Abstract
The recent developments in the market and craft beer industry in Italy have led to an increasing demand for local raw materials, such as barley malt and hops. Few works have been addressed to evaluate suitability and productivity of hop in semi-arid Italian environments. With this purpose, two experiments were carried out in 2018 and 2019, testing the suitability to cultivation of three commonly used hop varieties (Cascade, Chinook and Nuggett) in two typical semi-arid environments in Sicily. Phenological stages were also evaluated, and GDDs accumulated in vegetative and reproductive stages were calculated according to 9 different methods, dealing with three Tbase temperatures (0 °C, 5 °C and 10 °C) and with different adjustments of daily thermal sums for Tmax values >30 °C. The obtained hop cones were used to prepare small batches of beer (fresh hop American Pale Ale), further submitted to sensory analysis through a panel test.
The results have shown a high variability of yields and biometrical characteristics of the tested hop varieties according to the cropping management. However, a definite advantage showed up for the earliest maturing variety (Chinook), that allowed cones yield of 41 g per plant (d.m.) and biomass yield of 143 g per plant (d.m.). The sensory analyses assessed the excellent organoleptic characteristics of the obtained hop, as well as of the beer produced. Hop demonstrates to be a promising crop for semi-arid Mediterranean environments, although many aspects of cropping technique are still to be pointed out.
Keywords: Craft beer, Mediterranean agro-ecosystems, Thermal sums, Development stages, Agricultural science, Agronomy, Crop production, Crop quality, Crop yields
Craft beer; Mediterranean agro-ecosystems; Thermal sums; Development stages; Agricultural science; Agronomy; Crop production; Crop quality; Crop yields
1. Introduction
Hop (Humulus lupulus L.; fam. Cannabaceae) is a dioecious twining herb of ancient domestication and use, spread in the wild throughout almost all temperate climate ranges (Biancardi and Wagner, 1989). Although the species has a long history as an edible and medicinal plant in many areas of the world (di Tizio et al., 2012; Shishehgar et al., 2012), hop primary destination presently is brewing industry (Gresser, 2010). As a specialized culture, hop first established in central Europe, and then spread to almost all historically brewing countries. More recently, the dynamics of global trade have greatly encouraged the expansion of hop growing area, now spanning between the 35° and the 55° parallel of both hemispheres (Biancardi and Wagner, 1989). In the modern brewing industry, hop is a crucial element for the characterization of brewing products. In fact, besides giving beer the typical bitter and aromatic notes, hop is an important co-factor in several processes of stabilization of the finished product. Due to the reaction between bitter acids and wort proteins, hop allows for the clarification of the beer, stabilizes the foam, and is also a natural preservative (Gresser, 2010).
Hop breeding has historically been focused on improving alpha-acids content and resistance to the main diseases, starting from the clonal selections obtained from wild hops, and then crossed with European noble landraces (Patzak et al., 2010). Researches on hop breeding have also led to the requalification of taxonomic varieties (Knobloch et al., 1982; Turner et al., 2011), such as H. lupulus var. pubescens and var. neomexicanus. Those studies aimed at selecting new varieties able to satisfy the needs of organic and low-input systems (Turner et al., 2011). In fact, H. lupulus var. pubescens and var. neomexicanus are wild genotypes mainly diffused in the Midwestern US, even more utilized in breeding research due to the excellent characteristics of adaptability and productivity conferred to the new obtained cultivars to be grown in dry and warm environments. The adoption of these new varieties therefore seems to suggest the possibility to extend hop cultivation also to semi-arid and warm-arid environments, such as the US states of California, Arizona, Colorado and New Mexico (Carr, 2017).
In Italy, as reported by Pignatti (1982), wild hops are widely diffused in the Northern temperate areas. Thus, systematic studies have been made on the adaptability of hop commercial landraces to the growing conditions of Northern regions of the country. In fact, Northern Italy appeared particularly suitable to the cultivation of finishing, aromatic hops like Bramling Cross, Magnum, Marynka and Tettnanger (Mongelli et al., 2015, 2016). So, even though hop cultivation in Italy still covers a rather small area (Carbone and Cherubini, 2016), there is no shortage of examples of highly developed and virtuous hop production facilities. A recent research also confirmed the excellent adaptability of hops to the climatic conditions of central Italy, with special concern to the Hallerteau Magnum, Hallerteau Spat, Yeoman and Cascade varieties (Rossini et al., 2016). Moreover, with reference to the cv Cascade, Forteschi et al. (2019) have assessed that hops obtained from Sardinian hopyards were dealing with quality characteristics comparable to those from the United States.
Being hop a climbing species, it is grown by means of supporting structures (trellises), where the plants are allowed climbing on ropes or strings, usually 4 to 6–8 m high (Biancardi and Wagner, 1989). Such management enables hop plants, especially the newest ameliorated varieties, to fully express their growth (and ultimately yield) potential. However, besides being expensive to build, the traditional trellises are more labour-consuming in cultivation operations (such as pest control and harvest), and require a large amount of water and nutrients for a full plants' establishment. Hence, starting from the early 1990s, first in the US (Neve, 1991) and later on in the UK (Darby, 2005) new hop growing systems were developed with the aim of cultivating the traditional varieties on shorter (2–3 m) wireworks. The advantages of these new “low-trellis” facilities are many: besides being less expensive and labour-consuming, they can allow a more sustainable and environmentally friendly hop cultivation (Turner et al., 2011). Thanks to the higher plant wall density and reduced height, treatments against pests and diseases can be carried out more efficiently and with a reduced pesticide drift into the environment (Neve, 1991; Darby, 2005; Seigner et al., 2009; Turner et al., 2011); furthermore, in such facilities, a higher population density of useful arthropods was found (Lilley et al., 1999), making the biological control of many insects and mites easier and more effective (Darby, 2005). For these reasons, low-trellis hopyards are increasingly used in Europe, and breeding of specific “dwarf” varieties, expressly addressed to the cultivation in these systems, has been promoted in many countries (Seigner et al., 2009). In warm and semiarid climates of Sicily and southern Italy, where water shortage and severe climatic constraints often set a limit to the cultivation of many crops, and the high-yielding hop genotypes can unlikely express their full yield potential, a reduced input growing method seems particularly appealing.
Hence the need for further investigations, aimed at highlighting the potential of hop, in terms of productivity and adaptability, to the climatic conditions of the Italian southern regions.
Heat units, expressed as Growing Degree-Days (GDD) are one of the most widespread and useful methods to study and describe the onset and progress of development stages in crops (Gilmore and Rogers, 1958; McMaster and Wilhelm, 1997; Bonhomme, 2000). The method has the merit to merge air temperatures values (in °C) and duration of plant growth stages (in days) in a unique indicator, allowing a more accurate description of phenological events than the two measurements (temperature and duration) can do alone. Many examples of application of GDD calculation method are available in the literature, concerning a large number of crops including trees (Ruml et al., 2012; Reighard and Rauh, 2015) and field crops (Narwal et al., 1986; Rajput et al., 1987; Kumar et al., 2008; Islam and Sikder, 2011). In the USA, weather reports including GDDs are created and broadcast by the National Oceanic and Atmospheric Administration (Miller, 2018; NOAA, 2020). Much research has been carried out, furthermore, with the goal to use GDDs for practical purposes, including the assessment of plants' susceptibility to diseases and the prediction of pest activity (Herms, 2004). One of the most studied topics has concerned the individuation of the best Tbase value for each crop (Yang et al., 1995). Of course, the accuracy level of GDD calculations, hence their predictive ability, will vary according many factors, among which the studied species, the phenological stage of interest, and the chosen calculation method. Some methods, for example, try to consider the biological effect of temperatures higher than a definite threshold value, by simply excluding them from calculations, or by subtracting the exceeding number of degrees, that, in this way, do not contribute to the composition of heat units; the assumptions are that, in the first case, temperatures exceeding that threshold value do not induce any plant growth, or that, in the second case, they even can be detrimental to plant growth (Baldoni and Giardini, 2002).
The GDD method relies on the general concept that a linear relationship exists between air temperature and plant development rate (Roltsch et al., 1999). Actually, much research has demonstrated that this linearity does not always apply throughout the entire cycle of many crops, above all perennials or however characterized by a long cycle (Zhou and Wang, 2018), and this relationship may take sometimes different non-linear shapes according to different parameters among which the considered range of temperatures (Bonhomme, 2000). Hence, there is general agreement on the necessity to perform different calculations with varying the development stage in order to have the best fitting of data (Del Pozo et al., 1987; Slafer and Savin, 1991).
Unlikely many other crops, there are few studies related to the calculation of growing degree days (GDD) for hop's main phenological phases, and, as far as we know, none of them was devoted to the study of this topic in Mediterranean semi-arid environments. Srečec et al. (2008) used GDD calculations to discuss the effect of weather pattern on several aspects of hop production. Rossini et al. (2016) were interested in the evaluation of 20 commercial hop genotypes screened during three growing seasons (2013–2015) in an experimental hopyard in central Italy. The study aimed at analyzing the differences between the varieties, by taking into account the growth and yield parameters but also plant phenology. They found out a negative correlation between the cones yield and the GDD to harvest (r = −0.5, P ≤ 0.05). In addition to that, Ruggeri et al. (2018) investigated the possible correlation between the number of shoots per hop plant and the GDD to shoots emergence. In that experiment, the number of shoots was found to be negatively correlated to GDD's accumulation, but the authors brought up the interesting conclusion that early sprouting genotypes could be the most productive in the Mediterranean climatic conditions. Therefore, hop GDD accumulation pattern is confirmed a topic that still needs deepening, given the applicative possibilities derived from its use.
In the last decade, the production and consumption of craft beer in Italy have exhibited a remarkable increase (Assobirra, 2018). This has caused an increase in the number of the business operators, namely craft breweries, brewpubs and beerfirms. These enterprises have shown even deeper interest in the use of local raw materials, able to enhance product quality and the linkage to the territory. Indeed, the development of regionally adapted hops cultivars and suitable cropping techniques could give a strong impulse to the emerging craft brewing sector, meeting the demand for locally produced foods and associated gastrotourism opportunities.
Hence, research was conducted with the aim of evaluating the overall agronomic performance of hop cultivated in the southern Mediterranean environment, also giving information about some aspects of technical plant management, i.e. the quality of starting plant material and the cultivation method.
During the trials, all collected data about plants' development were used to identify a proper GDD accumulation model.
Finally, through the production of a small experimental batch of beer, the technological characteristics and quality of the Cascade and Chinook hops - locally harvested - were investigated.
2. Materials and methods
2.1. Experiment 1
The first experiment was carried out in 2018 and 2019 on three American hop varieties (Cascade, Chinook and Nugget). The trial took place at the “Orleans” farm (Palermo, Italy, 38°06′27″ N; 13°21′01″ E; 0 m a.s.l.), belonging to the Department of Agricultural, Food and Forest Sciences (D/SAAF) of the University of Palermo. The climatic patterns during the cultivation periods of both years were characterized by reduced rainfall and high summer temperatures (maximum values always >30 °C) (Figure 2.1.1).
Figure 2.
Ten-days values of rainfall and temperatures recorded at “Sparacia” farm (Cammarata, AG, Italy) throughout the trial.
For the purpose of the research a single hop row was built, with supporting structures represented by iron poles 2.5 m high. Support for vertical plant growth was provided by coconut fibre ropes. Five plants per variety, at the development stage of 4–6 true leaves (about 10 cm high), were used for the experiment. Each plant was placed in a 40-litre pot, filled with a brown peat and perlite (3:2) mixed substrate, with a deep draining layer composed of expanded clay and gravel (1:1). All plants were purchased from a specialized nursery based in Northern Italy (Mr Hops, San Martino di Lupari, PD). In the first year, the cultivation was started on April 9th 2018, while in the second year it was started on May 6th 2019. In both years, a supporting mineral fertilization was applied on plants by means of a ternary fertilizer (NPK 15:7:21), also containing microelements (Mg, Fe and B, at amounts of 2%, 0.1% and 0.01%, respectively). A localized irrigation system was set up with a single dripline along the row. The system was equipped with a closing valve and a pressure gauge to control the working pressure and water flow, allowing to supply the irrigation volumes according to crop requirements. Frequent shifts and reduced dispensing intensity were adopted in order to ensure available water amount close to field capacity.
2.2. Experiment 2
A second trial was conducted in 2019, with the purpose to compare the performance of one hop variety (Cascade), cultivated with two contrasting cropping systems (pots in Palermo, PA, and open field in Cammarata, AG). In both locations, micropropagated plants from two different sources were compared, in order to detect differences in growth response due to origin of plant material.
Two different experimental hopyards were built, being the first one in the “Orleans” didactic farm (Palermo, Italy, 38°06′27″ N; 13°21′01″ E; 0 m a.s.l.), and the second one in the “Sparacia” experimental farm (Cammarata, AG, Italy, 37°38′07″ N; 13°45′47″ E; 450 m a.s.l.), both belonging to the Department of Agricultural, Food and Forest Sciences (D/SAAF) of the University of Palermo. Both environments have a similar predominant climatic pattern, classed as warm Mediterranean (Rivas-Martínez et al., 2011), with rainfall usually distributed between winter and early spring, high summer temperatures (maximum values always >30 °C) and mild temperatures in winter (rarely falling below 0 °C). In the first location (Orleans), each plant was placed in an 8-litre pot, filled with a brown peat and perlite (3:2) mixed substrate, with a deep draining layer composed of expanded clay and gravel (1:1). Pots were aligned in a single row, with supporting structures represented by iron poles 2.5 m high. Support for vertical plant growth was provided by coconut fiber ropes. Ten healthy hop plants (cv Cascade), at the development stage of 4–6 true leaves (about 10 cm high) were used for the experiment. Five plants had been purchased from a specialized nursery based in Northern Italy (Mr Hops, San Martino di Lupari, PD), whereas the five plants left had been micropropagated, from plant material obtained in the preceding cultivation year, in the laboratories of the SAAF Department. The cultivation was started on June 24th 2019. A supporting mineral fertilization was applied by means of a ternary fertilizer (NPK 15:7:21), also containing microelements (Mg, Fe and B, at amount of 2%, 0.1% and 0.01%, respectively). In the “Sparacia” hopyard, plants were grown directly in field, using supporting structures made up of iron poles 2.6 m high, planted into the ground to set up two different rows. Distances of 3 m between rows and 1.5 m between plants in the row were adopted to allow mechanical weeding.
Soil texture analysis was carried out in the laboratories of the SAAF Department (UNIPA) using the gravimetric method. The soil was classified as an Aridic Haploxerert (Baldoni and Giardini, 2002), dealing with an average composition of 10%, 30% and 60% in sand, silt and clay, respectively (Table 1). Organic matter content was determined by the Tinsley method (Tinsley, 1950). The nitrogen content (N) of soil was determined by the Kjeldahl method (Helrich, 1990). The phosphorus (P) of the soil was obtained by extraction with sodium bicarbonate (Olsen et al., 1954). Water content at field capacity and wilting point were determined by the use of the Richards chamber (Baldoni and Giardini, 2002), by applying to the soil sample pressures of 0.33 kPa and 1500 kPa respectively. Hydraulic conductivity (ks) was obtained by the laboratory constant-head method (Stolte, 1997).
Table 1.
Main physical and chemical characteristics of the soil used for hop cultivation in Sparacia (Cammarata, AG, Italy) in 2019.
| Sand (%) | 10 % |
| Silt (%) | 30 % |
| Clay (%) | 60 % |
| Soil Bulk Density (g/l) | 1.04 g/l |
| Water Field Capacity (%) | 38.71 % |
| Permanent Wilting Point (%) | 22.00 % |
| Hydraulic conductivity (m/h) | 0.25 m/h |
| Organic matter (‰) | 7 ‰ |
| Total N (%) | 0.1% |
| Available P (ppm) | 76 ppm |
The study involved twenty hop plants (cv Cascade); in this case also, ten plants had been propagated by a specialized nursery in northern Italy, and the other ten plants had been micro-propagated in the D/SAAF laboratories. The cultivation began on June 21th 2019. A pre-transplant fertilization was carried out, burying about 5 kg of ripe bovine manure at the depth of 30–40 cm for each plant pocket. During the growing season, two shallow mechanical interventions (about 5 cm in depth) were done by means of a self-propelled tiller, in order to contain the development of weeds, mainly represented by Ecballium elaterium (L.) A. Rich, Picris echioides (L.) Holub, and Polygonum aviculare (L.).
In both localities, daily values of rainfall and temperatures (Figure 1; Figure 2) throughout the trial were obtained from the Agrometeorology Service Network of the Sicilian Region (SIAS, 2020). Due to the scarce rainfall throughout the hop growth season, a localized irrigation was necessary. Hence, in both hopyards, localized irrigation systems were set up with driplines for every row; each block was then equipped with a closing valve and a pressure gauge to control the working pressure. Irrigation was supplied according to crop requirements. Frequent shifts and reduced dispensing intensity were adopted in order to ensure a quantity of available water close to field capacity.
Figure 1.
Ten-days values of rainfall and temperatures recorded at “Orleans” (Palermo, PA, Italy) farm during 2018 and 2019 growing seasons.
2.3. Field measurements
Throughout both trials, the plants were periodically checked to monitor their phytosanitary and developmental conditions. The harvest period was assessed through the evaluation of the cones' dry matter content, proceeding to the harvest once they reached a 20% dry matter (d.m.) value (Calderwood and Post, 2015). At harvest time, the total biomass obtained for each plant was weighed, and sorted by useful biomass (hop cones) and residual epigeal biomass (annual stems and leaves). Representative samples of each plant fraction were dried in stove at 104 °C for 24 h, so obtaining the respective dry matter content, further reported in percent. All botanical fractions were therefore reported into dry matter values.
2.4. Phenological observations and calculation of GDDs
The beginning and end dates of the main phenological stages were determined by visual assessment according to the BBCH scale, as obtained from the German Federal biological Research Centre for Agriculture (Rossbauer et al., 1995, Table 2).
Table 2.
BBCH identification keys for development stages in hop (from Rossbauer et al., 1995, modif.).
| Growth stage | Code | Description |
|---|---|---|
| 1: leaf development | 1.1 | First pair of leaves unfolded |
| 1.2 | 2nd pair of leaves unfolded (beginning of twining) | |
| 1. | Stage continuous till… | |
| 1.9 | 9 and more pair of leaves unfolded | |
| 2: formation of side shoots | 2.1 | First pair of side shoots visible |
| 2.2 | 2nd pair of side shoots visible | |
| 2. | Stage continuous till… | |
| 2.9 | 9 and more pair of side shoots visible (secondary side shoots occur) | |
| 3: elongation of bines | 3.1 | Bines have reached 10% of top wire height |
| 3.2 | Bines have reached 20% of top wire height | |
| 3. | Stage continuous till… | |
| 3.8 | Plants have reached the top wire | |
| 3.9 | End of bine growth | |
| 5: inflorescence emergence | 5.1 | Inflorescence bud visible |
| 5.5 | Inflorescence bud enlarged | |
| 6: flowering | 6.1 | Beginning of flowering: about 10% of flowers open |
| 6.4 | About 40% of flowers open | |
| 6.5 | Full flowering: about 50% of flowers open | |
| 6. | Stage continuous till… | |
| 6.9 | End of flowering | |
| 7: development of cones | 7.1 | Beginning of cone development: 10% of inflorescences are cones |
| 7.5 | Cone development half way: all cones visible, cones soft, stigmas still present | |
| 7.9 | Cone development complete: nearly all cones have reached full size | |
| 8: maturity of cones | 8.1 | Beginning of maturity: 10% of cones are compact |
| 8.2 | 20% of cones are compact | |
| 8. | Stage continuous till… | |
| 8.5 | Advanced maturity: 50% of cones are compact | |
| 8.8 | 80% of cones are compact | |
| 8.9 | Cones ripe for picking: cones closed; lupulin golden; aroma potential fully developed |
For identification of development stages, surveys were taken every three days on all plants in both years and locations. For each development stage, the growing degree days (GDDs), were calculated accordingly, considering as the starting date of heat accumulation the day of completion of the phase 1.2 (according to BBCH scale, 2nd pair of leaves unfolded), set equal to the transplant date. All calculations were performed according to the general formula:
| (1) |
where:
i and k: starting and ending date of each growth stage, i.e. first and last days of measurement, respectively;
Tavg: daily average temperature, and Tbase: base temperature, i.e. temperature value below which plant growth is assumed to be zero.
Tavg was calculated based on daily temperature measurements, as:
| Tavg = (Tmax -Tmin)/2 | (2) |
where:
Tmin: minimum day temperature; when Tmin < Tbase, then Tbase should be used for calculation. This however never happened in either trial seasons, occurring in late spring and summer;
Tmax: maximum day temperature; a ceiling value of Tmax = 30 °C was set; in IV, VI and VIII calculation methods, when Tmax>30 °C, then 30 °C was used for calculation; in V, VII and IX methods, when Tmax>30 °C, then the value T = 30 – (Tmax-30) was used for summation.
The nine tested GDDs calculation methods are summarized in Table 3.
Table 3.
GDD's calculation methods adopted in the trials. Tbase: base temperature; Tmax: maximum daily temperature.
| Methods | Tbase | Tmax |
|---|---|---|
| I | 0 °C | |
| II | 5 °C | |
| III | 10 °C | |
| IV | 0 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C |
| V | 0 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C-(Tmax – 30 °C) |
| VI | 5 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C |
| VII | 5 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C-(Tmax – 30 °C) |
| VIII | 10 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C |
| IX | 10 °C | If Tmax ≤ 30 °C, then Tmax; if Tmax > 30 °C, then 30 °C- (Tmax - 30 °C) |
2.5. Sensory evaluation
The technological and quality characteristics of hops obtained from the cv Cascade and Chinook were investigated through the production of two small experimental batches of beer. Fresh cones were used to brew two single-hop Pale Ale.
The aim of the beer design was to enhance as much as possible the aromatic qualities of the hop cones used. Weyermann Pilsner malt was used only. The mash phase was carried out with a single-step temperature of 70 °C, followed by a boil of 60 min. Simcoe hops (0.5 g L−1 for 10 IBU) were added at the beginning of boil phase for the bitterness, while the fresh cones (100 g L−1 for an estimated IBU of 15) were finally added during the whirlpool phase. The worts were both chilled down to 20 °C and so fermented by a neutral strain of S. cerevisiae (Fermentis US-05) at 17 °C for 7 days. The maturation phase lasted two weeks at a temperature of 20 °C, followed by a lagering phase at 0 °C for 3 days, before proceeding with bottling. Both test batches were refermented in bottle with the addition of fresh yeast and sucrose (4.2 g L−1), until reaching 1.90 volumes of CO2. Thus, once reached the target volume of carbonation, two panel tests were set up for each type of beer.
The main parameters and information concerning the two batches are shown in Table 4.
Table 4.
Details of the experimental batches brewed for the sensory evaluation trials.
| Recipe details | |||||
| Batch size | Boil time | IBU | EBC | OG | FG |
| 20 l | 60′ | 25 IBUs | 12 EBC | 1.050 | 1.012 |
| Fermentables | Amount | % | |||
| Weyermann Pilsner | 5.12 kg | 100 % | |||
| Hops | |||||
| Name | Amount | Time | Use | Form | AA % |
| Simcoe | 0.5 g L−1 | 60′ | Bitterness | Pellet | 9.8 |
| Cascade/Chinook | 100 g L−1 | Whirlpool | Aroma | Fresh cones | n/d |
| Yeast | |||
| Name | Lab | Attenuation | Temperature |
| US-05 | Fermentis | 75–80 % | 17–22 °C |
| Water profile | |
| Minerals | Amount |
| Ca2+ | 146 ppm |
| Mg2+ | 2 ppm |
| Na+ | 10 ppm |
| SO42- | 144 ppm |
| Cl- | 155 ppm |
A panel test was then set up to determine the main aromatic and flavor traits. According to the experimental model proposed by Sortino et al. (2017), the experimental batch was administered to a group of 19 moderately trained panelists. The evaluation sheet has been divided into three parts (aroma, taste and aftertaste intensity) in order to carry out a screening of the main quality characteristics of the local hops adopted.
The following discontinuous scale of values was then adopted: 1 (absence of sensation), 2 (barely recognizable), 3 (very weak), 4 (weak), 5 (light), 6 (moderate), 7 (intense), 8 (very intense) and 9 (extremely intense) (Sortino et al., 2017).
2.6. Statistical analysis
All data collected, including yield and biometric measurements, and GDD values obtained by means of all calculation methods, were subjected to variance analysis (ANOVA) using the Minitab statistical software (version 19.2.0.0). The GLM (General Linear Model) procedure was used in all cases, setting as dependent variables the measured data (cones yield, plant height, and plant biomass) obtained in either experiment. In the first experiment, the factors “variety” (Cascade, Chinook, and Nugget) and “year” were set as independent variables, whereas in the second experiment the independent variables were “locality” (Sparacia and Orleans) and “origin of plant material” (purchased - “Extra” - and managed by the UNIPA laboratories – “SAAF”). When the ANOVA offered statistically significant results, the differences between mean values were appreciated through the Tukey's test (P ≤ 0.05) (Gomez and Gomez, 1984).
The studies on GDDs were made on pooled data. In order to identify the GDD methods with the best fitting with the hop phenological data, coefficients of variation (CV%) and coefficients of quartile variation (CQV%) for each method were determined. CV% was calculated according the classical formula CV% = σ/μ x 100, being σ: standard deviation and μ: mean. CQV% was calculated as CQV% = (Q3 – Q1)/(Q3+Q1) x 100, where Q3 and Q1 are the values corresponding to the 3rd and the 1st quartile, respectively, of each dataset, being the GDD values calculated by means of each method representing one dataset (Bonett, 2006). Moreover, the inherent variability of each method was explored by means of a bivariate ANOVA, including “variety” and “year” as fixed factors. With this purpose, since the distribution of data suggested non-normality, a preliminary Ryan-Joiner's test for normality was run for each GDD dataset, and whenever normality hypothesis was rejected, data were submitted to Box-Cox transformation according to the formula Y’ = 1/√(Y). All the above procedures are implemented in the Minitab package.
Regression analysis was used to study the trend of all calculated GDDs as a function of the observed durations of all vegetative and reproductive stages of hop.
Finally, in order to identify any significant relation between the different measured traits, a simple correlation matrix was built up, by calculating Pearson's correlation coefficients between yield of cones and total epigeal biomass, by one side, and the GDDs accumulated in each main phenological stage and during the entire crop cycle, by the other side.
3. Results and discussion
3.1. Varietal response
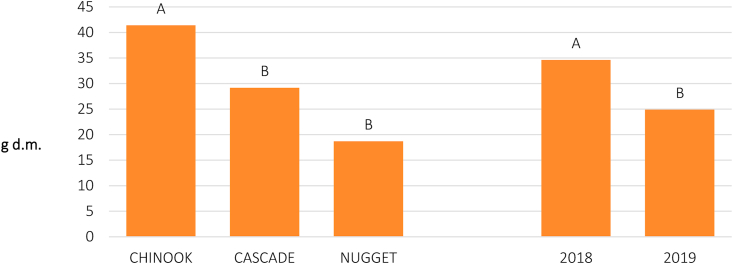
Dry cones' yield (d.m.) was significantly different both between the tested varieties (P ≤ 0.001) and between the two years of experimentation (P ≤ 0.05; Table 5). The ANOVA shows that both independent variables acted additively, since no significant “Variety x Year” interaction was detected. The cv Chinook, with an average production of 41 g of dry cones per plant (corresponding to 204.1 g of fresh material) was the most productive, whereas the cvs Nugget and Cascade showed lower production levels (18.7 and 29.1 g of d.m per plant, and 89.4 and 105.1 g of fresh weight, for the two varieties, respectively). In 2018, higher yields were recorded compared to 2019 (Figure 3).
Table 5.
Results of ANOVA (F values) for yield of dry cones (g d.m.) and one plant weight (g d.m.) of three hop varieties in a two-year trial at “Orleans” farm (Palermo, Italy).
| Source | DF | Dry Cones Yield (g d.m.) | Dry Plant Weight (g d.m.) |
|---|---|---|---|
| Variety | 2 | 12.21∗∗∗ | 24.88∗∗∗ |
| Year | 1 | 6.76∗ | 33.57∗∗∗ |
| Variety∗Year | 2 | 3.18 n.s. | 1.00 n.s. |
| Error | 24 | ||
| Total | 29 |
∗, ∗∗, ∗∗∗: significant differences at P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, respectively; n.s.: not significant.
Figure 3.
Cones yield (g of d.m.) obtained in a 2-year cultivation trial of 3 hop varieties at “Orleans” farm (Palermo, PA, Italy); mean values by variety (on the left) and by trial year (on the right). For each group of means, bars marked by the same letter are not significantly different at P ≤ 0.05 (Tukey's test).
The variance analysis carried out for the dry weight of the plants (annual stems and leaves) evidenced significant differences between varieties (P ≤ 0.001; Table 5; Figure 4). In fact, the plant biomass produced by the Chinook variety (143 g of dry matter per plant) was significantly higher than in the other two varieties. Also, compared to 2019, a significantly (P ≤ 0.001) higher biomass production was recorded in 2018 (Table 5; Figure 4).
Figure 4.
Plant weight (g of d.m.) obtained in a 2-year cultivation trial of 3 hop varieties at “Orleans” farm (Palermo, PA, Italy); mean values by variety (on the left) and by trial year (on the right). For each group of means, bars marked by the same letter are not significantly different at P ≤ 0.05 (Tukey's test).
Hence, it is possible to state that hop productivity in the Mediterranean environment is strongly influenced by the variety chosen. This conclusion is based on the significantly higher average cones yield and total epigeal biomass obtained by Chinook variety, while the remaining two genotypes showed significantly lower production levels (Figures 3 and 4).
3.2. Growth condition and propagation material
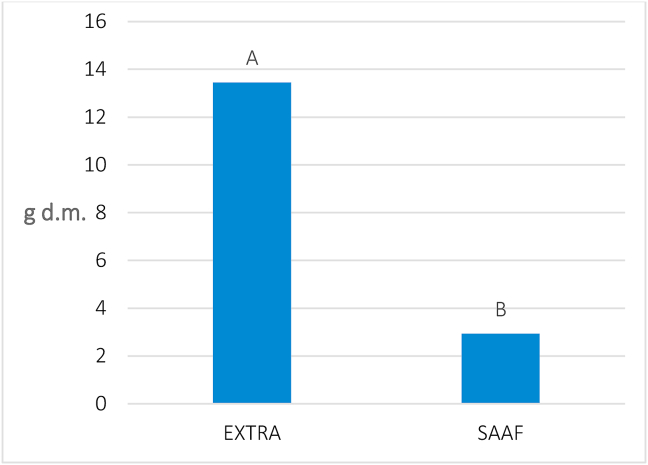
In 2019, the yield of dry cones appeared statistically different (P ≤ 0.05) as a function of the origin of the propagation material used (Table 6), whereas no difference showed up between the two localities. Irrespective of their origin, plants of the cv “Cascade” allowed obtaining 52.9 g of fresh cones in the “Orleans” farm and 45.4 g in the “Sparacia” farm, corresponding to 13.1 and 10.4 g of dry matter, respectively. Otherwise, the purchased plants produced, on average, more than four times cone yield than the micropropagated plants of the same variety obtained from the SAAF laboratories (13.5 vs 3.3 g d.m., respectively; Figure 5).
Table 6.
Results of ANOVA (F values) for yield of dry cones (g d.m.) and one plant weight (g d.m.) of hop variety “Cascade” cultivated in 2019 in pots at “Orleans” farm (Palermo, Italy) and in open field at “Sparacia” farm (Cammarata, Italy).
| Source | DF | Dry Cone Yield (g d.m.) | Dry Plant Weight (g d.m.) |
|---|---|---|---|
| Locality | 2 | <1 n.s. | 24.57∗∗∗ |
| Origin of plant material | 1 | 7.92∗ | 12.45∗∗∗ |
| Locality∗Origin | 2 | - | 5.08∗ |
| Error | 23 | 28 | |
| Total | 25 | 31 |
∗, ∗∗, ∗∗∗: significant differences at P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, respectively; n.s.: not significant.
Figure 5.
Cones yield (g of d.m.) obtained in a cultivation trial of hop variety “Cascade” in 2019. Mean values of the two localities, by the origin of propagation material (“Extra”: purchased; “SAAF”: managed by the UNIPA laboratories). Different letters above the bars indicate significant difference at P ≤ 0.05 (Tukey's test).
The unitary plant weight, composed by annual stems and leaves (cones excluded), was significantly influenced by the locality (P ≤ 0.001) and by the origin of the used propagation material (P ≤ 0.05), as well as by the interaction of these two factors (P ≤ 0.05; Table 6; Figure 6). As shown, a definite trend appears between localities, with “Sparacia” farm always allowing higher plant biomass values and, with a lesser extent, plants from “Extra” origin always heavier than “SAAF” plants. Although plant height was not relevantly different in the two conditions (data not shown), hop seems capable to reach its highest yield and biomass potentialities when grown in open field, whereas pot cultivation represents a constraint to its full development. Cultivation in pot is economically viable only if sufficient volumes are guaranteed for the growth of the plants' root systems. Further studies are required to determine root expansion in both conditions.
Figure 6.
Unitary dry weight of plants (g of d.m., cones excluded) obtained in a cultivation trial of hop variety “Cascade” in 2019. Mean values of the interaction “locality” x “origin of propagation material” (Orleans: plants cultivated in pots at “Orleans” farm (Palermo, Italy); Sparacia: plants cultivated in open field at “Sparacia” farm (Cammarata, Italy); “Extra”: purchased; “SAAF”: managed by the UNIPA laboratories). Bars marked by the same letter are not significantly different at P ≤ 0.05 (Tukey's test).
The relevant yield and vegetative differences between plants according to the source of propagation material demonstrates that some aspects of the micropropagation protocol adopted for hop, although successful for many other crops, do not fit perfectly to the obtainment of healthy, vigorous and ready-to-cultivation hop plants. This topic needs further technical deepening, because the pointing out of efficient protocols for micropropagation represents a necessary step in order to put at farmers' disposal satisfactory amounts of selected plant material for cultivation.
3.3. Phenological observations and calculation of GDDs
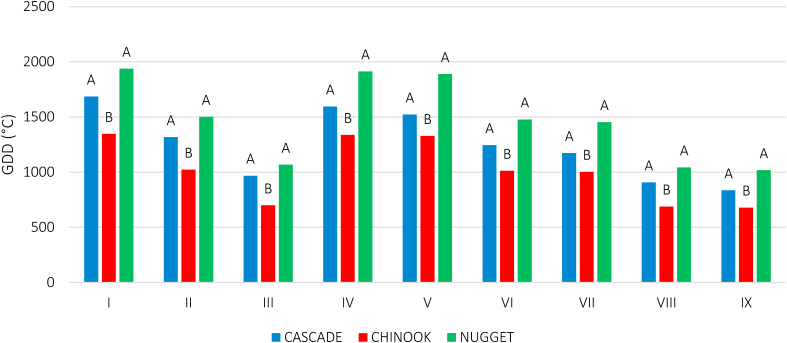
In 2018, all varieties reached harvest moment (stage 8.9) after 130–147 days from transplant (Figure 7), i.e. approximately between the end of August and the first days of September. In 2019, total cycle duration was on average a little shorter (from 108 dd, cv Chinook, to 136 dd, cv Nugget). In rather all cases, exception made for the cv Chinook in the second trial year, most of plants' cycle was composed by the vegetative stages (1.10–5.1), sharing more than 50% of total cycle duration.
Figure 7.
Development stages according to BBCH scale in 3 hop varieties cultivated at “Orleans” farm, Palermo, Italy, in 2018 and 2019, and at “Sparacia” farm, Cammarata, Italy, in 2019 only. Vegetative stages (1.10–5.1) are filled with green hues, and reproductive stages (5.1–8.9) with red hues.
GDD values calculated by means of the different methods expressed a wide variability (Figure 8; Table 7). In all GDDs calculated for vegetative stages, median was slightly higher than mean, showing a certain asymmetry of data towards higher values. Oppositely, the distribution of GDD values for reproductive stages exhibited a certain skewness towards lower values.
Figure 8.
Box-and-whiskers plot of the GDDs calculated using nine methods (I to IX) for the vegetative (a) and reproductive (b) stages in several cultivation trials of hop. For each group, box borders indicate the 25–75% percentiles, median is shown as horizontal line, and the mean as a X. The whiskers are drawn from the top of the box up to the largest data point less than 1.5 times the box height, and similarly below the box. Outliers are shown as circles.
Table 7.
Coefficients of variation (CV%), coefficients of quartile variation (CQV%), angular coefficient (m) and coefficient of determination (R2) of the regression line GDD vs days, for the GDD datasets calculated using nine methods (I to IX) for the vegetative (1.2–5.1 BBCH) and reproductive (5.1–8.9 BBCH) stages in several cultivation trials of hop.
| Methods | Vegetative Stages (from 1.2 to 5.1 BBCH) |
Reproductive Stages (from 5.1 to 8.9 BBCH) |
||||||
|---|---|---|---|---|---|---|---|---|
| CV% | CQV% | m | R2 | CV% | CQV% | m | R2 | |
| I | 27.3 | 24.9 | 15.3 | 0.433 | 35.1 | 28.1 | 29.8 | 0.993 |
| II | 27.4 | 23.3 | 10.7 | 0.348 | 34.7 | 29.3 | 23.5 | 0.950 |
| III | 28.9 | 20.5 | 7.1 | 0.267 | 36.7 | 31.3 | 18.9 | 0.952 |
| IV | 27.5 | 25.0 | 14.7 | 0.423 | 33.5 | 28.7 | 27.4 | 0.945 |
| V | 28.6 | 25.7 | 15.1 | 0.441 | 33.4 | 29.3 | 26.7 | 0.940 |
| VI | 28.1 | 26.6 | 11.1 | 0.387 | 34.6 | 30.1 | 22.8 | 0.946 |
| VII | 29.4 | 26.8 | 11.5 | 0.414 | 34.6 | 31.0 | 22.1 | 0.939 |
| VIII | 30.0 | 25.9 | 8.2 | 0.357 | 38.2 | 32.4 | 19.1 | 0.979 |
| IX | 31.3 | 29.0 | 8.6 | 0.406 | 38.2 | 33.7 | 18.4 | 0.973 |
All methods showed a rather similar range distribution according to the used Tbase; hence, a similar fashion can be observed among the methods termed I, IV and V (Tbase = 0 °C), II, VI and VII (Tbase = 5 °C) and III, VIII and IX (Tbase = 10 °C). The same grouping is evident in the corresponding regression lines (Figure 9).
Figure 9.
Regression lines of accumulated GDD (°C) on the corresponding vegetative (on the left) and reproductive (on the right) stage durations (days) according to nine calculation techniques (I to IX) in several cultivation trials of hop.
Variation coefficients (CV%) and quartile variation coefficients (CQV%) were used to study of the GDD's calculation methods (Table 7). Each of them offered a different point of view on examined datasets: in GDDs calculated for the vegetative stages, the variation coefficients ranged from 27.3 (I) to 31.3 % (IX), taking higher values with passing from the lowest (0 °C) to the highest Tbase value (10 °C). CQV %, that, being based on median rather than on mean, is a more robust estimator than CV%, ranged from 20.5% (III) to 29.0% (IX). In this case also, the highest variability was found on the IX method, built up with a Tbase = 10 °C and a correction for high temperatures. The slope of regression lines (Figure 9; Table 7) as indicated by the angular coefficient m was higher in the methods with a Tbase = 0 (I, IV and V), and lower for the corresponding lines with a Tbase = 10 °C (III, VIII and IX). Coefficients of determinations (R2) ranged from 0.267 (III) and 0.433 (I), both calculated on different Tbase values but without any correction for Tmax>30.
In reproductive stages, CV% values were generally higher than in the preceding case, spanning from 33.4% (V) to 38.2% (VIII and IX). Similarly, CQV% ranged from 28.1% (I) to 38.2% (IX). Slopes were higher than in the case of vegetative stages; as shown in Figure 9, all curves had a negative intercept, but similarly to those calculated for vegetative stages, they assumed the highest values in I, IV and V methods, i.e. those with a Tbase = 0 °C. However, the GDDs on reproductive stages showed a better fit on observed data. Differently from the vegetative stages, R2 values were always higher than 0.9, meaning that more than 90% of the observed variability could, in this case, be explained by the model's input.
A Tbase value = 5 °C has been suggested for hops by some authors (Srečec et al., 2008; Rossini et al., 2016), who however did not operate any distinction between vegetative and reproductive stages. In our experiment, none of the applied methods clearly performed better than the others; a certain advantage, as expressed by the lower general variability, may be attributed to the adoption of lower Tbase values (0 °C and 5 °C), although the highest correlation between calculated GDD values and duration of development stages was ensured by the adoption of a Tbase = 10 °C.
No advantage was definitely assessed by the use of correction for Tmax > 30 °C, whose adoption led always to the highest variability and the lowest R2 values.
Finally, the variability observed in each GDD calculation method was analyzed by means of ANOVA, separately performed on transformed data of vegetative (1.2–5.1 BBCH scale) and reproductive (5.1–8.9 BBCH scale) stages (Tables 8 and 9). As shown, the factor “variety” had a significant effect in the ANOVA carried out on vegetative stages, irrespective of the chosen calculation method (Figure 10), whereas, in the analysis performed on reproductive stages, locality shows up as the major variability source (Figure 11). It is worth noting, however, that in the first case also, the factor “locality” was significant in two out of nine datasets (V and VII), dealing with different Tbase values, but both calculated by correcting Tmax values higher than 30 °C. No significant interaction between variety and locality showed up in either cases and, interestingly, the factor “year” resulted irrelevant in both analyses carried out for vegetative and reproductive stages.
Table 8.
Results of ANOVA (F values) for the GDD (°C) calculated with 9 methods (I to IX) for vegetative stages (1.2–5.1 BBCH) of 3 hop varieties cultivated in 2018 and 2019 in 2 localities (n = 54).
| Source | DF | I | II | III | IV | V | VI | VII | VIII | IX |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1 | 1.37 n.s. | <1 n.s. | <1 n.s. | 1.94 n.s. | 2.80 n.s. | 1.25 n.s. | 2.15 n.s. | <1 n.s. | <1 n.s. |
| Locality | 1 | <1 n.s. | <1 n.s. | <1 n.s. | 2.84 n.s. | 5.20∗ | 2.06 n.s. | 4.76∗ | <1 n.s. | 3.70 n.s. |
| Variety | 2 | 6.66∗∗ | 7.66∗∗∗ | 7.78∗∗∗ | 6.78∗∗ | 6.22∗∗ | 7.21∗∗ | 6.61∗∗ | 7.54∗∗∗ | 6.78∗∗ |
| Year x variety | 2 | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. |
| Error | 47 | |||||||||
| Total | 53 |
Table 9.
Results of ANOVA (F values) for the GDD (°C) calculated with 9 methods (I to IX) for reproductive stages (5.1–8.9 BBCH) of 3 hop varieties cultivated in 2018 and 2019 in 2 localities (n = 53).
| Source | DF | I | II | III | IV | V | VI | VII | VIII | IX |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1 | <1 n.s. | 1.40 n.s. | 1.32 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. |
| Locality | 1 | 27.25∗∗∗ | 28.71∗∗∗ | 31.86∗∗∗ | 27.11∗∗∗ | 29.23∗∗∗ | 29.81∗∗∗ | 32.79∗∗∗ | 35.87∗∗∗ | 41.16∗∗∗ |
| Variety | 2 | 1.29 n.s. | 1.63 n.s. | 1.68 n.s. | 1.37 n.s. | 1.36 n.s. | 1.38 n.s. | 1.36 n.s. | 1.26 n.s. | 1.25 n.s. |
| Year x variety | 2 | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. | <1 n.s. |
| Error | 46 | |||||||||
| Total | 52 |
Figure 10.
Cumulated values of GDDs calculated with 9 methods (I to IX) for the vegetative stages (1.2–5.1 BBCH) obtained on 3 hop varieties; mean values of 2 years and 2 locations. In each GDDs calculation method, bars marked by the same letter are not significantly different at P ≤ 0.05 (Tukey's test).
Figure 11.
Cumulated values of GDDs calculated with 9 methods (I to IX) for the reproductive stages (5.1–8.9 BBCH) obtained on hop in 2 localities; mean values of 2 years and 3 varieties. In each GDDs calculation method, letters above the bars indicate a significant difference at P ≤ 0.05 (Tukey's test).
In addition, the analysis of correlation among GDDs, dry cones yield and dry plant biomass (Table 10) showed that cones yield (g d.m.) and dry plant weight (g d.m.) were negatively correlated to heat accumulation (GDDs) to the onset of flowering (phase 5.1 according to BBCH). On the contrary, dry cones weight showed to be positively correlated to GDDs accumulation along the reproductive stages (from the onset of flowering to full ripening).
Table 10.
Pearson's correlation coefficients (r) between dry weight of cones per plant and unitary plant mass and calculated GDDs for the vegetative development stages, the reproductive development stages and the entire cycle length of hop.
| Methods | Vegetative Stages (from 1.2 to 5.1 BBCH) |
Reproductive Stages (from 5.1 to 8.9 BBCH) |
Total (from 1.2 to 8.9 BBCH) |
|||
|---|---|---|---|---|---|---|
| Dry Cones Yield (g d.m.) | Dry Plant Weight (g d.m.) | Dry Cones Yield (g d.m.) | Dry Plant Weight (g d.m.) | Dry Cones Yield (g d.m.) | Dry Plant Weight (g d.m.) | |
| I | -0.326 | -0.483 | 0.583 | -0.220 | 0.252 | -0.594 |
| II | -0.362 | -0.486 | 0.575 | -0.227 | 0.232 | -0.606 |
| III | -0.456 | -0.447 | 0.585 | -0.236 | 0.194 | -0.624 |
| IV | -0.228 | -0.529 | 0.565 | -0.236 | 0.283 | -0.583 |
| V | -0.153 | -0.547 | 0.562 | -0.252 | 0.310 | -0.572 |
| VI | -0.273 | -0.521 | 0.571 | -0.246 | 0.273 | -0.591 |
| VII | -0.180 | -0.547 | 0.567 | -0.265 | 0.307 | -0.577 |
| VIII | -0.371 | -0.482 | 0.591 | -0.258 | 0.254 | -0.604 |
| IX | -0.254 | -0.527 | 0.586 | -0.282 | 0.301 | -0.585 |
Vegetative stages: n = 54; reproductive stages: n = 53; total: n = 53.
These findings corroborate the results obtained by Rossini et al. (2016), who assessed the existence of a negative correlation between the cones yield and the GDD requirements. The small values of correlation coefficients calculated for the entire cycle may find an explanation in the evident presence of negative correlation for the first stages and a positive correlation for the second stages, which counterbalance their effects when the entire cycle is on stake. As a matter of fact, hop biomass production (plant weight) seemed to be maximized with smaller accumulation of thermal units (GDDs). This leads to the conclusion that the most productive hop genotypes might also show a more efficient response to the growth environment's conditions, by having a rapid and vigorous vegetative development.
3.4. Sensory analysis
The sensory analysis through panel test defined the main aromatic and sensory notes given to the beer by Cascade and Chinook fresh cones. Although less analytical than that shown by Forteschi et al. (2019), spider plots in Figures 12a and 13a highlight the excellent aroma and flavor characteristics obtainable from hop productions in the Mediterranean environment. The single-hop test batch produced with Cascade hops showed an aroma and flavor essentially oriented towards the citrus descriptor, followed by fruity (tropical/peach/apricot), floral and spicy descriptors (Figure 12a).
Figure 12.
Spider plot of sensory evaluation of two Pale Ale single hop batch beers, crafted using the locally-grown hop cv. Chinook (a) and American hop cv. Chinook (Schott, 2018b).
Figure 13.
Spider plot of sensory evaluation of two Pale Ale single hop batch beers, crafted using the locally-grown hop cv. Cascade (a) and Californian hop cv. Cascade (Schott, 2018a).
In addition, comparing the spider plots obtained from the sensory evaluation of the Pale Ale single-hop produced with locally harvested Cascade and the Pale Ale single-hop with American Cascade (Schott, 2018a), interesting analogies emerged (Figure 12). Also, the single-hop test batch produced with Chinook hops showed an aroma and flavor essentially fruit-forward (tropical/peach/apricot), followed by spicy, citrus and herbal descriptors (Figure 13a). Moreover, comparing the spider plots obtained from the sensory evaluation of the Pale Ale single-hop produced with locally harvested Chinook and the Pale Ale single-hop with American Chinook (Schott, 2018b), common traits were noted (Figure 13). However, a small difference could be seen with reference to the spicy descriptor. In fact, it seemed that Sicilian Chinook hops gave to the beer a unique spiciness that is not detected in the other single-hop batch compared with. That might be addressed to the technological brewing process, that might have been a little different, but of course might also be linked to the specific growth environment's conditions.
4. Conclusions
Although preliminary, this work confirms the good potentialities of hop cultivation also in Mediterranean semi-arid environments. Cones yield was, on average, in line with the reference values obtained in different environments, and the high retrieved variability according to the tested agronomic factors testifies that there are still wide margins of improvement of yield levels, provided a proper cropping technique is pointed out. Significant differences showed up in cones yield according to the variety, that confirms being a key factor in determining yield. In our trial, the Chinook variety performed better than the other genotypes, most probably due to its higher precocity. Hence, the best genotype seems to be individuated as that having the shortest vegetative stage and, comparatively, a longer reproductive stage. The correlation analysis, that showed the existence of a negative relationship between crop productivity and thermal requirements (GDDs) of the early phenological stages, is a further demonstration of this assessment. This conclusion is also confirmed in the studies carried out by Ruggeri et al. (2018) with reference to the higher productivity of the earliest sprouting genotypes, that showed a more efficient response to those growth environment's conditions.
The trial also confirmed the crucial importance of selecting good quality starting material for hop's cultivation. Also, this aspect was capable of conditioning the production levels, ultimately making the hops' plantings sustainable or not. The availability of good quality commercial material has been however verified, and this might represent a good chance for farmers.
Sensory analysis has shown important similarities between batches obtained with local and US hops. The cultivation environment had however a greater impact on the aromatic bouquet of Chinook cones than found for the Cascade variety. Together with the analogies for the main “fruity”, “herbaceous” and “floral” descriptors, more “spicy” notes were detected in the single-hop Pale Ale with Sicilian Chinook compared to the batch with US hops. Besides the fact that only two varieties were tested, it is clear, however, the need of deepening these studies, extending to a larger number of genotypes the comparison for the main aromatic and sensorial characteristics.
Declarations
Author contribution statement
Roberto Marceddu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Alessandra Carrubba: Conceived and designed the experiments, Analyzed and interpreted the data, Wrote the paper.
Mauro Sarno: Conceived and designed the experiments, Contributed resources, Revised the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Assobirra . 2018. Annual Report. Rome IT.https://www.assobirra.it/wp-content/uploads/2019/06/AnnualReport_2018.pdf Available at: [Google Scholar]
- Baldoni R., Giardini L. Pàtron; Bologna: 2002. Coltivazioni Erbacee. (in Italian) [Google Scholar]
- Biancardi E., Wagner T. Il luppolo da birra in Italia. Annali dell'Istituto Sperimentale per le Colture Industriali. 1989;XXI (in Italian) [Google Scholar]
- Bonett D.G. Confidence interval for a coefficient of quartile variation. Comput. Stat. Data Anal. 2006;50:2953–2957. [Google Scholar]
- Bonhomme R. Bases and limits to using ‘degree day’ units. Eur. J. Agron. 2000;13(1):1–10. [Google Scholar]
- Calderwood L., Post J. University of Vermont Extension Northwest Crops and Soils Program; Burlington, Vermont, USA: 2015. Hop Harvest Timing in the Northeast.https://www.uvm.edu/sites/default/files/media/Hop_Harvest_Determination_factsheet.pdf last accessed. [Google Scholar]
- Carbone K., Cherubini D. Atti del convegno - Criticità e Opportunità per lo Sviluppo Sostenibile della Filiera Brassicola. Roma, 26 October. 2016. Il Luppolo fresco “made in Italy”: qualità e competitività per l’industria brassicola. (in Italian) [Google Scholar]
- Carr N. 2017. Medusa hops: the mythical multihead hop variety.https://learn.kegerator.com/medusa-hops/ Available at: [Google Scholar]
- Darby P. The history of hop breeding and development. Brewery History. 2005;121:94–112. http://www.breweryhistory.com/journal/archive/121/bh-121-094.htm Available at: [Google Scholar]
- Del Pozo A.H., García-Huidobro J., Novoa R., Villaseca S. Relationship of base temperature to development of spring wheat. Exp. Agric. 1987;23(1):21–30. [Google Scholar]
- di Tizio A., Łuczaj Ł., Quave C.L., Redžić S., Pieroni A. Traditional food and herbal uses of wild plants in the ancient South-Slavic diaspora of Mundimitar/Montemitro (Southern Italy) J. Ethnobiol. Ethnomed. 2012;8:21. doi: 10.1186/1746-4269-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forteschi M., Porcu M.C., Fanari M., Zinellu M., Secchi N., Buiatti S., Pretti L. Quality assessment of cascade hop (Humulus lupulus L.) grown in Sardinia. Eur. Food Res. Technol. 2019;245(4):863–871. [Google Scholar]
- Gilmore C., Rogers J.S. Heat units as a method of measuring maturity in corn. Agron. J. 1958;50(10):611–615. [Google Scholar]
- Gomez K.A., Gomez A.A. John Wiley & sons Inc; New York: 1984. Statistical Procedures for Agricultural Research. [Google Scholar]
- Gresser A. Fachverlang Hans Carl; Nurnberg: 2010. Il Manuale del Birraio Pratico. Teoria e Pratica della Preparazione del Malto e della Fabbricazione della Birra; p. 1030. (in Italian) [Google Scholar]
- Helrich K. fifteenth ed. 1990. AOAC Official Methods of Analysis. Arlington, Virginia (US) [Google Scholar]
- Herms D.A. 58–07645. Minnesota Agricultural Experiment Station Publication; 2004. Using degree-days and plant phenology to predict pest activity; pp. 49–59. (IPM (Integrated Pest Management) of Midwest Landscapes). [Google Scholar]
- Islam M.R., Sikder S. Phenology and degree days of rice cultivars under organic culture. Bangladesh J. Bot. 2011;40(2):149–153. [Google Scholar]
- Knobloch K., Paulini H., Eley C., Eley J.H., Ziegler E., Brandauer H., Vostrowsky O. On the essential oil components from Humulus lupulus L. Var. neomexicanus nels. & cockerell. I. Contribution. Z. Naturforsch. C Biosci. 1982;37(7-8):565–569. [Google Scholar]
- Kumar A., Pandey V., Shekh A.M., Kumar M. Growth and yield response of soybean (Glycine max L.) in relation to temperature, photoperiod and sunshine duration at Anand, Gujarat, India. Am.-Eurasian J. Agron. 2008;1(2):45–50. [Google Scholar]
- Lilley R., Campbell C.A.M., Ridout M.S. Vertical dispersal of the two-spotted spider mite Tetranychus urticae, and the predatory mite Phytoseiulus persimilis on dwarf hops. Agric. For. Entomol. 1999;1(2):111–117. doi: 10.1046/j.1461-9563.1999.00015.x. [DOI] [Google Scholar]
- McMaster G.S., Wilhelm W.W. Growing degree-days: one equation, two interpretations. Agric. For. Meteorol. 1997;87:291–300. [Google Scholar]
- Miller P. Montana State University Extension, report MT200103AG; 2018. Using Growing Degree Days to Predict Plant Stages; p. 8. [Google Scholar]
- Mongelli A., Rodolfi M., Ganino T., Marieschi M., Dall’Asta C., Bruni R. Italian hop germplasm: characterization of wild Humulus lupulus L. genotypes from Northern Italy by means of phytochemical, morphological traits and multivariate data analysis. Ind. Crop. Prod. 2015;70:16–27. [Google Scholar]
- Mongelli A., Rodolfi M., Ganino T., Marieschi M., Caligiani A., Dall’Asta C., Bruni R. Are Humulus lupulus L. ecotypes and cultivars suitable for the cultivation of aromatic hop in Italy? A phytochemical approach. Ind. Crop. Prod. 2016;83:693–700. [Google Scholar]
- Narwal S.S., Poonia S., Singh G., Malik D.S. Influence of sowing dates on the growing degree days and phenology of winter maize (Zea mays L.) Agric. For. Meteorol. 1986;38(1-3):47–57. [Google Scholar]
- Neve R.A. Springer-Science + Business Media, B.V., Southport; 1991. Hops. [Google Scholar]
- NOAA . 2020. National Weather Service - Climate Prediction Center. CFSR/GFS Based Growing Degree Days (GDD)https://www.cpc.ncep.noaa.gov/products/wesley/cfsr/GDD.html Available at. [Google Scholar]
- Olsen S.R., Cole C.V., Watanabe F.S., Dean L.A. Vol. 939. U.S. Department of Agriculture, Circular; Washington D.C (US): 1954. (Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate). [Google Scholar]
- Patzak J., Nesvadba V., Krofta K., Henychova A., Marzoev A.I., Richards K. Evaluation of genetic variability of wild hops (Humulus lupulus L.) in Canada and the Caucasus region by chemical and molecular methods. Genome. 2010;53(7):545–557. doi: 10.1139/g10-024. [DOI] [PubMed] [Google Scholar]
- Pignatti S. Vol. 1. Edagricole; Bologna: 1982. (Flora d’Italia). (in Italian) [Google Scholar]
- Rajput R.P., Deshmukh M.R., Paradkar V.K. Accumulated heat units and phenology relationships in wheat as influenced by planting dates under late sown conditions. J. Agron. Crop Sci. 1987;159(5):345–348. [Google Scholar]
- Rivas-Martínez S., Rivas Sáenz S., Penas A. Worldwide bioclimatic classification system. Global Geobotany. 2011;1:1–634. [Google Scholar]
- Reighard G.L., Rauh B. Predicting peach fruit size potential from GDD 30 days post-bloom. Acta Hortic. 2015;1084:753–758. [Google Scholar]
- Roltsch W.J., Zalom F.G., Strawn A.J., Strand J.F., Pitcairn M.J. Evaluation of several degree-day estimation methods in California climates. Int. J. Biometeorol. 1999;42:169–176. [Google Scholar]
- Rossbauer G., Buhr L., Hack H., Hauptmann S., Klose R., Meier U., Stauss R., Weber E. Phänologische Entwicklungsstadien von KulturHopfen (Humulus lupulus L.). Nachrichtenblatt des Deutschen Planzenschutzdienstes. In: Meier U., editor. Vol. 47. 1995. pp. 249–253.https://www.politicheagricole.it/flex/AppData/WebLive/Agrometeo/MIEPFY800/BBCHengl2001.pdf (Growth Stages of Mono- and Dicotyledonous Plants, BBCH Monograph, 2001, Federal Biological Research Centre for Agriculture and Forestry). Available at: [Google Scholar]
- Rossini F., Loreti P., Provenzano M.E., De Santis D., Ruggeri R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016;11(3):746. [Google Scholar]
- Ruggeri R., Loreti P., Rossini F. Exploring the potential of hop as a dual purpose crop in the Mediterranean environment: shoot and cone yield from nine commercial cultivars. Eur. J. Agron. 2018;93:11–17. [Google Scholar]
- Ruml M., Vuković A., Vujadinović M., Djurdjević V., Ranković-Vasić Z., Atanacković Z., Petrović N. On the use of regional climate models: implications of climate change for viticulture in Serbia. Agric. For. Meteorol. 2012;158:53–62. [Google Scholar]
- Schott M. 2018. The Hop Chronicles – California Cascade, 2018.http://brulosophy.com/2018/12/13/the-hop-chronicles-california-cascade-2018/ last accessed. [Google Scholar]
- Schott M. 2018. The Hop Chronicles - California Chinook, 2018.http://brulosophy.com/2018/09/13/the-hop-chronicles-california-chinook-2018/ last accessed. [Google Scholar]
- Seigner E., Lutz A., Oberhollenzer K., Seidenberger R., Seefelder S., Felsenstein F. Breeding of hop varieties for the future. Acta Hortic. 2009;848:49–57. [Google Scholar]
- Shishehgar R., Rezaie A., Nazeri M. Study of sedation, pre-anesthetic and anti-anxiety effects of hop (Humulus lupulus L.) extract compared with diazepam in rats. J. Anim. Vet. Adv. 2012;11:2570–2575. [Google Scholar]
- SIAS . 2020. Servizio Informativo Agrometeorologico Siciliano.http://www.sias.regione.sicilia.it/ last accessed. [Google Scholar]
- Slafer G.A., Savin R. Developmental base temperature in different phenological phases of wheat (Triticum aestivum) J. Exp. Bot. 1991;42(8):1077–1082. [Google Scholar]
- Sortino G., Allegra A., Farina V., Inglese P. Postharvest quality and sensory attributes of ‘Pesca di Bivona’ peaches (Prunus persica L.) during storage. Bulg. J. Agri. Sci. 2017;23(6):939–946. [Google Scholar]
- Srečec S., Kvaternjak I., Kaučić D., Špoljar A., Erhatić R. Influence of climatic conditions on accumulation of α-acids in hop cones. Agric. Conspectus Sci. 2008;73(3):161–166. [Google Scholar]
- Stolte J. Determination of the saturated hydraulic conductivity using the constant head method. In: Stolte J., editor. Manual for Soil Physical Measurements, Version 3. Wageningen, DLO-Staring Centre. Technisch Document/Technical Document 37; 1997. pp. 27–32. [Google Scholar]
- Tinsley J. Vol. 1. 1950. The determination of organic carbon in soils by dichromate mixtures; pp. 161–164. (Transactions 4th International Congress of Soil Sciences). [Google Scholar]
- Turner S.F., Benedict C.A., Darby H., Hoagland L.A., Simonson P., Sirrine J.R., Murphy K.M. Challenges and opportunities for organic hop production in the United States. Agron. J. 2011;103(6):1645–1654. [Google Scholar]
- Yang S., Logan J., Coffey D.L. Mathematical formulae for calculating the base temperature for growing degree days. Agric. For. Meteorol. 1995;74(1-2):61–74. [Google Scholar]
- Zhou G., Wang Q. A new nonlinear method for calculating growing degree days. Sci. Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-28392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]