Abstract
Background
Data are sparse concerning the sequential use of multiple anaplastic lymphoma kinase (ALK) inhibitors for ALK-positive locally advanced or metastatic non-small cell lung cancer (NSCLC).
Objective
This study investigated sequencing and outcomes among patients receiving multiple ALK inhibitors.
Patients and Methods
This was a retrospective observational cohort study of adult patients with ALK-positive NSCLC treated with available first- and second-generation ALK inhibitors from 1 September 2011 to 31 December 2017. Duration of therapy (DOT) and overall survival (OS) were assessed with the Kaplan–Meier method. A multivariable linear regression analysis was performed to assess if DOT with a preceding ALK inhibitor was predictive of DOT for subsequent ALK inhibitor treatments.
Results
A total of 410 patients were analyzed: 57% received 1 ALK inhibitor; 35%, 2 ALK inhibitors; and 8%, 3–4 ALK inhibitors. Among those receiving > 1 ALK inhibitor (n = 177), 60% received a crizotinib-led sequence and 39% an alectinib-led sequence. Nearly 60% of the overall population received chemotherapy prior to their first ALK inhibitor. Median OS for the study population was 28 months, 15 months in patients who received 1 ALK inhibitor, 42 months in patients who received 2 ALK inhibitors, and 56 months in patients who received 3–4 ALK inhibitors. Longer DOT of the first ALK inhibitor was associated with increased DOT of the second (p < 0.0001), and longer DOT of the second ALK inhibitor was associated with increased DOT of the third (p < 0.0001).
Conclusions
This study provides initial information on real-world treatment patterns following the introduction of new ALK inhibitors, and supports the use of sequential ALK therapies.
Key Points
| This study characterizes the real-world treatment patterns and outcomes for patients treated with ALK inhibitors within the timeline of new ALK inhibitor drug approvals and indications. |
| Patients received 1–4 ALK inhibitors during the study period. In patients who were able to receive multiple lines of treatment, longer therapy duration and survival were observed in patients who were able to receive sequential ALK inhibitors. |
| A considerable proportion of the patients were treated with chemotherapy before ALK inhibitor treatment, suggesting the opportunity to improve biomarker testing strategies to identify the patients who would benefit from ALK inhibitors. |
Introduction
Lung cancer is the leading cause of cancer-related deaths in the US. It is estimated that there were 228,150 new cases and 142,670 deaths due to lung cancer in 2019 [1]. Non-small cell lung cancer (NSCLC) comprises around 80–85% of all lung cancers and commonly includes adenocarcinoma, large-cell, and squamous cell histologies [2]. Although NSCLCs occur most often in smokers, adenocarcinomas are the most common type of lung cancer seen in nonsmokers [3].
A genetic alteration of the anaplastic lymphoma kinase (ALK) gene is present in 3–5% of NSCLCs. Patients with this gene alteration are most often younger in age, female, and nonsmokers with adenocarcinoma [4, 5]. The FDA approved the first ALK inhibitor, crizotinib, for ALK-positive locally advanced or metastatic NSCLC in 2011. Accelerated approval was based on two single-arm trials demonstrating objective response rates of 50% and 61% and median response durations of 42 and 48 weeks [6–8]. In 2013, crizotinib received regular FDA approval based on confirmation of clinical benefit and improved progression-free survival (PFS) and tumor responses compared with chemotherapy in patients with metastatic NSCLC who carry the ALK gene rearrangement [9–11]. Second-generation ALK inhibitors have since been approved in the US, including ceritinib (2014) [12], alectinib (2015) [13], and brigatinib (2017) [14]. These were initially approved for use following progression on crizotinib. Subsequent data from phase III trials have demonstrated efficacy with alectinib and with ceritinib in the front-line setting versus crizotinib and versus chemotherapy, respectively [15–17], and both were approved for first-line treatment in 2017. Recently, lorlatinib, a third-generation ALK, was approved in the US [18]. Studies with second- and third-generation ALK inhibitors are ongoing [19, 20].
Despite the availability of new ALK inhibitors, data on the sequential use of multiple ALK inhibitors is limited. This study aimed to understand ALK inhibitor therapy sequencing and outcomes among patients receiving multiple agents, including duration of ALK inhibitor therapy (DOT) and overall survival (OS) among patients with ALK-positive NSCLC.
Materials and Methods
This was a retrospective observational cohort study of adult patients with ALK-positive NSCLC who received treatment with available first-generation (crizotinib) and second-generation (alectinib, brigatinib, ceritinib) ALK inhibitors from 1 September 2011 to 31 December 2017. The study included patients who received care in a US Oncology Network (USON) clinic utilizing the iKnowMed (iKM) electronic health record (EHR). The USON is affiliated with approximately 1,400 physicians in more than 60 community oncology practices across 25 states in the US. Structured data were collected via programmatic queries of the iKM EHR. Included patients were ≥ 18 years of age at first diagnosis of NSCLC, had at least 2 office visits (to exclude patients who may have only had second opinions in the USON, and therefore to include patients with some continuity of care), initiated treatment with a prescription date for an ALK inhibitor during the study period, and had documented ALK-positive status. The index date was defined as the date on which the patient was first prescribed treatment with an ALK inhibitor. The baseline period represented the last known value available prior to treatment with the first ALK inhibitor. Duration of follow-up was variable. The study received Institutional Review Board approval.
Descriptive statistics (mean, median, ranges) were examined for patient demographic and clinical characteristics, with patients stratified into those receiving 1, 2, or 3–4 unique ALK inhibitors overall. Treatment patterns assessed the total number of unique ALK inhibitors received per patient and the ALK treatment sequencing among patients who received more than one ALK inhibitor. Results in subgroups with fewer than five patients were aggregated.
DOT was calculated for the overall ALK treatment sequence from the first ALK inhibitor prescription date until the documented ALK treatment discontinuation date, the last prescription date of the last ALK inhibitor, or the start date of a subsequent non-ALK inhibitor therapy (as an indicator of ALK inhibitor discontinuation). Patients with no evidence of discontinuation were censored at the last visit date or the end of the study period, whichever came first.
The time to non-ALK inhibitor treatment was assessed. The time from the first ALK inhibitor to treatment with a non-ALK inhibitor therapy after the last ALK inhibitor was calculated among patients who received uninterrupted sequential ALK inhibitors, and among patients who had other intervening non-ALK inhibitor treatments in between the ALK inhibitors. Only patients who received a subsequent non-ALK inhibitor were included in the calculations.
OS was calculated from the first ALK inhibitor treatment (first prescription date) until death or censoring at the last visit date or the end of the study period, whichever came first. DOT and OS were assessed using the Kaplan–Meier method.
A multivariable linear regression analysis was performed to assess if the DOT with the preceding ALK inhibitor was associated with the DOT of the subsequent ALK inhibitor treatment. The analyses were conducted using SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
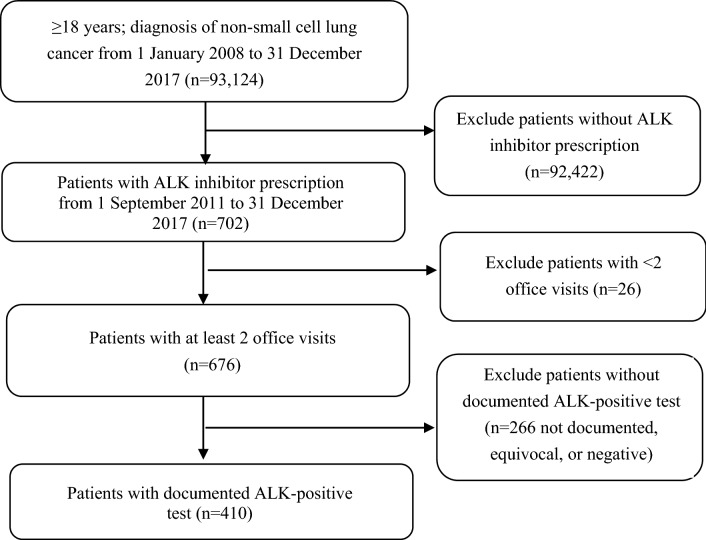
Over 93,000 patients with NSCLC were identified in the EHR database during the study period, of whom 410 patients known to have ALK-positive NSCLC were included (Fig. 1).
Fig. 1.
Sample attrition. ALK anaplastic lymphoma kinase
Table 1 presents the baseline demographics and clinical characteristics for the study population overall and stratified by the number of ALK inhibitors received. In the overall population, the median age at initiation of the first ALK was 62 years, 78% were Caucasian, and 87% had adenocarcinoma histology. There were more women than men in each of the groups, and most patients in all groups were never smokers. Most patients had an Eastern Cooperative Oncology Group (ECOG) status of 0–1. Approximately 90% of the overall population had advanced (stage III–IV) disease at initial NSCLC diagnosis, and nearly 60% of the population had been treated with chemotherapy prior to their first ALK inhibitor. The most common sites of metastases in the overall population prior to initiation of ALK inhibitor therapy were bone (22%), brain (13%) and liver (9%). Epidermal growth factor receptor (EGFR) mutation status was known in 74% (n = 304) of the patients; 97% of those were negative. ROS mutation status was known in 18% (n = 73) of the patients; 3 patients were positive. BRAF mutation status was not known in 96% of the patients. Programmed death-ligand 1 (PD-L1) expression was available in 19% (n = 77) of the patients, of whom the majority (n = 46) had an expression of ≥ 1%.
Table 1.
Demographic and clinical characteristics at baseline for the overall study population (n = 410) and stratified by number of ALK inhibitors received
| Overall (n = 410) | 1 ALK inhibitor (n = 233) | 2 ALK inhibitors (n = 144) | 3–4 ALK inhibitors (n = 33) | |
|---|---|---|---|---|
| Age at index, years | ||||
| Median (min, max) | 62 (20, 89) | 64 (27, 88) | 59 (20, 85) | 58 (30, 79) |
| Age distribution, n (%) | ||||
| 18–65 years | 238 (58.0) | 125 (53.6) | 91 (63.2) | 22 (66.7) |
| > 65 years | 172 (42.0) | 108 (46.4) | 53 (36.8) | 11 (33.3) |
| Sex, n (%) | ||||
| Female | 222 (54.1) | 126 (54.1) | 79 (54.9) | 17 (51.5) |
| Male | 188 (45.9) | 107 (45.9) | 65 (45.1) | 16 (48.5) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 47 (11.5) | 24 (10.3) | 20 (13.9) | 3 (9.1) |
| Not Hispanic or Latino | 330 (80.5) | 187 (80.3) | 113 (78.5) | 30 (90.9) |
| Unknown | 33 (8.0) | 22 (9.4) | 11 (7.6) | 0 (0.00) |
| Race, n (%) | ||||
| Black or African American | 23 (5.6) | 14 (6.0) | 8 (5.6) | 1 (3.0) |
| Caucasian | 318 (77.6) | 182 (78.1) | 107 (74.3) | 29 (87.9) |
| Other | 26 (6.3) | 9 (3.9) | 14 (9.7) | 3 (9.1) |
| Missing | 43 (10.5) | 28 (12.0) | 15 (10.4) | 0 (0.00) |
| Smoking status at index, n (%) | ||||
| Current | 33 (8.0) | 24 (10.3) | 7 (4.9) | 2 (6.1) |
| Former | 151 (36.8) | 89 (38.2) | 51 (35.4) | 11 (33.3) |
| Never | 220 (53.7) | 117 (50.2) | 84 (58.3) | 19 (57.6) |
| Not recorded | 6 (1.5) | 3 (1.3) | 2 (1.4) | 1 (3.0) |
| ECOG status at index, n (%) | ||||
| 0 | 52 (12.7) | 25 (10.7) | 20 (13.9) | 7 (21.2) |
| 1 | 243 (59.3) | 135 (57.9) | 86 (59.7) | 22 (66.7) |
| 2 | 60 (14.6) | 41 (17.6) | 16 (11.1) | 3 (9.1) |
| 3 | 5 (1.2) | 4 (1.7) | 1 (0.7) | 0 (0.00) |
| 4 | 1 (0.2) | 0 (0.00) | 1 (0.7) | 0 (0.00) |
| Unknown | 49 (12.0) | 28 (12.0) | 20 (13.9) | 1 (3.0) |
| Disease stage at initial NSCLC diagnosis, n (%) | ||||
| IA | 11 (2.7) | 7 (3.0) | 3 (2.1) | 1 (3.0) |
| IB | 11 (2.7) | 5 (2.1) | 6 (4.2) | 0 (0.00) |
| IIA | 13 (3.2) | 11 (4.7) | 1 (0.7) | 1 (3.0) |
| IIB | 6 (1.5) | 5 (2.1) | 1 (0.7) | 0 (0.00) |
| IIIA | 37 (9.0) | 20 (8.6) | 15 (10.4) | 2 (6.1) |
| IIIB | 31 (7.6) | 18 (7.7) | 11 (7.6) | 2 (6.1) |
| IV | 291 (71.0) | 158 (67.8) | 106 (73.6) | 27 (81.8) |
| Unknown | 10 (2.4) | 9 (3.9) | 1 (0.7) | 0 (0.00) |
| Histology, n (%) | ||||
| Adenocarcinoma | 357 (87.1) | 192 (82.4) | 134 (93.1) | 31 (93.9) |
| Adenosquamous | 7 (1.7) | 4 (1.7) | 1 (0.7) | 2 (6.1) |
| Bronchioloalveolar | 3 (0.7) | 3 (1.3) | 0 (0.00) | 0 (0.00) |
| Squamous cell | 14 (3.4) | 11 (4.7) | 3 (2.1) | 0 (0.00) |
| Unknown | 14 (3.4) | 12 (5.2) | 2 (1.4) | 0 (0.00) |
| Unspecified NSCLC | 6 (1.5) | 4 (1.7) | 2 (1.4) | 0 (0.00) |
| Other | 9 (2.2) | 7 (3.0) | 2 (1.4) | 0 (0.00) |
| Chemotherapy prior to first ALKa, n (%) | ||||
| Yes | 243 (59.3) | 128 (54.9) | 83 (57.6) | 32 (96.9) |
| No | 167 (40.7) | 105 (45.1) | 61 (42.4) | 1 (3.0) |
ALK anaplastic lymphoma kinase, ECOG Eastern Cooperative Oncology Group, NSCLC non-small cell lung cancer
aPresence of any systemic antineoplastic chemotherapy prior to first ALK inhibitor
Treatment Patterns
Table 2 describes the order of treatment with ALK inhibitors and the proportion of patients treated with each sequence. Most patients (57%) received 1 ALK inhibitor, with the majority receiving crizotinib; 35% received 2 ALK inhibitors, and 8% received 3–4 ALK inhibitors. Of the patients who received more than 1 ALK (n = 177), the largest proportion (60%, n = 106) received a crizotinib-led sequence, followed by an alectinib-led sequence (39%, n = 69). Only 2 patients started with ceritinib, and 22% of the overall study population received ceritinib at any time. There were no patients who started with brigatinib, and only 4% of the overall study population received brigatinib at any time.
Table 2.
ALK inhibitor sequences and proportions of patients
| n (%) of overall population (N = 410) | |
|---|---|
| 1 ALK inhibitor | 233 (56.8) |
| Crizotinib only | 205 |
| Alectinib only | 20 |
| Ceritinib only | 5 |
| Brigatinib only | 3 |
| 2 ALK inhibitors | 144 (35.1) |
| Crizotinib → ceritinib | 59 |
| Crizotinib → alectinib | 21 |
| Crizotinib → brigatinib | 3 |
| Alectinib → crizotinib | 60 |
| Alectinib → brigatinib | 1 |
| 3 ALK inhibitors | 31 (7.6) |
| Crizotinib → alectinib → ceritinib | 23 |
| Alectinib → crizotinib → brigatinib | 6 |
| Ceritinib → crizotinib → brigatinib | 2 |
| 4 ALK inhibitors | 2 (0.5) |
| Alectinib → crizotinib → brigatinib → ceritinib | 2 |
ALK anaplastic lymphoma kinase
More than half (59%) of all patients received chemotherapy prior to their first ALK, for a median duration of 6.6 months. The most common regimens were bevacizumab + pemetrexed combination therapy (30%), paclitaxel monotherapy (23%), and pemetrexed monotherapy (18%). Only four patients received immunotherapy with a checkpoint inhibitor prior to the ALK inhibitor.
Fifty-five percent (226 of 410) of patients ended ALK inhibitor therapy and received a non-ALK inhibitor chemotherapy as their therapy immediately after treatment with their last ALK inhibitor. An additional ten patients received immunotherapy. Similar to the chemotherapy regimens used prior to starting an ALK inhibitor, the most common regimens were bevacizumab + pemetrexed (n = 37, 17%) and paclitaxel protein-bound (n = 36, 17%).
Among the 226 patients who ended ALK inhibitor therapy and received a subsequent non-ALK inhibitor, 162 patients had an uninterrupted treatment sequence of only ALK inhibitors, and the median time to non-ALK inhibitor treatment was 21 months (Table 3). There were also 54 patients who received another intervening non-ALK inhibitor treatment between ALK inhibitors, and the median time to non-ALK inhibitor treatment after the last ALK in the sequence was 26 months. Overall, the time increased with number of ALK inhibitor treatments received.
Table 3.
Time to non-ALK inhibitor chemotherapy among patients receiving uninterrupted sequential ALK inhibitors
| Overall | 1 ALK inhibitor | 2 ALK inhibitors | 3 ALK inhibitors | |
|---|---|---|---|---|
| Number of patients who received a subsequent non-ALK inhibitor | n = 162 | n = 109 | n = 42 | n = 11 |
| Median (95% CI), months | 21.4 (5.9, 30.2) | 17.2 (7.6, 29.1) | 20.2 (8.5, 30.2) | 22.4 (7.5, 32.5) |
ALK anaplastic lymphoma kinase, CI confidence interval
Outcomes
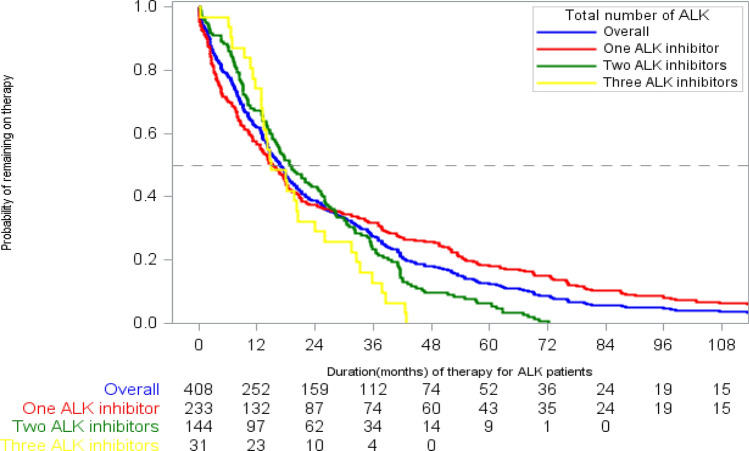
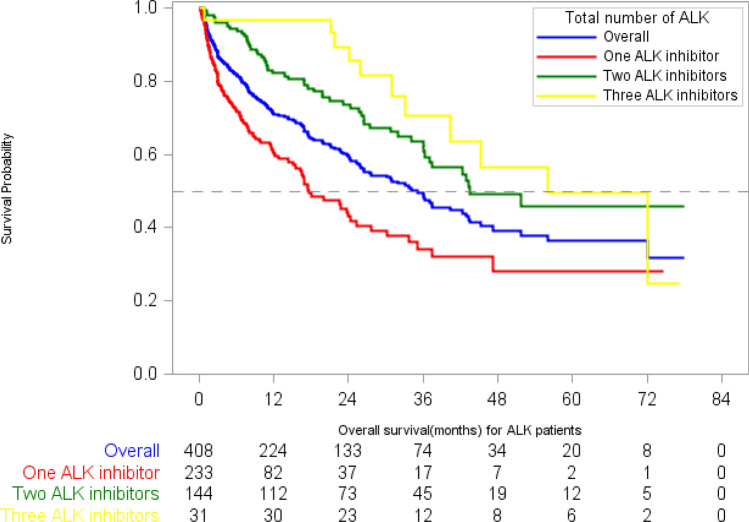
The median cumulative ALK inhibitor DOT in the study population, regardless of line of therapy or sequence, was 16 months [95% confidence interval (CI) 6, 19], with a median of 15 (95% CI 8, 22), 19 (95% CI 5, 20), and 15 (95% CI 9, 30) months in patients receiving 1, 2, and 3 ALK inhibitors, respectively (Fig. 2). The median OS for the study population was 28 months (95% CI 24, 36), with a median of 15 months (95% CI 10, 22) in patients who received 1 ALK inhibitor, 42 months (95% CI 38, 60) in patients who received 2 ALK inhibitors, and 56 months (95% CI 31, 72) in patients who received 3–4 ALK inhibitors (Fig. 3, Table 4). The unadjusted median OS was not significantly different in patients treated with or without chemotherapy prior to their first ALK inhibitor (26.1 months vs. 22.2 months; log-rank p = 0.89).
Fig. 2.
Kaplan–Meier estimates for duration of therapy, stratified by number of ALK inhibitors. ALK anaplastic lymphoma kinase
Fig. 3.
Kaplan–Meier estimates for overall survival, stratified by number of ALK inhibitors. ALK anaplastic lymphoma kinase
Table 4.
Kaplan–Meier estimates for overall survival, stratified by number of ALK inhibitors
| Overall population | 1 ALK inhibitor | 2 ALK inhibitors | 3 ALK inhibitors | |
|---|---|---|---|---|
| N = 410 | N = 233 | N = 144 | N = 31 | |
| Median OS (95% CI) | 27.6 (23.8, 36.1) | 15.1 (10.5, 22.5) | 42.4 (38.5, 60.3) | 56.0 (31.0, 72.0) |
ALK anaplastic lymphoma kinase, CI confidence interval, OS overall survival
In a multivariable linear regression analysis using age, race, sex, ECOG performance status, and smoking status, a longer DOT of the first ALK inhibitor was significantly associated with a longer DOT of the second ALK inhibitor (parameter estimate 0.61; p < 0.0001), and a longer second ALK inhibitor DOT was associated with a longer DOT of the third in a similar fashion (parameter estimate 0.46; p < 0.0001).
Discussion
This study describes the treatment patterns and outcomes for patients treated with ALK inhibitors at any time within a 6-year span from 2011 to 2017. As with previously published studies of patients with ALK-positive NSCLC in the US, the population consisted of predominantly female Caucasian nonsmokers with adenocarcinoma. Most patients (57%) were treated with only one ALK inhibitor, and crizotinib was the most frequently utilized agent. These results are consistent with the study time period, as crizotinib was the first FDA-approved ALK inhibitor in 2011 and was the only ALK inhibitor available until 2014. Since that time, the newer second- and third-generation agents have been approved, and new indications have emerged, shifting the utilization patterns for these targeted therapies.
In this study, among those patients who received two or more sequential ALK inhibitors, we observed that most patients started with crizotinib followed by alectinib. Among those patients with an alectinib-led sequence, most patients subsequently received crizotinib. The alectinib-to-crizotinib treatment sequence was a surprising finding. It is possible that some of these patients received alectinib first on a clinical trial before the front-line indication and received subsequent crizotinib if they had not had prior exposure. Due to improved central nervous system activity with alectinib, the presence of brain metastases may have influenced the use of alectinib-led sequences, as approximately 10–15% of patients in this study had brain metastases.
Nearly 60% of all patients had exposure to chemotherapy for advanced disease before treatment with an ALK inhibitor. With a median duration of 6 months of chemotherapy before initiation of the ALK inhibitor, it is possible that these patients had started chemotherapy before they knew they were ALK positive. It is not known whether the delay in starting ALK inhibitor therapy was due to a desire to start treatment while waiting for biomarker testing results or whether such testing was not performed initially. Only a small percentage of the population that received chemotherapy had non-adenocarcinoma histology, for which ALK testing may be less common. These observations suggest an opportunity to improve biomarker testing strategies to ensure early identification of patients who may be eligible and would benefit from treatment with these targeted therapies. The most common non-ALK inhibitor therapy received among those completing an ALK sequence was chemotherapy, with only a small number of patients receiving immunotherapy. Second-line immunotherapy became available in early 2015, and there is little evidence of efficacy of immunotherapy in patients with ALK-positive disease [21]. Guidelines state that PD-1/PD-L1 inhibitor monotherapy is less effective, irrespective of PD-L1 expression, in EGFR-positive and ALK-positive NSCLC [21].
Regardless of the line of therapy or number of ALK inhibitors received, the median ALK inhibitor DOT observed in this study in the overall population was approximately 16 months. These real-world outcomes are consistent with and in the range of prior published median durations of response in the registrational trials of approximately 10–12 months with crizotinib [7, 10], 7–12 months with ceritinib [22] or alectinib [23] following progression on crizotinib, and a median PFS of 16–25 months with ceritinib [17] or alectinib [15] in the first line. They also support the use of sequential ALK inhibitors. In this study, patients were observed to receive a range of 1–4 ALK inhibitors, and patients had a median DOT of approximately 9–12 months with each subsequent ALK therapy. The observation that a longer DOT for the first TKI was associated with a longer DOT for subsequent TKIs likely reflects tumor biology and sensitivity to TKI treatment.
Strategies promoting the sequential use of ALK inhibitors are feasible and result in a clinically meaningful OS. The median OS in the overall population was 28 months, and an increase in OS was observed upon increasing the number of ALK inhibitors used, suggesting that the use of multiple sequential ALK inhibitors was feasible in patients with different disease biologies who were able to live long enough to receive multiple lines of treatment. However, the best strategy for therapy sequencing is not known for patients with ALK-positive NSCLC. Alectinib is the current guideline-recommended front-line therapy, and with the availability of multiple ALK inhibitors, studies aimed at better understanding the optimal subsequent sequencing strategies are underway. In our study, the initial ALK inhibitor was likely a first-generation ALK inhibitor given the time frame of the study. Treatment guidelines currently recommend the use of multiple ALK inhibitors [21, 24]. Our findings support sequencing through ALK therapies before proceeding to chemotherapy.
Important considerations when interpreting the results of this study include the 6-year study period and the development of new ALK inhibitors and emerging new data during that time. The time frame also restricts our ability to interpret the impact of new immunotherapy treatments on this study population. Changes in indications for later-line vs. front-line use over time may affect treatment patterns. In addition, no minimum duration of follow-up time was required, so the DOT varied across patients and was influenced by the date of patient initiation on alectinib therapy for entry into the study. While most patients showed good performance status at the time of ALK inhibitor treatment initiation, the rate of disease progression or disease severity was not known, which could have influenced the ability of patients to receive additional therapy.
Strengths of this study include the large, multicenter population of patients with ALK-positive NSCLC, given the rarity of patients with this known gene rearrangement, and insights into the real-world, community-based practice patterns and outcomes of these patients. A limitation of this study is that it was a database study performed by programmatic EHR data extraction, so there was the potential for documentation omissions or errors. In this study, fewer than 1% of the initial NSCLC population had known ALK-positive disease, which is less than the estimated 3–5% in the general population. As this was a database study, the low rate of ALK-positive disease in the initial NSCLC population does not necessarily reflect all patients tested, but only those for whom results were known. We expect that there were additional patients who were noted to have ALK-positive status in the unstructured progress notes or in scanned PDF pathology reports, but those notes or reports were not available for this study. There is a need for more data surrounding biomarker ordering and utilization of results, and follow-up studies to investigate this are ongoing. Prior treatments received outside of the practice may not have been captured by the EHR. Additionally, ALK inhibitors are oral therapies, and while the EHR allows insight into prescribing patterns, fulfilment or compliance could not be confirmed.
Conclusion
Patients received 1–4 ALK inhibitors during the study period. Crizotinib-led sequences were the most common, reflecting the approval history of ALK inhibitors during the study period. Many patients received chemotherapy prior to ALK-directed therapy, and there was no survival benefit from receiving chemotherapy first. Longer DOT and OS were observed in patients receiving multiple ALK inhibitors. This study provides an initial view of treatment patterns following the emergence of new ALK inhibitors, and supports the use of sequential ALK therapies. Testing for ALK rearrangements early and then starting and maintaining treatment with ALK inhibitors may be the most efficient strategy to optimize patient care. Follow-up studies will improve understanding of the outcomes of patients treated with second-generation-led sequences.
Acknowledgements
Portions of the research described in this article were presented at the 20th World Conference on Lung Cancer (WCLC), September 7–10, 2019, Barcelona, Spain. The authors would like to acknowledge Lisa Kaspin-Powell, PhD, ELS, an employee of McKesson Life Sciences, for editorial assistance, which was funded by Pfizer, Inc.
Author Contributions
All authors contributed to the concept and research design of the study; BB contributed to the assembly of data and analysis; and all authors participated in the interpretation of results and contributed to manuscript development.
Compliance with Ethical Standards
Ethical Approval
This study received Institutional Review Board approval.
Funding
This study was sponsored by Pfizer, Inc. and conducted by McKesson Life Sciences.
Conflict of Interest
Elizabeth T. Masters, Marc D. Chioda, and Jack Mardekian are employees of Pfizer, Inc. Janet L. Espirito, Bismark Baidoo, and Nicholas J. Robert are employees of and David M. Waterhouse was contracted by McKesson Life Sciences, who were paid consultants to Pfizer in connection with the development of this manuscript. The authors report no other conflicts of interest in this work.
Availability of Data and Material
The raw data used for this analysis are not publicly available due to privacy or ethical restrictions.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Petersen I. The morphological and molecular diagnosis of lung cancer. Dtsch Arztebl Int. 2011;108(31–32):525–531. doi: 10.3238/arztebl.2011.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PDQ® Adult Treatment Editorial Board. PDQ non-small cell lung cancer treatment. Bethesda, MD: National Cancer Institute. 2019. https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq. Accessed 24 May 2019 (PMID: 26389304).
- 4.Du X, Shao Y, Qin H-F, Tai Y-H, Gao H-J. ALK-rearrangement in non-small-cell lung cancer (NSCLC) Thorac Cancer. 2018;9:423–430. doi: 10.1111/1759-7714.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantuejoul S, Rouquette I, Blons H, et al. French multicentric validation of ALK rearrangement diagnostic in 547 lung adenocarcinomas. Eur Respir J. 2015;46:207–218. doi: 10.1183/09031936.00119914. [DOI] [PubMed] [Google Scholar]
- 6.Malik SM, Maher VE, Bijwaard KE, et al. US Food and Drug Administration approval: crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Oncologist. 2014;19:e5–e11. doi: 10.1634/theoncologist.2014-0241. [DOI] [PubMed] [Google Scholar]
- 7.Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(Suppl):Abstr 2501. doi: 10.1200/jco.2011.29.15_suppl.2501. [DOI] [Google Scholar]
- 8.Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(Suppl):Abstr 7514. doi: 10.1200/jco.2011.29.15_suppl.7514. [DOI] [Google Scholar]
- 9.Shaw AT, Kim D, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 10.Solomon BJ, Mok T, Kim D, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 11.Kazandjian D, Blumenthal GM, Chen HY, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19:e5–11. doi: 10.1634/theoncologist.2014-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21:2436–2439. doi: 10.1158/1078-0432.CCR-14-3157. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. US Food and Drug Administration. Alectinib approved for (ALK) positive metastatic non-small cell lung cancer (NSCLC). 2017. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm584082.htm. Accessed 20 Sept 2018.
- 14.US Department of Health and Human Services. US Food and Drug Administration. Brigatinib. 2017. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555841.htm. Accessed 20 Sept 2018.
- 15.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 16.Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK + NSCLC. J Clin Oncol. 2018;36(Suppl. 15):9043. doi: 10.1200/JCO.2018.36.15_suppl.9043. [DOI] [Google Scholar]
- 17.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 18.Pfizer, Inc. Prescribing information for Lorbrena (lorlatinib). 2018. https://www.labeling.pfizer.com/ShowLabeling.aspx?id=11140. Accessed 29 July 2019.
- 19.Clinicaltrials.gov. A study of lorlatinib versus crizotinib in first line treatment of patients with ALK-positive NSCLC. ClinicalTrials.gov Identifier: NCT03052608. Last verified: May 2019. https://www.clinicaltrials.gov/ct2/show/NCT03052608. Accessed 24 May 2019.
- 20.Clinicaltrials.gov. Biomarker/ALK inhibitor combinations in treating patients with stage IV ALK positive non-small cell lung cancer (the NCI-NRG ALK master protocol). Last verified: April 2019. https://www.clinicaltrials.gov/ct2/show/NCT03737994. Accessed 24 May 2019.
- 21.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer. Version 4.2019—April 29, 2019. https://www.nccn.org/professionals/physician_gls/default.aspx#nscl. Accessed 24 May 2019.
- 22.Kiura K, Imamura F, Kagamu H, et al. Phase 3 study of ceritinib vs chemotherapy in ALK-rearranged NSCLC patients previously treated with chemotherapy and crizotinib (ASCEND-5): Japanese subset. Jpn J Clin Oncol. 2018;48:367–375. doi: 10.1093/jjco/hyy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant non-small-cell lung cancer: a single-group, multicenter, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melosky B, Cheema P, Agulnik J, et al. Canadian perspectives: update on inhibition of ALK-positive tumors in advanced NSCLC. Curr Oncol. 2018;25:317–328. doi: 10.3747/co.25.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]