Calcium dysregulation is a universal characteristic of heart failure (HF) and reduced sarco/endoplasmic reticulum calcium-ATPase (SERCA) activity plays a central role in disease progression1. Hence, increasing SERCA activity has been pursued as a clinical approach for treating HF and significant evidence supports its therapeutic potential1,2. Recently, we discovered the novel muscle-specific micropeptide dwarf open reading frame (DWORF), which enhances SERCA activity by displacing the SERCA-inhibitory peptide phospholamban3,4. In mice, cardiac-specific transgenic overexpression of DWORF (DWORF Tg) enhanced SERCA activity, increased calcium-cycling and contractility4, and rescued a genetic model of dilated cardiomyopathy (DCM)3, suggesting that DWORF overexpression might be used to achieve SERCA activation in HF to normalize calcium homeostasis and prevent disease progression.
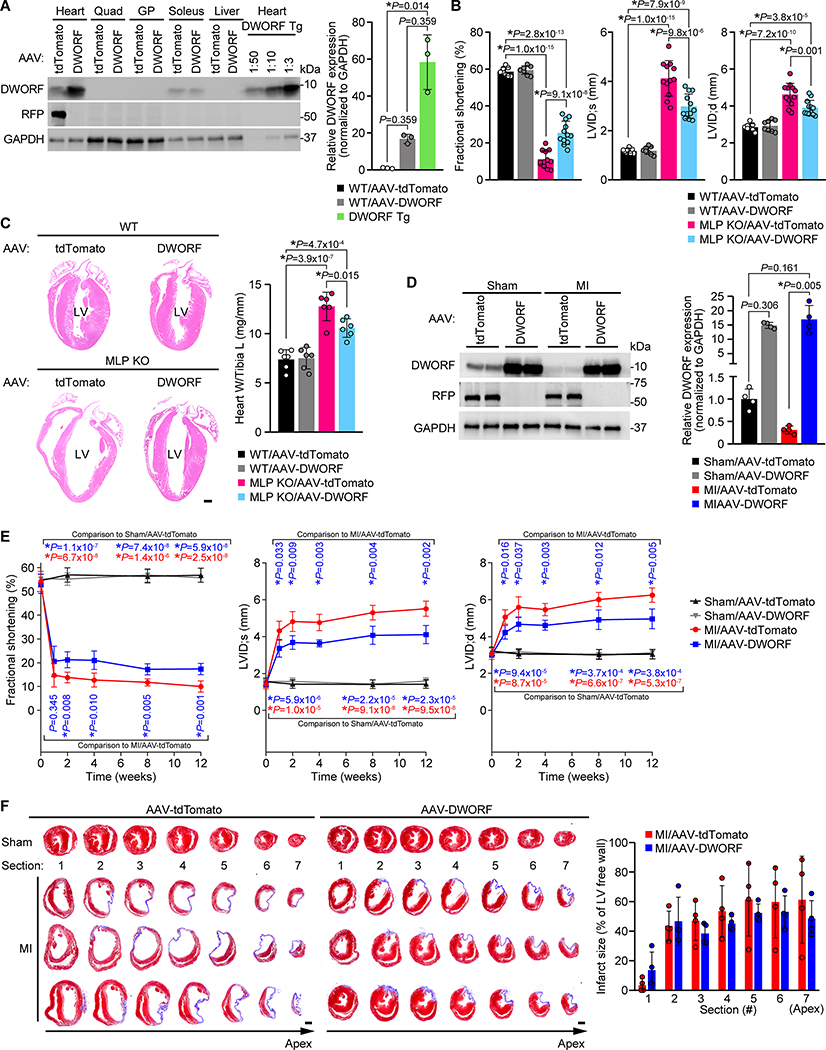
To explore the therapeutic potential of DWORF gene therapy in HF, we developed an adeno-associated virus (AAV) approach using cardiotropic serotype 9 (AAV9) and the cardiac troponin-T (cTnT) promoter (Addgene plasmid #69915)5. All animal procedures were conducted in accordance with institutional guidelines and were approved by the IACUC. Both male and female mice were used. AAV9-cTnT-DWORF (AAV-DWORF) and control AAV9-cTnT-tdTomato (AAV-tdTomato) were validated in mice by delivery at postnatal day 5 (P5) by intraperitoneal injection at 5×1013 viral genomes/kilogram and Western blot analysis revealed cardiac-specific overexpression at P28 (Figure A). The efficacy of AAV-DWORF gene therapy was assessed in a mouse model of DCM caused by gene deletion of muscle-specific LIM protein (MLP KO). Consistent with the cardioprotection previously observed through transgenic overexpression of DWORF in MLP KO mice3, echocardiography of mice that received AAV-DWORF at P5 showed significantly enhanced ventricular function compared to control MLP KO/AAV-tdTomato mice at 8-weeks (Figure B) and adverse cardiac remodeling was attenuated (Figure B,C). The degree of cardioprotection observed in MLP KO/AAV-DWORF mice was diminished compared to MLP KO/DWORF Tg mice3, likely due to the reduced level of DWORF overexpression achieved by AAV-delivery (16.9±2.4-fold) compared to DWORF Tg overexpression (58.5±14.7-fold)(Figure A). Nevertheless, these results indicate that enhancing SERCA activity via DWORF gene therapy is a viable therapeutic strategy.
FIGURE. Development of DWORF gene therapy as a HF therapeutic.
A, Western blot analysis and DWORF quantification of lysates from mice 4-weeks post-AAV-delivery (n=3/group). GP, gastrocnemius plantaris. DWORF Tg heart lysates were diluted to compare the level of AAV-DWORF-mediated overexpression with DWORF Tg mice. B, Echocardiography analysis of LV fractional shortening and internal diameter (LVID) during systole (s) and diastole (d). n=8 (WT/AAV-tdTomato, WT/AAV-DWORF) or n=12 (MLP KO/AAV-tdTomato, MLP KO/AAV-DWORF). C, Left: Representative H&E staining of heart sections (n=4/group). Scale=1mm. Right: Heart weight (W) to tibia length (L) measurements for n=6/group. D, Western blot analysis and quantification of heart lysates 12-weeks after sham/MI surgery (n=4/group). E, Echocardiography analysis of fractional shortening and LVID at baseline (0 weeks) and post-sham/MI surgery for n=5 sham mice (sham/AAV-tdTomato, sham/AAV-DWORF) or n=6 MI mice (MI/AAV-tdTomato, MI/AAV-DWORF). F, Left: Masson’s trichrome staining on serial heart sections taken at 0.5mm increments starting at the suture (section 1) and numbered (1–7) for quantification. Scale=1mm. Right: Quantification of infarct size of each section calculated as the length of the scar/length of total LV free wall for n=4 mice/group.
Statistics: Data shown as mean±standard deviation. The Shapiro-Wilk normality test was used for distribution. Statistical analysis included nonparametric Kruskal-Wallis test with Dunn-multiple comparison (A, D) or Mann-Whitney test (F), and two-way ANOVA with Tukey post hoc test (B, C) or mixed-effects analysis with Geisser-Greenhouse correction and Tukey post hoc test (E). P-values are corrected for multiple testing with P<0.05 considered statistically significant.
Next, we tested the potential of DWORF gene therapy in preventing adverse cardiac outcomes in a myocardial infarction (MI) model of HF. Mice received AAV-DWORF or AAV-tdTomato at P5 and were subjected to sham surgery or MI by permanent ligation of the left coronary artery at 8-weeks of age and HF induction and progression were monitored for 12-weeks. Consistent with previous observations in other HF models3,4, endogenous DWORF protein expression was reduced in response to MI (3.4±1.0-fold)(Figure D), likely contributing to the reduced SERCA activity underlying HF. Western blot analysis also indicated sustained AAV-mediated overexpression of DWORF in both sham (14.9±1.0-fold) and MI samples (17.0±4.8-fold) 12-weeks post-surgery (Figure D). Cardiac function was assessed in mice by echocardiography at baseline and post-MI and MI/AAV-DWORF mice showed significantly enhanced ventricular function (Figure E) and reduced cardiac dilation compared to MI/AAV-tdTomato mice (Figure E,F). Histological analysis of hearts with Masson’s trichrome staining indicated no significant difference in infarct size between groups (Figure F).
Collectively, the data presented here indicates that DWORF gene therapy holds promise as a novel HF therapeutic. Future studies will continue to address the direct clinical relevance of DWORF gene therapy in established HF to build on the success of our prevention-based approach.
ACKNOWLEDGMENT
We thank J. Cabrera for graphics, W. Tan for echocardiography and surgeries, and the UTSW Molecular Pathology Core for histology.
SOURCES OF FUNDING
Supported by grants from the NIH (HL-130253, HL-138426, HD-087351, AR-067294, HL-141630), Leducq Foundation and Robert A. Welch Foundation (1–0025).
Footnotes
DISCLOSURES
C.A.M., R.B.D. and E.N.O. are co-inventors on a patent regarding the use of DWORF for treatment of HF (US 10,570,183).
Data Availability.
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Kranias EG and Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a r regulatome. Circ Res. 2012;110:1646–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penny WF and Hammond HK. Randomized Clinical Trials of Gene Transfer for Heart Failure with Reduced Ejection Fraction. Hum Gene Ther. 2017;28:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarewich CA, Munir AZ, Schiattarella GG, et al. The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife.2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson BR, Makarewich CA, Anderson DM, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z, von Gise A, Zhou P, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]