Abstract
Trophic downgrading in coastal waters has occurred globally during recent decades. On temperate rocky reefs, this has resulted in widespread kelp deforestation and the formation of sea urchin barrens. We hypothesize that the intact kelp forest communities are more spatially variable than the downgraded urchin barren communities, and that these differences are greatest at small spatial scales where the influence of competitive and trophic interactions is strongest. To address this, benthic community surveys were done in kelp forests and urchin barrens at nine islands spanning 1230 km of the Aleutian Archipelago where the loss of predatory sea otters has resulted in the trophic downgrading of the region’s kelp forests. We found more species and greater total spatial variation in community composition within the kelp forests than in the urchin barrens. Further, the kelp forest communities were most variable at small spatial scales (within each forest) and least variable at large spatial scales (among forests on different islands), while the urchin barren communities followed the opposite pattern. This trend was consistent for different trophic guilds (primary producers, grazers, filter feeders, predators). Together, this suggests that Aleutian kelp forests create variable habitats within their boundaries, but that the communities within these forests are generally similar across the archipelago. In contrast, urchin barrens exhibit relatively low variability within their boundaries, but these communities vary substantially among different barrens across the archipelago. We propose this represents a shift from small-scale biological control to large-scale oceanographic control of these communities.
Subject terms: Biogeography, Ecosystem ecology
Introduction
Trophic downgrading occurs when apex predators have been extirpated over large geographic regions, which can lead to important consequences for ecosystem functioning due to both direct and indirect cascading effects1. This has been observed globally across a variety of terrestrial and marine ecosystems2–7. Often, trophic downgrading triggers increases in herbivore populations, thereby changing overall community structure6,8,9 and altering patterns of ecosystem productivity10–12. Trophic downgrading can be especially important if it ultimately affects ecosystem engineers that provide habitat, which modifies the physical environment, regulates primary production and energy flow, and generally supports high biodiversity. For example, the extirpation of gray wolves from Yellowstone National Park, USA in the early 1900s resulted in reduced predation on elk and increased herbivory on forest-forming trees13. This ultimately led to changes in the morphology and hydrology of the region’s river systems and its riparian plant communities14,15. Similarly, the loss of sea otters from the nearshore habitats of the Aleutian Archipelago during the 1980s and 1990s resulted in reduced predation on herbivorous sea urchins and a subsequent overgrazing of the regions kelp forests2. This led to reduced biodiversity16, altered food web dynamics17, and reduced benthic ecosystem productivity12 in the coastal environment. Further, when a community has been downgraded, its resilience (i.e., recovery and stability after a disturbance) can decrease in comparison to intact communities3. This has been an important consideration in the design of marine protected areas (MPAs) and terrestrial parks and reserves, which typically protect apex predators to maintain diversity and normal ecosystem functioning7,18–20.
Spatial and temporal variability in community structure are important components of ecological systems, and understanding how variability changes in space and time can infer a wide range of ecological processes21–27. For example, resistance to biological invasions is strongly correlated with variability in environmental conditions28 and community structure29, with less variable communities being more resistant to invasion than highly variable communities. However, the loss of foundation species can increase susceptibility to biological invasions30,31. Moreover, patterns of variability can themselves change at different temporal and spatial scales coincident with environmental and demographic forcing factors21,25,26,32,33. Indeed, Levin21 noted that the problem of pattern and scale is the central problem in ecology and that it is important to find ways to quantify patterns of variability in space and time, understand how patterns change with scale, and understand the causes and consequences of pattern. This can be especially important in ecosystems where different forcing factors affect communities across a range of spatial scales33–35.
Kelp forests are benthic, biogenic habitats that include highly productive primary producers rivaling those of cultivated agricultural fields and tropical rainforests in productivity36–39. This productivity and the associated formation of complex, three-dimensional biogenic habitat enhances local biodiversity and secondary productivity16,40,41. However, kelp forests in many areas of the world have been trophically downgraded as their apex predators have been removed42,43. For example, in Tasmania, the loss of predatory lobsters has led to increases in sea urchin abundance and an increased risk of catastrophic shifts to widespread sea urchin barrens44. Such deforestation of kelp forests due to sea urchin grazing is becoming more common in mid-latitudes45–47 (see also citations in Steneck et al.9). This generally results in a loss of biodiversity and associated ecosystem services, as kelp forests generally support more species16,48 (this study) and exhibit greater primary productivity and habitat complexity12,49,50 than sea urchin barrens. Consequently, we expected there would be more combinations of species that could spatially differentiate the kelp forest communities than the sea urchin barren communities. This would be especially important at small spatial scales where the influence of biological interactions (e.g., competition, grazing, and predation) in spatial structuring of communities can be strongest and potentially obscure large-scale variability due to climate or oceanographic variability51–55.
The shallow subtidal regions of the Aleutian Archipelago (Fig. 1) are a well-studied simple system under top-down control56. Here, sea otters that were previously hunted to near extinction by the fur trade began to recover in the early 1900s57. This recovery continued through the 1980s when the removal of sea otters by killer whales became apparent by the 1990s56,57. Following the removal of otters, the abundance and biomass of their primary prey, herbivorous sea urchins, dramatically increased2. These hyper-abundant sea urchins quickly overgrazed the macroalgal communities, causing widespread deforestation of the region’s kelp forests, and increases in the prevalence and extent of urchin barrens. These urchin barrens are largely devoid of all fleshy macroalgae but instead are dominated by sea urchins and coralline red algae12,16. They can be of varying ages depending on the timing of sea otter recovery and subsequent sea otter removal, but once formed they are stable over periods of at least several years58. Here, we use this trophically-mediated deforestation to examine spatial patterns of community variability in kelp forests and urchin barrens throughout the Aleutian Archipelago. We ask if non-downgraded communities with more species (i.e., intact kelp forests) exhibit greater spatial variability compared to downgraded communities with fewer species (i.e., urchin barrens)16. We then compare how these patterns of variability are distributed across different spatial scales, from meters to hundreds of kilometers. We do this for the whole communities and for different trophic guilds (i.e., primary producers, grazers, filter feeders, and predators). Based on previous observations we have made during numerous visits to the archipelago, we hypothesized that (1) the community state with higher biodiversity (intact kelp forests) will have greater overall spatial variability in community structure than the community state with lower biodiversity (downgraded urchin barrens), (2) patterns of variability will be greatest at small spatial scales for the kelp forests but greatest at large spatial scales for the urchin barrens, and (3) these patterns will be consistent among different trophic guilds.
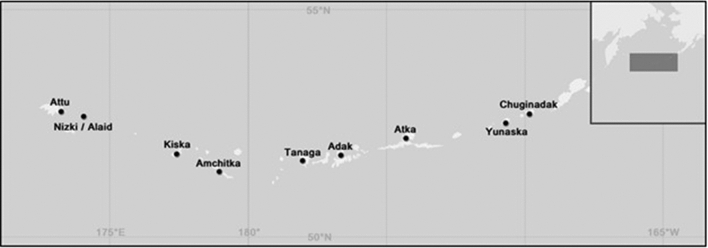
Figure 1.
Map of study area showing the nine islands sampled across the Aleutian Archipelago (inset shows portion of archipelago where islands are located). Coordinates in decimal degrees for approximate sampling locations are: Attu: 52.92°, 173.20°; Nizki/Alaid: 52.74°, 174.00°; Kiska: 51.97°, 177.58°; Amchitka: 51.41°, 179.28°; Tanaga: 51.81°, − 177.94°; Adak: 51.87°, − 176.66°; Atka: 52.10°, − 174.69°; Yunaska: 52.66°, − 170.74°; and Chuginadak: 52.84°, − 169.75°. Image from Metzger et al. 2019.
Results
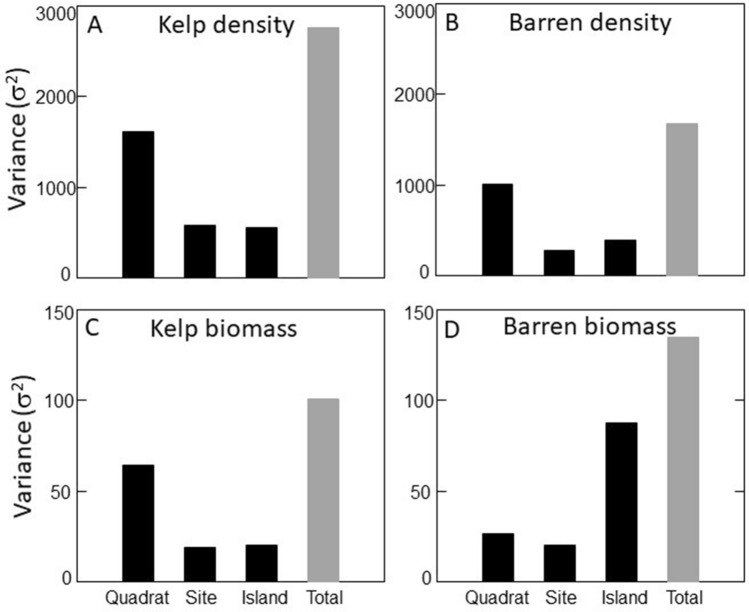
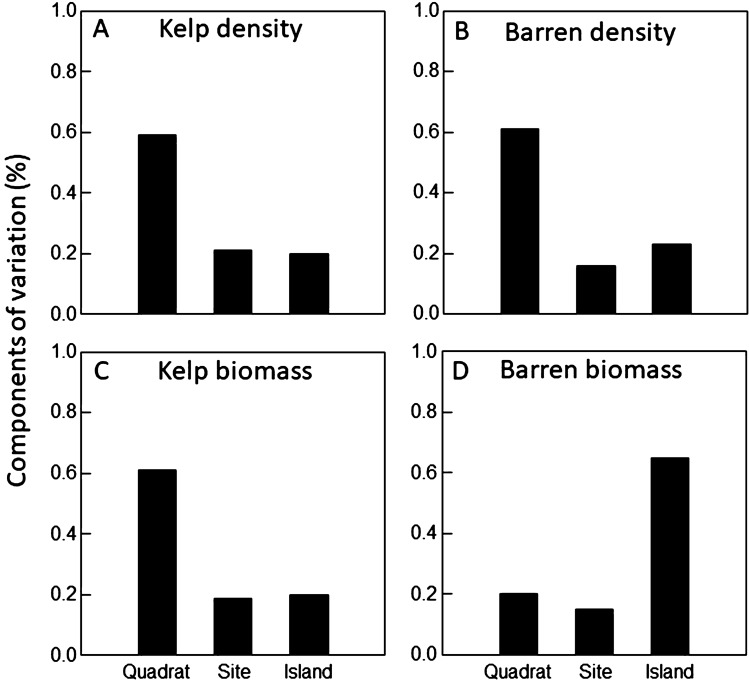
We identified a total of 217 species in the kelp forests and 153 species in the urchin barrens using both density and biomass based data. Overall, both data types indicated that benthic community composition varied significantly among the nine islands, and between the two sites within each island in both the kelp forests and urchin barrens (Supplementary Table S1). Further, we found 1.6 times more total spatial variation in community structure in the kelp forests than the urchin barrens when using density data, but 1.3 times more total spatial variation in the urchin barrens when using biomass data (Fig. 2). This variation generally followed a scaling pattern within the kelp forests, with the largest amount of variation (59–61%) in the PERMANOVA models observed at the smallest spatial scale (i.e., among quadrats within each site), while much less of the variation (19–20%) was observed at the largest scale (i.e., among islands) (Fig. 3). Variation at the intermediate scale (i.e., among sites within each island) was similar to that of the largest scale, accounting for approximately 19–21% of the variation in the models. In contrast, when these patterns were examined within the urchin barrens, the amount of variation associated with each spatial scale in our PERMANOVA models depended on the type of data used. Here, the patterns were similar to those in the kelp forests when density data were used, with most of the variation in the model (61%) observed at the smallest spatial scale, and much less of the variation observed at the intermediate (16%) and largest (23%) scales (Fig. 3). In contrast, these patterns followed the opposite scaling pattern in the urchin barrens when biomass data were used. Specifically, the largest amount of variation (65%) in the PERMANOVA model was observed at the largest scale, while much less of the variation was observed at the intermediate (15%) and smallest (20%) scales (Fig. 3). Consequently, the kelp forest communities were 1.6 times more variable than the urchin barren communities at the smallest scale (among quadrats within each site), 2.1 times more variable at the intermediate scale (among sites within each island), and 1.4 times more variable at the largest scale (among islands) when based on density data (Figs. 2, 4). However, when based on biomass data, the kelp forest communities were 2.3 times more variable than the urchin barren communities at the smallest scale, but they were approximately the same at the intermediate scale, and the urchin barren communities were 1.3 times more variable than the kelp forest communities at the largest scale (Figs. 2, 5). It is important to note, however, that these components of variation are not independent of each other, as they are conditional on both the underlying amount of total variation in each PERMANOVA model and by each factor’s degrees of freedom within the model59, and care should therefore be taken when comparing them among PERMANOVA models.
Figure 2.
Bar graphs showing variance components (σ2) associated with each spatial scale in the PERMANOVA models for both density-based and biomass-based data. Gray bars on the right show total amount of variation in each model.
Figure 3.
Bar graphs showing magnitude of effects (% of total variation) that is associated with each spatial scale in each PERMAOVA model for both density-based and biomass-based data.
Figure 4.
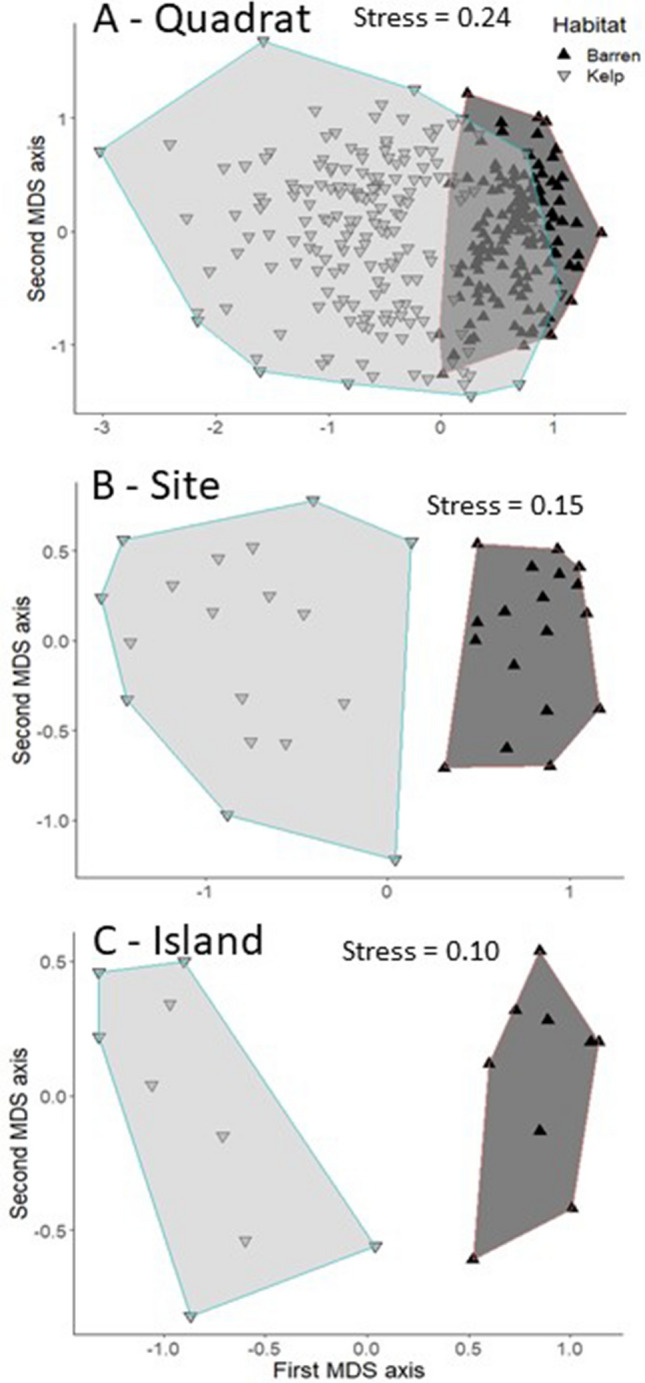
Density data-based nMDS plots comparing relative similarities in barren and kelp forests communities at different spatial scales; (A) among quadrats within each site, (B) among sites within each island, and (C) among islands. Data were square root transformed prior to analyses, and each resemblance matrix was based on zero inflated Bray–Curtis similarities. Shaded areas represent two-dimensional convex hulls representing areas connecting exterior data points.
Figure 5.
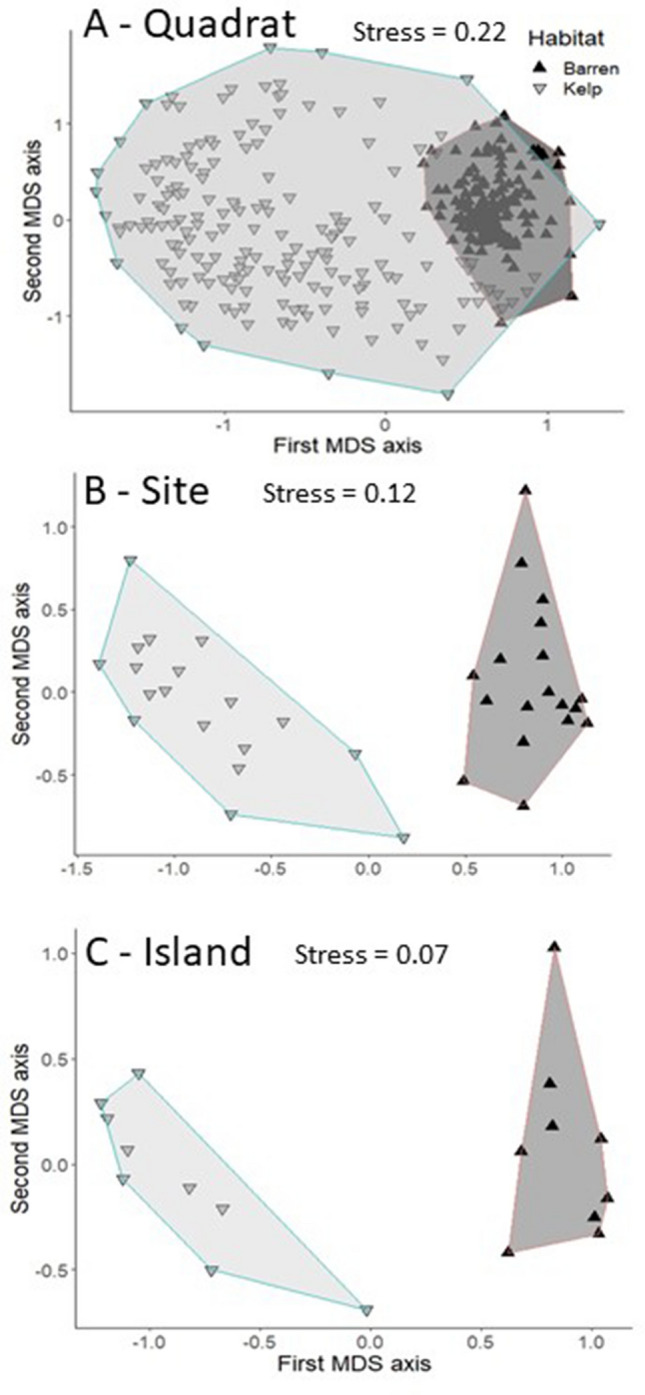
Biomass data-based nMDS plots comparing relative similarities in barren and kelp forests communities at different spatial scales; (A) among quadrats within each site, (B) among sites within each island, and (C) among islands. Data were square root transformed prior to analyses, and each resemblance matrix was based on zero inflated Bray–Curtis similarities. Shaded areas represent two-dimensional convex hulls representing areas connecting exterior data points.
Independent estimates of variability in community structure at each spatial scale were determined for each data type based on analyses of multivariate dispersions60. Doing this revealed that the kelp forest communities were more variable than the urchin barrens, and variation in kelp forest community structure followed the same scaling pattern as observed in the PERMANOVA models. In particular, for both density and biomass-based data, the greatest amount of variability in kelp forest community composition was observed at the smallest scale (among quadrats within each site) (MvDisp = 1.281 and 1.403, respectively), followed by the intermediate scale (among sites within each island) (MvDisp = 1.233 and 1.053, respectively), and then by the largest scale (among islands) (MvDisp = 1.205 and 1.024, respectively) (Table 1). In contrast, variation in community composition within the urchin barrens followed the opposite scaling pattern, with the greatest amount of variation observed at the largest scale for both density and biomass data (MvDisp = 0.795 and 0.976, respectively), followed by the intermediate scale (MvDisp = 0.767 and 0.947, respectively), and then by the smallest scale (MvDisp = 0.728 and 0.606, respectively) (Table 1). This resulted in the communities within the kelp forests being about 1.8 and 2.3 times more variable than those in the urchin barrens when examined at the smallest scale using density and biomass data, respectively. When examined at the intermediate scale, the communities in the kelp forests were 1.6 and 1.1 times more variable than those in the urchin barrens, respectively. At the largest scale, they were 1.5 and 1.1 times more variable than in the urchin barrens, respectively (Table 1).
Table 1.
Multivariate dispersions (variances) in the composition of whole communities, and primary producer, grazer, filter feeder and predator assemblages observed in each habitat type (kelp forests and urchin barrens) at each spatial scale (quadrats, sites, and islands) for both (A) density-based and (B) biomass-based data. Data were square root transformed prior to analysis, and each resemblance matrix was based on zero inflated Bray–Curtis similarities.
| Habitat | Scale | Communities | Primary producers | Grazers | Filter feeders | Predators |
|---|---|---|---|---|---|---|
| (A) Density data | ||||||
| Kelp | Quadrat | 1.281 | 1.499 | 1.176 | 1.289 | 1.200 |
| Site | 1.233 | 1.466 | 1.151 | 1.377 | 1.125 | |
| Island | 1.205 | 1.467 | 1.072 | 1.457 | 1.068 | |
| Barren | Quadrat | 0.728 | 0.512 | 0.828 | 0.717 | 0.805 |
| Site | 0.767 | 0.534 | 0.849 | 0.623 | 0.875 | |
| Island | 0.795 | 0.533 | 0.928 | 0.543 | 0.932 | |
| (B) Biomass data | ||||||
| Kelp | Quadrat | 1.403 | 1.440 | 1.115 | 1.391 | 1.291 |
| Site | 1.053 | 0.660 | 1.070 | 0.768 | 0.745 | |
| Island | 1.024 | 0.618 | 1.076 | 0.715 | 0.713 | |
| Barren | Quadrat | 0.606 | 0.570 | 0.888 | 0.618 | 0.716 |
| Site | 0.947 | 1.340 | 0.930 | 1.232 | 1.255 | |
| Island | 0.976 | 1.382 | 0.924 | 1.285 | 1.287 | |
Multivariate dispersions were estimated using the MvDisp routine in Primer-E.
When patterns of variability were examined for the different trophic guilds (primary producers, grazers, filter feeders, and predators), similar patterns to those of the whole-communities were identified. First, we observed more species of primary producers (11 vs. 6), grazers (15 vs. 13), filter feeders (30 vs. 26), and predators (52 vs. 40) in the kelp forests than in the urchin barrens respectively, when using density data, though the differences in overall species numbers were only marginally significant (paired t test: t = 2.644, df = 3, P = 0.077). Likewise, we observed more species of primary producers (66 vs. 36), herbivores (18 vs. 12), filter feeders (71 vs. 54), and predators (54 vs. 45) in the kelp forests than in the urchin barrens, respectively, when using biomass data, but the differences in overall species numbers were again only marginally significant (paired t test: t = 2.89, df = 3, p = 0.063). Second, when density data were used, the assemblages making up the different trophic guilds were each more variable within the kelp forest than the urchin barrens at all spatial scales examined (Table 1). Third, primary producer, grazer, and predator densities within the kelp forests were each most variable at the smallest spatial scale and this variability decreased at the larger scales (Table 1). In contrast, primary producer, grazer, and predator densities within the urchin barrens followed the opposite pattern in that they were all least variable at the smallest scale and this variability increased at the larger spatial scales. The filter feeders, however, were an exception. Within the kelp forests, filter feeder densities were most variable at the largest scale and least variable at the smallest scale, but these followed the opposite pattern within the urchin barrens (Table 1). When biomass data were used, primary producer, grazer, filter feeder, and predator assemblages within the kelp forests were all most variable at the smallest scale (among quadrats within each site) and this variability decreased at the larger scales, while the opposite pattern was again observed within the urchin barrens (Table 1). Further, biomass of the assemblages belonging to the different trophic guilds were all more variable within the kelp forests than within the urchin barrens at the smallest scale, but they were generally more variable within the urchin barrens at the larger scales (Table 1). The exception to this were the grazers, which were more variable within the kelp forests than the urchin barrens at all spatial scales.
Discussion
Trophically downgraded ecosystems are becoming more common worldwide, which is leading to conservation concerns due to the associated losses of biodiversity, productivity, and community resilience1,9. Downgrading often results in the formation of alternate stable states4,6,8 in which herbivores become hyper-abundant and autotrophs become rare. When this affects the distribution and abundance of ecosystem engineers, such as forest-forming trees and kelps that create habitat and supply energy for their communities, ecosystem function can be altered12 and biodiversity reduced16. Such ecosystem changes, however, can vary among different spatial scales due to a variety of forcing factors21,24,32,33. Here, we demonstrate that the intact kelp forests in the Aleutian Archipelago have more species than the trophically downgraded urchin barrens, and that this is consistent using both biomass and density data. Further, the intact kelp forest communities were most variable at the smallest spatial scale examined (among quadrats within each forest) and least variable at the largest spatial scale (among islands). This suggests that kelp forests create a variable habitat within their boundaries with high potential for species interactions (e.g., competition, grazing, predation), which are important drivers of small-scale variability54,55 and which can mask the influence of large-scale climate or oceanographic factors51–53. These habitat characteristics are then repeated over larger spatial scales (i.e., different kelp forests typically contain similar species assemblages and biological interactions), which reduces larger-scale variability.
Following trophic downgrading that resulted in reduced biodiversity as the kelp forests transitioned to urchin barrens16, the number of potential species interactions also declined. Although the timing of these changes varied among islands, the actual changes were consistent across the archipelago, which led to a transformation of the landscape61. This also led to changes in spatial variability in community structure and how it was distributed among the different spatial scales, but this depended on the type of data used. Specifically, when species abundances were estimated based on their densities, there was an overall reduction in total spatial variability in community structure at each spatial scale, and the barrens remained most variable at the smallest spatial scale and least variable at the larger spatial scales. In contrast, when species abundances were estimated based on their biomasses, variability in community structure again decreased at the smallest spatial scale, but it increased substantially at the larger spatial scales. This resulted in the urchin barren communities being least variable at the smallest spatial scale and most variable at the largest spatial scale. It also resulted in the urchin barrens exhibiting more total spatial variability in community structure than the kelp forests. These differences between the two data types appeared largely due to how each estimated the abundance of filter feeders and grazers (discussed below). Regardless, we believe this increase in large-scale variability reflected variation in oceanographic, topographic and hydrodynamic conditions, which can mask the importance of small-scale biological influences33,62–64.
Environmental conditions can vary among the islands that make up the Aleutian Archipelago. For example, island size and coastline morphology, differences in the dimensions and depths of the many oceanic passes that separate them, their associated shelf bathymetries, and their water mass properties can also influence the environment65. Currents influencing water masses in this region are complex. In the central and western Aleutians, the Alaska Stream flows westward along the Aleutian shelf break, providing Bering Sea in flow from Samalga Pass to Near Strait66,67. These waters are relatively cool, saline, and nutrient rich66. In contrast, the Aleutian North Slope Current flows along the Bering side of the islands from Amchitka Pass eastward68,69. These waters become warmer, fresher, and more nutrient poor to the east due to the influence of the Alaska Coastal Current67. An example of the complexities of the Aleutian environment can be seen at Samalga Pass, a well-described biogeographic break for some cold-water corals, zooplankton, demersal fishes, seabirds, and deep marine faunal communities65, especially for benthic invertebrate and macroalgal assemblages on coastal rocky reefs70. We propose that these large-scale oceanographic influences play a larger role in structuring the trophically downgraded urchin barrens, while prior to downgrading, the Aleutian kelp forest were presumably locally controlled by biological interactions.
When the different taxa within the two habitats were organized into their respective trophic guilds, we found similar results to those observed for the whole communities, with the exception of filter feeders. In particular, when abundances were estimated using density data, each of the trophic guilds was more variable in the kelp forest than the urchin barrens. Further, primary producer, grazer and predator assemblages were each most variable at the smallest spatial scale and least variable at the largest spatial scale within the kelp forests, but they followed the opposite pattern in the urchin barrens. In contrast, filter feeder assemblages were most variable at the largest spatial scale in kelp forests but at the smallest spatial scales in urchin barrens. However, given that many of the benthic filter feeders in this study (i.e., sponges and tunicates) exhibit indeterminate growth, a wide range in body sizes, and encrusting morphologies, we believe that estimates of variability based on density are insufficient to properly describe their abundances or spatial variation. Consequently, when abundances were estimated based biomass data, variability in filter feeder assemblages followed the same patterns observed for the whole communities and the for other feeding guilds; they were each most variable at the smallest scale and least variable at the largest scale in the kelp forests, but followed the opposite pattern in the urchin barrens. We believe that the differences in filter feeder scaling patterns between the two data types are likely not a sampling artifact but rather because biomass is the better measure of filter-feeder abundance and variation. Indeed, other studies have found discrepancies in community structure and distribution patterns for sponges when examining both density and biomass71,72. Likewise, the grazers were dominated by gastropods, whose heavy calcium carbonate shells may have dominated their biomass estimates, and thus were likely better described by their densities. In other words, the two sampling methods assess different taxa in the communities and this is important to how these communities are organized spatially. Consequently, we propose that each trophic guild followed the same scaling patterns as did the whole communities, and thus were likely affected by the same forcing factors. Specifically, they were each most variable at the smallest spatial scale within the kelp forests, which again is likely due to enhanced biological interactions51,53,64. As the kelp forests transitioned to urchin barrens and species diversity was reduced, the effects of these interactions became less important at larger spatial scales and the importance of physical factors and oceanographic control increased35,62,73–75.
Our findings are in agreement with previous studies in coastal marine systems that have found variability in the structure of biological communities to exist at multiple spatial scales21,26,33–35. These patterns are often due to different forcing factors that operate across a range of scales21,32,33,54. For example, variation in biological interactions can drive variation at small scale (< 10 m) patterns, while habitat heterogeneity can drive local scale (< 10 km) differences in community structure33,76,77. At mesoscales (10–100 km) or regional scales (> 100 s km), coastal marine communities may differ across oceanographic boundaries where multiple physical parameters change simultaneously35,78,79. This can have important consequences to our understanding of the relative importance of the factors that structure these communities, as small-scale biological interactions can mask the influence of large-scale oceanographic and climate factors51–53, while large-scale factors can mask the influence of small-scale biological interactions33,62–64. Here, we show that variation in Aleutian kelp forest communities generally follows a scaling pattern, which is similar to patterns observed for giant kelp, Macrocystis pyrifera, populations along the coasts of California, USA and Baja California, MEX33,35. There, as with our current study, the greatest amount of variability was observed at spatial scales that encompassed 10 s of meters (i.e., among samples within each kelp forest) compared to scales that encompassed 100 s to 1000 s of kilometers (i.e., among geographic regions). However, that scaling pattern was altered by large-scale changes in ocean temperature and wave intensity during the 1997–1998 ENSO event, which affected the kelp populations at large (100 s–1000 s km) spatial scales and masked patterns of small-scale variability27. Specifically, this resulted in a reduction in small-scale variability and a shift towards large-scale variability33. Given the Aleutian nearshore ecosystem is a top-down driven system where, in general, environmental variables play a small role in determining community structure and the presence of alternate stable states56, we suggest the effects of trophic downgrading in the Aleutian kelp forests were similar to the ENSO effects in the California and Mexico kelp populations in that they reduced overall spatial variability in community structure and shifted this variability from small scales (i.e., within forests) to large scales (among geographic regions), and propose that this occurred due to a shift in the relative importance of biological interactions towards oceanographic forcing.
Ecological theory predicts that global warming will increase the importance of foundation species in maintaining ecosystem function because they can ameliorate environmental stress79–81. In the Aleutian Archipelago, we have demonstrated that the trophic downgrading that has greatly eliminated foundational kelp species has also reduced variability in overall community structure and in the structure of trophic guilds. While functional redundancies among predators and herbivores can make diverse systems more stable7,9, simple food webs, such as those found in the Aleutian Archipelago, do not have the functional redundancies at higher trophic levels needed to maintain stability. Trophic rewilding (i.e., the restoration of apex predators) has been suggested for systems that have been downgraded82 and may be one way that Aleutian kelp forests and their spatial variability can be restored. However, this may also be problematic for the Aleutian ecosystem given that killer whales still may be hunting sea otters there. Further, the establishment of a successful sea urchin fishery (i.e. human predation) to reduce sea urchin numbers may also not be feasible because the urchins in these barrens are generally characterized as having small gonads of poor quality due to a lack of macroalgal food83. But, as ocean temperatures in this region continue to rise with climate change84, the possibility of urchin disease may increase85,86, which could overtake predation as the primary source of urchin mortality87 and result in substantial declines in urchin populations88–90. This can ultimately lead to a restoration of the ecosystem as seen in other areas of the world80,88,91,92. Regardless, we believe some mechanism of widespread urchin mortality may be necessary to return normal variability in ecosystem structure.
Materials and methods
Study region and sites
The Aleutian Archipelago is a volcanic mountain chain that extends approximately 1900 km over 25° of longitude and 4° of latitude. The islands separate the Bering Sea to the north from the Pacific Ocean to the south, with the eastern extent occurring at the Alaska Peninsula, USA and the western extent occurring at the Kamchatka Peninsula, RUS. Island sizes are variable (27–2500 km2), as is the extent of the continental shelf (i.e., the 200 m isobath) around these islands (10–100 km). Oceanographic passes separate many of the island groups and facilitate water exchange between the Bering Sea and Pacific Ocean88. Net water flow through the passes is northward, but the direction and water flow rate through these passes depend on pass width and depth66,93, which vary across the island chain94. Because of these conditions, the hydrodynamic environments around the different islands can vary.
We sampled nine islands spanning ~ 1230 km of the Aleutian Archipelago, from Attu to Chuginadak (Fig. 1), during two research cruises aboard the R/V Oceanus in July 2016 and June 2017. Specifically, we sampled Adak, Chugidinadak and Tanaga in 2016, and Amchitka, Atka, Attu, Kiska, Nizki, and Yunaska in 2017. Previous analyses of community data by our research group did not identify any differences in community structure between the two sample years12,16. Two 6–8 m deep sites belonging to each habitat type (kelp forest and urchin barren) were sampled at each island using SCUBA. Depending on location and accessibility, the sites within each island were separated by hundreds of meters to several kilometers. The kelp forests were identified as having canopy-forming kelp (Eualaria fistulosa; hereafter Eualaria) and a mixed sub-canopy comprised of kelps and other brown and red fleshy macroalgae. In contrast, the urchin barrens lacked nearly all canopy and sub-canopy fleshy macroalgae, but had an abundance of green sea urchins (Strongylocentrotus spp.) (see Metzger et al.16 for more detailed descriptions of community compositions).
Community structure sampling methods
We estimated the density and biomass of benthic invertebrates and macroalgae occurring within each habitat type at each site. For this, all epibenthic organisms that occurred within ten haphazardly placed 0.25 m2 quadrats, except those strongly adhered to the substrate (e.g., barnacles and encrusting coralline algae), were scraped from the substrate and placed in fine mesh (< 1 mm) collection bags for shipboard processing. In addition, Eualaria sporophytes were counted within three haphazardly placed 10 m × 2 m swaths. Also along these swaths, all conspicuous and sparsely distributed large mobile invertebrates (e.g., sea stars, crabs, and large gastropods) whose densities were assumed to be less than 1.0 per 2.5 m2 (the total area covered by the quadrats in each site) were collected in fine mesh bags for shipboard processing. All collected organisms were identified to the lowest possible taxonomic level, counted if the taxon had discrete individuals, and weighed (to the nearest 0.005 kg) for biomass using hanging spring scales. Ambiguous or difficult to identify individuals were preserved in 10% formalin (for invertebrates) or pressed on herbarium paper (for algae) for later identification.
Following identification, the organisms were grouped according to their primary feeding method. Working with density and biomass based data separately, each taxon was assigned to one of four trophic guilds; namely primary producers, herbivores, filter feeders, or predators. Specifically, all macroalgae were classified as primary producers, and all ascidians, sponges, and cnidarians were classified as filter feeders. Taxa that also fed on detritus were classified according to their other primary feeding method (e.g., those that both grazed on algae and fed on detritus were classified as grazers, and those that were both predatory and fed on detritus were classified as predators). Taxa that were extremely rare (i.e., only a single individual occurred in one or two of our samples) were not included in this analysis.
Community structure statistical analyses
All statistical analyses were done in Primer-E (Version 7) and R-Studio (Version 1.1.463). Quadrat and swath data were combined into density and biomass datasets within each habitat separately (kelp forests and urchin barrens). Consequently, our sampling method resulted in four discrete data sets; density and biomass estimates for organisms occurring in both kelp forests and sea urchin barrens. Density and biomass data were square root transformed prior to analysis to remove the effects of numerically dominant taxa. Four separate zero-inflated Bray–Curtis resemblance matrices assessing dissimilarities in community assemblages among quadrats within each site, between sites within each island, and among islands were generated; one for density-based data and one for biomass-based data within each of the two habitats. Variation in community structure was then evaluated among the different spatial scales within each of the two habitat types using four separate three-factor fully-nested PERMANOVAs (for each data type and habitat). Along with the PERMANOVAs, we used variance partitioning59 to compare patterns of variability among the different spatial scales and between the habitat types within each PERMANOVA model. Additionally, multivariate dispersions (i.e., variances in community assemblages) were determined for each spatial scale and within each habitat type using the MvDisp routine in Primer-E. Lastly, nMDS (nonmetric metric multidimensional scaling) plots were generated to visually display similarities in community assemblages at each spatial scale for each data type. The MDS axis values were imported into R-Studio where they were re-graphed with the exterior polygon points connected, which allowed us to evaluate the area encompassed by each habitat (i.e. convex hulls). Following analyses for the community assemblages, variation in the assemblage structures making up each feeding guild were similarly examined at each spatial scale using the MvDisp procedure in Primer-E as described above.
Supplementary information
Acknowledgements
This study was funded by the National Science Foundation (Award Number: 1435205). We thank S. Lamerdin and the captain and crew of the R/V Oceanus for excellent ship support. We are grateful to J. Estes for introductions to the Aleutian kelp ecosystem. We thank A. Bland, S. Gabara, M. Good, T. McHugh, J. Metzger, A. Ravelo, S. Small, P. Spector, G. Sullaway, S. Traiger, and B. Weitzman for field assistance. We also thank the Alaska Maritime National Wildlife Refuge for logistical support.
Author contributions
M.S.E. and B.K. were equally responsible for project conception, funding acquisition, carrying out the research, data analyses, writing, and editing of the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75117-2.
References
- 1.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 2.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton SL, Caselle JE. Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. R. Soc. B Biol. Sci. 2015;282:20141817. doi: 10.1098/rspb.2014.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Power ME. Effects of fish in river food webs. Science. 1990;250:811–814. doi: 10.1126/science.250.4982.811. [DOI] [PubMed] [Google Scholar]
- 5.Saleem M. Loss of microbiome ecological niches and diversity by global change and trophic downgrading. Microbiome Commun. Ecol. 2015;20:89–113. [Google Scholar]
- 6.Risch AC, et al. Size-dependent loss of aboveground animals differentially affects grassland ecosystem coupling and functions. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-06105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisaguirre JH, et al. Trophic redundancy and predator size class structure drive differences in kelp forest ecosystem dynamics. Ecology. 2020;101:e02993. doi: 10.1002/ecy.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stromayer KA, Warren RJ. Are overabundant deer herds in the eastern United States creating alternate stable states in forest plant communities? Wildl. Soc. Bul. 1997;25:227–234. [Google Scholar]
- 9.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Cons. 2002;29:436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- 10.Strickland MS, Hawlena D, Reese A, Bradford MA, Schmitz OJ. Trophic cascade alters ecosystem carbon exchange. Proc. Natl. Acad. Sci. 2013;110:11035–11038. doi: 10.1073/pnas.1305191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atwood TB, et al. Predator-induced reduction of freshwater carbon dioxide emissions. Nat. Geosci. 2013;6:191–194. doi: 10.1038/ngeo1734. [DOI] [Google Scholar]
- 12.Edwards MS, et al. Marine deforestation leads to widespread loss of ecosystem function. PLoS One. 2020 doi: 10.1371/journal.pone.0226173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ripple WJ, Becshta RL. Hardwood tree decline following large carnivore loss on the Great Plains, USA. Front. Ecol. Environ. 2004;5:241–246. doi: 10.1890/1540-9295(2007)5[241:HTDFLC]2.0.CO;2. [DOI] [Google Scholar]
- 14.Ripple WJ. Wolves and the ecology of fear: Can predation risk structure ecosystems. Bioscience. 2004;54:55–766. doi: 10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2. [DOI] [Google Scholar]
- 15.Beschta RL, Ripple WJ. Recovering riparian plant communities with wolves in northern Yellowstone, USA. Rest. Ecol. 2010;18:380–389. doi: 10.1111/j.1526-100X.2008.00450.x. [DOI] [Google Scholar]
- 16.Metzger JR, Konar B, Edwards MS. Assessing a macroalgal foundation species: Community variation with shifting algal assemblages. Mar. Biol. 2019;166:156. doi: 10.1007/s00227-019-3606-1. [DOI] [Google Scholar]
- 17.Gabara, S, Konar, B. & Edwards, M. Biodiversity loss leads to reductions in community-wide trophic complexity. Ecosphere. (in press).
- 18.Hamilton SL, Caselle JE, Malone DP, Carr MH. Incorporating biogeography into evaluations of the Channel Islands marine reserve network. Proc. Natl. Acad. Sci. 2010;107:18272–18277. doi: 10.1073/pnas.0908091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer H, et al. Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. Proc. Natl. Acad. Sci. 2015;112:14894–14899. doi: 10.1073/pnas.1500664112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellin C, MacNeil AM, Cheal AJ, Emslie MJ, Caley JM. Marine protected areas increase resilience among coral reef communities. Ecol. Lett. 2016;19:629–637. doi: 10.1111/ele.12598. [DOI] [PubMed] [Google Scholar]
- 21.Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. doi: 10.2307/1941447. [DOI] [Google Scholar]
- 22.Bengtsson J, Baillie SR, Lawton J. Community variability increases with time. Oikos. 1997;78:249–256. doi: 10.2307/3546291. [DOI] [Google Scholar]
- 23.Connell JH, Hughes TP, Wallace CC. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol. Monogr. 1997;67:461–488. doi: 10.1890/0012-9615(1997)067[0461:AYSOCA]2.0.CO;2. [DOI] [Google Scholar]
- 24.Deutschman DH, Levin SA, Devine C, Buttel LA. Scaling from trees to forests: Analysis of a complex simulation model. Science. 1997;277:1688. doi: 10.1126/science.277.5332.1688. [DOI] [Google Scholar]
- 25.Brown BL. Spatial heterogeneity reduces temporal variability in stream insect communities. Ecol. Lett. 2003;6:316–325. doi: 10.1046/j.1461-0248.2003.00431.x. [DOI] [Google Scholar]
- 26.Hughes TP, et al. Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature. 1999;397:59–63. doi: 10.1038/16237. [DOI] [Google Scholar]
- 27.Edwards MS, Estes JA. Catastrophe, recovery, and range limitation in NE Pacific kelp forests: A large-scale perspective. Mar. Ecol. Prog. Ser. 2006;320:79–87. doi: 10.3354/meps320079. [DOI] [Google Scholar]
- 28.Parepa M, Fischer M, Bossdorf O. Environmental variability priomotes plant invasion. Nat. Commun. 2013;4:1604. doi: 10.1038/ncomms2632. [DOI] [PubMed] [Google Scholar]
- 29.Dunstan PK, Johnson CR. Linking richness, community variability, and invasion resisteance with patch size. Ecology. 2006;87:2842–2850. doi: 10.1890/0012-9658(2006)87[2842:LRCVAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Prevéy JS, Germino MJ, Huntly NJ, Inouye RS. Exotic plants increase and native plants decrease with loss of foundation species in sagebrush steppe. Plant Ecol. 2010;207:39–51. doi: 10.1007/s11258-009-9652-x. [DOI] [Google Scholar]
- 31.Marks LM, Reed DC, Obaza AK. Assessment of control methods for the invasive seaweed Sargassum horneri in California, USA. Manag. Biol. Invasions. 2017;8:205–213. doi: 10.3391/mbi.2017.8.2.08. [DOI] [Google Scholar]
- 32.Wiens JA. Spatial scaling in ecology. Funct. Ecol. 1989;3:385–397. doi: 10.2307/2389612. [DOI] [Google Scholar]
- 33.Edwards MS. Estimating scale-dependency in disturbance impacts: El Niños and giant kelp forests in the northeast Pacific. Oecologia. 2004;138:436–447. doi: 10.1007/s00442-003-1452-8. [DOI] [PubMed] [Google Scholar]
- 34.Dayton PK, Tegner MJ. The importance of scale in community ecology: A kelp forest example with terrestrial analogs. In: Price PW, Slobodchikoff CN, Gaud WS, editors. A New Ecology: Novel Approaches To Interactive Systems. New York: Wiley; 1984. [Google Scholar]
- 35.Jenkinson RS, Hovel KA, Dunn RP, Edwards MS. Biogeographical variation in the distribution, abundance, and interactions among key species on rocky reefs of the northeast Pacific. Mar. Ecol. Prog. Ser. 2020;648:51–65. doi: 10.3354/meps13437. [DOI] [Google Scholar]
- 36.Mann KH. Seaweeds: Their productivity and strategy for growth: The role of large marine algae in coastal productivity is far more important than has been suspected. Science. 1973;182:975–981. doi: 10.1126/science.182.4116.975. [DOI] [PubMed] [Google Scholar]
- 37.Leith H, Whittaker RH. Primary Productivity of the Biosphere. Berin: Springer; 1975. [Google Scholar]
- 38.Reed DC, Brzezinski MA. Kelp forests. In: Laffoley D, Grimsditch G, editors. The Management of Natural Coastal Carbon Sinks. Gland: Springer; 2009. p. 31. [Google Scholar]
- 39.Spector M, Edwards MS. Modelling the impacts of kelp deforestation on benthic primary production on temperate rocky reefs. Algae. 2020;35:1–16. doi: 10.4490/algae.2020.35.8.19. [DOI] [Google Scholar]
- 40.Dayton PK. Ecology of kelp communities. Ann. Rev. Ecol. Syst. 1985;16:215–245. doi: 10.1146/annurev.es.16.110185.001243. [DOI] [Google Scholar]
- 41.Krumhansl KA, Scheibling RE. Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 2012;467:281–302. doi: 10.3354/meps09940. [DOI] [Google Scholar]
- 42.Estes JA, et al. Complex trophic interactions in kelp forest ecosystems. Bull. Mar. Sci. 2004;74:621–638. [Google Scholar]
- 43.Krumhansl KA, et al. Global patterns of kelp forest change over the past half-century. Proc. Natl. Acad. Sci. 2016;113:13785–13790. doi: 10.1073/pnas.1606102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kriegisch N, Reeves SE, Johnson CR, Ling SD. Top-down sea urchin overgrazing overwhelms bottom-up stimulation of kelp beds despite sediment enhanncement. J. Exp. Mar. Biol. Ecol. 2019;514(515):48–58. doi: 10.1016/j.jembe.2019.03.012. [DOI] [Google Scholar]
- 45.Schiebling RE, Hennigar AW, Balch T. Destructive grazing, epiphytism, and disease: The dynamics of sea urchin—kelp interactions in Nova Scotia. Can. J. Fish. Sci. Aquat. 1999;56:2300–2314. doi: 10.1139/f99-163. [DOI] [Google Scholar]
- 46.Fagerli CW, Norderhaug KM, Christie HC. Lack of sea urchin settlement may explain kelp forest recovery in overgrazed areas in Norway. Mar. Ecol. Prog. Ser. 2012;488:119–132. doi: 10.3354/meps10413. [DOI] [Google Scholar]
- 47.Filbee-Dexter K, Scheibling RE. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 2014;495:1–25. doi: 10.3354/meps10573. [DOI] [Google Scholar]
- 48.Ling SD, Johnson CR, Frusher SD, Ridgway KR. Overfishing reduces resilience of kelp beds to climate-drivebn catastrophic phase shift. Proc. Natl. Acad. Sci. 2009;106:22341–22345. doi: 10.1073/pnas.0907529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simenstad CA, Estes JA, Kenyon KW. Aleuts, sea otters, and alternate stable state communities. Science. 1978;200:403–411. doi: 10.1126/science.200.4340.403. [DOI] [PubMed] [Google Scholar]
- 50.Christie H, Norderhaug KM, Fredriksen S. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 2009;396:221–233. doi: 10.3354/meps08351. [DOI] [Google Scholar]
- 51.Greig-Smith P. Pattern in vegetation. J. Ecol. 1979;67:755–779. doi: 10.2307/2259213. [DOI] [Google Scholar]
- 52.Clark WC. Scales of climate impacts. Clim. Change. 1985;7:5–27. doi: 10.1007/BF00139438. [DOI] [Google Scholar]
- 53.Woodward FI. Climate and Plant Distribution. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- 54.Levin SA. Multiple scales and the maintenance of biodiversity. Ecosystems. 2000;3:498–506. doi: 10.1007/s100210000044. [DOI] [Google Scholar]
- 55.Edwards, M.S. Scale-dependent patterns of community regulation in giant kelp forests. Ph.D. dissertation, University of California Santa Cruz (2001).
- 56.Estes JA. Serendipity: An Ecologists Quest to understand Nature. California: University of California Press; 2016. [Google Scholar]
- 57.Doroff AM, et al. Sea otter population declines in the Aleutian archipelago. J. Mammal. 2003;84:55–64. doi: 10.1644/1545-1542(2003)084<0055:SOPDIT>2.0.CO;2. [DOI] [Google Scholar]
- 58.Konar BK, Edwards MS, Estes JA. Biological interactions maintain the boundaries between kelp forests and urchin barrens in the Aleutian Archipelago. Hydrobiol. 2014;724:91–107. doi: 10.1007/s10750-013-1727-y. [DOI] [Google Scholar]
- 59.Graham MH, Edwards MS. Statistical significance versus factor fit: Estimating the importance of individual factor in ecological analysis of variance. Oikos. 2001;93:505–513. doi: 10.1034/j.1600-0706.2001.930317.x. [DOI] [Google Scholar]
- 60.Anderson MJ, Gorley RN, Clarke KR. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth: K PRIMER-E; 2006. [Google Scholar]
- 61.Estes JA, Duggins DO. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol. Monogr. 1995;65:75–100. doi: 10.2307/2937159. [DOI] [Google Scholar]
- 62.Levin SA. Challenges in the development of a theory of ecosystem structure and function. In: Roughgarden J, May RM, Levin SA, editors. Perspectives in Ecological Theory. Princeton University Press: Princeton; 1989. pp. 242–255. [Google Scholar]
- 63.Tegner MJ, Dayton PK, Edwards PB, Riser KL. Large-scale, low-frequency oceanographic effects on kelp forest successions: A tale of two cohorts. Mar. Ecol. Prog. Ser. 1997;146:17–134. doi: 10.3354/meps146117. [DOI] [Google Scholar]
- 64.Karlson RH, Cornell HV. Scale-dependent variation in local vs regional effects on coral species richness. Ecol. Monogr. 1998;68:259–274. doi: 10.1890/0012-9615(1998)068[0259:SDVILV]2.0.CO;2. [DOI] [Google Scholar]
- 65.Reed RK, Stabeno PJ. The recent return of the Alaskan Stream to Near Strait. J. Mar. Res. 1993;51:515–527. doi: 10.1357/0022240933224025. [DOI] [Google Scholar]
- 66.Ladd C, Hunt GL, Mordy CW, Salo SA, Stabeno PJ. Marine environment of the eastern and central Aleutian Islands. Fish. Oceanogr. 2005;14:22–38. doi: 10.1111/j.1365-2419.2005.00373.x. [DOI] [Google Scholar]
- 67.Reed RK, Stabeno PJ. Dynamics of the Bering Sea. Alaska: University of Alaska Sea Grant; 1999. The Aleutian North slope current; pp. 177–191. [Google Scholar]
- 68.Stabeno PJ, Reed RK. A major circulation anomaly in the western Bering Sea. Geophys. Res. Let. 1992;19:1671–1674. doi: 10.1029/92GL01688. [DOI] [Google Scholar]
- 69.Hunt GL, Stabeno PJ. Oceanography and ecology of the Aleutian Archipelago: Spatial and temporal variation. Fish. Oceanogr. 2005;14:292–306. doi: 10.1111/j.1365-2419.2005.00378.x. [DOI] [Google Scholar]
- 70.Konar, et al. A swath across the great divide: Kelp forests across the Samalga Pass biogeographic break. Cont. Shelf Res. 2017;143:78–88. doi: 10.1016/j.csr.2017.06.007. [DOI] [Google Scholar]
- 71.Wilkinson CR, Cheshire AC. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs. 1989;8:127–134. doi: 10.1007/BF00338268. [DOI] [Google Scholar]
- 72.Wilkinson CR, Cheshire AC. Comparisons of sponge populations across the Barrier Reefs of Australia and Belize: Evidence for higher productivity in the Caribbean. Mar. Ecol. Prog. Ser. 1990;67:285–294. doi: 10.3354/meps067285. [DOI] [Google Scholar]
- 73.Dayton PK, Tegner MJ, Edwards PB, Riser KL. Temporal and spatial scales of kelp demography: The role of oceanographic climate. Ecol. Monogr. 1999;69:219–250. doi: 10.1890/0012-9615(1999)069[0219:TASSOK]2.0.CO;2. [DOI] [Google Scholar]
- 74.Konar B, Edwards M, Efird T. Local habitat and regional oceanographic influence on fish distribution patterns in the diminishing kelp forests across the Aleutian Archipelago. Environ. Biol. Fish. 2015;98:1935–1951. doi: 10.1007/s10641-015-0412-6. [DOI] [Google Scholar]
- 75.Blanchette CA, Broitman BR, Gaines SD. Intertidal community structure and oceanographic patterns around Santa Cruz Island, CA, USA. Mar. Biol. 2006;149:689–701. doi: 10.1007/s00227-005-0239-3. [DOI] [Google Scholar]
- 76.García-Charton JA, Pérez-Ruzafa Á, Sánchez-Jerez P, Bayle-Sempere JT, Reñones O, Moreno D. Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar. Biol. 2004;144:161–182. doi: 10.1007/s00227-003-1170-0. [DOI] [Google Scholar]
- 77.Hewitt JE, Thrush SF, Dayton PD. Habitat variation, species diversity and ecological functioning in a marine system. J. Exp. Mar. Biol. Ecol. 2008;366:116–122. doi: 10.1016/j.jembe.2008.07.016. [DOI] [Google Scholar]
- 78.Bland A, Konar B, Edwards M. Spatial trends and environmental drivers of epibenthic shelf community structure across the Aleutian Islands. Cont. Shelf Res. 2019;175:12–29. doi: 10.1016/j.csr.2019.01.006. [DOI] [Google Scholar]
- 79.Bruno JF, Petes LE, Drew Harvell C, Hettinger A. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 2003;6:1056–1061. doi: 10.1046/j.1461-0248.2003.00544.x. [DOI] [Google Scholar]
- 80.Halpern BS, Selkoe KA, Micheli F, Kappel CV. Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Cons. Biol. 2007;21:1301–1315. doi: 10.1111/j.1523-1739.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman G, et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS One. 2011;20:20. doi: 10.1371/journal.pone.0028983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svenning JC, et al. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc. Natl. Acad. Sci. 2016;113:898–906. doi: 10.1073/pnas.1502556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stewart N, Konar B. Kelp forests versus urchin barrens: Alternate stable states and their effect on sea otter prey quality in the Aleutian Islands. J. Mar. Sci. 2012 doi: 10.1155/2012/492308. [DOI] [Google Scholar]
- 84.Rogachev KA, Shlyk NV. The role of the Aleutian eddies in the Kamchatka current warming. Russ. Meteorol. Hydrol. 2018;43:43–48. doi: 10.3103/S1068373918010065. [DOI] [Google Scholar]
- 85.Scheibling RE, Hennigar AW. Recurrent outbreaks of disease in sea urchins Strongylocentrotus droebachiensis in Nova Scotia: Evidence for a link with large-scale meterologic and oceanographic events. Mar. Ecol. Prog. Ser. 1997;152:155–165. doi: 10.3354/meps152155. [DOI] [Google Scholar]
- 86.Girard D, Clemente S, Toledo-Guedes K, Brito A, Hernández JC. A mass mortality of subtropical intertidal populations of the sea urchin Paracentrotus lividus: Analysis of potential links with environmental conditions. Mar. Ecol. 2012;33:377–385. doi: 10.1111/j.1439-0485.2011.00491.x. [DOI] [Google Scholar]
- 87.Feehan CJ, Scheibling RE. Disease as a control of sea urchin populations in Nova Scotian kelp beds. Mar. Ecol. Prog. Ser. 2014;500:149–158. doi: 10.3354/meps10700. [DOI] [Google Scholar]
- 88.Hagen NT. Sea urchin outbreaks and nematode epizootics in Vestfjorden, northern Norway. Sarsia. 1987;72:213–229. doi: 10.1080/00364827.1987.10419719. [DOI] [Google Scholar]
- 89.Shimizu M, Nagakura K. Acid phosphatase activity in the body wall of the sea urchin, Strongylocentrotus intermedius, cultured at varying water temperatures. Comp. Biochem. Physiol. 1993;106B:303–307. [Google Scholar]
- 90.Wang Y, et al. Isolation and characterization of bacteria associated with a syndrome disease of sea urchin Strongylocentrotus intermedius in North China. Aquacult. Res. 2013;44:691–700. doi: 10.1111/j.1365-2109.2011.03073.x. [DOI] [Google Scholar]
- 91.Behrens MD, Lafferty KD. Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar. Ecol. Prog. Ser. 2004;279:129–139. doi: 10.3354/meps279129. [DOI] [Google Scholar]
- 92.Feehan CJ, Scheibling RE. A mass mortality of subtropical intertidal populations of the sea urchin Paracentrotus lividus: Analysis of potential links with environmental conditions. Mar. Biol. 2014;161:1467–1485. doi: 10.1007/s00227-014-2452-4. [DOI] [Google Scholar]
- 93.Stabeno PJ, Kachel DG, Kachel NB, Sullivan ME. Observations from moorings in the Aleutian Passes: Temperature, salinity and transport. Fish. Oceanogr. 2005;14:39–54. doi: 10.1111/j.1365-2419.2005.00362.x. [DOI] [Google Scholar]
- 94.Favorite F. Flow into the Bering Sea through the Aleutian Passes. In: Hood DW, Kelly EJ, editors. Oceanography of the Bering Sea with Emphasis on Renewable Resources. Fairbanks: Institute of Marine Science University of Alaska; 1974. pp. 3–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.