Abstract
Rationale:
Prospective cohort studies question the value of HDL-C for stroke risk prediction.
Objective:
Investigate the relationship between long-term functional recovery and HDL proteome and function.
Methods and Results:
Changes in HDL protein composition and function (cholesterol efflux capacity, or CEC) in patients after acute ischemic stroke at two time points (24 h, 35 patients; 96 h, 20 patients) and in 35 control subjects were measured. The recovery from stroke was assessed by 3 month The National Institute of Health Stroke Scale (NIHSS) and Modified Rankin scale (mRS) scores. When compared to control subject after adjustments for sex and HDL-C levels, twelve proteins some of which participate in acute phase response and platelet activation (APMAP, GPLD1, APOE, IHH, ITIH4, SAA2, APOA4, CLU, ANTRX2, PON1, SERPINA1, and APOF) were significantly (adj. p<0.05) altered in stroke HDL at 96h. The first eight of these proteins were also significantly altered at 24h. Consistent with inflammatory remodeling, CEC was reduced by 32% (P<0.001) at both time points. Baseline stroke severity adjusted regression model showed that changes within 96 hours post stroke in APOF, APOL1, APMAP, APOC4, APOM, PCYOX1, PON1, and APOE correlate with stroke recovery scores (R2=0.38–0.73, adjusted p<0.05). APOF (R2=0.73), and APOL1 (R2=0.60) continued to significantly correlate with recovery scores after accounting for tPA treatment.
Conclusion:
Changes in HDL proteins during early acute phase of stroke associate with recovery. Monitoring HDL proteins may provide clinical biomarkers that inform on stroke recuperation.
Keywords: Stroke, HDL, proteomics, NIHSS, stroke recovery, HDL proteome, HDL function, recovery biomarkers
Subject Terms: Lipids and Cholesterol, Ischemic Stroke
Graphical Abstract
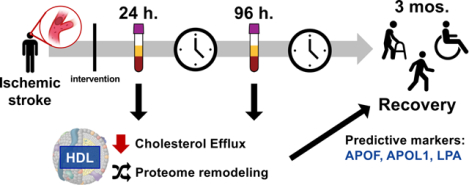
INTRODUCTION
Stroke is the second leading cause of death worldwide and a major cause of adult disability[1,2]. Although survival rates have improved with the current treatment options, patients often face the burden of post-stroke permanent disability[2]. Reliable recovery prediction models are needed to improve recovery and reduce post-stroke disability rates.
High density lipoproteins (HDL) are a heterogeneous group of lipid-protein complexes. HDL-cholesterol (HDL-C) levels exhibit strong inverse correlations with coronary artery disease (CAD) in many populations[3,4], and HDL’s main atheroprotective actions are believed to be the promotion of reverse cholesterol transport[5,6] and the suppression of inflammatory responses[7,8]. Some prospective cohort studies support an inverse association between HDL-C levels and ischemic stroke risk[9–14] while meta-analyses examining the relationship between HDL-C and stroke indicate either no relationship [15,16] or even a direct one[17]. These studies cast doubt on the validity of HDL-C as a marker for stroke risk prediction. HDL metrics other than cholesterol content have been scarcely studied with regard to stroke. Baseline HDL size was significantly larger among cases vs. controls in the Northern Manhattan Study (NOMAS)[18] and in the Women’s Health Initiative [19]. HDL cholesterol efflux capacity (CEC) describes HDL’s ability to promote cholesterol efflux from macrophages[20], the initial step in reverse cholesterol transport. HDL CEC has been shown to be associated with prevalent and incident cardiovascular disease, even after adjustment for levels of both HDL cholesterol and APOA1 (the major structural protein of HDL)[10,21–23]. In an isolated report, greater HDL CEC, independent of HDL-C and APOA1 levels, was found associated with increased stroke risk[11]. The relationship between CEC, stroke events, and recovery remains to be established.
The human HDL proteome consists of over 100 proteins that highly controlled by genetics and modulated by health status[24–28]. Most changes in HDL function are likely a reflection of changes in the HDL proteome[24,25,29]. HDL proteome changes have been previously associated with diverse functional outcomes in human cohorts. For example, a recent study identified PON1 and APOC2 enrichment with HDL particles from fenofibrate-treated diabetics which displayed reduced sterol efflux[26]. HDL associated proteins such as SAA1, APOE, and PLTP have been implicated in increased CHD risk [24,25]. Specifically, HDL associated APOE levels strongly predicted new clinical events in the secondary prevention CARE trial[30]. Furthermore, in a small study, HDL from stroke subjects was shown to have reduced APOA1 and PON1 protein levels when compared to controls [31]. While valuable, these approaches are reductionist and do not capture the global proteomic changes due to stroke event and the continued remodeling in early acute phase of stroke.
Our previous studies suggest that HDL is composed of highly co-regulated and genetically controlled core proteins that primarily associate with lipid metabolism and peripheral, environmentally regulated proteins linked to the acute inflammatory response[32]. The latter group of proteins seem to reflect the local environment interacting with the HDL particle. We propose that an acute stroke event will remodel HDL’s protein cargo to incorporate proteins associated with stroke characteristics, due to HDL particle-stroke milieu interaction.
A reproducible and valid method for quantification of neurological deficits that occur after stroke is essential for monitoring patients’ functional recovery[33]. Stroke scales represent a useful tool for estimating the severity of stroke at onset and for assessing prognostic information in clinic. The National Institute of Health Stroke Scale (NIHSS) is the most frequently used stroke deficit scale in routine clinical practice and clinical trials[33]. The modified Rankin scale (mRS) and Barthel index (BI) are widely used functional impairment and disability scales, which have proven to be valid and reliable in defining outcome of stroke patients[34]. While some studies show strong agreement between NIHSS and mRS, others suggest that NIHSS is more sensitive than mRS[33]. We have used both the NIHSS and mRS in our analyses, as they are routine stroke scales most commonly used in clinic for more than two decades[35]. We predicted that HDL protein cargo will reflect the stroke event and associate with the patient’s recovery scores.
We tested the hypothesis that changes in HDL-associated proteome after an acute ischemic stroke associate with physiological responses and predict the functional recovery from stroke events. Plasma samples were collected at two time points, 24 hour and 96 hours post-stroke intervention, and protein cargo of isolated HDL particles was analyzed by quantitative parallel reaction monitoring mass spectrometry. The baseline stroke severity and 3 month functional and neurological recovery was assessed by NIHSS and mRS scoring. We describe robust time dependent changes in HDL proteome consistent with an acute inflammatory event and show that these changes predict the functional recovery from stroke (see graphical abstract).
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Patient Cohort and Study Design.
All participants signed an IRB-approved informed consent. Acute ischemic stroke patients presenting to Oregon Health & Science University within 12 hours of stroke event were asked for consent to be included in the stroke study OHSU IRB: 6333. ~75% of the approached patients consented (n=35). Plasma was collected by venipuncture into EDTA tubes at 24 (n=35) and 96 hours (n=20) post-tissue plasminogen activator (tPA) delivery or mechanical thrombectomy, or 24- and 96-hours post-admission for non-treatment patients. The 96-hour sample volume was limited and available only for 20 patients. The subgroup adequately represents the parent cohort as seen in Table 1. Neurological assessment was performed using NIH Stroke Scale (NIHSS) following admission for a stroke event, and again at 3 months post ischemic stroke event [33]. Recovery was also assessed at 3 months by modified Rankin score (mRS)[34]. Two patients expired before the 3-month recovery assessment visit and were excluded from the recovery association analyses. One patient did not indicate their sex. The stroke cohort was age-matched to a non-stroke control cohort (n=35) (Table 1) with the following selection criteria: no significant cognitive impairment, no history of clinically significant stroke or of poorly controlled vascular risk factors. Less than 30% of subjects were on statin treatment, equally represented between groups. Plasma was collected on non-stroke subjects (n=35) as a part of the Oregon Alzheimer’s Disease Center Biorepository (IRB00006845: Layton Center and ORCATECH Research Repository). Subjects were free of significant cognitive impairment based on history, examination by a neurologist specializing in cognitive disorders, and interview with a collateral informant. Subjects with a history of clinically significant stroke or with poorly controlled vascular risk factors were excluded from the non-stroke patient group. Applying the health selection criteria and the age matching resulted in, 8 vs. 21 males in non-stroke vs. stroke respectively.
Table 1. Cohort Characteristics.
Stroke samples obtained 24-h poststroke intervention (n=35) and for some patients additionally at 96 h (n=20) are compared with nonstroke control samples free of history of stroke or cognitive impairment (n=35).
| Variable | Non-stroke | Stroke | ||
|---|---|---|---|---|
| Total | P-value | 24–96 h paired Subset | ||
| (N=35) | (N=35) | Stroke vs Non | (N=20) | |
| Male sex - no. (%) | 8 (23.5) | 21 (60.0) | 0.005 | |
| Statin use - no. (%) | 6 (19.4) | 10 (28.6) | 0.559 | |
| Age at blood collection - yr | 63.93 (3.42) | 68.20 (10.51) | 0.131 | 67.40 (10.98) |
| Lipid levels in mg/dL - mean (SD) | ||||
| Total Cholesterol | 208.34 (43.98) | 148.83 (40.54) | 1.37E-07 | 140.65 (40.83) |
| High-density lipoprotein cholesterol | 63.86 (20.92) | 43.09 (15.99) | 1.62E-05 | 38.80 (9.30) |
| Low-density lipoprotein cholesterol | 169.26 (52.22) | 128.43 (49.47) | 0.001 | 124.84 (55.22) |
| Triglycerides | 123.89 (86.49) | 113.46 (80.64) | 0.854 | 114.95 (75.76) |
| Ischemic stroke type - no. (%) | ||||
| Cardioembolic | NA | 8 (22.9) | 4 (20.0) | |
| Large Vessel | NA | 22 (62.9) | 14 (70.0) | |
| Small Vessel | NA | 5 (14.3) | 2 (10.0) | |
| tPA administration - no. (%) | NA | 16 (45.7) | 9 (45.0) | |
| Mechanical thrombectomy - no. (%) | NA | 15 (42.9) | 8 (40.0) | |
| TICI2B+ - no. (%) | NA | 10 (28.6) | 3 (15.0) | |
| Stroke severity scoring - median [IQR] | ||||
| NIHSS score at baseline | NA | 15.00 [9.00, 20.00] | 17.00 [10.75, 20.00] | |
| NIHSS score at 3 months post stroke | NA | 6.00 [1.00, 13.00] | 8.5 [1.75, 13.75] | |
| Modified Rankin score | NA | 3.00 [2.00, 4.00] | 4.0 [3.0, 4.75] | |
| Cholesterol efflux capacity - mean (SD) | 15.56 (4.45) | 10.55 (2.93) | 6.91E-07 | 9.98 (2.56) |
IQR indicates interquartile range; NA, not available; NIHSS, National Institutes of Health Stroke Scale; and tPA, tissue-type plasminogen activator.
Plasma lipid analysis.
Cholesterol, Triglycerides, HDL-C, and Lp(a) levels in plasma were measured at the clinical lipid laboratory at Oregon Health & Science University using a Hitachi 704 Chemistry Analyzer with Roche Diagnostics Reagents for Cholesterol, Triglycerides, HDL-C measurements or Medtest reagents for Lp(a) measurements. Cholesterol, Triglycerides, HDL-C, Friedewald’s LDL-C are reported as the lipid concentration (mg/dL). Lp(a) is reported as mass concentration (mg/dL)[36] .
Cholesterol efflux capacity (CEC) measurement.
Macrophage-mediated sterol efflux was measured using J774 cells as previously described[37]. Macrophages were radiolabeled with [3H]cholesterol and stimulated with a cAMP analogue. Efflux of [3H]cholesterol was measured after a 4-hour incubation in medium with serum depleted of APOB (2.8% v/v) by polyethylene glycol and calcium chloride. Cholesterol efflux was calculated as the percentage of radiolabel in the medium of the cells at the end of the incubation divided by the total radioactivity of the medium and cells.
HDL isolation and digestion.
Plasma was quickly thawed at 37°C, and 100 μL used to isolate HDL (density 1.063–1.210 g/mL) by sequential ultracentrifugation[32,38]. Total protein concentration in HDL was measured using the bicinchoninic assay (BCA) with bovine serum albumin as the standard. HDL was digested as previously described, with additional description in the online supplement[32,39]. Please see the Major Resources Table in the Supplemental Materials
HDL proteome analysis by mass spectrometry.
HDL proteome was analyzed by data-dependent acquisition (DDA) mass spectrometry to determine what proteins were present in the experimental HDL samples, and to construct a library of proteins and peptides for further targeted analysis by parallel reaction monitoring (PRM) mass spectrometry. Peptides for 41 proteins detected by DDA analysis were selected from previously published targeted assays[17]. Assay performance and detailed methods can be found in the online supplemental data.
Data and software availability.
The MS/MS datasets produced in this study are available in the PRIDE consortium PXD015001, and in Panorama https://panoramaweb.org/pamirstroke.url. A github page that contains source files and R analyses is found at https://github.com/dlplubell/StrokeHDLproteins.
Statistical analysis.
Stroke and cholesterol efflux association was evaluated by linear regression (stroke as binary dependent variable) to adjust for sex, age, HDL-C and LDL-C. Protein abundance is reported as the surrogate peptide normalized area under the curve for the top 5 intense fragment ion chromatographic peaks, as described above, and was log2 transformed. Significantly changing log2 protein abundance between stroke and non-stroke patients was determined through linear regression with adjustment for sex and HDL-C, with change in log2-protein abundance as the outcome, followed by Benjamini-Hochberg (BH) correction. For paired stroke samples from 24h and 96 hour post-intervention, significant changes in protein abundance was determined by a paired two-sided t-test, followed by Benjamini-Hochberg correction. Correlation between protein fold change or other patient characteristics and plasma lipid measures was determined by Pearson correlation coefficient with Benjamini-Hochberg correction. The association of change in protein abundance with recovery score was evaluated by multiple linear regression, adjusting for baseline stroke severity, or adjusting for baseline stroke severity and tPA status, sex (Male = 1, Female = 0), or age.
Power analysis.
The study was powered at 80% with FDR controlled at 5% to detect a difference in mean stroke recovery score between groups (n1=20 and n2=35) of 1 times the standard deviation in each group, assuming 38 proteins were tested and 13 had true differences at least that large. Due to the adjusted analyses losing some degrees of freedom in estimation, the detected effect size is likely larger than a mean difference of 1.
RESULTS
Cohort demographics and plasma lipid profiles.
Our study was designed to investigate the changes in HDL proteome and function following ischemic stroke event, during the early stages of the acute phase (Figure 1A). Plasma was collected from subjects with acute ischemic stroke presenting to OHSU after tPA (16 of the 35 participants) or mechanical thrombectomy intervention (15 of the 35 participants) within 12 hours of the stroke event. 5 participants received both tPA and thrombectomy. Of the 15 participants that received thrombectomy, 10 were TICI2b+. Blood was collected at 24 hours (n=35) and again at 96 hours post stroke intervention (n=20, of which 9 received t-PA treatment, 3 received successful thrombectomy) (Figure 1A). HDL was isolated and subjected to tryptic digest followed by MS/MS analysis (Figure 1B).
Figure 1. Experimental design for HDL characterization following stroke event.

a) Plasma was acquired from non-stroke patients and from stroke patients 24 h after intervention post ischemic stroke event, and additionally at 96 h after intervention post ischemic stroke event. Plasma drawn from patients were used to measure lipid levels, and processed to measure cholesterol efflux capacity and HDL proteome components. Stroke severity was assessed upon patient presentation to clinic, and recovery assessed at 3 months post-stroke event. b) For HDL proteomics, particles were isolated by stepwise ultracentrifugation, digested, and analyzed first by tandem mass spectrometry to determine whole proteome composition, followed by targeted parallel reaction monitoring mass spectrometry to quantify changes in 41 protein components of HDL.
Plasma HDL-C, total cholesterol, and triglycerides were measured (and LDL-C was calculated) for all patients at each time point (Table 1 and Online Table I). While the lipid metrics did not differ between the two post-stroke time points, plasma HDL-C, LDL-C, and total cholesterol levels were significantly lower (35, 30, and 40%, respectively) in stroke patients compared to non-stroke controls. The lower lipid levels were maintained between 24- and 96-hours post stroke intervention (Table 1).
A majority of stroke in this cohort were caused by large vessel ischemia (n=22, 62.9%), followed by cardioembolic causes (n=8, 22.9%) and small vessel ischemia (n=5, 14.3%). Half of the stroke patients received tPA (n=16) within the first 24 hours of stroke event. Baseline assessment of stroke severity by NIHSS score ranged from 9 to 20 with a median score of 15. The NIHSS score 3 months post-stroke ranged from 1 to 13, with a median of 6. The median change in NIHSS score from initial assessment to 3 months post-stoke decreased 9 points. Modified Rankin scores (mRS) 3 months post-stroke ranged from 2 to 4 with a median of 3 (Table 1).
The HDL proteome remodels over time following stroke events.
Stroke-driven changes in HDL proteome were investigated by preliminary discovery based semi-quantitative shotgun proteomics (Online Table II), followed by more accurate targeted parallel reaction monitoring quantification. HDL was isolated from subjects in three groups: non-stroke control (n=35), 24-hour post stroke intervention (n=35) and 96-hour post stroke intervention (n=20). Samples were analyzed by shotgun proteomics to identify global protein changes, peptide sequence detection, retention time, charge state of peptides, and intensity distributions of fragment ions in the MS/MS spectra. These parameters were used to develop parallel reaction monitoring assays for a subset of identified proteins as depicted in Figure 1B. Ninety-seven proteins were identified by shotgun proteomics on pooled HDL samples across control, 24-hour and 96-hour post-stroke samples (Online Table II presents the proteins identified and their relative abundance). Of these, 79 were detected with at least 2 unique peptides. The results from DDA analysis of the pooled samples were used to select peptides to target in the PRM experiments. The analysis of DDA experiments are presented in the online supplemental information (Online Table II).
Subsequent targeted analyses were performed on a subset of 41 proteins known for their established association with HDL[39,40]. The quantification of these proteins was carried out by parallel reaction monitoring assays. The sample preparation and targeted assay performance was assessed by the peptide percent coefficient of variation between replicate reference samples (Online Figure I). Three of the 41 proteins were removed from further statistical analyses due to high variance (%CV>50) post-normalization.
For the 38 remaining proteins, the sex and HDL-C adjusted abundance of 8 proteins was significantly altered between non-stroke and stroke groups at both 24-hour and 96-hour post-stroke (Figure 2 presents significantly different proteins between non-stroke and stroke subjects; Table 2 presents all significantly different proteins among all groups). Of these 8 proteins, 3 were reduced (APOA4, IHH, ITIH4) and 5 were increased (APMAP, APOE, CLU, GPLD1, SAA2) in stroke samples (P<0.05). Four additional proteins (ANTRX2, APOF, PON1, SERPINA1) (Table 2) were significantly altered in the 96-hour group compared to controls. Gene enrichment analysis indicated that the differentially expressed proteins are involved in lipid metabolism and acute phase response (Online Figure II) [24,25,32,40,41]. There were no significant changes between groups in terms of APOA1 levels (Online Table I).
Figure 2. Differentially abundant HDL proteins between stroke and non-stroke.
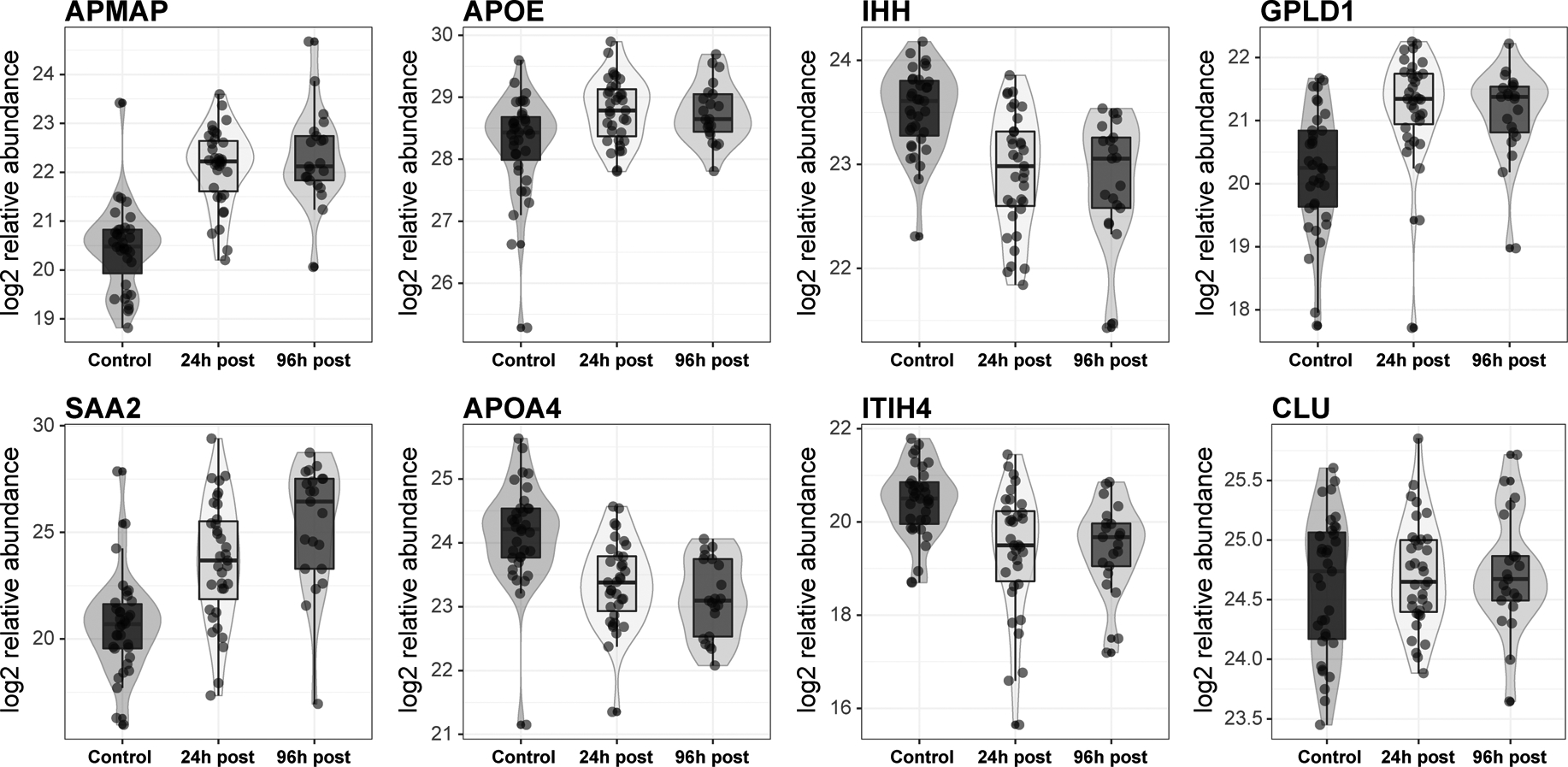
HDL isolated from control (n=35), stroke patients at 24 h (n=35), and 96 h post stroke intervention (n=20) were analyzed by parallel reaction monitoring for the target proteins. From the quantifiable proteins, 8 have significantly different log2 relative abundance between healthy patients and stroke subjects at 24 h after adjustment for sex and HDL-C levels.
Table 2. Significantly Different Protein Abundance by Parallel Reaction Monitoring.
A surrogate peptide was used for estimating protein level abundance for targeted proteins. Both 24 h poststroke intervention (n=35) vs nonstroke samples (n=35) and 96 h poststroke intervention (n=20) comparisons describe the log2 fold change of proteins in stroke subjects compared with nonstroke control subjects. Differential abundance was determined for between nonstroke and stroke time points by linear model adjusted for sex and HDL-C. Differential protein abundance for paired samples at 24 and 96 h was determined by paired 2-sided t test. For the paired measures, 3 proteins were not tested due to missing values (LPA, ITIH4, and SAA1). BenjaminiHochberg correction was applied for each comparison, with 38 tests for both nonstroke and stroke time point comparisons, and 35 tests for the paired comparison. Compared with healthy nonstroke HDL proteome, 8 proteins were significantly different in 24 h postintervention stroke patient HDL, and 4 additional proteins were significantly different in 96 h postintervention stroke patient HDL. Three proteins were significantly different from 24 to 96 h measurements.
| 24h vs. non-stroke | 96h vs. non-stroke | 96h vs. 24h (paired) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Abbrev. | log2FC | Adj. P value | log2FC | Adj. P value | log2FC | Adj. P value | ||
| adipocyte plasma membrane-associated protein | APMAP | 1.61 | 4.68E-08 | 1.84 | 9.04E-08 | 0.10 | 0.750 | ||
| phosphate inositol-glycan specific phospholipase D | GPLD1 | 1.47 | 6.23E-08 | 1.47 | 3.94E-06 | −0.01 | 0.963 | ||
| apolipoprotein E | APOE | 0.76 | 3.51E-04 | 0.73 | 0.003 | −0.06 | 0.750 | ||
| indian hedgehog protein | IHH | −0.59 | 0.001 | −0.68 | 0.001 | −0.12 | 0.557 | ||
| inter-alpha-trypsin inhibitor chain H4 | ITIH4 | −1.12 | 0.003 | −1.03 | 0.018 | − | - | ||
| serum amyloid A2 | SAA2 | 2.48 | 0.010 | 4.15 | 0.000 | 1.62 | 0.032 | ||
| apolipoprotein A-IV | APOA4 | −0.56 | 0.028 | −0.70 | 0.013 | −0.07 | 0.848 | ||
| clusterin | CLU | 0.36 | 0.041 | 0.42 | 0.029 | −0.02 | 0.954 | ||
| anthrax toxin receptor 2 | ANTXR2 | −0.25 | 0.253 | −0.81 | 1.77E-04 | −0.51 | 0.015 | ||
| alpha-1-antitrypsin | SERPINA1 | 0.21 | 0.474 | 0.70 | 0.013 | 0.42 | 0.163 | ||
| apolipoprotein F | APOF | −0.06 | 0.765 | 0.40 | 0.028 | 0.41 | 0.015 | ||
| serum paraoxonase/arylesterase 1 | PON1 | 0.15 | 0.253 | 0.28 | 0.037 | 0.16 | 0.174 | ||
Comparison; significance testing. 24 h vs nonstroke; linear model: ≈sample group + sex + HDL-C; 96 h vs nonstroke; linear model: ≈sample group + sex + HDL-C; and 96 vs 24 h; paired 2-sided t test. HDL indicates high-density lipoprotein; ITIH4, inter-alpha-trypsin inhibitor chain H4; and SAA1, serum amyloid A1.
Three proteins were significantly altered between 24-hour and 96-hour post-stroke intervention measurements. Anthrax toxin receptor 2 (ANTXR2) was decreased 1.5 fold (adjusted p=0.015), apolipoprotein F (APOF) was increased 1.4 fold (adjusted p=0.015), and serum amyloid A2 (SAA2) was increased 3.1 fold (adjusted p=0.032 Table 2). Because SAA2 is non-normally distributed by the Shapiro-Wilk test, in addition paired t-test, we have we have applied Wilcoxon signed rank test to assess changes in SAA2 levels between 24 and 96 hour. Benjamini-Hochberg adjusted p-values were comparable by the two methods, 0.0368 vs 0.0319 (the details of the analysis are in the online supplemental html and rmd files). Of the 20 individuals with both 24-hour and 96-hour measurements, 19 had decreased ANTXR2 at 96-hours, 16 had increased APOF at 96-hours, and 14 had increased SAA2 at 96-hours (Figure 3). APOF targeted mass spectrometry peptide measurements agree with immunoaffinity (western blotting) based quantification (Online Figure III).
Figure 3. HDL protein changes following stroke.

Log2 relative abundance of three proteins are significantly different between 24 h and 96 h post-stroke intervention by paired analysis. Lines connect the protein measures of the 20 patients with plasma drawn at both 24 h and 96 h time points.
Changes in HDL protein abundance correlate with functional recovery from stroke.
Using paired 24-hour and 96-hour sample measurements, we investigated if the time-dependent remodeling of the HDL proteome correlates with HDL function and stroke-related metrics. A total of 1326 correlations were calculated, of which 40 had Benjamini-Hochberg corrected |r| values >0.5 and P<0.05 (Figure 4A, correlation r and p values in Online Table IV). Hierarchical clustering analysis (Figure 4B) identified both expected (between APOA1 and APOA2; Cholesterol and LDL-C) and novel correlations. While baseline stroke severity associated with platelet binding protein (PPBP), platelet factor 4 (PF4), long-term stroke recovery associated with APOF. The hierarchical clustering analysis revealed that changes in APOF, LPA, APOL1, PCYOX1, APOC3, and PON1 abundance between 24- and 96-hour time points correlate with at least one measure of stroke functional recovery (Figure 4B, the entire analysis presented in Online Table IV). The change in protein expression levels did not correlate with age or t-PA treatment as shown by the global correlation plot (Figure 4A).
Figure 4. Relationship between stroke related patient metrics, HDL proteome, and function.
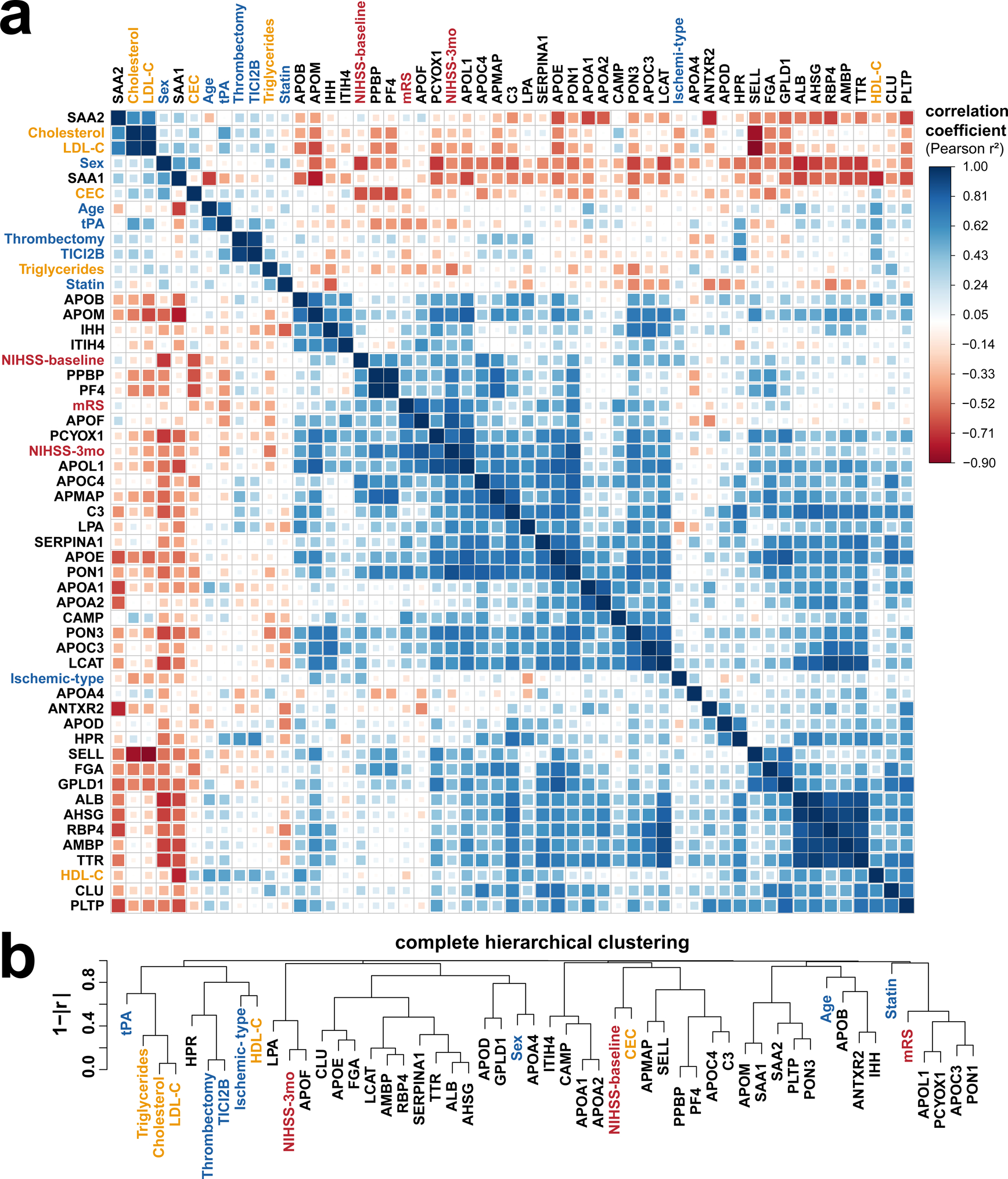
(a). Pearson correlations (positive in blue, negative in red) and (b) hierarchical clustering show stroke severity and recovery scores (in red) associating with HDL protein changes (black) more strongly than with other patient covariates (blue) or plasma lipid levels (gold).
Studies have shown that 3-month recovery scores associate with baseline stroke severity [42,43]. Therefore, we used a baseline stroke severity adjusted linear regression model to assess the association between changes in protein abundance and stroke recovery. In the regression model NIHSS score at 3 months is an outcome variable, and NIHSS score at baseline and log2FC in protein levels were predictor variables (Model: NIHSS-3mos~ NIHSS baseline + Protein log2FC, Table 3). The simple linear model examining the relationship between log2FC protein levels and NIHSS 3-month stroke recovery is presented in Online Table V. Changes in nine proteins (APOE, APOF, APOL1, APMAP, APOC4, APOM, LPA, PCYOX1, PON1) significantly correlated (adj.R2= 0.380–0.734 P<0.05) with NIHSS scoring at 3 months post stroke after adjusting for baseline stroke severity (Table 3 and Online Table V). APOF, APOL1, and LPA maintained their association with 3-month NIHSS recovery scores after including t-PA treatment in the regression model (Online Table V). APOF and LPA remained significantly associated after including sex and age in the model.
Table 3. Linear Regression Model for Differentially Expressed HDL Proteins and Stroke Recovery Swcores.
A model adjusting for NIHSS baseline scores was used to determine whether HDL protein changes from 24 to 96 h poststroke intervention are related to stroke recovery, as assessed at 3 mo poststroke by NIHSS. Benjamini-Hochberg correction was performed to correct for multiple testing (38 tests). Nine proteins corelated significantly with stroke recovery.
| Protein | Adj. R2 | Intercept | (SE) | F statistic | (DF) | P value | Adj. P value |
|---|---|---|---|---|---|---|---|
| APOF | 0.734 | −6.51 | (3.32) | 24.41 | (15) | 1.92E-05 | 7.30E-04 |
| APOL1 | 0.599 | 0.78 | (4.08) | 13.72 | (15) | 4.10E-04 | 0.008 |
| APMAP | 0.413 | 1.80 | (5.13) | 6.99 | (15) | 0.007 | 0.041 |
| LPA | 0.599 | −8.40 | (5.22) | 9.96 | (10) | 0.004 | 0.041 |
| APOC4 | 0.411 | 6.15 | (5.93) | 6.93 | (15) | 0.007 | 0.041 |
| APOM | 0.399 | −1.38 | (4.91) | 6.64 | (15) | 0.009 | 0.041 |
| PCYOX1 | 0.452 | 1.26 | (4.86) | 8.01 | (15) | 0.004 | 0.041 |
| PON1 | 0.401 | −1.76 | (4.89) | 6.68 | (15) | 0.008 | 0.041 |
| APOE | 0.380 | 1.22 | (5.23) | 6.21 | (15) | 0.011 | 0.046 |
Model: NIHSS-3 months ≈NIHSS baseline + protein Log2FC. APMAP indicates adipocyte plasma membrane-associated protein; APOE, apolipoprotein E; APOF, apolipoprotein F; HDL, high-density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; and PON1, serum paraoxonase/ arylesterase.
Further, we find that 3-month mRS scores correlate with changes in APOL1 and PCYOX1 in baseline and/or tPA adjusted models however, these correlations don't survive the multiple comparison adjustments (Online Table V).
Cholesterol efflux capacity is reduced for stroke patients.
To understand if the changes in proteome correlate with changes in HDL function, we measured CEC in J774 murine macrophages [5]. CEC was significantly decreased (32%, P<6.9×10^-7) in stroke patients at 24-hours compared to controls and did not significantly change between 24- and 96-hours post stroke (Figure 5, Table 1). The linear regression model with CEC as an outcome variable and Stroke as a predictor, adjusted for HDL-C identified stroke as a strong predictor of CEC (β=-3.44, p=0.001, adjusted R2=0.391; Model a: CEC ~ Stroke(Stroke=1, Control=0) + HDL-C). The significant association was maintained after further adjustments for sex, age and LDL-C (β=-2.45, p=0.014, adjusted R2=0.490, Model b: CEC~ Stroke(Stroke=1, Control=0) + HDL-C + LDL-C + Age + Sex (Male=1, Female=0) (Table 4).
Figure 5. Cholesterol efflux capacity is reduced in stroke patients.

Macrophage cholesterol efflux capacity after stimulation with cAMP was measured for control (n=35) and stroke patients at 24 h (n=35) and 96 h (n=20) post stroke event. Significance determined by two-sided t-test.
Table 4. Linear Regression Model for MacrophageMediated Cholesterol Efflux Capacity.
Two models were used to evaluate the relation between the cholesterol efflux capacity and stroke in 24 h poststroke intervention samples compared with nonstroke controls. Both models adjust for multiple patient covariates which may affect cholesterol efflux capacity.
| Dependent variable: | ||
|---|---|---|
| Cholesterol efflux capacity | ||
| (1) | (2) | |
| Stroke status | −3.435 (−5.330, −1.540) | −2.447 (−4.329, −0.565) |
| p = 0.001*** | p = 0.014** | |
| Sex (male) | 1.708 (0.014, 3.402) | |
| p = 0.053* | ||
| Age at blood collection | 0.002 (-0.093, 0.096) | |
| p = 0.971 | ||
| High-density lipoprotein cholesterol | 0.076 (0.031, 0.121) | 0.088 (0.043, 0.132) |
| p = 0.002*** | p = 0.0003*** | |
| Low-density lipoprotein cholesterol | 0.030 (0.015, 0.045) | |
| p = 0.0003*** | ||
| Constant | 10.717 (7.619, 13.815) | 4.217 (-3.706, 12.139) |
| p = 3.7E-09*** | p = 0.302 | |
| Observations | 70 | 65 |
| R2 | 0.409 | 0.53 |
| Adjusted R2 | 0.391 | 0.49 |
| Residual Std. Error | 3.521 (df = 67) | 3.001 (df = 59) |
| F Statistic | 23.172*** (df = 2; 67) | 13.299*** (df = 5; 59) |
Model 1 adjusts for HDL-C; Model 2 adjusts for HDL-C, LDL-C, sex, and age. HDL indicates high-density lipoprotein; and LDL, low-density lipoprotein.
There was no correlation between CEC, total cholesterol, LDL-C, triglyceride, or HDL-C levels and stroke severity, as assessed by baseline NIHSS, 3-month NIHSS or mRS scores (Online Table VI). Of the proteins that significantly correlate with stroke recovery, only PPBP associated with CEC and total cholesterol, but this did not reach significance after multiple testing correction (Online Table VII). All other proteins showed no significant association to either CEC, HDL-C, LDL-C, total cholesterol, or triglycerides (Online Table VIII). A relationship between CEC and tPA treatment was observed for patient samples at 96 hours post stoke intervention, but not at 24 hours post (Online Figure VI). Patients who received tPA treatment had increased CEC at 96 hours compared to non-tPA patients. No significant difference was seen in any other lipid measurement based on tPA treatment status (Online Table VIII).
DISCUSSION
Although the interactions between stroke and HDL-C have been studied before, the relationship between stroke and HDL function and proteome is undefined. We hypothesized that HDL harbors proteins reflective of the biology and the severity of a stroke event, and predictive of recovery. We conducted a time-dependent deep phenotyping of HDL proteome and function in stroke subjects and investigated how these phenotypes correlate with 3-month recovery from stroke measured as NIHSS and mRS scores. We report reduced sterol efflux capacity and both qualitative and quantitative differences in the protein cargos of HDL between healthy and stroke subjects. We show that changes within 96 hours post stroke in APOF, APOL1, APMAP, APOC4, APOM, PCYOX1, PON1, APOE, and PPBP correlate with stroke recovery scores even after being adjusted for baseline stroke severity. APOF and APOL1 continued to significantly correlate with recovery scores after adjustments for tPA treatment.
HDL particles are a heterogeneous group of lipid-protein complexes, and HDL-C levels exhibit strong inverse correlations with CAD rates in many populations [3,4]. However, whether HDL-C levels associate with stroke remains to be determined as existing results are conflicting [9,12,14–17]. Promotion of reverse cholesterol transport [44,45] and suppression of inflammatory responses [46–48] are thought to be the mechanisms through which HDL protects against disease [6–8]. In addition, HDL’s protein cargo is involved in a variety of immune and regulatory functions [27,49–51]. Although the interactions between stroke and HDL-C have been studied before, the relationship between stroke, HDL function, and HDL proteome is undefined. We hypothesized that HDL harbors proteins reflective of the biology and the severity of a stroke event, and therefore could be predictive of recovery.
The active remodeling of HDL proteome and subsequent changes in HDL function during early acute phase of a stroke event has not been previously studied. We describe differences in proteins participating in inflammation (SAA2, SERPINA1, ITIH4), and lipid metabolism (APOE, APOF, APOA4, GPLD1, CLU, PON1) in post-stroke HDL. All of these proteins have been previously shown to be associated with HDL [24–26,28,40]. We explored the possibility that protein changes could provide information about the biology of the stroke event, which in turn can be used to predict recovery. We conducted a time-dependent phenotyping of HDL proteome and function in stroke subjects and investigated how these phenotypes correlate with recovery from stroke. We show that while CEC is reduced throughout the study period in stroke patients, the HDL protein cargo continues to remodel for 96hours after stroke intervention. We additionally show that changes in HDL proteins within 96-hours post-stroke intervention associate with functional and neurological recovery, but do not associate with CEC and HDL-C levels. Multiple linear regression models adjusting for baseline stroke severity and t-PA treatment identified that APOF, APOL1, and LPA correlate strongly with 3-month stroke recovery scores. In these models, CEC and HDL-C did not correlate with 3-month stroke recovery scores, which argues for the specificity of the HDL proteome remodeling post stroke event.
Acute ischemic stroke is an inflammatory event manifested by immediate increase in plasma CRP levels in response to tissue injury and infection, proportionate to the severity of the acute event [52]. In the post-acute phase, inflammatory status is maintained as CRP remains elevated compared with control subjects, with the greatest elevation in patients with large-artery stroke mechanisms. Accordingly, the remodeling of HDL proteome during early acute phase of stroke is mostly of inflammatory nature, as captured by changes in proteins with inflammatory functions (i.e. SAA2, ITIH4, SERPINA1). In recent studies, plasma ITIH4 levels have been shown to be reduced in stroke patients and proposed to be a biomarker for stroke [53]. Accordingly, with our unbiased orthogonal approach, we detected up to 50% reduction in HDL associated ITIH4 at both 24- and 96-hour time points after stroke intervention. Kashyap and colleagues examined plasma ITIH4 levels up to 144-hour post stroke, showing plasma levels return to normal as patients improve. In our study, HDL associated ITIH4 levels associated with baseline stroke severity, 3-month NIHSS, and mRS; however, the significance of these associations failed to survive multiple testing adjustments.
HDL’s inflammatory remodeling continued in the post-acute phase, with SAA2 increased about 5-fold between the 24- and 96-hour time points. The human SAA protein family comprises the acute phase SAA1/SAA2, known to activate a large set of innate and adaptive immune cells, and the constitutive SAA4[54]. Inflammatory remodeling of HDL with SAA1 has been previously shown in mice to be associated with reduced CEC [55–57]In agreement with these studies, we find that patients who have suffered a stroke have reduced HDL CEC at 24- and 96-hours —maintained after adjustments for HDL-C levels. The reduction in CEC correlated with a pro-inflammatory proteome profile, but not with recovery from stroke. Further, none of the proteins that correlated with recovery from stroke, correlated with CEC. Our findings suggest that HDL proteome remodeling during the early acute phase of stroke captures biomarkers that do not modulate CEC. These findings are consistent with a recent report that measured CEC in 1642 samples from the MESA (Multi-Ethnic Study of Atherosclerosis)[23]. Shea et al. show that HDL-mediated CEC is an atheroprotective mechanism for coronary heart disease but not stroke. Higher cholesterol mass efflux capacity level was associated with lower risk of incident coronary heart disease events, but no association was found with risk of stroke.
ANTRX2, a membrane protein involved in extracellular matrix adhesion, collagen IV and laminin binding, and collagen VI homeostasis. ANTRX2 has been previously shown to be associated with human and mouse ultracentrifugation density isolated HDL [32,57–61]. The mechanisms of ANTXR2 function in stroke is not well understood, but in multiple gene expression based human stroke studies, it has been proposed as a marker of stroke in blood [62–64]. Collectively, the relative expression of ANTXR2 increased in patients with stroke, as measured at the time of admission to the hospital. We report no significant change in HDL associated ANTXR2 between non-stroke and stroke subjects, however we observe a significant decrease in HDL associated ANTXR2 in 96-hour vs. 24-hour post-stroke intervention. This change significantly associated with functional recovery from stroke even when adjusted for baseline stroke severity. While time-dependent change in plasma ANTRX2 levels was not monitored in previous studies, our findings suggest that HDL-associated ANTRX2 levels may behave differently than plasma levels. Further understanding of the biology requires additional studies to determine the relationship between plasma ANTXR2 levels and HDL-bound ANTXR2 in stroke, and to further explore the time-dependent nature of this response.
Prognostic studies of outcome after stroke have concentrated on predicting outcomes at a specific time point such as 3 or 6 months after stroke [65]. This type of prediction does not aid clinical decisions about whether to continue an intervention, such as a rehabilitation program, or the identification of causes of failure to recover. It has been emphasized that normal patterns of recovery from stroke should be established as a guide to monitoring recovery of future patients [66,67]. However, carefully designed multivariate statistical models for recovery patterns fail to model a personalized recovery trajectory. The personalization of clinical care requires better understanding and prediction of recovery from stroke. Our discovery that changes in levels of HDL-associated proteins such as APOF and APOL1 correlate with recovery regardless of baseline stroke severity or tPA treatment, may be used to develop biomarkers for recovery prediction. Future studies powered to analyze the impact of stroke type on these associations are required.
In conclusion, we propose that the protein changes detected herein may be indicative of the distinct stroke biology each patient experiences. Further, we show that changes in several HDL specific proteins predict functional and neurological stroke recovery. Our data supports the idea that HDL particles associate with proteins relevant to stroke biology. We show that time-dependent association with inflammation associated proteins likely echo the severity of stroke event. There is a potential to utilize the proteome of HDL particles to understand the severity and biological characteristics of a stroke event. Validation of our findings in a larger stroke cohort with statistical power to account for common risk factors may provide novel strategies to improve prediction of stroke recovery in the clinic. Our work raises the exciting possibility that monitoring changes in HDL proteome in acute stroke phase may provide better biomarkers for stroke recovery than relying on baseline stroke severity scores alone.
Study Limitations.
The stroke cohort size limits stroke-type related statistical analyses. One center rather than multi center participation limits generalizing the conclusions. Later time point blood collection would have allowed to asses if the observed difference return to basal levels. The study design is discovery and exploration rather than rigorous development of a robust targeted assay; therefore, peptide CVs are not calculated from triplicate calibration curves. Even though our analyses adjusted for sex, the inherent variability in sex distribution between stroke and control cohorts could be responsible for some of the differences observed. We applied parametric statistics as 84% of the proteins were normally distributed, it is possible that remaining 16% contains false negatives.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Plasma high density lipoprotein cholesterol levels do not associate with stroke recovery.
HDL protein composition is a dynamic process which dictates HDL function.
HDL protein levels and HDL function both associate with cardiovascular disease
What New Information Does This Article Contribute?
The HDL proteome is remodeled during the early acute phase of recovery after a stroke.
Changes in HDL proteins associate with stroke recovery.
While plasma HDL cholesterol levels do not associate with the incidence or prevalence of stroke in most studies, nor do they predict recovery from stroke, we know little about how HDL’s proteome and function relate to stroke and how they are modulated during recovery from a stroke. This study of stroke patients and age-matched controls revealed that the HDL proteome remodels during the early acute phase of stroke, and that changes in protein abundance associate with three month stroke recovery scores but not with the cholesterol efflux function of HDL. This study shows for the first time that changes in HDL protein cargo are linked to the acute inflammatory response to stroke. Tracking HDL’s proteome in the clinical setting for prediction of recovery would offer a novel and exciting approach likely to improve the health of stroke victims.
ACKNOWLEDGMENT
The authors are grateful to all the providers enrolling patients into the study registry, and to all patients who generously contributed blood samples to our study.
SOURCES OF FUNDING
NP and NAZ were partially supported by R01HL136373.
Nonstandard Abbreviations and Acronyms:
- HDL-C
High density lipoprotein cholesterol
- CEC
Cholesterol efflux capacity
- PSM
Peptide Spectra Matches
- PRM
Parallel Reaction Monitoring
- NIHSS
The National Institute of Health Stroke Scale
- mRS
Modified Rankin scale
Footnotes
DISCLOSURES
None.
SUPPLEMENTAL MATERIALS
Datasets: Online Tables I–IV
REFERENCES
- [1].Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res 2017;120:439–448. doi: 10.1161/circresaha.116.308413. [DOI] [PubMed] [Google Scholar]
- [2].MEERS WG, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Executive Summary: Heart Disease and Stroke Statistics—2010 Update. Circulation 2010;121:948–954. doi: 10.1161/circulationaha.109.192666. [DOI] [PubMed] [Google Scholar]
- [3].Castelli WP, Anderson K. A population at risk: Prevalence of high cholesterol levels in hypertensive patients in the framingham study. The American Journal of Medicine 1986;80:23–32. doi: 10.1016/0002-9343(86)90157-9. [DOI] [PubMed] [Google Scholar]
- [4].Gordon D, Medicine R-B of. High-density lipoprotein—the clinical implications of recent studies 1989. [DOI] [PubMed]
- [5].Llera-Moya M de la, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology 2010;30:796–801. doi: 10.1161/atvbaha.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-Density Lipoprotein Function, Dysfunction, and Reverse Cholesterol Transport. Arteriosclerosis, Thrombosis, and Vascular Biology 2018;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. n.d.;114. doi: 10.1161/CIRCRESAHA.114.300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nature Reviews Immunology 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reina SA, Llabre MM, Allison MA, Wilkins JT, Mendez AJ, Arnan MK, et al. HDL cholesterol and stroke risk: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2015;243:314–319. doi: 10.1016/j.atherosclerosis.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. n.d.;371. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li X-MM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arteriosclerosis, Thrombosis, and Vascular Biology 2013;33:1696–1705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tirschwell D, Smith N, Heckbert S, Lemaitre R, Longstreth W, Psaty B. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. n.d.;63. doi: 10.1212/01.WNL.0000144282.42222.DA. [DOI] [PubMed] [Google Scholar]
- [13].Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, et al. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): A Multicenter, Randomized, Open-label, Parallel-group Study. Ebiomedicine 2015;2:1071–1078. doi: 10.1016/j.ebiom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Collaboration PS. Cholesterol, diastolic blood pressure, and stroke: 13 000 strokes in 450 000 people in 45 prospective cohorts. Lancet 1995;346:1647–1653. doi: 10.1016/s0140-6736(95)92836-7. [DOI] [PubMed] [Google Scholar]
- [15].Woodward M, Barzi F, Feigin V, Gu D, Huxley R, Nakamura K, et al. Associations between high-density lipoprotein cholesterol and both stroke and coronary heart disease in the Asia Pacific region. European Heart Journal 2007;28:2653–2660. doi: 10.1093/eurheartj/ehm427. [DOI] [PubMed] [Google Scholar]
- [16].Bots ML, Elwood PC, Nikitin Y, Salonen JT, Concalves AF de, Inzitari D, et al. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. Journal of Epidemiology and Community Health 2002;56:i19–i24. doi: 10.1136/jech.56.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amarenco P, Labreuche J, Touboul P-J. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: A systematic review. Atherosclerosis 2008;196:489–496. doi: 10.1016/j.atherosclerosis.2007.07.033. [DOI] [PubMed] [Google Scholar]
- [18].Willey JZ, Xu Q, Boden-Albala B, Paik MC, Moon Y, Sacco RL, et al. Lipid profile components and risk of ischemic stroke: the Northern Manhattan Study (NOMAS). Archives of Neurology 2009;66:1400–1406. doi: 10.1001/archneurol.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berger J, McGinn A, Howard B, the … K-L of. LIPID AND LIPOPROTEIN BIOMARKERS AND THE RISK OF ISCHEMIC STROKE IN POSTMENOPAUSAL WOMEN 2010. [DOI] [PMC free article] [PubMed]
- [20].Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50:S189–S194. doi: 10.1194/jlr.r800088-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 2015;3:507–513. doi: 10.1016/s2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khera AV, Cuchel M, Llera-Moya M de la, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. New Engl J Medicine 2011;364:127–135. doi: 10.1056/nejmoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shea S, Stein JH, Jorgenson NW, McClelland RL, Tascau L, Shrager S, et al. Cholesterol Mass Efflux Capacity, Incident Cardiovascular Disease, and Progression of Carotid Plaque. Arteriosclerosis Thrombosis Vasc Biology 2018;39:89–96. doi: 10.1161/atvbaha.118.311366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Green PS, Vaisar T, Pennathur S, Kulstad JJ, Moore AB, Marcovina S, et al. Combined statin and niacin therapy remodels the high-density lipoprotein proteome. n.d.;118. doi: 10.1161/CIRCULATIONAHA.108.770669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. n.d;117. doi: 10.1172/jci26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ronsein GE, Reyes-Soffer G, He Y, Oda M, Ginsberg H, Heinecke JW. Targeted Proteomics Identifies Paraoxonase/Arylesterase 1 (PON1) and Apolipoprotein Cs as Potential Risk Factors for Hypoalphalipoproteinemia in Diabetic Subjects Treated with Fenofibrate and Rosiglitazone. Mol Cell Proteomics 2016;15:1083–1093. doi: 10.1074/mcp.m115.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon SM, Remaley AT. High density lipoproteins are modulators of protease activity: Implications in inflammation, complement activation, and atherothrombosis. Atherosclerosis 2017;259:104–113. doi: 10.1016/j.atherosclerosis.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic Characterization of Human Plasma High Density Lipoprotein Fractionated by Gel Filtration Chromatography. Journal of Proteome Research 2010;9:5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ronsein GE, Vaisar T. Inflammation, remodeling, and other factors affecting HDL cholesterol efflux. Curr Opin Lipidol 2017;28:52–59. doi: 10.1097/mol.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sacks F, Alaupovic P, Moye L, Cole T, Circulation SB. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial 2000. [DOI] [PubMed]
- [31].Ortiz-Munoz G, Couret D, Lapergue B, Bruckert E, Meseguer E, Amarenco P, et al. Dysfunctional HDL in acute stroke. Atherosclerosis 2016;253:75–80. doi: 10.1016/j.atherosclerosis.2016.08.035. [DOI] [PubMed] [Google Scholar]
- [32].Pamir N, Pan C, Plubell DL, Hutchins PM, Tang C, Wimberger J, et al. Genetic control of the mouse HDL proteome defines HDL traits, function, and heterogeneity. Journal of Lipid Research 2019:jlr.M090555. doi: 10.1194/jlr.m090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke J Cereb Circulation 1997;28:307–310. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- [34].Uyttenboogaart M, Stewart RE, Vroomen PCAJ, Keyser JD, Luijckx G-J Optimizing Cutoff Scores for the Barthel Index and the Modified Rankin Scale for Defining Outcome in Acute Stroke Trials. Stroke 2005;36:1984–1987. doi: 10.1161/01.str.0000177872.87960.61. [DOI] [PubMed] [Google Scholar]
- [35].Ghandehari K Challenging comparison of stroke scales. J Res Medical Sci Official J Isfahan Univ Medical Sci 2013;18:906–910. [PMC free article] [PubMed] [Google Scholar]
- [36].Friedewald W, Levy R, chemistry F-D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge 1972. [PubMed]
- [37].Pamir N, Hutchins PM, Ronsein GE, Wei H, Tang C, Das R, et al. Plasminogen promotes cholesterol efflux by the ABCA1 pathway. JCI Insight 2017;2. doi: 10.1172/jci.insight.92176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pamir N, Hutchins P, Ronsein G, Vaisar T, Reardon CA, Getz GS, et al. Proteomic analysis of HDL from inbred mouse strains implicates APOE associated with HDL in reduced cholesterol efflux capacity via the ABCA1 pathway. Journal of Lipid Research 2016;57:246–257. doi: 10.1194/jlr.M063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ronsein GE, Pamir N, Haller PD von, Kim DS, Oda MN, Jarvik GP, et al. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J Proteomics 2015;113:388–399. doi: 10.1016/j.jprot.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davidson WS, Silva RAGD, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic Analysis of Defined HDL Subpopulations Reveals Particle-Specific Protein Clusters. Arteriosclerosis Thrombosis Vasc Biology 2009;29:870–876. doi: 10.1161/atvbaha.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gordon SM, Deng J, Tomann AB, Shah AS, Lu JL, Davidson SW. Multi-dimensional Co-separation Analysis Reveals Protein–Protein Interactions Defining Plasma Lipoprotein Subspecies. Molecular & Cellular Proteomics 2013;12:3123–3134. doi: 10.1074/mcp.M113.028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Choi JC, Kim BJ, Han M-K, Lee SJ, Kang K, Park J-M, et al. Utility of Items of Baseline National Institutes of Health Stroke Scale as Predictors of Functional Outcomes at Three Months after Mild Ischemic Stroke. J Stroke Cerebrovasc Dis 2017;26:1306–1313. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.027. [DOI] [PubMed] [Google Scholar]
- [43].Adams HP, Davis PH, Leira EC, Chang K-C, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke. Neurology 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- [44].Rosenson R, Jr BH, Davidson W, Circulation F-Z. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport 2012. [DOI] [PMC free article] [PubMed]
- [45].Heinecke JW. The not-so-simple HDL story: A new era for quantifying HDL and cardiovascular risk? Nature Medicine 2012;18:1346–1347. doi: 10.1038/nm.2930. [DOI] [PubMed] [Google Scholar]
- [46].Feingold K, Grunfeld C. The acute phase response inhibits reverse cholesterol transport n.d.;51. doi: 10.1194/jlr.E005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Feingold KR, Grunfeld C. The role of HDL in innate immunity. n.d.;52. doi: 10.1194/jlr.E012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rosenson RS, Brewer HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nature Reviews Cardiology 2016;13:48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barter PJ, Nicholls S, Rye K-A, Anantharamaiah G, Navab M, Fogelman AM. Antiinflammatory properties of HDL. n.d;95. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- [50].Papachristou NI, Blair HC, Kypreos KE, Papachristou DJ. High-density lipoprotein (HDL) metabolism and bone mass. n.d.;233. doi: 10.1530/JOE-16-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Navab M, Reddy ST, Lenten BJV, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nature Reviews Cardiology 2011;8:222. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- [52].Napoli MD, Schwaninger M, Cappelli R, Ceccarelli E, Gianfilippo GD, Donati C, et al. Evaluation of C-Reactive Protein Measurement for Assessing the Risk and Prognosis in Ischemic Stroke. Stroke 2005;36:1316–1329. doi: 10.1161/01.str.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- [53].Kashyap RS, Nayak AR, Deshpande PS, Kabra D, Purohit HJ, Taori GM, et al. Inter-α-trypsin inhibitor heavy chain 4 is a novel marker of acute ischemic stroke. Clin Chim Acta 2009;402:160–163. doi: 10.1016/j.cca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- [54].Jumeau C, Awad F, Assrawi E, Cobret L, Duquesnoy P, Giurgea I, et al. Expression of SAA1, SAA2 and SAA4 genes in human primary monocytes and monocyte-derived macrophages. Plos One 2019;14:e0217005. doi: 10.1371/journal.pone.0217005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vaisar T, Hippe D, Neradilek M, Pollisar N, Chait A, O’Brien K, et al. HDL-associated serum amyloid A (SAA) may be a predictor of cardiovascular risk independent of HDL- or LDL-cholesterol. Atherosclerosis 2017;263:e218. doi: 10.1016/j.atherosclerosis.2017.06.709. [DOI] [Google Scholar]
- [56].Han CY, Tang C, Guevara ME, Wei H, Wietecha T, Shao B, et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J Clin Invest 2016;126:796–796. doi: 10.1172/jci86401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vaisar T, Tang C, Babenko I, Hutchins P, Wimberger J, Suffredini AF, et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J Lipid Res 2015;56:1519–1530. doi: 10.1194/jlr.m059089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Oberbach A, Adams V, Schlichting N, Heinrich M, Kullnick Y, Lehmann S, et al. Proteome profiles of HDL particles of patients with chronic heart failure are associated with immune response and also include bacteria proteins. Clin Chim Acta 2016;453:114–122. doi: 10.1016/j.cca.2015.12.005. [DOI] [PubMed] [Google Scholar]
- [59].Okada T, Ohama T, Takafuji K, Kanno K, Matsuda H, Sairyo M, et al. Shotgun Proteomic Analysis Reveals Proteome Alterations in HDL of Patients with Cholesteryl Ester Transfer Protein Deficiency. J Clin Lipidol 2019;13:317–325. doi: 10.1016/j.jacl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- [60].Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, et al. Altered Activation of Endothelial Anti- and Proapoptotic Pathways by High-Density Lipoprotein from Patients with Coronary Artery Disease. Circulation 2013;127:891–904. doi: 10.1161/circulationaha.112.108753. [DOI] [PubMed] [Google Scholar]
- [61].Weichhart T, Kopecky C, Kubicek M, Haidinger M, Döller D, Katholnig K, et al. Serum Amyloid A in Uremic HDL Promotes Inflammation. J Am Soc Nephrol 2012;23:934–947. doi: 10.1681/asn.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].O’Connell GC, Chantler PD, Barr TL. Stroke-associated pattern of gene expression previously identified by machine-learning is diagnostically robust in an independent patient population. Genom Data 2017;14:47–52. doi: 10.1016/j.gdata.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].O’Connell GC, Petrone AB, Treadway MB, Tennant CS, Lucke-Wold N, Chantler PD, et al. Machine-learning approach identifies a pattern of gene expression in peripheral blood that can accurately detect ischaemic stroke. Npj Genom Medicine 2016;1:16038. doi: 10.1038/npjgenmed.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang Q, Chen W, Chen S, Li S, Wei D, He W. Identification of key genes and upstream regulators in ischemic stroke. Brain Behav 2019;9:e01319. doi: 10.1002/brb3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wade DT, Skilbeck CE, Hewer RL. Predicting Barthel ADL score at 6 months after an acute stroke. Arch Phys Med Rehab 1983;64:24–28. [PubMed] [Google Scholar]
- [66].Tilling K, Sterne JAC, Rudd AG, Glass TA, Wityk RJ, Wolfe CDA. A New Method for Predicting Recovery After Stroke. Stroke 2001;32:2867–2873. doi: 10.1161/hs1201.099413. [DOI] [PubMed] [Google Scholar]
- [67].Partridge CJ, Johnston M, Edwards S. RECOVERY FROM PHYSICAL DISABILITY AFTER STROKE: NORMAL PATTERNS AS A BASIS FOR EVALUATION. Lancet 1987;329:373–375. doi: 10.1016/s0140-6736(87)91739-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


