting nutritional forms of vitamin D; consequently very high doses were used[109]. Adding oral phosphate was helpful, but oral phosphate alone was ineffective at treating the rickets in growing children[110]. In addition, phosphate dosing increases the risk for hyperparathyroidism. Multiple investigators subsequently demonstrated that addition of active forms of vitamin D was necessary to treat rickets and osteomalacia in patients with XLH[111–113], and this has been the conventional form of therapy for over 40 years. Thus, treatment with active forms of vitamin D and phosphate salts address the downstream biochemical consequences of excess FGF23. However, such treatment does not improve the TmP/GFR.
Published recommendations vary widely for both phosphate and active vitamin D doses. This is in part due to varied individual patient response to a given dose, side effects of therapy and a lack of systematic trials to determine the optimal dose regimen. However, in general starting doses of phosphate are recommended between 20–60 mg/kg per day and must be given in doses divided 4 or 5 times a day, while calcitriol doses range from 20–40 ng/kg per day and alfacalcidol 30–50 ng/kg per day with titrations depending on effect, safety parameters and tolerability[24, 30]. Overall treatment must be individualized. Some children may do well on smaller doses while some may require higher doses to heal rickets and osteomalacia. Gastrointestinal tolerability including a laxative effect of phosphate necessitate starting at lower doses and titrating upward generally.
Dosing is also limited by important side effects including hyperparathyroidism and nephrocalcinosis[114–117]. Secondary hyperparathyroidism occurs in about 83% of patients, while about 4% of children and 30% of adults develop tertiary hyperparathyroidism[114]. Consequently, vigilant monitoring of laboratory values including calcium, phosphate, creatinine and PTH are necessary to guide titration for safe administration of therapy for XLH. Further, patients should be monitored for development of hypercalciuria, which would necessitate a decrease in the dose of active vitamin D. Patients should also be monitored with renal ultrasounds for development of nephrocalcinosis every 1 to 2 years. Radiographs should be conducted to follow for changes in rickets and limb deformities.
Studies have demonstrated that in growing children conventional therapy improves growth and rickets, especially when imitated within the first 1 to 2 years after birth [35, 118]. However, it is also clear that skeletal outcomes are highly variable, and at the end of growth the mean height is < −2 SDS compared to population normative values[23]. Assessment of the results of these studies would indicate these growth deficits appear first around 1–2 years of age shortly after beginning ambulation and worsen again during puberty. Despite appropriate therapy, many children require corrective surgeries for lower extremity deformities[30, 36].
As XLH is the most common congenital hypophosphatemic condition, its treatment has been the most robustly studied, and generally medical treatment of FGF23 mediated hypophosphatemia has followed the conventional therapy for XLH. Thus, patients with ADHR, ARHR, fibrous dysplasia or linear sebaceous nevus syndrome have been managed with phosphate plus active vitamin D. However, as described above, recent studies in ADHR have indicated that the ADHR clinical phenotype is driven by iron deficiency, and that both FGF23 and phosphate can be normalized in ADHR patients by repleting their iron stores with oral iron supplementation[19, 60, 61]. In contrast, several hypophosphatemic conditions that are not mediated by FGF23 have a component of hypercalciuria (such as HHRH), in which case active vitamin D treatment is avoided as it may worsen the hypercalciuria.
Anti-FGF23 antibody for XLH
Recently burosumab, a fully human monoclonal antibody to FGF23 was approved for treatment of XLH in multiple countries[119, 120]. Burosumab is administered subcutaneously in children over 6 months of age at doses of 0.8 to 1.2 mg/kg every 2 weeks, with maximum doses of 90 mg. In adults with XLH, burosumab is given at 1 mg/kg every 4 weeks. Burosumab is described in detail in an accompanying article by Dr. Insogna in this issue.
By binding FGF23, burosumab blocks the effects of FGF23 resulting in increased TmP/GFR and hence serum phosphate in both adults and children [121–123]. Burosumab also increases production of 1,25(OH)2D. Three trials have been conducted in children: two phase 2 uncontrolled open labeled trials to establish doses in children ages 1–4 years old[124] and in children ages 5–12 years old[121], and an open label randomized controlled trial comparing to conventional therapy among children ages 1–12 at enrollment[125]. The initial trial was conducted in children ages 5–12 years of age over 64 weeks of treatment and established effective doses and identified more stable improvements of serum phosphate better improvements in rickets with burosumab dosing every 2 weeks compared to every 4 weeks[121]. The second trial confirmed appropriate dosing, changes in serum phosphate and rachitic improvements in children ages 1–4 years[124].
The phase 3 trial in children sought to determine the comparative effectiveness of switching children from conventional therapy to burosumab versus continuing on conventional therapy[125]. To date this has been the only trial of burosumab in adults or children to include a comparison group treated with conventional therapy. Out of 122 children screened 55 (45%) failed screening due to insufficient severity of rickets, and 61 were randomized. At baseline the randomized groups had received prior conventional therapy for a mean of 3.3 and 4.3 years. In order to qualify for the trial, patients had to have evidence of active rickets with a Thacher rickets severity score of at least 2 (where scores ranged from 0–10 with 10 being the worst and 0 being no active rickets). Each radiograph was assessed by three radiologists blinded to treatment assignment and the scores averaged. Rickets was assessed using two radiographic methods at the wrists and knees: the Thacher rickets severity score (where decreasing score from baseline was better) and the Radiographic Global Impression of Change (RGI-C, which is an ordinal scale ranging from negative scores indicating worsening, 0 indicating no change, +1 minimal healing, +2 substantial healing and + 3 complete healing).
Both treatment groups demonstrated improvements of rickets by both scales. However, the magnitude of improvement was greater in the group treated with burosumab than in the conventional therapy group, including the primary outcome of RGI-C at 40 weeks [+1.9 (SE 0.1) vs +0.8 (SE 0.1) (p<0.0001)], and also at 64 weeks [+2.1 (SE 0.1) vs +1.0 (SE 0.1) (p<0.0001)]. The Thacher rickets severity score decreased by −2.0 (SE 0.1) in the burosumab group and by −0.7 (SE 0.1) in the conventional therapy group at 40 weeks (p<0.0001). Those treated with burosumab had increases in fasting serum phosphate and in TmP/GFR compared to those receiving conventional therapy, as well as greater decreases in alkaline phosphatase, and increases in length/height Zscore.
Treatment requires careful blood and urine laboratory monitoring similarly to conventional therapy, except burosumab is titrated to achieve serum phosphate within the lower end of the normal range. Hyperphosphatemia should still be avoided, and would necessitate dose decrease. During the initial titration phase, measuring serum laboratory tests every 2 to 4 weeks for calcium, creatinine and phosphate can help guide therapy. Alkaline phosphatase and PTH are measured approximately every 3 months along with assessments of urine calcium excretion. Further, renal ultrasounds should still be conducted to screen or follow nephrocalcinosis.
The primary and most common adverse events in the burosumab trials in children were transient injection site reactions[121, 124, 125]. The risk for nephrocalcinosis was similar between groups during the trials[125, 126]. More tooth abscesses occurred during the study period in the burosumab group than the conventional therapy group in children (over 64 weeks) or the placebo group in adults (over 24 weeks) [125, 126]. In an adult trial, those treated with burosumab reported restless legs syndrome (11.8%) more frequently than placebo treated adults (7.6%)[41].
The long term effects of burosumab on adult height reached in growing children, limb deformities, need for skeletal surgery, tooth abcesses, nephrocalcinosis, hyperparathyroidism and other complications of XLH remain to be determined. Importantly the clinical trials for burosumab to date have only included XLH and tumor induced osteomalacia, and it is only approved for XLH.
Additional treatment considerations.
Extensive laboratory monitoring during treatment of hypophosphatemia is necessary for both efficacy and for safety to ensure patients do not develop excessive hypercalciuria, impaired kidney function or hyperparathyroidism. Serum and urine aboratory testing should be conducted approximately every 3 months. Renal ultrasounds are monitored annually for development of nephrocalcinosis. In the setting of nephrocalcinosis or chronic kidney disease, it is useful to include monitoring by a pediatric nephrologist, if one is not already involved.
An orthopedic surgeon with experience managing patients with congenital hypophosphatemia is a critical member of the care team. Radiographs are needed to follow for improvements in rickets, and changes in skeletal deformities to guide surgical intervention or to evaluate areas of bone pain. Surgery may be needed to correct skeletal deformities including bowing and torsion and resultant gait abnormalities, using osteotomies or guided growth procedures. Orthopedic care may also be needed to treat fractures or pseudofractures, though these seem to be a larger problem for adults. Physiotherapy is can help increase mobility, especially during recovery from orthopedic injuries or surgery.
Dental abscesses are especially common among XLH patients, as well as periodontitis, but dental complications also occur in other hypophosphatemic diseases. Good dental hygiene and care with a dental provider are important for prevention and addressing dental complications. Hearing loss is an under-recognized complication of XLH, including among children, and patients may benefit from audiology evaluation. Obesity can contribute to decreased mobility in patients with skeletal deformities. Involvement of a dietician to provide guidance to assist weight management may be useful. Social work can help patients address psychosocial aspects of their disease burden or to access resources. Patient support groups provide an important source of information, advocacy and encouragement for families.
Summary
There are several forms of congenital hypophosphatemias, with overlapping clinical features especially regarding skeletal effects. These conditions though overall rare, result in considerable medical burden to those affected both in childhood and as adults. Treatment should be guided by whether conditions are mediated by FGF23 or by other mechanisms. Recognition of hypercalciuria as part of the underlying disease in conditions such as HHRH and Dent’s disease, or as a complication of therapy, is critical to safe management. For XLH therapeutic options have been expanded to include conventional therapy (with phosphate and active vitamin D) or monotherapy with burosumab, though the long term benefits and risks of burosumab are not fully known. For ADHR evidence suggests that normalizing iron stores with oral iron is therapeutic correcting the high FGF23. In all of the congenital hypophosphatemic conditions, the treatment must be carefully monitored and individualized.
Acknowledgments
Funding: This work was supported by NIH grant P30AR072581.
Footnotes
Declarations
Conflicts of interest/Competing interests: EAI has received research funding from Ultragenyx Pharmaceuticals and participated in advisory boards.
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable.
This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Terms of use and reuse: academic research for non-commercial purposes, see here for full terms. https://www.springer.com/aam-terms-v1
References
- 1.Imel EA, Econs MJ (2012) Approach to the hypophosphatemic patient. J Clin Endocrinol Metab 97:696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imel EA, Carpenter TO (2018) Rickets: The skeletal disorders of impaired calcium or phosphate availability In: Radovick Sally, Misra M (eds) Pediatric Endocrinology: A Practical Clinical Guide, 3rd edition Springer International Publishing AG, New York, New York, p 497–524 [Google Scholar]
- 3.Demay MB, Sabbagh Y, Carpenter TO (2007) Calcium and vitamin D: what is known about the effects on growing bone. Pediatrics 119 Suppl 2:S141–144 [DOI] [PubMed] [Google Scholar]
- 4.Tiosano D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27:392–401 [DOI] [PubMed] [Google Scholar]
- 5.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC (2000) Radiographic scoring method for the assessment of the severity of nutritional rickets. Journal of tropical pediatrics 46:132–139 [DOI] [PubMed] [Google Scholar]
- 6.DiMeglio LA, Imel EA (2019) Calcium and Phosphate: Hormonal Regulation and Metabolism In: Burr DB, Allen MR (eds) Basic and Applied Bone Biology. Elsevier, Academic Press, New York, p 257–282 [Google Scholar]
- 7.Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2:309–310 [DOI] [PubMed] [Google Scholar]
- 8.Stark H, Eisenstein B, Tieder M, Rachmel A, Alpert G (1986) Direct measurement of TP/GFR: a simple and reliable parameter of renal phosphate handling. Nephron 44:125–128 [DOI] [PubMed] [Google Scholar]
- 9.El-Maouche D, Dumitrescu CE, Andreopoulou P, Gafni RI, Brillante BA, Bhattacharyya N, Fedarko NS, Collins MT (2016) Stability and degradation of fibroblast growth factor 23 (FGF23): the effect of time and temperature and assay type. Osteoporos Int 27:2345–2353 [DOI] [PubMed] [Google Scholar]
- 10.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663 [DOI] [PubMed] [Google Scholar]
- 11.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D (2012) Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Annals of clinical biochemistry 49:546–553 [DOI] [PubMed] [Google Scholar]
- 12.Smith ER, Cai MM, McMahon LP, Holt SG (2012) Biological variability of plasma intact and Cterminal FGF23 measurements. J Clin Endocrinol Metab 97:3357–3365 [DOI] [PubMed] [Google Scholar]
- 13.Gkentzi D, Efthymiadou A, Kritikou D, Chrysis D (2014) Fibroblast growth factor 23 and Klotho serum levels in healthy children. Bone 66:8–14 [DOI] [PubMed] [Google Scholar]
- 14.Ali FN, Josefson J, Mendez AJ, Mestan K, Wolf M (2016) Cord Blood Ferritin and Fibroblast Growth Factor-23 Levels in Neonates. J Clin Endocrinol Metab 101:1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmlund-Suila E, Viljakainen H, Ljunggren O, Hytinantti T, Andersson S, Makitie O (2016) Fibroblast Growth Factor 23 Concentrations Reflect Sex Differences in Mineral Metabolism and Growth in Early Infancy. Horm Res Paediatr 85:232–241 [DOI] [PubMed] [Google Scholar]
- 16.Holmlund-Suila E, Enlund-Cerullo M, Valkama S, Hauta-Alus H, Rosendahl J, Helve O, Hytinantti T, Viljakainen H, Andersson S, Makitie O (2017) Sex and Iron Modify Fibroblast Growth Factor 23 (FGF23) Concentration in 1-Year-Old Children. J Clin Endocrinol Metab [DOI] [PubMed] [Google Scholar]
- 17.Imel EA, Hui SL, Econs MJ (2007) FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22:520–526 [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed] [Google Scholar]
- 19.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ (2011) Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 96:3541–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folsom LJ, Imel EA (2015) Hyperphosphatemic familial tumoral calcinosis: genetic models of deficient FGF23 action. Curr Osteoporos Rep 13:78–87 [DOI] [PubMed] [Google Scholar]
- 21.Bergwitz C, Miyamoto KI (2019) Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Archiv : European journal of physiology 471:149–163 [DOI] [PubMed] [Google Scholar]
- 22.Hohenfellner K, Rauch F, Ariceta G, Awan A, Bacchetta J, Bergmann C, Bechtold S, Cassidy N, Deschenes G, Elenberg E, Gahl WA, Greil O, Harms E, Herzig N, Hoppe B, Koeppl C, Lewis MA, Levtchenko E, Nesterova G, Santos F, Schlingmann KP, Servais A, Soliman NA, Steidle G, Sweeney C, Treikauskas U, Topaloglu R, Tsygin A, Veys K, R VV, Zustin J, Haffner D (2019) Management of bone disease in cystinosis: Statement from an international conference. J Inherit Metab Dis 42:1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zivicnjak M, Schnabel D, Billing H, Staude H, Filler G, Querfeld U, Schumacher M, Pyper A, Schroder C, Bramswig J, Haffner D, Hypophosphatemic Rickets Study Group of Arbeitsgemeinschaft fur Padiatrische E, Gesellschaft fur Padiatrische N (2011) Age-related stature and linear body segments in children with X-linked hypophosphatemic rickets. Pediatr Nephrol 26:223–231 [DOI] [PubMed] [Google Scholar]
- 24.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 26:1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez Ballesteros LF, Ma NS, Gordon RJ, Ward L, Backeljauw P, Wasserman H, Weber DR, DiMeglio LA, Gagne J, Stein R, Cody D, Simmons K, Zimakas P, Topor LS, Agrawal S, Calabria A, Tebben P, Faircloth R, Imel EA, Casey L, Carpenter TO (2017) Unexpected widespread hypophosphatemia and bone disease associated with elemental formula use in infants and children. Bone 97:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creo AL, Epp LM, Buchholtz JA, Tebben PJ (2018) Prevalence of Metabolic Bone Disease in Tube-Fed Children Receiving Elemental Formula. Horm Res Paediatr 90:291–298 [DOI] [PubMed] [Google Scholar]
- 27.Upgraded Neocate Junior [Internet]. Neocate Syneo. [cited 2019Nov15]. Available from: https://www.neocate.com/junior/. In:
- 28.Carpenter TO, Mitnick MA, Ellison A, Smith C, Insogna KL (1994) Nocturnal hyperparathyroidism: a frequent feature of X-linked hypophosphatemia. J Clin Endocrinol Metab 78:1378–1383 [DOI] [PubMed] [Google Scholar]
- 29.Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK (2009) Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol 160:491–497 [DOI] [PubMed] [Google Scholar]
- 30.Haffner D, Emma F, Eastwood DM, Duplan MB, Bacchetta J, Schnabel D, Wicart P, Bockenhauer D, Santos F, Levtchenko E, Harvengt P, Kirchhoff M, Di Rocco F, Chaussain C, Brandi ML, Savendahl L, Briot K, Kamenicky P, Rejnmark L, Linglart A (2019) Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nature Reviews Nephrology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK (2008) Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest 118:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onishi T, Umemura S, Shintani S, Ooshima T (2008) Phex mutation causes overexpression of FGF23 in teeth. Arch Oral Biol 53:99–104 [DOI] [PubMed] [Google Scholar]
- 33.Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T, Matsumoto T (2008) Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone 42:1235–1239 [DOI] [PubMed] [Google Scholar]
- 34.Boukpessi T, Hoac B, Coyac BR, Leger T, Garcia C, Wicart P, Whyte MP, Glorieux FH, Linglart A, Chaussain C, McKee MD (2017) Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia. Bone 95:151–161 [DOI] [PubMed] [Google Scholar]
- 35.Makitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E (2003) Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 88:3591–3597 [DOI] [PubMed] [Google Scholar]
- 36.Gizard A, Rothenbuhler A, Pejin Z, Finidori G, Glorion C, de Billy B, Linglart A, Wicart P (2017) Outcomes of orthopedic surgery in a cohort of 49 patients with X-linked hypophosphatemic rickets (XLHR). Endocr Connect 6:566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biosse Duplan M, Coyac BR, Bardet C, Zadikian C, Rothenbuhler A, Kamenicky P, Briot K, Linglart A, Chaussain C (2017) Phosphate and Vitamin D Prevent Periodontitis in X-Linked Hypophosphatemia. Journal of dental research 96:388–395 [DOI] [PubMed] [Google Scholar]
- 38.Connor J, Olear EA, Insogna KL, Katz L, Baker S, Kaur R, Simpson CA, Sterpka J, Dubrow R, Zhang JH, Carpenter TO (2015) Conventional Therapy in Adults With X-Linked Hypophosphatemia: Effects on Enthesopathy and Dental Disease. J Clin Endocrinol Metab 100:3625–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Econs MJ, Samsa GP, Monger M, Drezner MK, Feussner JR (1994) X-Linked Hypophosphatemic Rickets - a Disease Often Unknown to Affected Patients. Bone and mineral 24:17–24 [DOI] [PubMed] [Google Scholar]
- 40.Beck-Nielsen SS, Brusgaard K, Rasmussen LM, Brixen K, Brock-Jacobsen B, Poulsen MR, Vestergaard P, Ralston SH, Albagha OM, Poulsen S, Haubek D, Gjorup H, Hintze H, Andersen MG, Heickendorff L, Hjelmborg J, Gram J (2010) Phenotype presentation of hypophosphatemic rickets in adults. Calcified tissue international 87:108–119 [DOI] [PubMed] [Google Scholar]
- 41.Insogna KL, Briot K, Imel EA, Kamenicky P, Ruppe MD, Portale AA, Weber T, Pitukcheewanont P, Cheong HI, Jan de Beur S, Imanishi Y, Ito N, Lachmann RH, Tanaka H, Perwad F, Zhang L, Chen CY, Theodore-Oklota C, Mealiffe M, San Martin J, Carpenter TO, Investigators A (2018) A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults With X-Linked Hypophosphatemia: Week 24 Primary Analysis. J Bone Miner Res 33:1383–1393 [DOI] [PubMed] [Google Scholar]
- 42.Reid IR, Hardy DC, Murphy WA, Teitelbaum SL, Bergfeld MA, Whyte MP (1989) X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine (Baltimore) 68:336–352 [PubMed] [Google Scholar]
- 43.Polisson RP, Martinez S, Khoury M, Harrell RM, Lyles KW, Friedman N, Harrelson JM, Reisner E, Drezner MK (1985) Calcification of entheses associated with X-linked hypophosphatemic osteomalacia. N Engl J Med 313:1–6 [DOI] [PubMed] [Google Scholar]
- 44.Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM (2009) Survey of the enthesopathy of Xlinked hypophosphatemia and its characterization in Hyp mice. Calcified tissue international 85:235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills ES, Iorio L, Feinn RS, Duignan KM, Macica CM (2019) Joint replacement in X-linked hypophosphatemia. Journal of orthopaedics 16:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothenbuhler A, Fadel N, Debza Y, Bacchetta J, Diallo MT, Adamsbaum C, Linglart A, Di Rocco F (2019) High Incidence of Cranial Synostosis and Chiari I Malformation in Children With X-Linked Hypophosphatemic Rickets (XLHR). Journal of Bone and Mineral Research 34:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gjorup H, Beck-Nielsen SS, Haubek D (2017) Craniofacial and dental characteristics of patients with vitamin-D-dependent rickets type 1A compared to controls and patients with X-linked hypophosphatemia. Clinical oral investigations [DOI] [PubMed] [Google Scholar]
- 48.Gjorup H, Sonnesen L, Beck-Nielsen SS, Haubek D (2014) Upper spine morphology in hypophosphatemic rickets and healthy controls: a radiographic study. Eur J Orthod 36:217–225 [DOI] [PubMed] [Google Scholar]
- 49.Davies M, Kane R, Valentine J (1984) Impaired hearing in X-linked hypophosphataemic (vitamin-Dresistant) osteomalacia. Ann Intern Med 100:230–232 [DOI] [PubMed] [Google Scholar]
- 50.Fishman G, Miller-Hansen D, Jacobsen C, Singhal VK, Alon US (2004) Hearing impairment in familial X-linked hypophosphatemic rickets. European journal of pediatrics 163:622–623 [DOI] [PubMed] [Google Scholar]
- 51.Chesher D, Oddy M, Darbar U, Sayal P, Casey A, Ryan A, Sechi A, Simister C, Waters A, Wedatilake Y, Lachmann RH, Murphy E (2018) Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The_ADHR_Consortium;, White KE, Evans WE, O’Riordan JL, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Meitinger T, Strom TM (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 [DOI] [PubMed] [Google Scholar]
- 53.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomaldominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney international 60:2079–2086 [DOI] [PubMed] [Google Scholar]
- 54.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proceedings of the National Academy of Sciences of the United States of America 108:E1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82:674–681 [DOI] [PubMed] [Google Scholar]
- 56.Durham BH, Joseph F, Bailey LM, Fraser WD (2007) The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Annals of clinical biochemistry 44:463–466 [DOI] [PubMed] [Google Scholar]
- 57.Braithwaite V, Prentice AM, Doherty C, Prentice A (2012) FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int J Pediatr Endocrinol 2012:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Braithwaite V, Jones KS, Assar S, Schoenmakers I, Prentice A (2014) Predictors of intact and Cterminal fibroblast growth factor 23 in Gambian children. Endocr Connect 3:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imel EA, Liu Z, McQueen AK, Acton D, Acton A, Padgett LR, Peacock M, Econs MJ (2016) Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone 86:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imel EA, Liu Z, Coffman M, Acton D, Mehta R, Econs MJ (2020) Oral Iron Replacement Normalizes Fibroblast Growth Factor 23 in Iron-Deficient Patients With Autosomal Dominant Hypophosphatemic Rickets. J Bone Miner Res 35:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapelari K, Kohle J, Kotzot D, Hogler W (2015) Iron Supplementation Associated With Loss of Phenotype in Autosomal Dominant Hypophosphatemic Rickets. J Clin Endocrinol Metab 100:3388–3392 [DOI] [PubMed] [Google Scholar]
- 62.Imel EA, Gray AK, Padgett LR, Econs MJ (2014) Iron and fibroblast growth factor 23 in X-linked hypophosphatemia. Bone 60:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hum JM, Clinkenbeard EL, Ip C, Cass TA, Allen M, White KE (2017) The metabolic bone disease associated with the Hyp mutation is independent of osteoblastic HIF1alpha expression. Bone Rep 6:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schouten BJ, Doogue MP, Soule SG, Hunt PJ (2009) Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Annals of clinical biochemistry 46:167–169 [DOI] [PubMed] [Google Scholar]
- 65.Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG (2009) FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 94:2332–2337 [DOI] [PubMed] [Google Scholar]
- 66.Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, Fukumoto S, Sugimoto T, Fujita T (2009) Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 45:814–816 [DOI] [PubMed] [Google Scholar]
- 67.Bishay RH, Ganda K, Seibel MJ (2017) Long-term iron polymaltose infusions associated with hypophosphataemic osteomalacia: a report of two cases and review of the literature. Therapeutic advances in endocrinology and metabolism 8:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W (2018) Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf M, Koch TA, Bregman DB (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28:1793–1803 [DOI] [PubMed] [Google Scholar]
- 70.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM (2006) DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mäkitie O, Pereira RC, Kaitila I, Turan S, Bastepe M, Laine T, Kröger H, Cole WG, Jüppner H (2010) Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 25:2165–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turan S, Aydin C, Bereket A, Akcay T, Güran T, Yaralioglu BA, Bastepe M, Jüppner H (2010) Identification of a novel dentin matrix protein-1 (DMP-1) mutation and dental anomalies in a kindred with autosomal recessive hypophosphatemia. Bone 46:402–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD (2008) Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab 295:E254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nurnberg P (2003) Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34:379–381 [DOI] [PubMed] [Google Scholar]
- 76.Ruf N, Uhlenberg B, Terkeltaub R, Nürnberg P, Rutsch F (2005) The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI). Human mutation 25:98–98 [DOI] [PubMed] [Google Scholar]
- 77.Rutsch F, Boyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, Weissen-Plenz G, Fischer RJ, Mughal Z, Gregory JW, Davies JH, Loirat C, Strom TM, Schnabel D, Nurnberg P, Terkeltaub R, Group GS (2008) Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet 1:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R (2010) Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. American journal of human genetics 86:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. American journal of human genetics 86:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nitschke Y, Rutsch F (2017) Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Curr Osteoporos Rep 15:255–270 [DOI] [PubMed] [Google Scholar]
- 81.Mackenzie NC, Zhu D, Milne EM, van ‘t Hof R, Martin A, Darryl Quarles L, Millan JL, Farquharson C, MacRae VE (2012) Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PloS one 7:e32177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferreira CR, Ziegler SG, Gupta A, Groden C, Hsu KS, Gahl WA (2016) Treatment of hypophosphatemic rickets in generalized arterial calcification of infancy (GACI) without worsening of vascular calcification. American journal of medical genetics 170A:1308–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thumbigere-Math V, Alqadi A, Chalmers NI, Chavez MB, Chu EY, Collins MT, Ferreira CR, FitzGerald K, Gafni RI, Gahl WA, Hsu KS, Ramnitz MS, Somerman MJ, Ziegler SG, Foster BL (2018) Hypercementosis Associated with ENPP1 Mutations and GACI. Journal of dental research 97:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S, Fujitab T (2011) A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone 49:913–916 [DOI] [PubMed] [Google Scholar]
- 85.Mehta P, Mitchell A, Tysoe C, Caswell R, Owens M, Vincent T (2012) Novel compound heterozygous mutations in ENPP1 cause hypophosphataemic rickets with anterior spinal ligament ossification. Rheumatology (Oxford) 51:1919–1921 [DOI] [PubMed] [Google Scholar]
- 86.Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, Bjerknes R (2013) Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res 28:1378–1385 [DOI] [PubMed] [Google Scholar]
- 87.Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, Sun Y, Hao J, George A, Lu Y, Groppe J, Yuan B, Feng JQ, Qin C (2012) Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet 8:e1002708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE (2014) Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proceedings of the National Academy of Sciences of the United States of America 111:5520–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hartley I, Zhadina M, Collins MT, Boyce AM (2019) Fibrous Dysplasia of Bone and McCune-Albright Syndrome: A Bench to Bedside Review. Calcified tissue international 104:517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM (1991) Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. New England Journal of Medicine 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- 91.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, Wientroub S, Bianco P, Robey PG (2001) Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res 16:806–813 [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV, Gafni RI, Cherman N, Cho M, Hager GL, Collins MT (2012) Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res 27:1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menascu S, Donner EJ (2008) Linear nevus sebaceous syndrome: case reports and review of the literature. Pediatric neurology 38:207–210 [DOI] [PubMed] [Google Scholar]
- 95.Lim YH, Ovejero D, Sugarman JS, Deklotz CM, Maruri A, Eichenfield LF, Kelley PK, Juppner H, Gottschalk M, Tifft CJ, Gafni RI, Boyce AM, Cowen EW, Bhattacharyya N, Guthrie LC, Gahl WA, Golas G, Loring EC, Overton JD, Mane SM, Lifton RP, Levy ML, Collins MT, Choate KA (2014) Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia. Human molecular genetics 23:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffman WH, Jueppner HW, Deyoung BR, O’Dorisio M S, Given KS (2005) Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. American journal of medical genetics 134:233–236 [DOI] [PubMed] [Google Scholar]
- 97.Ovejero D, Lim YH, Boyce AM, Gafni RI, McCarthy E, Nguyen TA, Eichenfield LF, DeKlotz CM, Guthrie LC, Tosi LL, Thornton PS, Choate KA, Collins MT (2016) Cutaneous skeletal hypophosphatemia syndrome: clinical spectrum, natural history, and treatment. Osteoporos Int 27:3615–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuthiroly S, Yesodharan D, Ghosh A, White KE, Nampoothiri S (2017) Osteoglophonic Dysplasia: Phenotypic and Radiological Clues. J Pediatr Genet 6:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ (2005) Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. American journal of human genetics 76:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han X, Xiao Z, Quarles LD (2015) Membrane and integrative nuclear fibroblastic growth factor receptor (FGFR) regulation of FGF-23. J Biol Chem 290:10447–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu A-L, Feng B, Chen MZ, Kolumam G, Zavala-Solorio J, Wyatt SK, Gandham VD, Carano RAD, Sonoda J (2013) Antibody-mediated activation of FGFR1 induces FGF23 production and hypophosphatemia. PloS one 8:e57322-e57322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD (2014) Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PloS one 9:e104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dasgupta D, Wee MJ, Reyes M, Li Y, Simm PJ, Sharma A, Schlingmann KP, Janner M, Biggin A, Lazier J, Gessner M, Chrysis D, Tuchman S, Baluarte HJ, Levine MA, Tiosano D, Insogna K, Hanley DA, Carpenter TO, Ichikawa S, Hoppe B, Konrad M, Savendahl L, Munns CF, Lee H, Juppner H, Bergwitz C (2014) Mutations in SLC34A3/NPT2c are associated with kidney stones and nephrocalcinosis. J Am Soc Nephrol 25:2366–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dhir G, Li D, Hakonarson H, Levine MA (2017) Late-onset hereditary hypophosphatemic rickets with hypercalciuria (HHRH) due to mutation of SLC34A3/NPT2c. Bone 97:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cebeci AN, Zou M, BinEssa HA, Alzahrani AS, Al-Rijjal RA, Al-Enazi AF, Al-Mohanna FA, Cavalier E, Meyer BF, Shi Y (2019) Mutation of SGK3, a novel regulator of renal phosphate transport, causes autosomal dominant hypophosphatemic rickets. J Clin Endocrinol Metab [DOI] [PubMed] [Google Scholar]
- 106.Bhandaru M, Kempe DS, Rotte A, Capuano P, Pathare G, Sopjani M, Alesutan I, Tyan L, Huang DY, Siraskar B, Judenhofer MS, Stange G, Pichler BJ, Biber J, Quintanilla-Martinez L, Wagner CA, Pearce D, Foller M, Lang F (2011) Decreased bone density and increased phosphaturia in gene-targeted mice lacking functional serum- and glucocorticoid-inducible kinase 3. Kidney international 80:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K (2010) A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med 362:1102–1109 [DOI] [PubMed] [Google Scholar]
- 108.Devuyst O, Thakker RV (2010) Dent’s disease. Orphanet journal of rare diseases 5:28–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glorieux FH, Scriver CR, Reade TM, Goldman H, Roseborough A (1972) Use of phosphate and vitamin D to prevent dwarfism and rickets in X-linked hypophosphatemia. N Engl J Med 287:481–487 [DOI] [PubMed] [Google Scholar]
- 110.Stickler GB, Hayles AB, Rosevear JW (1965) Familial hypophosphatemic vitamin D resistant rickets: effect of increased oral calcium and phosphorus intake without high doses of vitamin D. American journal of diseases of children (1960) 110:664–667 [DOI] [PubMed] [Google Scholar]
- 111.Drezner MK, Lyles KW, Haussler MR, Harrelson JM (1980) Evaluation of a role for 1,25dihydroxyvitamin D3 in the pathogenesis and treatment of X-linked hypophosphatemic rickets and osteomalacia. The Journal of clinical investigation 66:1020–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costa T, Marie PJ, Scriver CR, Cole DE, Reade TM, Nogrady B, Glorieux FH, Delvin EE (1981) X-linked hypophosphatemia: effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J Clin Endocrinol Metab 52:463–472 [DOI] [PubMed] [Google Scholar]
- 113.Glorieux FH, Marie PJ, Pettifor JM, Delvin EE (1980) Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med 303:1023–1031 [DOI] [PubMed] [Google Scholar]
- 114.DeLacey S, Liu Z, Broyles A, El-Azab SA, Guandique CF, James BC, Imel EA (2019) Hyperparathyroidism and parathyroidectomy in X-linked hypophosphatemia patients. Bone 127:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goodyer PR, Kronick JB, Jequier S, Reade TM, Scriver CR (1987) Nephrocalcinosis and its relationship to treatment of hereditary rickets. J Pediatr 111:700–704 [DOI] [PubMed] [Google Scholar]
- 116.Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M (1991) Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med 325:1843–1848 [DOI] [PubMed] [Google Scholar]
- 117.Rivkees SA, el-Hajj-Fuleihan G, Brown EM, Crawford JD (1992) Tertiary hyperparathyroidism during high phosphate therapy of familial hypophosphatemic rickets. J Clin Endocrinol Metab 75:1514–1518 [DOI] [PubMed] [Google Scholar]
- 118.Quinlan C, Guegan K, Offiah A, Neill RO, Hiorns MP, Ellard S, Bockenhauer D, Hoff WV, Waters AM (2012) Growth in PHEX-associated X-linked hypophosphatemic rickets: the importance of early treatment. Pediatr Nephrol 27:581–588 [DOI] [PubMed] [Google Scholar]
- 119.EMA (2017) Crysvita : EPAR - Product Information. In, http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004275/hum an_med_002224.jsp&mid=WC0b01ac058001d124
- 120.FDA (2019) CRYSVITA® (burosumab-twza) injection, for subcutaneous use; Initial U.S. Approval: 2018; revised 9/2019. In: FDA; (ed), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761068Orig1s000TOC.cfm [Google Scholar]
- 121.Carpenter TO, Whyte MP, Imel EA, Boot AM, Hogler W, Linglart A, Padidela R, Van’t Hoff W, Mao M, Chen CY, Skrinar A, Kakkis E, San Martin J, Portale AA (2018) Burosumab Therapy in Children with X-Linked Hypophosphatemia. N Engl J Med 378:1987–1998 [DOI] [PubMed] [Google Scholar]
- 122.Carpenter TO, Imel EA, Ruppe MD, Weber TJ, Klausner MA, Wooddell MM, Kawakami T, Ito T, Zhang X, Humphrey J, Insogna KL, Peacock M (2014) Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest 124:1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Imel EA, Zhang X, Ruppe MD, Weber TJ, Klausner MA, Ito T, Vergeire M, Humphrey JS, Glorieux FH, Portale AA, Insogna K, Peacock M, Carpenter TO (2015) Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J Clin Endocrinol Metab 100:2565–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whyte MP, Carpenter TO, Gottesman GS, Mao M, Skrinar A, San Martin J, Imel EA (2019) Efficacy and safety of burosumab in children aged 1–4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol 7:189–199 [DOI] [PubMed] [Google Scholar]
- 125.Imel EA, Glorieux FH, Whyte MP, Munns CF, Ward LM, Nilsson O, Simmons JH, Padidela R, Namba N, Cheong HI, Pitukcheewanont P, Sochett E, Hogler W, Muroya K, Tanaka H, Gottesman GS, Biggin A, Perwad F, Mao M, Chen CY, Skrinar A, San Martin J, Portale AA (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, activecontrolled, open-label, phase 3 trial. Lancet 393:2416–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Insogna KL, Rauch F, Kamenicky P, Ito N, Kubota T, Nakamura A, Zhang L, Mealiffe M, San Martin J, Portale AA (2019) Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J Bone Miner Res 34:2183–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]