Graphical abstract
Keywords: Aortic dissection, Bicuspid aortic valve, Aorta
Highlights
-
•
BAV is associated with risk for dissection of the proximal aorta.
-
•
Thorough BAV investigation is required for any aortic dissection.
-
•
Familial forms of BAV are probably associated with an increased risk for aortopathy.
-
•
Familial screening is desirable to organize follow-up of each patient with BAV.
Introduction
Bicuspid aortic valve (BAV) disease is associated with increased risk for ascending thoracic aortic aneurysm. However, the risk for aortic dissection is considered to be moderate.1,2 This discrepancy could be explained by the high heterogeneity of vascular phenotypes associated with BAV disease, which depend on genetics, syndromic form, and valvular function modifying aortic hemodynamics. Although the genetic variants involved are only partially identified, the presence of a familial form of BAV disease appears to be associated with a higher risk for aortopathy.3 Familial forms represent 10% to 30% all BAV, with an autosomal genetic transmission and a variable penetrance. Familial screening is encouraged by current recommendations for early detection of aortic valve dysfunction and ascending thoracic aortic aneurysm.4
Case Presentation
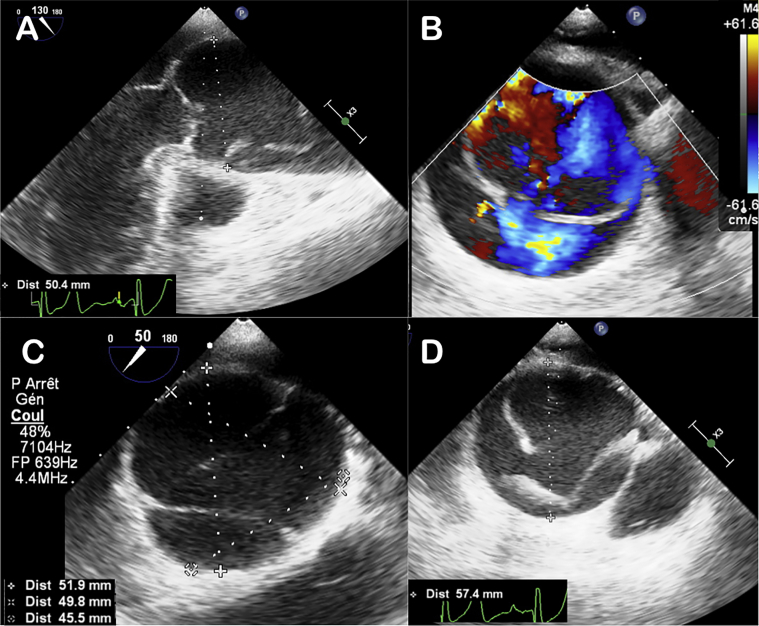
A 63-year-old patient without cardiovascular risk factors nor previous medical or surgical history was brought to the emergency department for sudden retrosternal chest pain radiating in the dorsolumbar region, associated with paresthesia of the right leg. On arrival, he had confusion, with a Glasgow scale of 15, and marked brachial blood pressure asymmetry. All pulses were detectable, except at the right femoral artery. A diastolic murmur at the aortic site was found on cardiac auscultation, radiating along the left sternal border. The per-critical electrocardiogram showed a regular sinus rhythm at 50 beats/min without repolarization disorder evocative of ischemia, apart from negative lateral T waves, suggestive of left ventricular hypertrophy. Initial biological tests showed moderate anemia (hemoglobin 117 g/L) and renal injury (creatinine 146 μmol/L). Troponin was increased to 27.9 ng/L (normal range, <19.8 ng/L) and d-dimer level was >10,000 ng/ml. Arterial gas analysis revealed metabolic acidosis (pH 7.32) and a lactate level of 2.8 mmol/L. The presence of asymmetric brachial blood pressure and migratory pain, in the absence of the right femoral pulse, led the physician to suspect an aortic dissection. Type A aortic dissection was confirmed by computed tomographic angiography, extending from the sinus of Valsalva to the femoral arteries (Figure 1). It was associated with dissection of the left upper polar renal artery, superior mesenteric artery, and right femoral artery. Once the aortic dissection diagnosis was made, the patient was directly transferred to surgery. Perioperative transesophageal echocardiography confirmed that the proximal entry of the dissection was located above the right aortic hemicusp. Also, it showed dissection flap descending to the sinus with patent coronary arteries (Figure 2, Video 1). The ascending aortic diameter was 51 mm at the sinus of Valsalva and 55 mm at the sinotubular junction and tubular aorta. The aortic valve was not remodeled, with moderate insufficiency due to dilatation and prolapse of the right hemicusp. On transesophageal echocardiography, a BAV was suspected, with partial fusion of the right and left coronary cusps (Video 1), of which the patient was not aware. These echocardiographic findings were then confirmed at surgery. The left ventricular function was normal with no disturbance of the segmental kinetics.
Figure 1.
Initial computed tomographic angiography. Dissection of the ascending aorta (A) with entry just above the right hemicusp (B) and extension of the false lumen along the aortic arch and the descending thoracic aorta (C).
Figure 2.
Perioperative transesophageal ultrasound. Visualization of the true and false aortic channel in longitudinal (A) and transverse (B) aortic section with the presence of the intimal tear above the aortic valve (A-D). Presence of a dilated sinus of Valsalva just below the aortic dissection.
The surgery consisted of a Bentall operation with placement of a CE-Perimount number 27 bioprosthesis with a Vascutek Valsalva tube number 30 and reimplantation of the right coronary artery, aortic arch replacement, and finally a bypass on the brachiocephalic trunk. Extracorporeal circulation (242 min) with cooling was set up after cannulation of the right axillary artery and vena cava, with discharge of the left cavities by cannulation of the right superior pulmonary vein. Antegrade blood cardioplegia by coronary ostia was repeated every 20 min.
The postoperative period was uneventful, and the aortic valve bioprosthesis and aortic tube echocardiographic control showed no abnormality. Antiplatelet therapy and bisoprolol were introduced, as well as ramipril for newly diagnosed arterial hypertension.
Gross analysis of the aortic valve confirmed the presence of BAV with a short, thick, and fibrous raphe connecting the left and right cusps (Figure 3). Ascending aortic pathology found media abnormalities, characterized by fairly extensive alterations of the extracellular matrix, including mucoid extracellular matrix accumulation, not specific but fully compatible with aortic injury associated with BAV5 (Figure 4).
Figure 3.
Gross analysis of the aortic valve (A, B). A two-fragment valve unit includes the normal-sized, noncalcified, noncoronary cusp. The second fragment corresponds to the left and right cusps joined by a short, thick and fibrous raphe, confirming the BAV. Histological section of the aortic valve (C; hematoxylin and eosin stain) with the presence of the raphe between the two cusps (blue asterisk). The black arrows indicate the fibrosa of the two left and right coronary cusps.
Figure 4.
Histologic features observed in the proximal aorta. Interlamellar degeneration with mucoid replacement (mucoid extracellular matrix accumulation; hematoxylin and eosin stain; original magnification ×10; A, B). Analysis of the elastic network reveals fragmentation and loss of the elastic fibers distributed heterogeneously, sometimes moderately altered (C) and with severe alterations in some areas (D; elastic stain; original magnification ×10).
At 3 months, control computed tomographic angiography revealed no perianastomotic anomaly of the aortic tube or implantation of the right coronary or the carotid arteries (Figure 5). Dissection of the left upper polar renal artery with delayed contrast enhancement of the upper part of the left kidney persisted, its lower part being supplied by a left lower polar renal artery originating from the true channel. The true aortic channel perfused the upper mesenteric and iliac arteries. Retrospectively, the patient indicated that his brother was diagnosed with a BAV decades ago. Family screening was suggested to first-degree relatives to look for other cases of BAV.
Figure 5.
Computed tomographic angiography at 3 months after surgery. Visualization of the aortic tube in sagittal section (A) and frontal section (B). Aortic dissection persists downstream from the tube, visible at the descending thoracic aortic level (A).
Discussion
BAV associated aortopathy is well known, but the risk for complication is very difficult to predict given the heterogeneity of profiles in terms of both valvular and associated aortic phenotype. The risk for aortic dissection is moderate, although higher than expected in young subjects. The prevalence of BAV reaches 9% of all aortic dissections before the age of 40, whereas the overall prevalence of BAV is estimated around 1%.6,7 The main prognostic factor for acute aortic syndrome is the increase in aortic diameters. New parameters, focusing on the aortic wall biomechanical properties, have been proposed to refine the prediction of these acute events in the life span of BAV.8,9 Moreover, family history of BAV is associated with higher risk for increasing ascending aortic size, highlighting the possible genetic involvement in the BAV-associated aortopathy.3 Gene variants such as SMAD6, TBX20, and ROBO4 have recently been identified as genetic factors contributing to this BAV-associated aortopathy.10, 11, 12 However, none of these genes has demonstrated any risk prediction for acute aortic events. Therefore, in the absence of systematic genetic screening, family surveys with transthoracic echocardiography remains the easiest way to identify family cases, which can help to better stratify the risk at the individual level.
For this reason, the patient could have benefited from a routine screening when BAV was diagnosed in his brother.4 Awareness of a BAV allows closer follow-up of aortic diameters, which can detect aneurysm earlier. In addition, the valve function can be controlled, as well as the blood pressure level. A thorough dental hygiene is proposed in case of BAV.
This case is particularly interesting because the patient had an incomplete raphe (forme fruste),13 rather than a complete raphe, which could be easily missed on transthoracic and even transesophageal echocardiography. The apparent tricuspid opening in short axis made the diagnosis complex, and histologic analysis of the valve allowed BAV confirmation. The presence of mini-raphe is nevertheless associated with aortic dilatation and aortic hemodynamic abnormalities.13,14
Conclusion
Although not very common, aortic dissection may occur in BAV-associated aortopathy.15,16 In case of aortic dissection, a thorough examination of the valve (intraoperative transesophageal echocardiography, surgical observation, and histologic examination) is necessary and may lead to BAV diagnosis. The consequences are not minor, because aortic dissection associated with BAV increases the risk for acute aortic events in case of familial BAV. In the absence of routine genetic screening, transthoracic echocardiography should be proposed to all first-degree relatives. Awareness of a familial BAV allows a better estimation of the risk for acute aortic events. It is also important to initiate follow-up of relatives with BAV.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.07.006.
Supplementary Data
Perioperative transesophageal echocardiography and immediate postsurgical evaluation.
References
- 1.Tzemos N., Therrien J., Yip J., Thanassoulis G., Tremblay S., Jamorski M.T. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Michelena H.I., Desjardins V.A., Avierinos J.F.F., Russo A., Nkomo V.T., Sundt T.M. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avadhani S.A., Martin-Doyle W., Shaikh A.Y., Pape L.A. Predictors of ascending aortic dilation in bicuspid aortic valve disease: a five-year prospective study. Am J Med. 2015;128:647–652. doi: 10.1016/j.amjmed.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R., Aboyans V., Boileau C., Bossone E., Di Bartolomeo R., Eggebrecht H. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 5.Halushka M.K., Angelini A., Bartoloni G., Basso C., Batoroeva L., Bruneval P. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: II. Noninflammatory degenerative diseases—nomenclature and diagnostic criteria. Cardiovasc Pathol. 2016;25:247–257. doi: 10.1016/j.carpath.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Januzzi J.L., Isselbacher E.M., Fattori R., Cooper J.V., Smith D.E., Fang J. Characterizing the young patient with aortic dissection: Results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–669. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 7.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aquaro G.D., Briatico Vangosa A., Toia P., Barison A., Ait-Ali L., Midiri M. Aortic elasticity indices by magnetic resonance predict progression of ascending aorta dilation. Eur Radiol. 2017;27:1395–1403. doi: 10.1007/s00330-016-4501-5. [DOI] [PubMed] [Google Scholar]
- 9.Goudot G., Mirault T., Bruneval P., Soulat G., Pernot M., Messas E. Aortic wall elastic properties in case of bicuspid aortic valve. Front Physiol. 2019;10:299. doi: 10.3389/fphys.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillis E., Kumar A.A., Luyckx I., Preuss C., Cannaerts E., van de Beek G. Candidate gene resequencing in a large bicuspid aortic valve-associated thoracic aortic aneurysm cohort: SMAD6 as an important contributor. Front Physiol. 2017;8:400. doi: 10.3389/fphys.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyckx I., Kumar A.A., Reyniers E., Dekeyser E., Vanderstraeten K., Vandeweyer G., MIBAVA Leducq Consortium Copy number variation analysis in bicuspid aortic valve-related aortopathy identifies TBX20 as a contributing gene. Eur J Hum Genet. 2019;27:1033–1043. doi: 10.1038/s41431-019-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould R.A., Aziz H., Woods C.E., Seman-Senderos M.A., Sparks E., Preuss C. ROBO4 variants predispose individuals to bicuspid aortic valve and thoracic aortic aneurysm. Nat Genet. 2019;51:42–50. doi: 10.1038/s41588-018-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling J.S., Lubat E. Forme fruste or “incomplete” bicuspid aortic valves with very small raphes: the prevalence of bicuspid valve and its significance may be underestimated. Int J Cardiol. 2015;184:1–5. doi: 10.1016/j.ijcard.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Guala A., Rodriguez-Palomares J., Galian-Gay L., Teixido-Tura G., Johnson K.M., Wieben O. Partial aortic valve leaflet fusion is related to deleterious alteration of proximal aorta hemodynamics. Circulation. 2019;139:2707–2709. doi: 10.1161/CIRCULATIONAHA.119.039693. [DOI] [PubMed] [Google Scholar]
- 15.Sengodan P., Kharazi A., Akhter S.A., Nekkanti R. Rare presentation of a ruptured sinus of Valsalva aneurysm. CASE (Phila) 2020;4:43–46. doi: 10.1016/j.case.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcos L.C., Medina H.M., Sandoval N., Gelves J., Salazar G. A case of sinus of Valsalva aneurysm rupture in a patient with bicuspid aortic valve. CASE (Phila) 2020;4:47–52. doi: 10.1016/j.case.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perioperative transesophageal echocardiography and immediate postsurgical evaluation.