Abstract
Background
Paracellular barriers play an important role in the pathogenesis of Inflammatory bowel disease (IBD) and maintain gut homeostasis. N-myc downstream-regulated gene 2 (NDRG2) has been reported to be a tumour suppressor gene and to inhibit colorectal cancer metastasis. However, whether NDRG2 affects colitis initiation and colitis-associated colorectal cancer is unclear.
Methods
Intestine-specific Ndrg2 deficiency mice (Ndrg2ΔIEC) were subjected to DSS- or TNBS-induced colitis, and AOM-DSS-induced colitis-associated tumour. HT29 cells, Caco2 cells, primary intestinal epithelial cells (IECs) from Ndrg2ΔIEC mice, mouse embryo fibroblasts (MEFs) from systemic Ndrg2 knockout mice, HEK293 cells and human UC and DC specimens were used to investigate NDRG2 function in colitis and colitis-associated tumour.
Findings
Ndrg2 loss led to adherens junction (AJ) structure destruction via E-cadherin expression attenuation, resulting in diminished epithelial barrier function and increased intestinal epithelial permeability. Mechanistically, NDRG2 enhanced the interaction of E3 ligase FBXO11 with Snail, the repressor of E-cadherin, to promote Snail degradation by ubiquitination and maintained E-cadherin expression. In human ulcerative colitis patients, reduced NDRG2 expression is positively correlated with severe inflammation.
Interpretation
These findings demonstrate that NDRG2 is an essential colonic epithelial barrier regulator and plays an important role in gut homeostasis maintenance and colitis-associated tumour development.
Funding
National Natural Science Foundation of China (No. 81770523, 31571437, 81672751), Creative Research Groups of China (No. 81421003), State Key Laboratory of Cancer Biology Project (CBSKL2019ZZ11, CBSKL201406, CBSKL2017Z08 and CBSKL2017Z11), Fund for Distinguished Young Scholars of ShaanXi province (2019JC-22).
Keywords: Colitis, NDRG2, Adherens junction, Colitis-associated colorectal cancer
Research in Context.
Evidence before this study
Paracellular barriers such as AJs play an important role in the pathogenesis of IBDs to maintain gut homeostasis. AJ dysfunction is closely associated with IBD, and expression attenuation of the major component E-cadherin could aggravate colitis.
Added value of this study
We demonstrated that intestinal-specific Ndrg2 knockout led to adherens junction structure destruction via E-cadherin reduction, resulting in diminished epithelial barrier function and enhanced gut permeability, caused mild spontaneous colitis with ageing, and aggravated colitis initiation and colitis-associated tumour development.
Implications of all the available evidence
Our study reveals that NDRG2 is an essential intestinal epithelial barrier regulator and plays important roles in gut homeostasis maintenance and colitis-associated tumour development.
Alt-text: Unlabelled box
1. Introduction
Inflammatory bowel disease (IBD) is a multifactorial chronic inflammatory disease mainly comprising Crohn's disease (CD) and ulcerative colitis (UC), which causes intestinal epithelial cell injury and relapsing chronic pathogenic rectal and colonic inflammation [1,2]. Intestinal epithelial homeostasis plays an important role in the intestinal tract. This bacteria-host homeostasis is maintained by an epithelial barrier, which includes tight junctions (TJs), adherens junctions (Ajs) and desmosomes [3,4]. Ajs are cell-cell adhesion complexes that participate in embryogenesis and tissue homeostasis [5]. The major AJ component E-cadherin or p120-catenin loss results in embryonic death [2]. Previous studies have demonstrated that AJ dysfunction is associated with IBD and that E-cadherin expression attenuation can aggravate colitis, possibly due to an increase in colonic epithelial barrier permeability [6].
We have previously identified N-myc downstream regulated gene 2 (NDRG2) as a novel tumour suppressor gene that plays a role in regulating the proliferation, differentiation and metastasis of multiple types of malignant tumours [7,8]. Notably, our recent data show that NDRG2 can inhibit colorectal cancer cell proliferation and promote cell differentiation [9]. Moreover, we have shown that decreased NDRG2 expression is a powerful and independent predictor of a poor prognosis in colorectal cancer [10,11]. However, whether NDRG2 participates in intestinal epithelial homeostasis and colitis initiation remains unknown.
In this study, we generated intestine-specific conditional Ndrg2 knockout mice and examined the roles of Ndrg2 in AJ structure and permeability regulation in the setting of spontaneous and experimentally induced colitis. Furthermore, we characterized the detailed function and mechanism of NDRG2 in intestinal epithelial inflammation and evaluated the effects of Ndrg2 deficiency on gut inflammation and colitis-associated tumour.
2. Materials and methods
2.1. Mice
Intestine-specific Ndrg2 knockout mice (Ndrg2ΔIEC) were generated by Shanghai Biomodel Organism Science and Technology Development Co., Ltd., and maintained on a C57BL6/J background. All animals were raised under specific-pathogen-free conditions. The wild-type (WT) and Ndrg2ΔIEC mice were age-matched, and all the mice that were used in the experiments were male. Typically, at least 8 mice were included each treatment group, and all experiments were repeated at least three times.
2.2. Spontaneous murine colitis analysis
To analyse spontaneous colitis development, 8 weeks and 36 weeks age Ndrg2ΔIEC mice were first given 1 g/L ampicillin (Sigma-Aldrich, A9393) water for one week, which was then replaced with normal water. The animals were raised under specific-pathogen-free conditions and sacrificed at the indicated time, and colon tissues were collected for further detection. Age-matched WT mice were used as a control. Throughout the experiment, the mice had free access to food and water. The mice were sacrificed in a CO2 chamber at a low-flow rate of 10-30% volume displacement per minute, followed by cervical dislocation.
2.3. DSS-induced colitis and colitis-associated colorectal cancer
For the DSS-induce colitis model, 8-week-old male mice were treated with 2.5% DSS (w/v, MW 36–50 kDa; MP Biomedicals, Solon, OH, 160110) in their drinking water for 5 days, followed by DSS-free water for another 5 days. Then sacrifice the mice and evaluate the inflammatory responses. Distal colon was obtained for histological analysis, and IECs and LPCs were separated with the total colon.
To induce colitis-associated colorectal cancer, we used the combination of the carcinogen azoxymethane (AOM, MedChemExpress, hy-111375) with repeated treatment with DSS in drinking water. Mice were treated with an intraperitoneal injection of a single dose of AOM (12 mg/kg). After 3 days, 2% DSS dissolved in the drinking water was administered for 6 days (the DSS solution was refreshed on day 3), followed by 15 days of regular drinking water. The DSS treatment was repeated for another two cycles. Mice were sacrificed 100 days after AOM injection, colons were removed and the colon tumours were dissected for further analysis.
2.4. TNBS-induced murine colitis
The 2,4,6-trinitrobenzenesulfonic acid (TNBS, 5%, w/v, Sigma-Aldrich, p2297)-induced experimental colitis was performed in C57BL/6 mice (B6) as previously described. Briefly, Ndrg2ΔIEC mice and their littermate controls were anaesthetized after fasting for 12 hours, after which 100 μl of 3 mg TNBS (dissolved in 50% ethanol) was slowly instilled into the colon using a 3.5 F catheter, which was inserted intra-rectally 4 cm from the anus. Mice were held in an inverted vertical position for 30 s after instillation to ensure an even distribution of TNBS throughout the entire colon and caecum. Mice from the respective control groups received PBS in a comparable volume via the same route.
2.5. Disease activity index (DAI) scores and Histological score determination
For each mouse, colonic tissue was cut into four segments and fixed in 4% neutral-buffered formalin, embedded in paraffin, cut into 5 μm-thick sections, and stained with H&E. The DAI score was composed of the sum of Diarrhea score (0-solid formed stool, 1-soft stool, and 2- loose formed stool), and rectal bleeding score (0-no blood, 1- the dimly visible streak of blood in stool, 2-gross bleeding from rectum). The histological score was evaluated in a blind manner that consisted of the sum of four parameters ranging from 0–3: cell hyperplasia (0, absent; 1, weak; 2, moderate; and 3, severe); epithelial damage (0, absent; 1, weak; 2, moderate; and 3, severe); inflammatory cell infiltration (0, rare inflammatory cells in lamina propria; 1, increased granulocytes in the lamina propria; 2, confluence of inflammatory cells extending into the submucosa; 3, transmural extension of the infiltrate); and crypt damage (0, intact crypt; 1, loss of basal one-third; 2, loss of basal two-thirds; 3, entire crypt loss; 4, erosion; 5, confluent erosion). The histological score for one mouse was the average of the summed scores from three biopsies of each of the four colon segments.
2.6. Isolation of intestinal epithelial cells (IECs) and lamina propria cells (LPCs)
Mice were sacrificed, and total colon tissues were dissected, opened longitudinally and washed extensively in cold PBS. The colon tissue was cut into 1 cm segments, which were washed with fresh PBS. The tissues were incubated with digestion buffer containing 8 mM EDTA (Sigma-Aldrich, E9884) and 1 mM DTT (Roche, 10197777001) at 37 °C for 20 min, which was then replaced with digestion buffer with cold PBS and shaken vigorously for 1 min. The process was repeated once. The supernatants were combined and centrifuged at 2000 rpm for 5 min at 4 °C. The pelleted cells were digested with dispase Ⅱ (0.2 mg/ml, Roche, 04942078001) in PBS at 37 °C for 5 min. The suspension was filtered with 70 μm and 40 μm nylon filters (BD biosciences) and centrifuged at 2000 rpm for 5 min at 4 °C, after which the cells were resuspended in cold PBS. To obtain LPCs, tissues incubated with EDTA were cut into small pieces and digested with a mixture of collagenase A (2 mg/ml, Roche, 10103586001) and DNase I (0.05 mg/ml, Sigma, D4263) in PBS at 37 °C for 35 min. After incubation, the enzyme mixture was replaced with cold PBS and shaken for 2 min. The process was repeated once. The suspension was combined and passaged through 70 μm and 40 μm nylon filters, followed by centrifugation at 4000 rpm for 5 min at 4 °C and resuspension of the pelleted cells in cold PBS. IECs were used for extraction of RNA and Western blot analysis. For quantification of immune cells, both IECS and LPCs were combined and used for subsequent FACS analysis immediately.
2.7. Analysis of intestinal permeability
Fluorescein isothiocyanate-conjugated (FITC) dextran with an average mol wt of 40,000 (Sigma-Aldrich, FD40) or an average mol wt of 500,000 (Sigma-Aldrich, FD500S) was dissolved at a concentration of 100 mg/ml in PBS. According to the manufacturer's instructions, all experimental mice were treated with water starvation for at least 10 hours; then, FITC-dextran was administered to each mouse (44 mg/100 g body weight) by oral gavage with a needle attached to a 1-ml syringe. After 4 hours, from mice anaesthetized was collected at least 400 μl of blood per mouse. Serum was separated and collected according to the manufacturer's instructions and stored in the dark at 4 °C. One hundred fifty microlitres of serum with an equal volume of PBS and 100 μl of the dilution were added to a 96-well microplate in duplicate. The concentration of FITC-dextran was determined using a spectrophotometry fluorometer (Tecan GENios Microplate Reader) with an excitation of 485 nm (20-nm band width) and an emission wavelength of 528 nm (20-nm band width). Serum from untreated mice was used to determine the background.
2.8. Flow cytometry staining
For the detection of monocytes, macrophages and neutrophils, the following fluorochrome-conjugated antibodies were used: anti-mouse CD11b FITC (eBioscience, 11-0112, AB_464933), anti-mouse Ly-6G (Gr-1) PE (eBioscience, 12-5931, AB_466045), anti-mouse F4/80 Antigen APC (eBioscience, 17-4801, AB_469452), and PE rat anti-mouse Siglec-F (BD Pharmingen™ 562068, AB_10896143). According to the reported methods, CD11b staining was used to detect monocytes, the combination of CD11b and Ly-6G (Gr-1) was used to detect neutrophils, and the combination of CD11b and Siglec-F was used to quantify eosinophils, while macrophages were detected with CD11b and F4/80. Both IECs and LPCs were combined for quantification of the immune cells. Total cells were resuspended in PBS containing 5% FBS, and at least 106 cells were used for the staining.
2.9. Analysis of adherens junction by transmission electron microscopy
Mice were perfused through the left atrium with a mixture of 4% paraformaldehyde (Servicebio, G1101) and 1% glutaraldehyde (Sigma-Aldrich, G5882). Pieces of colon tissue were fixed in 3% glutaraldehyde with 2% OsO4 (Sigma-Aldrich, 75633) for 6 hrs at 4 °C. The tissue was dehydrated at 4 °C through a series of acetone concentrations (50, 70, 90, 96, 100%) and embedded in Epon 812 epoxy resin (all from Epoxy Embedding Medium kit, Sigma-Aldrich, 45359). Sections were cut to a thickness of 70 nm and collected on 200-mesh, Formvar-coated copper grids. The grids were stained with uranyl acetate and lead citrate (both from Sigma-Aldrich, 45359), and micrographs were collected with a JEOL JEM 1230 electron microscope (JEOL, Japan).
2.10. RNA isolation and quantitative real-time PCR (qPCR)
Total IEC RNA was isolated from healthy and DSS-treated WT and Ndrg2ΔIEC mice using TRIzol reagent (Takara, Dalian, China, T9108) according to the manufacturer's instructions. Primer sequences for quantitative real-time polymerase chain reaction (qRT-PCR) are listed in Supplementary Tables 1 and 2. qRT-PCR data were analysed by the 2−ΔΔCT method, with GAPDH and β-actin as the housekeeping gene.
2.11. Co-immunoprecipitation assay
FLAG-tagged NDRG2 plasmids (full-length and truncated form named NDRG2/FL, NDRG2/ΔN, and NDRG2/ΔC) were co-transfected with Myc-Snail, HA-FBXO11 (gift from Dr. Yibin Kang, Princeton University) and V5-Ubiquitin or the indicated plasmids as described in the figures in 60-mm dishes using Lipofectamine™ 2000 Transfection Reagent (Invitrogen™, 11668019) according to the manufacturer's instructions. For the ubiquitination assay, cells were treated with 10 μM MG132 (Sigma-Aldrich, M8699) for the indicated times after 2 days of transfection. The cells were then harvested with lysis buffer (Beyotime, P0013), and the proteins were quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific, 23225) and adjusted to equal concentrations. Cell lysates were then immunoprecipitated with primary anti-FLAG (Sigma-Aldrich, F1804, AB_262044) or anti-Myc (Cell Signaling, 66004, AB_1549585) or anti-HA (CST, 3724, AB_1549585) or anti-V5 (Sigma, V8137) at 4 °C overnight. Then, 70 μL protein A/G sepharose (Abcam, ab193262) was added and incubated for 3 hrs. After 3 washes using NETN wash buffer, Tris 20 mM (pH 8.0), NaCl 100 mM, EDTA 1 mM, NP-40 0.5%, and sepharose beads were collected and re-suspended in 70 μL 2× SDS buffer. The samples pulled down with 5% input were resolved on SDS-PAGE gels and transferred to NC membranes. Then, the membranes were detected with appropriate antibodies and visualized using a Tanon 6200 Luminescent Imaging Workstation.
2.12. Western blot analysis
Total proteins were extracted from intestinal epithelial cells (IECs) according to the manufacturer's recommendations. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis were performed with 40 μg protein. The following antibodies were used: β-actin (Cell Signaling, 12262, AB_2566811), NDRG2 (Cell Signaling, 5667, AB_10829238), E-cadherin (Cell Signaling, 3195, AB_2291471), β-catenin (Cell Signaling, 8480, AB_11127855), P120 catenin (Proteintech, 12180, AB_2086267), α-catenin (Cell Signaling, 13-9700), Snail (Cell Signaling, 3879, AB_2255011), Slug (Cell Signaling, 9585, AB_2239535), and Claudin-1 (Cell Signaling, 13255, AB_2798163).
2.13. Immunofluorescence staining
For the murine studies, 8-μm frozen segments of colon from WT and ΔIEC mice were fixed in cold acetone for 15 min at −20 °C, and then 3% BSA was used for blocking at 25 °C for 1 h. Anti-E-cadherin (1:100), NDRG2 (1:80), β-catenin (1:100), P120 catenin (1:100) and ZO-1 (Cell Signaling, 8193, AB_10898025, 1:100) was used for incubation at 4 °C overnight, and Alexa-Fluor 488- or 647- conjugated antibody (Invitrogen, AB_2633280, AB_2633282, 1:2000) was used as the secondary antibody and incubated for 1 h at 25 °C. Incubation with 1 μg/ml of DAPI was performed for 15 min. Fluorescence analysis was performed with a laser scanning confocal microscope A1 (Nikon). For cellular protein distribution detection, cells were seeded in rat tail collagen-coated dishes and transfected with the indicated plasmid for 24 h, followed by gentle washing with PBS for 5 min and fixation in 4% paraformaldehyde for 30 min. Immunofluorescence staining was performed according to the above-described method. Anti-HA (1:500), anti-FLAG (1:500) and anti-SNAIL (1:200) were used for incubation at 4 °C overnight.
2.14. Membrane protein extraction
Membrane proteins were separated using a Mem-PER™ Plus Membrane Protein Extraction Kit (Thermo Scientific™, 89842) according to the manufacturer's instructions. Briefly, freshly isolated IECs from both Ndrg2ΔIEC mice and WT mice were washed with PBS and centrifuged at 300 × g for 5 min. The cell pellet was washed with 3 mL of Cell Wash Solution and centrifuged at 300 × g for 5 min. Then, the supernatant was discarded, and the cells were resuspend in 1.5 mL of Cell Wash Solution, centrifuged at 300 × g for 5 min and the supernatant discarded. We added 0.75 mL of Permeabilization Buffer to the cell pellet, vortexed it briefly and incubate it for 10 min at 4 °C with constant mixing. The permeabilized cells were centrifuged for 15 min at 16,000 × g. The supernatant containing cytosolic proteins was removed and transferred to a new tube. Then, 0.5 mL of Solubilization Buffer was added to the pellet, and the cells were suspended. The tubes were incubated at 4 °C for 30 min with constant mixing and centrifuged at 16,000 × g for 15 min at 4 °C. The supernatant containing solubilized membrane and membrane-associated proteins was transferred to a new tube. Both membrane and cytoplasm fractions could be used immediately or stored at −80 °C for future use.
2.15. Tissue samples and immunohistochemistry staining
A total of 19 ulcerative colitis cases and 5 Crohn's disease patient samples were obtained from Xijing Hospital from April 2013 to October 2016. These paraffin-embedded tissues were sectioned at 4-μm thickness and stained with HE or the indicated antibody using standard immunohistochemistry methods. The clinical information is shown in Supplementary Table 3.
2.16. Study approval and ethics statement
All animal procedures and patients sample study were reviewed and approved by the Animal Care and Ethic Committee of Fourth Military Medical University (No. KY20173182-1). All animals were housed, cared for, and used in compliance with the guidelines regarding the humane use and care of laboratory animals for biomedical research published by the National Institutes of Health (No. 85-23, revised 1996). All patients in the disease sample cohort provided full consent for the study.
2.17. Statistical analysis
Differences in parametric data were evaluated using the Student's t test or one-way analysis of variance (ANOVA). *p<0.05 and **p<0.01 were considered statistically significant.
3. Results
3.1. Intestinal Ndrg2 knockout mice develop mild spontaneous colitis
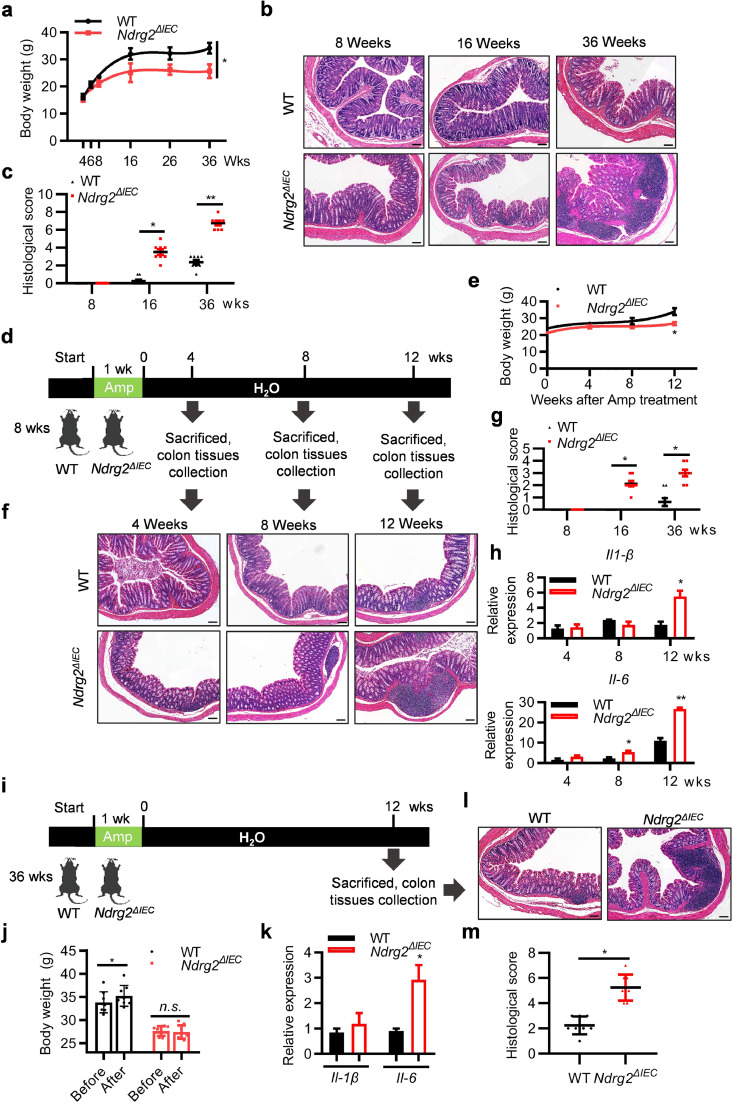
To determine the role of NDRG2 in colitis initiation, we generated intestine-specific conditional Ndrg2 knockout mice featuring intestinal epithelial cells lacking Ndrg2 (Supplementary Fig. 1(a)). NDRG2 expression was specifically and completely abolished in the intestinal epithelial cells (IECs) of Ndrg2ΔIEC mice (Supplementary Fig. 1(b), (c)). The mice were kept under specific-pathogen-free (SPF) conditions, and their body weight was monitored each week. Intriguingly, we observed significant growth retardation of Ndrg2ΔIEC mice compared with the wild type (WT) group, especially after 16 weeks age (Fig. 1(a)). However, there was no significant difference in the activity and diet between the two groups of mice. Thus, it was crucial for us to detect the alteration of intestinal tissues. As shown by the haematoxylin eosin (H&E) staining data in Fig. 1(b)–(c), increased intestinal inflammation was observed in Ndrg2ΔIEC mice that became more severe with ageing, which indicated the spontaneous colitis in Ndrg2ΔIEC mice.
Fig. 1.
Intestine-specific conditional Ndrg2 knockout mice (Ndrg2ΔIEC) spontaneously developed into mild colitis with ageing. (a) Body weight of WT and Ndrg2ΔIEC mice at the indicated time with specific-pathogen-free (SPF) feeding conditions (n=8/group; *p<0.05, (Student's t test)). (b) and (c) Histological score and H&E-staining of colon section from WT and Ndrg2ΔIEC mice of the indicated ages. Representative data of 3 independent experiments. Scale bar: 100 μm. (d) Schematic diagram to analyse the occurrence of spontaneous colitis in 8-week-old WT and Ndrg2ΔIEC mice after withdrawal of ampicillin treatment for 1 week. (e) Body weight of WT and Ndrg2ΔIEC mice at the indicated time after ampicillin withdrawal (n=8/group; *p<0.05, (Student's t test)). (f) and (g) Histological score and H&E-staining of colon tissue. Representative data of 3 independent experiments. Scale bar: 100 μm. (h) Quantitative real-time PCR (qRT-PCR) for Il-1β and Il-6 in IECs from WT and Ndrg2ΔIEC mice (n=8/group; *p<0.05, **p<0.01, (Student's t test)). (i) Schematic diagram to analyse the occurrence of spontaneous colitis in 36-week-old WT and Ndrg2ΔIEC mice after withdrawal of ampicillin treatment for 1 week. (j) Body weight of 36-week-old WT and Ndrg2ΔIEC mice before and after ampicillin withdrawal (48 weeks age)-induced spontaneous colitis (n=8/group; *p<0.05, (Student's t test)). (k) qRT-PCR for Il-1β and Il-6 in IECs of (G) (n=8/group; *p<0.05, (Student's t test)). (l) and (m) Histological score and H&E-staining of colon sections of WT and Ndrg2ΔIEC mice at the indicated times. Representative data of 3 independent experiments. Scale bar: 100 μm.
Next, to confirm that the growth retardation of Ndrg2ΔIEC mice was mainly due to increased intestinal inflammation, we treated the WT and Ndrg2ΔIEC mice (8 weeks age) with ampicillin (1 g/L) water for one week to alleviate any potential inflammation in digestive tract, and partially synchronize the gastrointestinal tract conditions between wild type and Ndrg2ΔIEC mice without disturbing weight and food intake, as mild intestinal inflammation might occurred during mice feeding under SPF condition and ampicillin is a broad-spectrum antibiotic that can effectively remove gram-positive and gram-negative bacteria (the major source of LPS) [12,13]. Then, we monitored the body weight in each group (Fig. 1(d)), as anticipated, there was a noticeable slower increase in body weight in the Ndrg2ΔIEC mice compared with the WT group (Fig. 1(e)). Additionally, more severe intestinal inflammation and increased IL-1β and IL-6 expression were observed in the Ndrg2ΔIEC mice (Fig. 1(f)–(h)), further supporting the increased spontaneous colitis in these mice. Then, we used the old age mice to further confirm this observation. As shown in Fig. 1(i), both the WT and Ndrg2ΔIEC mice aged 36 weeks were treated with ampicillin for 1 week and their body weight and intestinal inflammation analysed after 12 weeks, respectively. Compared with the increased body weight of WT mice after 12 weeks, there was no significant alteration of Ndrg2ΔIEC mice (Fig. 1(j)). Similar to the findings in young mice (Fig. 1(f)–(h)), increased intestinal inflammation and IL-1β and IL-6 expression were detected in the Ndrg2ΔIEC mice (Fig. 1(k)–(m)). Collectively, these data strongly suggested that intestinal Ndrg2 knockout facilitated the development of mild spontaneous colitis.
3.2. Intestinal Ndrg2 loss aggravates chemical-induced colitis
To further determine the role of NDRG2 in colitis initiation and progression, we treated the mice with DSS (dextran sodium sulphate) and TNBS (2,4,6-trinitrobenzenesulfonic acid), respectively, to mimic the colitis in vivo. We found that intestinal Ndrg2ΔIEC mice exhibited an increased body weight loss compared with WT mice with DSS treatment (Fig. 2(a)), as well as more severe colonic shortening than their counterparts (Fig. 2(b) and (c)). For histological analysis, Ndrg2ΔIEC mice exhibited severe colitis characterized by profound epithelial structural damage and robust inflammatory cell infiltration than the WT group (Fig. 2(d) and (e)), as well as higher disease activity index (DAI) scores (Fig. 2(f)). Alternatively, we observed similar results with more severe inflammation of Ndrg2ΔIEC mice in the TNBS-induced colitis model (Supplementary Fig. 2(a), (b)). For the survival analysis, Ndrg2ΔIEC mice exhibited increased susceptibility to lethal colitis induction (4% DSS for 7 days), leading to a shorter survival compared with WT mice (Fig. 2(g)). Taken together, our data demonstrated that intestine-specific Ndrg2 deficiency obviously aggravated chemical-induced colitis.
Fig. 2.
Ndrg2ΔIEC mice exhibited increased intestinal susceptibility to dextran sodium sulfate (DSS)-induced colitis. (a) Body weight change of 8-week-old WT and Ndrg2ΔIEC mice with 2.5% DSS treatment (n=10/group; *p<0.05, **p<0.01, (Student's t test)). (b) Colon lengths (left) and statistical results (right) on day 10 in the WT and Ndrg2ΔIEC mice (n=10/group; **p<0.01, (Student's t test)). (b) Representative images of the colon appearance at the end of the experiments. (n=10/group) (d) Representative H&E-staining of colon tissue. (n=10/group). Scale bar: 100 μm. (e) The histological scores of the WT and Ndrg2ΔIEC mice on days 0, 5, 7 and 10 of 2.5% DSS administration. (n=6/group; *p<0.05, n.s., no significance, (Student's t test)) (f) The DAI scores of the WT and Ndrg2ΔIEC mice on days 0, 5, 7 and 10 of 2.5% DSS administration. (n=6/group, *p<0.05, **p<0.01, (Student's t test)). (g) Survival rate analysis of 4% DSS-induced lethal colitis (n=15/group).
3.3. Intestinal Ndrg2 deficiency promotes inflammatory cell infiltration, chemokine and cytokine expression and LPS permeability
Inflammatory cell infiltration plays an important role in both UC and CD [14]. To characterize the role of NDRG2 in colonic inflammation, IECs and lamina propria cells (LPCs) were isolated from WT and Ndrg2ΔIEC mice treated with or without 2.5% DSS. Flow cytometry analysis showed that Ndrg2ΔIEC mice exhibited slight increases in monocyte and macrophage infiltration compared with WT mice under the DSS-free condition (Fig. 3(a), Supplementary Fig. 3(a)). Notably, Ndrg2ΔIEC mice exhibited significantly increased monocyte, macrophage and neutrophil infiltration with DSS treatment compared with WT mice (Fig. 3(b), supplementary Fig. 3(a)). Furthermore, DSS-treated Ndrg2ΔIEC mice exhibited dramatically increased chemokine expression of Cxl5 and Cxcl1/2, as well as pro-inflammatory cytokine expression of Il-1β, Tnf-α and Il-6 (Fig. 3(c), Supplementary Fig. 3(b)). In contrast, mRNA expression of the anti-inflammatory cytokine IL-10 was attenuated in DSS-treated Ndrg2ΔIEC mice (Fig. 3(c)), while the expression of Tgf-β, Il-12, Il-17 and Il-22 was comparable between the two groups (Supplementary Fig. 3(b)). These findings indicated that intestinal Ndrg2 deficiency aggravated DSS-induced colitis by increasing chemokine and pro-inflammatory cytokine expression and promoting inflammatory cell infiltration.
Fig. 3.
Ndrg2 deficiency enhanced inflammation in colon characterized by increased inflammatory cell infiltration and LPS permeability. WT and Ndrg2ΔIEC mice were treated with 2.5% DSS (n=12/group). Quantification of the infiltrated immunocytes on day 0 (a) and day 10 (b) detected by flow cytometry analysis. (c) qRT-PCR analysis of the chemokines Cxcl-1, Cxcl-2, Ccl-5 and pro-inflammatory cytokines Il-1β and Tnf-α, and the anti-inflammatory cytokines Il-10. (d) Detection of serum LPS concentrations in WT and Ndrg2ΔIEC mice with and without 2.5% DSS treatment (n=6/group). (e) Detection of serum LPS levels in WT and Ndrg2ΔIEC mice with 0.1 mg/kg LPS treatment with intrarectal administration after ampicillin treatment for 5 days, (n=4/group). (f) qRT-PCR analysis of Il-1β, Il-6, Tnf-α, Cxcl-1 and Cxcl-2 expression in IECs of WT and Ndrg2ΔIEC mice treated with 0.1 mg/kg LPS (n=4/group). (a), (b), (c), (d), (e), (f) Representative data of 3 independent experiments. Data are represented as the mean ± SEM. *p<0.05, **p<0.01, n.s., no significance (Student's t test).
The gut microbiota plays important roles in the pathogenesis of IBD, and lipopolysaccharide (LPS) is the main microflora-derived toxin that induces gut epithelial inflammation. Noticeably, the serum LPS concentration is dramatically increased in IBD patients [15]. As shown in Fig. 3(D), DSS-treated Ndrg2ΔIEC mice exhibited increased serum LPS levels compared with WT mice. Thus, we subsequently examined whether Ndrg2 deficiency facilitated LPS-induced inflammation. As expected, LPS induced significant inflammation characterized by dose-dependent increases in the levels of Il-1β, Il-6, Tnf-α and Cxcl1/2 expression (Supplementary Fig. 4(a)–(e)). Additionally, Ndrg2ΔIEC mice exhibited markedly higher LPS permeability and increased Il-1β, Il-6, Tnf-α, and Cxcl-1/2 expression (Fig. 3(e) and (f)), suggesting an enhanced inflammatory response. These data suggested that intestine-specific Ndrg2 deficiency increased LPS permeability and promoted LPS-induced inflammation and colitis progression.
3.4. Intestinal Ndrg2 loss disrupts adherens junction integrity of normal epithelium and increases colonic permeability
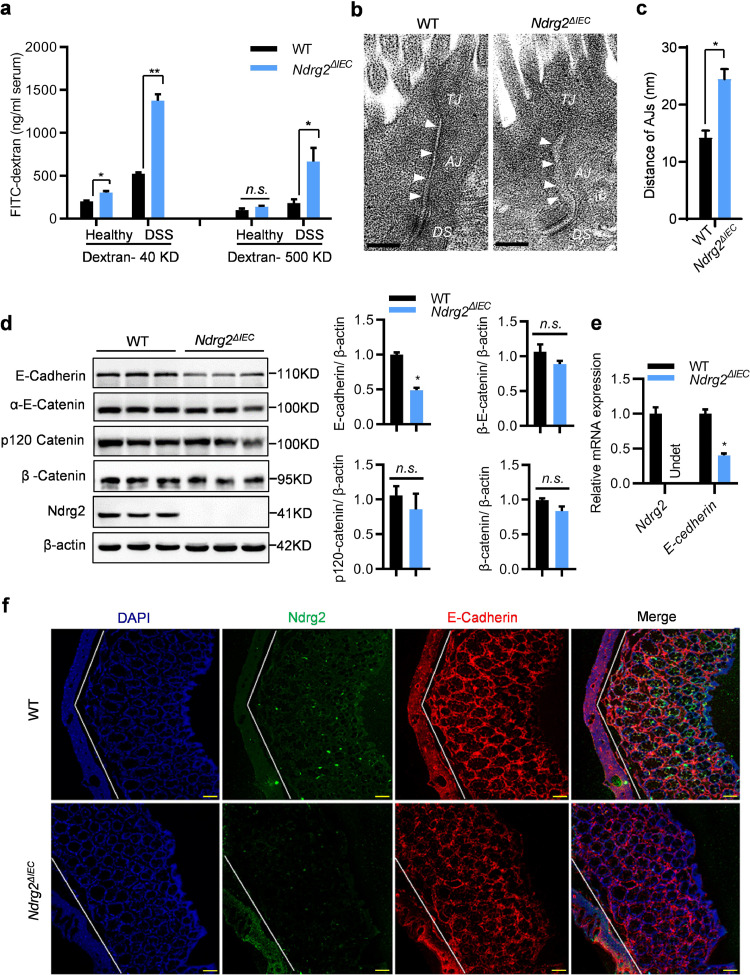
Epithelial barrier dysfunction increased gut permeability and aggravated the inflammatory response in IBD. To further investigate the role of Ndrg2 in DSS-induced colitis, we examined the gut permeability in WT and Ndrg2ΔIEC mice treated with or without DSS via FITC-dextran analysis. As shown in Fig. 4(a), even with DSS-free treatment, Ndrg2ΔIEC mice exhibited slightly increased permeability of low molecular weight dextran. As expected, DSS-treated Ndrg2ΔIEC mice showed extremely increased permeability of both low- and high-molecular-weight dextran, strongly suggesting epithelial barrier dysfunction in Ndrg2ΔIEC mice. Structural disruption of the adherens junction (AJ) and tight junction (TJ) resulted in epithelial barrier dysfunction, increased epithelial permeability and inflammation [16,17]. Next, it was very important for us to analyse the epithelial barrier structure difference between WT and Ndrg2ΔIEC mice. Using electron microscopy analysis, we noticed that Ndrg2ΔIEC mice exhibited significant AJ structural disruption but virtually no TJ structural alteration compared with WT mice (Fig. 4(b)). The widths of the AJs were markedly increased in the intestinal epithelium of Ndrg2ΔIEC mice (Fig. 4(c)), indicating intestinal epithelial AJ structure destruction in Ndrg2ΔIEC mice.
Fig. 4.
Ndrg2 deletion disrupted adherens junctions (AJs) integrity in the colonic epithelium. (a) Intestinal permeability was measured by determining serum FITC-dextran concentrations (40 KD and 500 KD, respectively) (n=8/group). (b) AJ structure detection in the colonic epithelium of WT and Ndrg2ΔIEC mice via electron microscopy. Representative image of 6 mice in each group. Bar: 200 nm. (c) Quantification of AJ width in the colonic epithelium of WT and Ndrg2ΔIEC mice (n=6/group). (d) Western blotting detection of E-Cadherin, α-E-Cadherin, p120 Cadherin, β-Catenin, Snail and Ndrg2 expression in the IECs of WT and Ndrg2ΔIEC mice (left panel, n=3/group), and band intensity quantification in the right panel. (e) qRT-PCR analysis of E-cadherin. (f) Immunofluorescence micrographs of NDRG2 (green) and E-cadherin (red) expression in the colons of WT and Ndrg2ΔIEC mice (n=6/group). Bar: 100 μm. (a), (c), (d), (e) Data are represented as the mean ± SEM. *p<0.05, **p<0.01, n.s., no significance, (Student's t test). Data are representative of 3 independent experiments.
The core components of AJ include E-cadherin and the catenin family members p120-catenin, β-catenin and α-catenin [18]. We are eager to understand the causes of the AJ destruction in the Ndrg2ΔIEC epithelium. As shown in Fig. 4(D), there was no obvious alteration of p120-catenin, β-catenin and α-catenin expression in Ndrg2ΔIEC mice (Fig. 4(d)). However, intestinal Ndrg2 loss caused a dramatic decrease in E-cadherin both in the protein and mRNA levels (Fig. 4(d) and (e)), suggesting the transcriptional regulation of E-cadherin by NDRG2. Beyond localization on AJs, cadherin and catenin family can be released from the cell membrane, undergo re-localization and function in the cytoplasm or nucleus in restricted cellular or developmental contexts. Consistent with the above results, we found that only E-cadherin was markedly decreased in the membrane component (without relocation in other cell compartment) of IECs from Ndrg2ΔIEC mice (Supplementary Figs 5 and 6). Moreover, there was no significant difference in the expression and distribution of ZO-1 (key component of TJs) (Supplementary Figs 5 and 6). Coincidently, the immunofluorescence analysis showed that E-cadherin was significantly decreased in the colonic epithelium of Ndrg2ΔIEC mice (Fig. 4(f)), suggesting that Ndrg2 loss resulted in E-cadherin downregulation in vivo. Remarkably, E-cadherin attenuation in Ndrg2ΔIEC mice was much more dramatic following spontaneous colitis with ageing (Supplementary Fig. 7(a) and (b)). These findings demonstrated that Ndrg2 deficiency disrupts AJ structural integrity in the colonic epithelium, most likely by suppressing E-cadherin expression, thus increasing colonic permeability and inflammation.
3.5. NDRG2 augments E-cadherin expression by promoting the ubiquitylation and degradation of Snail
The aforementioned results prompted us to uncover how Ndrg2 loss caused the transcriptional repression of E-cadherin. However, current studies do not support the concept of NDRG2 as a transcriptional regulator per se [9,19,20]. It is reasonable for us to further analyse whether Ndrg2 deficiency alters the expression of key transcriptional regulators of E-cadherin, such as Snail, Slug and ZEB1/2, among others [21,22]. Notably, Snail was one of the most changed regulators and increased significantly in Ndrg2-deficient IECs and mouse embryonic fibroblast (MEF) cells (Fig. 5(a)). We further confirmed the alteration of SNAIL and E-Cadherin in human colorectal cancer cells with NDRG2 knockdown (Supplementary Fig. 8(a), (b)). Next, by subjecting different FLAG-tagged NDRG2 truncations (Fig. 5(b), right panel) [9], we found that SNAIL expression was significantly decreased with full-length NDRG2 (NDRG2/FL) but abolished by NDRG2 N-terminal deletion (NDRG2/ΔN) (Fig. 5(b), left panel), suggesting a key role of N-terminal of NDRG2 for SNAIL regulation. With the chase assay of protein stability, we noticed that NDRG2/FL could markedly shorten the half-life of SNAIL protein (Fig. 5(c), (d)). In contrast, the protein stability of Snail was significantly increased in Ndrg2-deficient MEF cells (Fig. 5(e)). However, SNAIL suppression by NDRG2 was almost completely reversed by the proteasome inhibitor MG132 (Fig. 5(f)), strongly supporting that NDRG2 might promote Snail protein degradation through the ubiquitin-proteasome pathway.
Fig. 5.
NDRG2 augmented E-cadherin expression by promoting the ubiquitylation and degradation of SNAIL. (a) Western blot analysis of E-cadherin, Snail and Ndrg2 protein levels in intestinal epithelial cells (IECs) separated from WT and Ndrg2ΔIEC mice (left panel), and in mouse embryonic fibroblast (MEF) cells established from WT and CMV-Cre; Ndrg2ΔIEC mice (right panel). (b) Western blotting analysis of endogenous E-Cadherin and SNAIL protein levels in full-length NDRG2 (NDRG2/FL), N-terminal truncation (NDRG2/ΔN) and C-terminal truncation (NDRG2/ΔC) stably overexpressing HT-29 cells (left panel). Graphical representation of NDRG2/FL, NDRG2/ΔN and NDRG2/ΔC truncation mutants (right panel). (c) With 100 µg/ml cycloheximide (CHX) treatment, western blot detection of the SNAIL protein half-life in the above-mentioned cells, and quantification in (d) using ImageJ software. (e) Western blotting detection of Ndrg2 and Snail protein levels in MEF cells as mentioned above treated with CHX for the indicated times. (f) Endogenous SNAIL protein expression levels in NDRG2/FL and NDRG2/ΔN stably overexpressing HT-29 cells treated with 10 µM MG132 for the indicated time. (g) Using CHX (100 µg/ml) treatment and two siRNAs targeting FBXO11 with NC as a negative control, Western blotting analysis of SNAIL, FBXO11 and FLAG-NDRG2 protein expression was performed in parental or FLAG-NDRG2/FL overexpressing SW480 cells. (h) HEK293T cells were co-transfected with plasmids expressing HA-FBXO11, FLAG-NDRG2/FL and FLAG-NDRG2/ΔN as indicated, with EGFP as a control. The cell lysate was immunoprecipitated with FLAG antibody or IgG with anti-HA and anti-FLAG as primary antibodies for western blot analysis. (i) HEK293T cells were co-transfected with plasmids as indicated. Cell lysates were immunoprecipitated with anti-HA and anti-Myc/FLAG antibodies for western blot analysis. (j) HEK293T cells were co-transfected with plasmids expressing HA-FBXO11, Myc-Snail and V5-Ubiquitin together, together with either EGFP or FLAG-NDRG2/FL. Cells were treated with 10 µM MG132 for 6 hrs before the cell lysates were immunoprecipitated with anti-FLAG antibody, and the poly-ubiquitylated Snail protein was detected with anti-V5 antibody. All experiments were repeated at least 3 times independently.
Several reported E3 ligases are responsible for Snail ubiquitylation, including β-TRCP1/Fbxw1, Fbxo11, Fbxl14 and Fbxl5 [23,24]. Thus, we were curious to determine the E3 ligase responsible for Snail ubiquitylation mediated by NDRG2. We used an siRNA strategy to knockdown each of the E3 ligases and compared the protein stability of Snail in NDRG2 overexpression cells (data not shown). Remarkably, siRNA targeting of FBXO11 could significantly rescue the Snail protein stability inhibited by NDRG2 (Fig. 5(g), Supplementary Fig. 9). Based on this finding, we hypothesized that NDRG2 could enhance the interaction of FBXO11 and Snail to promote the ubiquitylation and degradation of Snail. The immunofluorescence assay demonstrated the cellular co-localization of NDRG2 with Snail and FBXO11, respectively (Supplementary Fig. 10). Additionally, as shown by the immunoprecipitation assay in Fig. 5(h), NDRG2/FL could interact with FBXO11 and significantly strengthen FBXO11 targeting to Snail (Fig. 5(i)) to further promote Snail ubiquitination (Fig. 5(j)). Our data demonstrated that NDRG2 could enhance E3 ligase FBXO11 with Snail to induce ubiquitin-mediated degradation of Snail, finally augmenting E-cadherin expression.
3.6. Ndrg2 loss in intestinal epithelial cells increases colitis-associated colorectal cancer
To further evaluate the role of Ndrg2 in colitis-associated tumour development, mice were subjected to azoxymethane (AOM) intraperitoneal injection followed by three cycles of the DSS-H2O rotation treatment (Fig. 6(a)). The mice were then sacrificed, and colon tissues were collected. After the treatment with AOM/DSS, both WT and Ndrg2ΔIEC mice developed colon tumours mainly in the distal to middle colon, and the Ndrg2ΔIEC mice showed a dramatic increase in tumour number and size compared with the WT mice (Fig. 6(b)). This phenotype was confirmed by HE staining, as typical cancerization was observed on the distal colon tissue slides (Fig. 6(c)). To further analyse carcinoma formation in WT and Ndrg2ΔIEC mice, we calculated the distribution, number and size of tumours and observed significant increases in tumours located in the middle and terminal colon (Fig. 6(d), (e), (f)). Moreover, the numbers of tumours with all volume scales (under 2 mm, 2–4 mm and above 4 mm) were increased in Ndrg2ΔIEC mice (Fig. 6(g), (h), (i)). Our data clearly demonstrated that intestinal Ndrg2 loss increased susceptibility to colitis-associated tumour development.
Fig. 6.
Intestinal deletion of Ndrg2 enhanced the initiation and progression of AOM-DSS-induced colorectal carcinoma. (a) The method used for the treatment of AOM-DSS in our study. (b) Tumour-bearing colon sections from WT and Ndrg2ΔIEC mice after AOM-DSS treatment. Representative images from WT (n=24) and Ndrg2ΔIEC (n=26) mice. (c) HE staining of normal epithelium and AOM-DSS induced colorectal carcinoma in WT and Ndrg2ΔIEC mice. Representative images from WT (n=24) and Ndrg2ΔIEC (n=26) mice. Bar: 100 μm. (d)–(f) Quantification analysis of tumour numbers from the whole, middle and distal colons of WT and Ndrg2ΔIEC mice. (g)–(i) Quantification analysis of tumour numbers with small size (<2 mm), middle size (2–4 mm) and big size (>4 mm) from whole colon of WT and Ndrg2ΔIEC mice. WT: n=24; Ndrg2ΔIEC: n=26. *p<0.05, **p<0.01, (Student's t test).
3.7. NDRG2 downregulation attenuates E-cadherin expression and enhances inflammation in human IBD patients
To confirm the role of NDRG2 in clinic IBD initiation, we further examined whether NDRG2 affected inflammation in human UC and CD patients (Supplementary Table 3). Interestingly, in UC patients, NDRG2 expression was significantly positively correlated with E-cadherin and negatively correlated with Snail (Fig. 7(a)(c)). Most importantly, NDRG2 expression was negatively correlated with inflammation levels and CD68+ macrophage recruitment. Higher NDRG2 expression was correlated with reduced inflammation and CD68+ macrophage recruitment at inflammatory foci, while decreases in NDRG2 expression enhanced inflammation and CD68+ macrophage infiltration (Fig. 7(a), (d), (e)).
Fig. 7.
NDRG2 positively correlated with E-cadherin and negatively correlated with Snail and inflammation in human IBD patients. (a) Immunohistochemistry of NDRG2, E-cadherin, Snail, CD68+ and HE staining of human UC tissues with mild, moderate and severe inflammation. Representative images from UC (n=19) and CD (n=5) patients. Scale bar: 100 μm. (b)–(e) Linear regression and Pearson's correlation analysis of NDRG2 with inflammation degree, E-cadherin and Snail in UC and CD samples, UC (n=19) and CD (n=5).
Taken together, our findings demonstrated that NDRG2 could positively enhance E-cadherin expression to maintain AJ structure and intestinal epithelial barrier integrity, thus suppressing colonic inflammation. However, intestinal Ndrg2 loss led to E-cadherin attenuation and AJ structure destruction, resulting in an increase in intestinal epithelial permeability, thus promoting colitis and colitis-associated tumour development (Fig. 8).
Fig. 8.
A schematic for the role of NDRG2 regulating Snail, E-cadherin and adherens junction structural integrity in colitis and colitis-associated tumour development. Intestinal Ndrg2 loss disrupts the AJ structure in the colonic epithelium by suppressing E-cadherin expression by abrogating Snail ubiquitination and degradation through E3 ligase FBXO11-dependent signalling, thus increasing colonic permeability, colitis and colitis-associated tumour development in Ndrg2ΔIEC mice.
4. Discussion
The gastrointestinal tract (GI) is chronically exposed to large numbers of foreign antigens, toxic molecules and microorganisms, and the epithelial barrier makes important contributions to GI health [4,25]. Epithelial barrier dysfunction is believed to contribute to IBD initiation and pathogenesis, mainly by damaging the structure of tight junctions (TJs) and/or adherens junctions (AJs). Previous reports have demonstrated that AJ and TJ disruption is associated with progressive colonic inflammation in human UC and CD due to increased permeability of LPS, peptidoglycan and N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP), which diffuse from the gut lumen to the lamina propria [26,27].
Our study explored the role of NDRG2 in intestinal inflammation pathogenesis and determined that NDRG2 modulated the AJ structure via FBXO11/Snail/E-cadherin signalling regulation in vivo. NDRG2 was initially discovered and demonstrated to be a tumour suppressor gene by our group. We found that NDRG2 played roles in cancer cell proliferation, differentiation and invasion [28,29]. NDRG2 expression was positively correlated with cancer prognosis and overall survival [10]. Furthermore, NDRG2 regulated EMT by controlling E-cadherin expression and participated in TGF-β-induced oncogenesis in late-stage CRC [30, 31].
E-cadherin is an important cytomembrane component and controls cell-cell adhesion by conjugating to β-catenin, α-catenin and p120-catenin complexes to modulate the spaces between epithelial cells [32,33]. Previous studies have confirmed that reduced E-cadherin aggravates DSS-induced colitis [6,34]. Our work demonstrated that Ndrg2 loss in intestinal epithelial cells caused AJ destruction but did not disrupt the tight junction structure, as determined via electron microscopic analysis. Moreover, we observed obvious reduced E-cadherin expression with no significant alterations of β-catenin or p120-catenin in Ndrg2ΔIEC mice, indicating that AJ destruction caused by Ndrg2 loss was mainly induced by E-cadherin suppression. Therefore, intestinal Ndrg2 deletion decreased E-cadherin expression and destroyed the integrity of adherens junctions to increase epithelial barrier permeability, resulting in spontaneous colitis with ageing, and increasing susceptibility to DSS and TNBS-induced colitis. Noticeably, we further found that intestinal Ndrg2 loss could promote colitis-associated tumour development, which is highly coincident with its tumour suppressor function.
Snail and Slug are believed to be the most important transcriptional repressors of E-cadherin in various types of solid tumours and tissues [35,36]. Our data showed that Snail significantly increased in Ndrg2-deficient IECs and MEF cells, suggesting that reduced E-cadherin expression by Ndrg2 loss might occur mainly through Snail upregulation. Furthermore, we first demonstrated that NDRG2 could strengthen the interaction of E3 ligase FBXO11 with Snail to promote the ubiquitination and degradation of Snail. Thus, as shown in Fig. 4 and 5, intestinal Ndrg2 deletion disrupted adherens junction structure in the intestinal epithelium by suppressing E-cadherin expression by promoting the FBXO11-Snail interaction for Snail ubiquitination and degradation, thus increasing colonic permeability, colitis and colitis-associated tumour development.
Inflammatory cell infiltration plays an important role in IBD [37], and we found that Ndrg2 loss facilitated inflammatory cell recruitment and that reduced NDRG2 expression was associated with the severe inflammatory state in clinical samples of UC patients. Most importantly, we confirmed the positive correlation of NDRG2 with E-cadherin and the negative correlation with Snail in UC samples. However, it was difficult to recognize this similar pattern in CD patients, partially due to the limited CD patient samples, which might also suggest the different function of NDRG2 in UC and CD. Ndrg2 loss-induced E-cadherin repression leads to an increase in intestinal permeability and pro-inflammatory cytokine production, thereby triggering a greater immune response to further aggravate colitis progression.
In summary, our work confirmed that NDRG2 played an important role in colitis initiation and pathogenesis by enhancing E-cadherin expression and regulating AJ structural integrity to maintain gut homeostasis. However, intestinal Ndrg2 deficiency resulted in attenuated E-cadherin expression and AJ structure disruption, finally promoting colitis and colitis-associated tumour development (Fig. 8). Thus, NDRG2 might be an important diagnostic and prognostic marker of colitis and colitis-associated colorectal cancer.
Contributors
Design of studies: JZ, JPL, LBY and KCW. Performance of the mice husbandry, DSS, AOM/DSS animal model and phenotyping: MYW, YZM, LLS. performed of the intestinal gene expression analysis and tissue dissection: YQX, ZHG, LJL, XB, HYQ. Clinical assessments: YQX, ZSL, ZW. Writing of the manuscript: JZ, MYW, YZM. All authors approved the manuscript.
Data sharing statement
Data are available upon request.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
We are thankful for the critical discussion and technical support from Professor Jing Ye.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103068.
Contributor Information
Jipeng Li, Email: jipengli1974@aliyun.com.
Jian Zhang, Email: biozhangj@fmmu.edu.cn.
Appendix. Supplementary materials
Supplementary Figure 1. Characteristics of intestine-specific Ndrg2 knockout mice (Ndrg2\elsamp #x0394;IEC). (a) Construction of the targeting vector and the wild-type allele, floxed allele and deleted allele of the mouse Ndrg2 gene. Loxp fragments were placed on both sides of exons 2 - 6 to delete them, and the neor cassette was identified for vector selection. (b) Western blot detection of Ndrg2 protein expression in brain, heart and liver tissue samples from WT and Ndrg2\elsamp #x0394;IEC mice. (c) Western blot detection of Ndrg2 protein expression in total colon tissue and IECs from WT and Ndrg2\elsamp #x0394;IEC mice respectively (n\elsamp #x003D;2/group). All experiments were repeated at least 3 times independently.
Supplementary Figure 2. Ndrg2 deficiency enhanced TNBS-induced colitis.
(a) WT and Ndrg2\elsamp #x0394;IEC mice was slowly instilled with 100\elsamp #x2009;\elsamp #x03BC;l of 3\elsamp #x2009;mg TNBS (dissolved in 50\elsamp #x0025; ethanol) into colon to induce colitis (n\elsamp #x003D;8/group). Body weight was monitored from Day 1 to Day 6. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05 (Student\elsamp #x0027;s t test). (b) Representative images of colon morphology of TNBS-induced colitis in WT and Ndrg2\elsamp #x0394;IEC mice.
Supplementary Figure 3. Ndrg2 deficiency enhanced inflammation in mice colon section. (a) WT and Ndrg2\elsamp #x0394;IEC mice were treated with 2.5\elsamp #x0025; DSS (n\elsamp #x003D;12/group). Flow cytometry analysis was performed on days 0 and 10 after DSS treatment. CD11b\elsamp #x002B; stained monocytes, CD11b\elsamp #x002B; F4/80\elsamp #x002B; stained macrophages, CD11b\elsamp #x002B; Gr-1\elsamp #x002B; stained neutrophils, and CD11b\elsamp #x002B; Siglec-F\elsamp #x002B; stained eosinophils. (b) qRT-PCR for the mRNA expression of indicated chemokines and cytokines. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05, **p\elsamp #x003C;0.01, n.s., no significance (Student\elsamp #x0027;s t test). All experiments were repeated at least 3 times independently.
Supplementary Figure 4. LPS induced inflammation in the colonic epithelium in a dose-dependent manner. WT mice were treated with ampicillin for 1 week before LPS administration, and then LPS was injected into the gut lumen at doses of 1 mg/kg and 0.1 mg/kg per mouse respectively. Intestinal epithelial cells (IECs) were isolated, and total RNA was extracted according to the manufacturer\elsamp #x0027;s instructions. (a-e) qRT-PCR for the mRNA expression of Il-1\elsamp #x03B2;, Il-6, Tnf-\elsamp #x03B1;, Cxcl-1, and Cxcl-2 expression (n\elsamp #x003D;4). Representative data of 3 independent experiments. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05, (Student\elsamp #x0027;s t test).
Supplementary Figure 5. Ndrg2 loss induces intestinal epithelial AJs (but not TJs) destruction in Ndrg2\elsamp #x0394;IEC mice. Colon coronal section from WT and Ndrg2\elsamp #x0394;IEC mice were staining for AJs marker E-cadherin (green), \elsamp #x03B2;-catenin (green), P120 Catenin (green), TJs marker ZO-1 (green) and Hoechst33258 (blue) represents nucleus. Representative data of 3 independent experiments. Scale bars indicate 10 \elsamp #x03BC;m.
Supplementary Figure 6. Ndrg2 loss caused E-cadherin attenuation in the membrane of epithelial cells without cytoplasm relocation in Ndrg2\elsamp #x0394;IEC mice. Western blotting for E-cadherin, \elsamp #x03B2;-catenin, P120 Catenin, ZO-1 and Ndrg2 expression in colon epithelial cells from WT and Ndrg2\elsamp #x0394;IEC mice. Representative data of 3 independent experiments.
Supplementary Figure 7. Intestinal Ndrg2 loss caused obvious E-cadherin attenuation in ageing mice with spontaneous colitis. (a) (Top) Schematic diagram for analyzing the occurrence of spontaneous colitis in 8 weeks age WT and Ndrg2\elsamp #x0394;IEC mice after withdrawal of ampicillin treatment for 1 week. (Bottom) Western blotting analysis of endogenous E-cadherin protein level in IECs from mice of WT and Ndrg2\elsamp #x0394;IEC mice with specific-pathogen-free (SPF) feeding condition. (b) (Top) Schematic diagram for analyzing the occurrence of spontaneous colitis in 36 weeks age WT and Ndrg2\elsamp #x0394;IEC mice after withdrawal of ampicillin treatment for 1 week. (Bottom) Western blotting analysis of endogenous E-cadherin protein level in the intestinal epithelial cells (IECs) of WT and Ndrg2\elsamp #x0394;IEC mice with specific-pathogen-free (SPF) feeding condition. Representative data of 3 independent biological replicates.
Supplementary Figure 8. NDRG2 knockdown caused the alteration of E-Cadherin and SNAILin HT-29 and Caco-2 cells. Western blotting for E-Cadherin, NDRG2 and SNAIL expression in HT-29 cells (a) and Caco-2 cells (b) with NDRG2 siRNA knockdown. Representative data of 3 independent experiments.
Supplementary Figure 9. SW480 cells were transfected using two siRNAs targeting to FBXO11 with NC as control. Western blotting was employed for the detection of endogenous SNAIL, FBXO11 and NDRG2 protein expression levels with MG132 (10 \elsamp #x00B5;M) treated for indicated time. Representative data of 3 independent experiments.
Supplementary Figure 10. NDRG2 co-localized with FBXO11 and SNAIL. FLAG-NDRG2/FL or FLAG-NDRG2/\elsamp #x0394;N were co-transfected with HA-FBXO11 or MYC-SNAIL into HEK293T cells respectively, empty vector were served as control. After transfection for 24 hours, cells were fixed and then subjected to Immunofluorescence with anti-HA antibody allowed FBXO11 visualization, anti-FLAG antibody represented NDRG2/FL or NDRG2/\elsamp #x0394;N, and anti-MYC antibody indicated SNAIL. Hoechst 33258 was used to visualize nuclei. Representative data of 3 independent experiments. Scale bars indicate 10 \elsamp #x03BC;m.
References
- 1.de Souza H S P, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14(12):739–749. doi: 10.1038/nrgastro.2017.110. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta M C, Bistritz L, Meddings J B. Alterations in intestinal permeability. Gut. 2006;55(10):1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein J, Ries J, Barrett K E. Disruption of intestinal barrier function associated with experimental colitis: possible role of mast cells. Am J Physiol. 1998;274(1 Pt 1):G203–G209. doi: 10.1152/ajpgi.1998.274.1.G203. [DOI] [PubMed] [Google Scholar]
- 4.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10(8):923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 5.Smalley-Freed W G, Efimov A, Burnett P E, Short S P, Davis M A, Gumucio D L. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Investig. 2010;120(6):1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grill J I, Neumann J, Hiltwein F, Kolligs F T, Schneider M R. Intestinal E-cadherin deficiency aggravates dextran sodium sulfate-induced colitis. Digest Dis Sci. 2015;60(4):895–902. doi: 10.1007/s10620-015-3551-x. [DOI] [PubMed] [Google Scholar]
- 7.Ha S D, Ng D, Pelech S L, Kim S O. Critical role of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3 signaling pathway in recovery from anthrax lethal toxin-induced cell cycle arrest and MEK cleavage in macrophages. J Biol Chem. 2007;282(50):36230–36239. doi: 10.1074/jbc.M707622200. [DOI] [PubMed] [Google Scholar]
- 8.Rembutsu M, Soutar M P, Van Aalten L, Gourlay R, Hastie C J, McLauchlan H. Novel procedure to investigate the effect of phosphorylation on protein complex formation in vitro and in cells. Biochemistry. 2008;47(7):2153–2161. doi: 10.1021/bi702030w. [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Qu X, Li H, Xu C, Wei M, Wang Q. NDRG2 facilitates colorectal cancer differentiation through the regulation of Skp2-p21/p27 axis. Oncogene. 2018 doi: 10.1038/s41388-017-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D, Zhang Z, Li Y, Wu L, Zhang J, Wang W. Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Mol Cancer Ther. 2011;10(1):47–56. doi: 10.1158/1535-7163.MCT-10-0614. [DOI] [PubMed] [Google Scholar]
- 11.Harris E S, Nelson W J. Adenomatous polyposis coli regulates endothelial cell migration independent of roles in beta-catenin signaling and cell-cell adhesion. Mol Biol Cell. 2010;21(15):2611–2623. doi: 10.1091/mbc.E10-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit: Int Med J Exp Clin Res. 2011;17(7):Ra164–Ra167. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani P D, Burcelin R G. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J: Off Publ Feder Am Soc Exp Biol. 2008;22(7):2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 14.Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y. Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell. 2019;179(5):1160–1176. doi: 10.1016/j.cell.2019.10.027. e1124. [DOI] [PubMed] [Google Scholar]
- 15.Pastorelli L, Dozio E, Pisani L F, Boscolo-Anzoletti M, Vianello E, Munizio N. Procoagulatory state in inflammatory bowel diseases is promoted by impaired intestinal barrier function. Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/189341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanan V, Nakata T, Desch AN, Levesque C, Boroughs A, Guzman G. C1orf106 is a colitis risk gene that regulates stability of epithelial adherens junctions. Science. 2018;359(6380):1161–1166. doi: 10.1126/science.aan0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J N, Li X, Qian J M, Lu X Q, Yang H. Effects of K-ras gene mutation on colon cancer cell line Caco-2 metastasis by regulating E-cadherin/beta-catenin/p120 protein complex formation and RhoA protein activity. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32(1):46–50. doi: 10.3881/j.issn.1000-503X.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Hartsock A, Nelson W J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Yin A, Sun X, Zhang M, Zhang J, Wang P. Deficiency of tumor suppressor NDRG2 leads to attention deficit and hyperactive behavior. J Clin Investig. 2017 doi: 10.1172/JCI94455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakahata S, Ichikawa T, Maneesaay P, Saito Y, Nagai K, Tamura T. Loss of NDRG2 expression activates PI3K-AKT signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5:3393. doi: 10.1038/ncomms4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Shi J, Chai K, Ying X, Zhou B P. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krebs A M, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 23.Diaz V M, de Herreros A G. F-box proteins: keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–79. doi: 10.1016/j.semcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Shen M, Zha Y L, Li W, Wei Y, Blanco M A. PKD1 phosphorylation-dependent degradation of SNAIL by SCF-FBXO11 regulates epithelial-mesenchymal transition and metastasis. Cancer Cell. 2014;26(3):358–373. doi: 10.1016/j.ccr.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson L W, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 26.Landy J, Ronde E, English N, Clark S K, Hart A L, Knight S C. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi K, Oyama S, Numata A, Rahman M M, Kumura H. Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions. PloS One. 2013;8(4):e62187. doi: 10.1371/journal.pone.0062187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao L, Zhang J, Liu X. NDRG2: a Myc-repressed gene involved in cancer and cell stress. Acta Biochim Biophys Sin. 2008;40(7):625–635. doi: 10.1111/j.1745-7270.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Lai X, Zhao Y, Zhang Y, Li M, Li D. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis. 2018;9(2):248. doi: 10.1038/s41419-018-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Qu X, Ma Y, Zheng J, Chu D, Liu B. Tumor suppressor NDRG2 tips the balance of oncogenic TGF-beta via EMT inhibition in colorectal cancer. Oncogenesis. 2014;3:e86. doi: 10.1038/oncsis.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y J, Kang H B, Yim H S, Kim J H, Kim J W. NDRG2 positively regulates E-cadherin expression and prolongs overall survival in colon cancer patients. Oncol Rep. 2013;30(4):1890–1898. doi: 10.3892/or.2013.2642. [DOI] [PubMed] [Google Scholar]
- 32.Bulgakova N A, Brown N H. Drosophila p120-catenin is crucial for endocytosis of the dynamic E-cadherin-Bazooka complex. J Cell Sci. 2016;129(3):477–482. doi: 10.1242/jcs.177527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler J M, Kim H C, Efstathiou J A, Ilyas M, Mortensen N J, Bodmer W F. Hypermethylation of the promoter region of the E-cadherin gene (CDH1) in sporadic and ulcerative colitis associated colorectal cancer. Gut. 2001;48(3):367–371. doi: 10.1136/gut.48.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral oncology. 2001;37(1):65–71. doi: 10.1016/s1368-8375(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 36.Hajra K M, Chen D Y, Fearon E R. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62(6):1613–1618. [PubMed] [Google Scholar]
- 37.Montagna E, Cancello G, Torrisi R, Rizzo S, Scarano E, Colleoni M. Lapatinib and metronomic capecitabine combination in an HER2-positive inflammatory breast cancer patient: a case report. Ann Oncol. 2010;21(3):667–668. doi: 10.1093/annonc/mdp563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Characteristics of intestine-specific Ndrg2 knockout mice (Ndrg2\elsamp #x0394;IEC). (a) Construction of the targeting vector and the wild-type allele, floxed allele and deleted allele of the mouse Ndrg2 gene. Loxp fragments were placed on both sides of exons 2 - 6 to delete them, and the neor cassette was identified for vector selection. (b) Western blot detection of Ndrg2 protein expression in brain, heart and liver tissue samples from WT and Ndrg2\elsamp #x0394;IEC mice. (c) Western blot detection of Ndrg2 protein expression in total colon tissue and IECs from WT and Ndrg2\elsamp #x0394;IEC mice respectively (n\elsamp #x003D;2/group). All experiments were repeated at least 3 times independently.
Supplementary Figure 2. Ndrg2 deficiency enhanced TNBS-induced colitis.
(a) WT and Ndrg2\elsamp #x0394;IEC mice was slowly instilled with 100\elsamp #x2009;\elsamp #x03BC;l of 3\elsamp #x2009;mg TNBS (dissolved in 50\elsamp #x0025; ethanol) into colon to induce colitis (n\elsamp #x003D;8/group). Body weight was monitored from Day 1 to Day 6. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05 (Student\elsamp #x0027;s t test). (b) Representative images of colon morphology of TNBS-induced colitis in WT and Ndrg2\elsamp #x0394;IEC mice.
Supplementary Figure 3. Ndrg2 deficiency enhanced inflammation in mice colon section. (a) WT and Ndrg2\elsamp #x0394;IEC mice were treated with 2.5\elsamp #x0025; DSS (n\elsamp #x003D;12/group). Flow cytometry analysis was performed on days 0 and 10 after DSS treatment. CD11b\elsamp #x002B; stained monocytes, CD11b\elsamp #x002B; F4/80\elsamp #x002B; stained macrophages, CD11b\elsamp #x002B; Gr-1\elsamp #x002B; stained neutrophils, and CD11b\elsamp #x002B; Siglec-F\elsamp #x002B; stained eosinophils. (b) qRT-PCR for the mRNA expression of indicated chemokines and cytokines. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05, **p\elsamp #x003C;0.01, n.s., no significance (Student\elsamp #x0027;s t test). All experiments were repeated at least 3 times independently.
Supplementary Figure 4. LPS induced inflammation in the colonic epithelium in a dose-dependent manner. WT mice were treated with ampicillin for 1 week before LPS administration, and then LPS was injected into the gut lumen at doses of 1 mg/kg and 0.1 mg/kg per mouse respectively. Intestinal epithelial cells (IECs) were isolated, and total RNA was extracted according to the manufacturer\elsamp #x0027;s instructions. (a-e) qRT-PCR for the mRNA expression of Il-1\elsamp #x03B2;, Il-6, Tnf-\elsamp #x03B1;, Cxcl-1, and Cxcl-2 expression (n\elsamp #x003D;4). Representative data of 3 independent experiments. Data are represented as the mean \elsamp #x00B1; SEM. *p\elsamp #x003C;0.05, (Student\elsamp #x0027;s t test).
Supplementary Figure 5. Ndrg2 loss induces intestinal epithelial AJs (but not TJs) destruction in Ndrg2\elsamp #x0394;IEC mice. Colon coronal section from WT and Ndrg2\elsamp #x0394;IEC mice were staining for AJs marker E-cadherin (green), \elsamp #x03B2;-catenin (green), P120 Catenin (green), TJs marker ZO-1 (green) and Hoechst33258 (blue) represents nucleus. Representative data of 3 independent experiments. Scale bars indicate 10 \elsamp #x03BC;m.
Supplementary Figure 6. Ndrg2 loss caused E-cadherin attenuation in the membrane of epithelial cells without cytoplasm relocation in Ndrg2\elsamp #x0394;IEC mice. Western blotting for E-cadherin, \elsamp #x03B2;-catenin, P120 Catenin, ZO-1 and Ndrg2 expression in colon epithelial cells from WT and Ndrg2\elsamp #x0394;IEC mice. Representative data of 3 independent experiments.
Supplementary Figure 7. Intestinal Ndrg2 loss caused obvious E-cadherin attenuation in ageing mice with spontaneous colitis. (a) (Top) Schematic diagram for analyzing the occurrence of spontaneous colitis in 8 weeks age WT and Ndrg2\elsamp #x0394;IEC mice after withdrawal of ampicillin treatment for 1 week. (Bottom) Western blotting analysis of endogenous E-cadherin protein level in IECs from mice of WT and Ndrg2\elsamp #x0394;IEC mice with specific-pathogen-free (SPF) feeding condition. (b) (Top) Schematic diagram for analyzing the occurrence of spontaneous colitis in 36 weeks age WT and Ndrg2\elsamp #x0394;IEC mice after withdrawal of ampicillin treatment for 1 week. (Bottom) Western blotting analysis of endogenous E-cadherin protein level in the intestinal epithelial cells (IECs) of WT and Ndrg2\elsamp #x0394;IEC mice with specific-pathogen-free (SPF) feeding condition. Representative data of 3 independent biological replicates.
Supplementary Figure 8. NDRG2 knockdown caused the alteration of E-Cadherin and SNAILin HT-29 and Caco-2 cells. Western blotting for E-Cadherin, NDRG2 and SNAIL expression in HT-29 cells (a) and Caco-2 cells (b) with NDRG2 siRNA knockdown. Representative data of 3 independent experiments.
Supplementary Figure 9. SW480 cells were transfected using two siRNAs targeting to FBXO11 with NC as control. Western blotting was employed for the detection of endogenous SNAIL, FBXO11 and NDRG2 protein expression levels with MG132 (10 \elsamp #x00B5;M) treated for indicated time. Representative data of 3 independent experiments.
Supplementary Figure 10. NDRG2 co-localized with FBXO11 and SNAIL. FLAG-NDRG2/FL or FLAG-NDRG2/\elsamp #x0394;N were co-transfected with HA-FBXO11 or MYC-SNAIL into HEK293T cells respectively, empty vector were served as control. After transfection for 24 hours, cells were fixed and then subjected to Immunofluorescence with anti-HA antibody allowed FBXO11 visualization, anti-FLAG antibody represented NDRG2/FL or NDRG2/\elsamp #x0394;N, and anti-MYC antibody indicated SNAIL. Hoechst 33258 was used to visualize nuclei. Representative data of 3 independent experiments. Scale bars indicate 10 \elsamp #x03BC;m.