Abstract
Background
The efficacy of gliclazide has been reported in clinical trials in India. However, real-world data on the effectiveness of gliclazide in India is unavailable.
Objective
To provide real-world evidence regarding the effectiveness of gliclazide or gliclazide + metformin fixed-dose combination or separate medications, used either as monotherapy or as the latest add-on to other antihyperglycemic agents in reducing glycated hemoglobin (HbA1c) levels in Indian patients with type 2 diabetes mellitus (T2DM).
Methods
Electronic medical record data of adult patients who were diagnosed with T2DM who were newly initiated on or had been prescribed gliclazide or gliclazide + metformin combination for < 30 days as monotherapy or as add-on therapy to other antihyperglycemic agents, and had HbA1c ≥ 6.5% were retrospectively analyzed. Mean change in HbA1c from baseline was the primary endpoint. Secondary endpoints were assessment of dosages and formulations of gliclazide or gliclazide + metformin prescribed in the HbA1c spectrum and antihyperglycemic agents to which gliclazide or gliclazide + metformin was added as an adjunct. Readings were obtained before initiating gliclazide or gliclazide + metformin and after at least 90 days of treatment with gliclazide or gliclazide + metformin.
Results
Included patients (n = 498) were categorized into gliclazide only (n = 66), gliclazide + metformin only (n = 179), gliclazide add-on (n = 169), and gliclazide + metformin add-on (n = 84) groups. Mean (95% confidence interval [CI]) change in HbA1c among patients with baseline HbA1c > 7% was − 0.8% (− 1.26, − 0.34) in gliclazide only group; − 1.6% (− 1.89, − 1.31; p < 0.001) in gliclazide + metformin group; − 1.2% (− 1.50, − 0.90; p < 0.001) in add-on gliclazide group; and − 1.4% (− 1.75, − 1.05; p < 0.001) in add-on gliclazide + metformin group. Gliclazide once daily was the most prescribed regimen in the gliclazide only group (72.7%), with 60 mg being the most prescribed modified-release dose (62.5%). Gliclazide + metformin twice daily was the most prescribed regimen in the gliclazide + metformin group (69.3%) with 80 mg + 500 mg being the most prescribed immediate-release dose (62.9%). Gliclazide and gliclazide + metformin were most added as an adjunct to existing prescriptions of biguanides (83.4%) or insulin (64.3%), respectively.
Conclusion
Gliclazide or gliclazide + metformin prescribed as mono- or add-on therapy during routine clinical practice effectively reduced HbA1c in Indian patients with T2DM, thus validating the use of gliclazide and gliclazide + metformin for managing T2DM in India.
Key Points
| The effectiveness of gliclazide and gliclazide + metformin prescribed to Indian patients with type 2 diabetes mellitus during routine clinical practice in India was assessed using electronic medical record data. |
| Gliclazide or gliclazide + metformin was found to be effective in reducing HbA1C in Indian patients with type 2 diabetes mellitus. |
Introduction
The International Diabetes Federation (IDF), ninth Edition reports 9.3% (463 million) of the global population aged 20–79 years to be currently living with diabetes [1]. In India, this population amounts to 77 million and is projected to increase to 134.2 million by 2045. The mortality attributed to diabetes and diabetes-related complications is immense, with > 1 million deaths reported in India alone. Diabetes has also been reported to detrimentally affect quality of life [2–6] and increase the economic burden [7–10].
Multiple guidelines such as the American Diabetes Association 2019 guidelines [11], IDF clinical practice recommendations for managing Type 2 Diabetes Mellitus (T2DM) in primary care 2017 [12], and Research Society for the Study of Diabetes in India—Endocrine Society of India clinical practice recommendations for the management of T2DM 2020 [13] recommend oral antihyperglycemic drugs (OADs) such as sulfonylureas to be used as monotherapy (if metformin is not tolerated) or as combination therapy. Moreover, a network meta-analysis reported the new sulfonylureas glimepiride and gliclazide to be associated with a lower risk of all-cause (risk ratio [RR] 0.65; 95% confidence interval [CI] 0.53, 0.79) and cardiovascular-related mortality (RR 0.60; 95% CI 0.45, 0.84) than other sulfonylureas [14]. Another network meta-analysis reported gliclazide to be the only OAD that significantly reduced left ventricular mass (an important factor leading to cardiovascular disease) compared to placebo (standardized mean difference [SMD] − 1.09, 95% CI − 1.62, − 0.57) [15].
Several studies have reported the high usage of sulfonylureas in India [16–19], with glimepiride and gliclazide being the most commonly prescribed sulfonylureas. Efficacy of gliclazide in improving the glycemic control in T2DM patients has been proven in several clinical trials [20–23]. However, real-world studies on the effectiveness of gliclazide in India are unavailable. Therefore, we conducted this study to evaluate the effectiveness of gliclazide or gliclazide + metformin (fixed-dose combination as well as separate medications) among Indian T2DM patients in a real-world setting.
Methods
Data Source(s)
Analysis was performed from an Indian electronic software owned and administered by HealthPlix Technologies PRV. This software has been in operation since 2016 and fulfils the clinical needs of 12 medical specialties across 150 + cities in 20 states. Longitudinal information including demographics, diagnoses, medications, investigations and procedures conducted, functional status, and other data elements obtained from the software were used to conduct the analysis.
Applicable national regulatory laws and guidelines were followed and the study protocol was approved by Suraksha independent ethics committee on 3 December 2019. Patient confidentiality was maintained at all times as the study was performed using anonymized information only.
Study Design
This retrospective, longitudinal, observational study assessed OAD prescriptions using EMR data of Indian patients diagnosed with T2DM from January 2016 to October 2019. Each patient was required to possess two valid HbA1c readings in the EMR. Visit 1 (baseline) reading was defined as the HbA1c reading taken before initiation date of gliclazide or gliclazide + metformin or latest HbA1c reading taken in those who were on gliclazide or gliclazide + metformin for < 30 days either as monotherapy or as the most recent add-on to other antihyperglycemic agents. Visit 2 reading was defined as the next immediate HbA1c assessment available after at least 90 days of treatment with gliclazide or gliclazide + metformin as monotherapy or as latest add-on to other antihyperglycemic agents.
Inclusion Criteria
The study included adult patients (≥ 18 years old) who were diagnosed with T2DM either based on plasma glucose criteria specified in the American Diabetes Association 2019 guidelines [24] or by the treating physician, or were prescribed medications to treat T2DM; who were newly initiated on or had been prescribed gliclazide or gliclazide + metformin for < 30 days as monotherapy or as add-on therapy to other antihyperglycemic agents; and had glycated hemoglobin (HbA1c) ≥ 6.5%. Patients diagnosed with T1DM were excluded from the study.
Study Endpoints
The primary endpoint was evaluation of mean change in HbA1c from baseline to after at least 90 days of treatment with gliclazide or gliclazide + metformin combination as monotherapy or as latest add-on to other antihyperglycemic agents. The secondary endpoints were to assess the dosages and formulations of gliclazide or gliclazide + metformin prescribed in the HbA1c spectrum (6.5–8%, 8.1–10%, and > 10%) and to assess the antihyperglycemic agents to which gliclazide or gliclazide + metformin were added as an adjunct.
Assessments
Demographic characteristics, co-morbidities, concomitant medications used (identified as per the prescriptions), and HbA1c level were collected at baseline. Data regarding concomitant medications and HbA1c level were collected at the next visit. Co-morbidities such as hypertension and dyslipidemia were confirmed based on the prescribed concomitant medications. Cardiovascular events were confirmed based on diagnosis of ischemic heart disease, coronary heart disease/coronary artery disease, myocardial infarction; presence of a cerebrovascular accident; or a record of percutaneous transluminal coronary angioplasty or coronary artery bypass graft surgery. Chronic kidney disease (CKD) was confirmed based on the Kidney Disease Improving Global Outcomes 2012 guidelines [25] or if CKD was diagnosed by the treating physician.
Statistical Analysis
Descriptive statistics were used to summarize the study variables. Mean and standard deviations were reported for continuous variables while frequency and percentages were reported for categorical variables. Change in continuous variables were reported as mean change with 95% CI. p values were calculated using the Altman and Bland method [26] and p < 0.05 was considered statistically significant.
Results
Baseline Characteristics
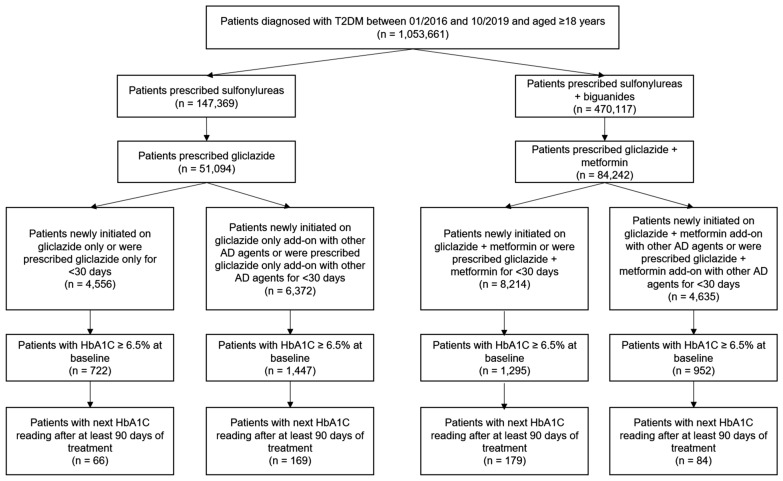
Among the 23,777 patients who were on gliclazide either as monotherapy or in combination with other oral antidiabetic drugs, 498 met the inclusion criteria and were included in the study (Fig. 1). These patients were categorized into gliclazide only (n = 66), gliclazide + metformin only (n = 179), gliclazide add-on (n = 169), and gliclazide + metformin add-on (n = 84) groups. Table 1 shows the baseline characteristics for individual groups. The overall mean ± SD age of the patients was 57.8 ± 12.6 years. Age-wise proportion of patients was 10.4% in < 40 years, 50.8% in 40–60 years, and 38.9% in the > 60 years category. The proportion of men was greater than women (54.2% vs. 45.6%). Dyslipidemia was the most common co-morbid condition (57.1%) followed by hypertension (50.3%), cardiovascular events (7.4%), and chronic kidney disease (CKD; 2.8%). Among the 11 patients with CKD, estimated glomerular filtration rate values were available for six patients (stage 2—two patients, stage 3A—two patients, stage 3B and stage 4—one patient each). The majority of the patients in all the groups reported HbA1c from 6.5% to 8%.
Fig. 1.
Patient flowchart. HbA1c glycated hemoglobin, T2DM type 2 diabetes mellitus
Table 1.
Demographic and clinical characteristics at baseline
| Gliclazide only (n = 66) | Gliclazide + metformin only (n = 179) | Gliclazide add-on (n = 169) | Gliclazide + metformin add-on (n = 84) | |
|---|---|---|---|---|
| Age (mean ± SD) | 60.6 (14.0) | 56.9 (12.9) | 57.4 (12.4) | 59.7 (10.9) |
| Age group [n (%)] | ||||
| < 40 years | 8 (12.3)* | 19 (10.6) | 20 (11.8) | 4 (4.7) |
| 40–60 years | 26 (40.0)* | 96 (53.6) | 83 (49.1) | 47 (55.9) |
| > 60 years | 31 (47.7)* | 64 (35.8) | 66 (39.0) | 33 (39.2) |
| Females [n (%)] | 31 (47.7) | 86 (48.0) | 75 (44.4) | 36 (42.9) |
| Males [n (%)] | 34 (52.3) | 93 (52.0) | 94 (55.6) | 48 (57.1) |
| Co-morbid conditions | ||||
| Dyslipidemia | 32 (48.5) | 93 (52.0) | 104 (61.5) | 56 (66.7) |
| Hypertension | 32 (48.5) | 87 (48.6) | 87 (51.5) | 48 (57.1) |
| CV events# | 4 (8.0) | 8 (6.0) | 12 (8.5) | 6 (7.1) |
| CKD# | 1 (2.0) | 0 (0.0) | 9 (6.4) | 1 (1.2) |
| HbA1c levels | ||||
| 6.5–8% | 37 (56.1) | 82 (45.8) | 83 (49.1) | 33 (39.3) |
| 8.1–10% | 24 (36.4) | 52 (29.1) | 61 (36.1) | 35 (41.7) |
| > 10% | 5 (7.5) | 45 (25.1) | 25 (14.8) | 16 (19.0) |
CKD chronic kidney disease, CV cardiovascular, HbA1c glycated hemoglobin
*One patient did not mention their age. Hence, sample size was considered as 65
#The sample sizes for assessment of number of patients with CV events and CKD are different from the sample size of the group as a limited number of patients had the diagnosis mentioned in the diagnosis field. Hence, the sample sizes were: Gliclazide only (n = 50), Gliclazide + metformin only (n = 133), Gliclazide add-on (n = 141), and Gliclazide add-on to metformin + other antihyperglycemics (n = 24). CV events comprise coronary artery disease, myocardial infarction, and stroke
Mean Change in HbA1c due to Gliclazide or Gliclazide + Metformin
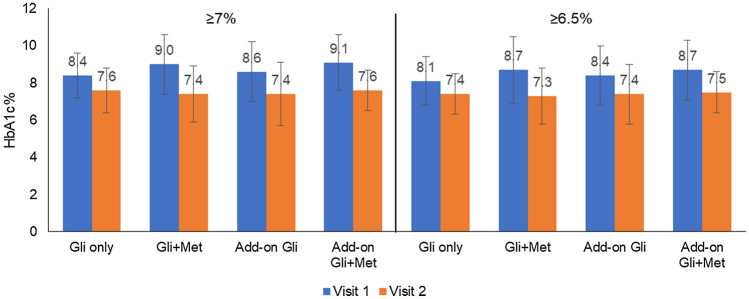
Initiation of gliclazide or gliclazide + metformin reduced HbA1c in all patients (Fig. 2). Among patients with an HbA1c ≥ 7% at baseline, the mean (95% CI) change in HbA1c after therapy was − 0.8% (95% CI − 1.26, − 0.34) in the gliclazide only group; − 1.6% (95% CI − 1.89, − 1.31; p < 0.001) in the gliclazide + metformin group; − 1.2% (95% CI − 1.50, − 0.90; p < 0.001) in the add-on gliclazide group; and − 1.5% (95% CI − 1.75, − 1.05; p < 0.001) in the add-on gliclazide + metformin group.
Fig. 2.
Effect of gliclazide or gliclazide + metformin on HbA1c%. The figure shows the HbA1c% levels after at least 90 days of treatment. The data are divided into patients with HbA1c ≥ 7% and ≥ 6.5% at baseline. Mean ± SD values are represented. Gli gliclazide, HbA1c glycated hemoglobin, Met metformin, SD standard deviation
Among patients with an HbA1c ≥ 6.5% at baseline, the mean (95% CI) change in HbA1c after therapy was − 0.62% (95% CI − 1.01, − 0.23) in the gliclazide only group; − 1.4% (95% CI − 1.56, − 1.04; p < 0.001) in the gliclazide + metformin group; − 1.0% (95% CI − 1.39, − 0.81; p < 0.001) in the add-on gliclazide group; and − 1.2% (95% CI − 1.52, − 0.88; p < 0.001) in the add-on gliclazide + metformin group (Fig. 3).
Fig. 3.
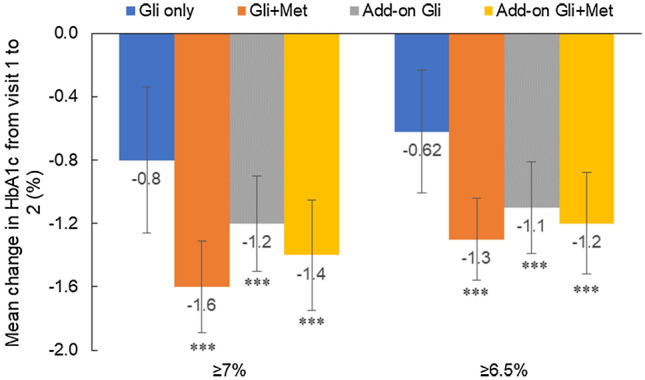
Gliclazide- or gliclazide + metformin-induced mean change in HbA1c%. The figure shows the mean (95% CI) change in HbA1c% levels from visit 1 to visit 2 after at least 90 days of treatment. Visit 2 readings were statistically compared against Visit 1 readings. The data are divided into patients with HbA1c ≥ 7% and ≥ 6.5% at baseline. ***p < 0.001. CI confidence interval, Gli gliclazide, HbA1c glycated hemoglobin, Met metformin
Dosages and Formulation of Gliclazide Administered as per HbA1c Spectrum
In the gliclazide only group, the majority of the patients across the spectrum were prescribed gliclazide once daily (HbA1c 6.5–8%: 70.3%; 8.1–10%: 79.2%; and > 10%: 60.0%). Among all the patients, 27.3% were on immediate-release (IR) tablets and the most prescribed gliclazide IR dose was 40 mg (72.2%). The 40 mg dose was also highly prescribed across the spectrum (HbA1c 6.5–8%: 81.8%; 8.1–10%: 40.0%; and > 10%: 100.0%). Among all the patients, 72.7% were on modified-release (MR) tablets and the most prescribed MR dose was 60 mg (62.5%). The 60 mg dose was also highly prescribed across the spectrum (HbA1c 6.5–8%: 53.8%; 8.1–10%: 68.4%; and > 10%: 100.0%; Table 2).
Table 2.
Frequency and dose of gliclazide administered as per HbA1c levels
| Baseline HbA1c levels | |||
|---|---|---|---|
| 6.5–8% | 8.1–10% | > 10% | |
| Gliclazide only (n) | 37 | 24 | 5 |
| Dose frequency (n, %) | |||
| Once daily (extended release) | 26 (70.3) | 19 (79.2) | 3 (60.0) |
| Twice daily (immediate release) | 11 (29.7) | 5 (20.8) | 2 (40.0) |
| Prescribed formulation (n, %) | |||
| Immediate release (n) | 11 | 5 | 2 |
| 40 mg | 9 (81.8) | 2 (40.0) | 2 (100.0) |
| 80 mg | 1 (9.1) | 2 (40.0) | – |
| 160 mg | 1 (9.1) | 1 (20.0) | – |
| Extended release (n) | 26 | 19 | 3 |
| 30 mg | 12 (46.2) | 6 (31.6) | – |
| 60 mg | 14 (53.8) | 13 (68.4) | 3 (100.0) |
| Gliclazide + metformin (n) | 82 | 52 | 45 |
| Dose frequency (n, %) | |||
| Once daily (extended release) | 30 (36.6) | 11 (21.2) | 14 (31.1) |
| Twice daily (immediate release) | 52 (63.4) | 41 (78.8) | 31 (68.9) |
| Prescribed formulation (n, %) | |||
| Immediate release (n) | 52 | 41 | 31 |
| 40 mg + 500 mg | 16 (30.8) | 14 (34.1) | 16 (51.6) |
| 80 mg + 500 mg | 36 (69.2) | 27 (65.9) | 15 (48.4) |
| Extended release (n) | 30 | 11 | 14 |
| 30 mg + 500 mg | 9 (30.0) | 2 (18.2) | 3 (21.4) |
| 60 mg + 500 mg | 21 (70.0) | 9 (81.8) | 11 (78.6) |
HbA1c glycated hemoglobin
In the gliclazide + metformin group, gliclazide + metformin twice daily was prescribed the most to patients across the spectrum (HbA1c 6.5–8%: 63.4%; 8.1–10%: 78.8%; and > 10%: 68.9%). Among all the patients, 69.3% were on gliclazide IR + metformin tablets and the most prescribed IR dose was 80 mg + 500 mg (62.9%). The 80 mg + 500 mg dose was also highly prescribed across the spectrum (HbA1c 6.5–8%: 69.2%; 8.1–10%: 65.9%; and > 10%: 48.4%). Among all the patients, 30.7% were on gliclazide MR + metformin tablets and the most prescribed MR dose was 60 mg + 500 mg (74.5%). The 60 mg + 500 mg dose was also highly prescribed across the spectrum (HbA1c 6.5–8%: 70.0%; 8.1–10%: 81.8%; and > 10%: 78.6%; Table 2).
Break-Up of Existing Antihyperglycemic Agents
Gliclazide was added on to an average of 2.5 antihyperglycemic medications and gliclazide + metformin was added on to an average of 2.4 antihyperglycemic medications. The majority of patients in the gliclazide add-on group had an existing prescription of biguanides (83.4%), dipeptidyl peptidase-4 (DPP4) inhibitors (78.7%), or insulin (42.0%). On the other hand, majority of patients in the gliclazide + metformin add-on group had an existing prescription of insulin (64.3%), DPP4 inhibitors (57.1%), or biguanides (51.2%) (Table 3).
Table 3.
Break down of antihyperglycemic agents to which gliclazide or gliclazide + metformin was added
| Antihyperglycemic drug class (n, %) | Gliclazide add-on (n = 169) | Gliclazide + metformin add-on (n = 84) |
|---|---|---|
| Biguanides | 141 (83.4) | 43 (51.2) |
| DPP4 inhibitors | 133 (78.7) | 48 (57.1) |
| Insulin | 71 (42.0) | 54 (64.3) |
| SGLT2 inhibitor | 25 (14.8) | 22 (26.2) |
| Alpha glucosidase inhibitors | 5 (3.0) | 4 (4.8) |
| Thiazolidinediones | 5 (3.0) | 3 (3.6) |
| Other sulfonylureas | 3 (1.8) | 3 (3.6) |
Some patients were on more than one drug class simultaneously; in these cases, the patient was counted in all the drug classes separately
DPP4 dipeptidyl peptidase-4, SGLT2 sodium glucose co-transporter 2
Discussion
Sulfonylureas are a potent class of OADs and have been an important part of the antidiabetic therapeutic armamentarium for decades [27]. Glimepiride and gliclazide are one of the most commonly prescribed sulfonylureas in India [17–19]. Gliclazide is a modern sulfonylurea with good effectiveness [22, 28, 29] and a good tolerability profile in terms of low risk of hypoglycemia [28–31] and weight gain [29, 30], cardiovascular neutrality [14, 32], and long-term reduction of end-stage kidney disease (ESKD) [33]. The common adverse effects of gliclazide or gliclazide + metformin combination include hypoglycemia, neuropathy, fatigue, headache, abdominal pain, bronchitis, and vision disorders [21–23, 28–31]. Gliclazide has also been recommended as an effective and tolerable first- or second-line agent (based on patient status) for the management of T2DM [27]. Despite the benefits of gliclazide usage, real-world evidence on the effectiveness of gliclazide in India was unavailable. The current study addresses this data gap. The current study is probably the first pan-India study to assess the real-world impact of gliclazide on patients withT2DM using EMR data. It provides new evidence supporting the effectiveness of gliclazide in a real-world scenario. In the present study, usage of gliclazide or gliclazide + metformin as monotherapy or as recent add-on to other antihyperglycemic agents resulted in an overall HbA1c reduction of 1.1% in patients with baseline HbA1c ≥ 6.5% after at least 90 days of treatment.
Mean reduction in HbA1c was impressive across the groups, both in patients with HbA1c ≥ 6.5% (1.1%) and ≥ 7% (1.3%). Gliclazide-induced 0.8% reduction in HbA1c observed among patients with baseline HbA1c ≥ 7% was relatively lower than the 1.6% reduction reported in a Chinese double-blind, randomized, multicenter study by Lu et al. [28], wherein a similar population was treated with gliclazide or gliclazide MR. However, the larger reduction could be attributed to the greater treatment duration of the Lu et al. study (20 weeks) versus the present study (at least 12 weeks), difference in type of study (randomized clinical trial vs. real-world study), as well as to the differing treatment doses: up-titration from gliclazide 80 mg or gliclazide MR 30 mg every 4 weeks in the Lu et al. study versus constant dose or up-titration as and when required, in the present study. We could not capture data on up-titration as the study was not designed to capture this information.
The mean reduction in HbA1c (1.6%) obtained on usage of gliclazide + metformin in patients with HbA1c ≥ 7% is consistent with previous results reported by Lu et al. [28] and Pareek et al. [22]. Lu et al. reported a 1.4% reduction in HbA1c after administering metformin (existing prescription dose) in combination with gliclazide twice daily or gliclazide MR once daily for 20 weeks. Pareek et al. conducted a prospective, open-labeled, multicentric study wherein Indian patients with HbA1c ≥ 7% were treated with an initial dose of gliclazide 80 mg + metformin 500 mg followed by up-titration every 4 weeks to a maximum dose of gliclazide 320 mg + metformin 2000 mg. Here, a 1.16% reduction in HbA1c was observed after administering treatment for 90 days.
In the current study, gliclazide effectively reduced HbA1c in individuals with baseline HbA1c ≥ 6.5% and ≥ 7%. This property of reducing HbA1c regardless of the baseline HbA1c level gives gliclazide an edge over relatively newer antidiabetic agents such as DPP4 inhibitors or sodium glucose co-transporter 2 (SGLT2) inhibitors. DPP4 inhibitors result in modest reduction in HbA1c when used as monotherapy (~ 0.5–1%) or in combination with metformin (~ 0.6–1.1%) [34]. SGLT2 inhibitors have been reported to reduce HbA1c by 0.5–0.8% when used as monotherapy or add-on therapy [35], which makes them an appropriate choice as add-on agents in patients with baseline HbA1c > 7% [35, 36].
Renally compromised patients are recommended to avoid sulfonylureas or adjust the dose before initiation [37]. However, the Kidney Disease Outcomes Quality Initiative Clinical Practice Guideline does not recommend any dose adjustment for gliclazide, even for patients with severe chronic kidney disease or ESKD [37], thus alleviating issues regarding dose adjustment. Moreover, gliclazide is available in various formulations (IR and MR) and various dosages (IR—40 mg and 80 mg, MR—30 mg and 60 mg), which allows a range of doses to be prescribed as per the patient’s requirement. Fixed-dose combinations (FDCs) of gliclazide/gliclazide MR with metformin are also available which offer convenience and improve patient adherence [27].
Gliclazide MR was the medication of choice among patients on gliclazide only, irrespective of the HbA1c spectrum, while the IR tablet was favored in patients prescribed gliclazide + metformin. The MR characteristics allow a once-daily dosing regimen to be prescribed, which has been associated with higher adherence rate (odds ratio [OR] 3.07; 95% CI 1.80, 5.23, p < 0.001) and compliance rate (OR 3.50; 95% CI 1.73, 7.08, p < 0.001) compared with more than once-daily dosing, irrespective of the disease, in a meta-analysis by Srivastava et al. [38]. Positive outcomes were also observed in diabetes-specific studies [39, 40].
Limitations
The retrospective design of the study is a major limitation. Also, unlike clinical trials where drug administration is supervised, patients in the current study were responsible for their own medication. As EMR records only contain prescription data, it is possible that some patients may not have adhered to the prescription. The relatively low number of patients can be attributed to: (a) the study design wherein patients who were treatment naïve or who were on gliclazide or gliclazide + metformin for < 30 days either as monotherapy or as latest add-on to other antihyperglycemic agents were only included; (b) absence of HbA1c ≥ 6.5% at baseline; and (c) lack of a second HbA1c reading after 90 days in the database. While the reasons for absence of the second HbA1c reading were not captured, they may include lack of follow-up, change in the treating physician, or change in treatment. Indeed, loss to follow-up/care is a major concern in India, as reported by Prenissl et al. [41], wherein 75% of the participants were “lost” to care. The proportion of patients on gliclazide only (66/498) was lower than other groups probably due to low prescription of gliclazide as monotherapy in the database used. Finally, we were unable to evaluate adverse events such as hypoglycemia as the database does not capture adverse events.
Conclusion
The current study is probably the first pan-India EMR-based study that assessed the real-world effectiveness of gliclazide or gliclazide + metformin, used either as monotherapy or as the latest add-on to other antihyperglycemic agents in reducing HbA1c levels in Indian patients with T2DM. We observed a good reduction in HbA1c in all the treatment regimens.
Acknowledgements
The authors thank Leo J. Philip Tharappel (SIRO Clinpharm Pvt Ltd.) for providing medical writing assistance.
Author Contributions
NKP, RK, AM, and SS developed the concept and performed the study. SS performed data analysis. NKP and RK drafted the manuscript. All authors reviewed the manuscript and gave final approval.
Compliance with Ethical Standards
Funding
This study was funded by Dr. Reddy’s Laboratories.
Conflict of interest
NKP, RK, AM, and SM are employees of Dr. Reddy’s Laboratories and may own stock. SS is an employee of Healthplix Ltd, which received consultancy fees from Dr. Reddy’s Laboratories to perform the study. BS and RKS are members of the scientific advisory board for Dr. Reddy’s Laboratories and have received an honorarium for the same. UP and SR do not have any conflicts of interest.
Ethics approval
The study protocol was approved by an independent ethics committee on 3 December, 2019.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.IDF Diabetes Atlas. 9th edition. Brussels, Belgium: International Diabetes Federation. 2019. https://www.diabetesatlas.org. 09/04/2020.
- 2.John R, Pise S, Chaudhari L, Deshpande PR. Evaluation of quality of life in type 2 diabetes mellitus patients using quality of life instrument for Indian diabetic patients: a cross-sectional study. J Midlife Health. 2019;10(2):81–88. doi: 10.4103/jmh.JMH_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PrasannaKumar HR, Mahesh MG, Menon VB, Srinath KM, Shashidhara KC, Ashok P. Patient Self-reported quality of life assessment in Type 2 diabetes mellitus: a pilot study. Niger J Clin Pract. 2018;21(3):343–349. doi: 10.4103/njcp.njcp_433_16. [DOI] [PubMed] [Google Scholar]
- 4.Goel M, Dhuldhule S, Prakash A, Ghotekar LH. Assessing health-related quality of life in patients with diabetes mellitus at a Tertiary Care Center in Central Delhi. Indian J Commun Med. 2019;44(2):171–172. doi: 10.4103/ijcm.IJCM_273_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santosh Kumar A, Koppad R, Chandrashekar SV. Quality of life of type 2 diabetes patients in a tertiary care hospital in southern part of India, Shimoga, Karnataka: a cross-sectional study. Int J Commun Med Pub Health3. 2016;3(7):1723–8.
- 6.Thunla PR, Gundepogu UJ, Thumma P, Bairi R. An observational study on health related quality of life in diabetes mellitus patients. Val Health. 2016;19(7):A901. [Google Scholar]
- 7.Yesudian CA, Grepstad M, Visintin E, Ferrario A. The economic burden of diabetes in India: a review of the literature. Global Health. 2014;10:80. doi: 10.1186/s12992-014-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra P, Gogate B, Gogate P, Thite N, Mutha A, Walimbe A. Economic burden of diabetes in urban indians. Open Ophthalmol J. 2014;8:91–94. doi: 10.2174/1874364101408010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansode B, Jungari DS. Economic burden of diabetic patients in India: a review. Diabetes Metab Syndr. 2019;13(4):2469–2472. doi: 10.1016/j.dsx.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Prajapati A, Kothari N, Ganguly B. Economic burden of diabetes mellitus in western India: a hospital based study. Int J Basic Clin Pharmacol. 2016;5(6):2572–2580. doi: 10.18203/2319-2003.ijbcp20164126. [DOI] [Google Scholar]
- 11.American Diabetes A. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90-S102. 10.2337/dc19-S009 [DOI] [PubMed]
- 12.Federation ID. Recommendations For Managing Type 2 Diabetes In Primary Care. 2017. www.idf.org/managing-type2-diabetes.
- 13.Clinical Practice Recommendations for the Management of Type 2 Diabetes Mellitus 2020. 2020. https://www.rssdi.in/newwebsite/index.php.
- 14.Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 2015;3(1):43–51. doi: 10.1016/S2213-8587(14)70213-X. [DOI] [PubMed] [Google Scholar]
- 15.Ida S, Kaneko R, Murata K. Effects of oral antidiabetic drugs on left ventricular mass in patients with type 2 diabetes mellitus: a network meta-analysis. Cardiovasc Diabetol. 2018;17(1):129. doi: 10.1186/s12933-018-0773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singla R, Bindra J, Singla A, Gupta Y, Kalra S. Drug prescription patterns and cost analysis of diabetes therapy in India: audit of an endocrine practice. Indian J Endocrinol Metab. 2019;23(1):40–45. doi: 10.4103/ijem.IJEM_646_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokta J, Mokta K, Ranjan A, Joshi I, Garg M. Diabetes drug prescription pattern and awareness among health care providers in Sub-Himalayan Region of India: a population based study. J Assoc Phys India. 2017;65(5):50–54. [PubMed] [Google Scholar]
- 18.Agarwal AA, Jadhav PR, Deshmukh YA. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J Basic Clin Pharm. 2014;5(3):79–83. doi: 10.4103/0976-0105.139731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satpathy SV, Datta S, Upreti B. Utilization study of antidiabetic agents in a teaching hospital of Sikkim and adherence to current standard treatment guidelines. J Pharm Bioallied Sci. 2016;8(3):223–228. doi: 10.4103/0975-7406.175975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra ST, Priya G, Khurana ML, Jyotsna VP, Sreenivas V, Dwivedi S, et al. Comparison of gliclazide with insulin as initial treatment modality in newly diagnosed type 2 diabetes. Diabetes Technol Ther. 2008;10(5):363–368. doi: 10.1089/dia.2008.0045. [DOI] [PubMed] [Google Scholar]
- 21.Mohan V, Chopra V, Sanyal D, Jain S, Jayaprakashsai J. Treatment of type 2 diabetes with a breakable extended release Gliclazide formulation in primary care: The Xrise Study. J Assoc Phys India. 2015;63(12):26–29. [PubMed] [Google Scholar]
- 22.Pareek A, Chandurkar N, Zawar S, Agrawal N. Evaluation of efficacy and tolerability of gliclazide and metformin combination: a multicentric study in patients with type 2 diabetes mellitus uncontrolled on monotherapy with sulfonylurea or metformin. Am J Ther. 2010;17(6):559–565. doi: 10.1097/MJT.0b013e3181c6c0f9. [DOI] [PubMed] [Google Scholar]
- 23.Kalra S, Das AK. Epidemiologic surveillance of glycemic response to a scored, breakable, extended release, fixed dose combination of gliclazide and metformin in persons with type 2 diabetes. J Assoc Phys India. 2017;65(6):38–41. [PubMed] [Google Scholar]
- 24.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–28. 10.2337/dc19-S002. [DOI] [PubMed]
- 25.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30. 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed]
- 26.Altman DG, Bland JM. How to obtain the P value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 27.Kalra S, Bahendeka S, Sahay R, Ghosh S, Md F, Orabi A, et al. Consensus recommendations on sulfonylurea and sulfonylurea combinations in the management of type 2 diabetes mellitus—International Task Force. Indian J Endocrinol Metab. 2018;22(1):132–157. doi: 10.4103/ijem.IJEM_556_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CH, Chang CC, Chuang LM, Wang CY, Jiang YD, Wu HP. Double-blind, randomized, multicentre study of the efficacy and safety of gliclazide-modified release in the treatment of Chinese type 2 diabetic patients. Diabetes Obes Metab. 2006;8(2):184–191. doi: 10.1111/j.1463-1326.2005.00501.x. [DOI] [PubMed] [Google Scholar]
- 29.Drouin P, Standl E, Diamicron MRSG. Gliclazide modified release: results of a 2-year study in patients with type 2 diabetes. Diabetes Obes Metab. 2004;6(6):414–421. doi: 10.1111/j.1462-8902.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 30.Drouin P. Diamicron MR once daily is effective and well tolerated in type 2 diabetes: a double-blind, randomized, multinational study. J Diabetes Complicat. 2000;14(4):185–191. doi: 10.1016/s1056-8727(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 31.Leiter LA, Shestakova MV, Satman I. Effectiveness of gliclazide MR 60 mg in the management of type 2 diabetes: analyses from the EASYDia trial. Diabetol Metab Syndr. 2018;10:30. doi: 10.1186/s13098-018-0331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. 10.1056/NEJMoa0802987. [DOI] [PubMed]
- 33.Wong MG, Perkovic V, Chalmers J, Woodward M, Li Q, Cooper ME, et al. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- 34.Makrilakis K. The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: when to select, what to expect. Int J Environ Res Public Health. 2019 doi: 10.3390/ijerph16152720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikhail N. Place of sodium-glucose co-transporter type 2 inhibitors for treatment of type 2 diabetes. World J Diabetes. 2014;5(6):854–859. doi: 10.4239/wjd.v5.i6.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lisenby KM, Meyer A, Slater NA. Is an SGLT2 inhibitor right for your patient with type 2 diabetes? J Fam Pract. 2016;65(9):587–593. [PubMed] [Google Scholar]
- 37.National KF. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434. doi: 10.2147/PPA.S44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezii CM, Kawabata H, Tran M. Effects of once-daily and twice-daily dosing on adherence with prescribed glipizide oral therapy for type 2 diabetes. South Med J. 2002;95(1):68–71. doi: 10.1097/00007611-200295010-00014. [DOI] [PubMed] [Google Scholar]
- 40.Guillausseau PJ. Compliance and optimisation of oral antidiabetic therapy. A longitudinal study. Presse Med. 2004;33(3):156–160. doi: 10.1016/s0755-4982(04)98512-0. [DOI] [PubMed] [Google Scholar]
- 41.Prenissl J, Jaacks LM, Mohan V, Manne-Goehler J, Davies JI, Awasthi A, et al. Variation in health system performance for managing diabetes among states in India: a cross-sectional study of individuals aged 15 to 49 years. BMC Med. 2019;17(1):92. doi: 10.1186/s12916-019-1325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]