Abstract
The thermal physiology of non-avian dinosaurs, especially the endothermic/ectothermic nature of their metabolism, inferred indirectly using body mass, biophysical modelling, bone histology and growth rate, has long been a matter of debate. Clumped isotope thermometry, based on the thermodynamically driven preference of 13C-18O bond in carbonate minerals of fossilized eggshells, yields temperature of egg formation in the oviduct and can delineate the nature of thermoregulation of some extinct dinosaur taxa. In the present study, the clumped isotope thermometry was applied to the eggshells of a few species of modern birds and reptiles to show that it is possible to obtain the body temperatures of these species in most of the cases. We then used this method to the fossil eggshells of Late Cretaceous sauropods and theropods recovered from western and central India. The estimated body temperatures varied between 29 °C and 46 °C, with an overall average of 37 °C, significantly higher than the environmental temperature (about 25 °C) of this region during the Late Cretaceous. The results also show that the theropod species with low body masses (~800 kg) had high body temperature (~38 °C), while some gigantic (~20000 kg) sauropods had low body temperatures that were comparable to or slightly higher than the environmental temperature. Our analyses suggest that these Late Cretaceous giant species were endowed with a capacity of variable thermoregulation to control their body temperature.
Keywords: Earth sciences, Paleobiology, Geochemistry, Dinosaur, Eggshell carbonate, Clumped isotopes, Body temperature, Thermoregulation
Earth sciences, Paleobiology, Geochemistry, Dinosaur, Eggshell carbonate, Clumped isotopes, Body temperature, Thermal physiology.
1. Introduction
The mechanism by which animals maintain their body temperatures has a direct bearing on some of the fundamental issues of evolutionary biology. In this context, the body temperature estimates of dinosaurs provide crucial information in tracing the biological evolution of metabolism and thermoregulation. Since the establishment of the taxonomic group of dinosaurs in 1842 CE, there have been many discussions about the nature of their metabolism, particularly who were warm blooded (endothermic) and who were cold blooded (ectothermic) (Farlow, 1990; Amiot et al., 2006; Grady et al., 2014; Eagle et al., 2011, 2015; Dawson et al., 2020). Endothermic mammals and birds maintain stable body temperatures, irrespective of the environmental temperature, by internal metabolic heat production but ectothermic animals fail to do so. Until recently, all dinosaurs were believed to be ectothermic, similar to modern day reptiles that derive heat from external environment to maintain their body temperatures. However, contrarian suggestions for endothermic metabolism of dinosaurs arose from observations of their behaviour, paleogeographic distribution and anatomy (Russell, 1965; Bakker, 1972; de Ricqlès, 1974). Two specific arguments were given in favour of endothermic metabolism: (1) aggressive physical performance such as high running speed estimated from preserved tracks and (2) a favourable predator/prey ratio determined by comparing the fossil records with the number ratio in modern ecosystems (Bakker, 1972; Ostrom, 1980; Farlow, 1990). These arguments and the manner of investigations have been widely discussed and revisited based on an extensive study of dinosaur thermoregulation using biophysical modelling (O'Conner and Dodson, 1999; Seebacher, 2003; Gillooly et al., 2006; Pontzer et al., 2009), bone histology (Chinsamy, 1993), growth rate analysis (Erickson, 2005; Klein and Sander, 2008; Woodward and Lehman, 2009; Sander et al., 2011), anatomical observations (Bakker, 1972; Ruben et al., 1997), oxygen isotope paleothermometry (Fricke and Rogers, 2000; Amiot et al., 2006), and clumped isotope thermometry (Eagle et al., 2010, 2011, 2015; Löffler et al., 2017; Dawson et al., 2020).
A multiple physiology hypothesis was proposed recently which envisaged that dinosaurs possessed a range of metabolic abilities to match the broad ecological niches they occupied (Clarke, 2013; Werner and Griebeler, 2014). This means that a simple division into endothermic and ectothermic species could be an oversimplified categorization, and the actual thermal physiology was more complex. Modelling of heat flow inferred that heavier dinosaurs maintained stable and often higher than environmental temperatures (Spotila, 1980; O'Conner and Dodson, 1999; Seebacher, 2003). This is consistent with the bone isotopic data, which indicated a relatively uniform body temperature in larger dinosaurs (Barrick et al., 1996, 1997). Some limited clumped isotope analysis of dinosaur eggshells, recovered mainly from high latitudes, also suggested variable thermoregulation for at least some of the species, if not all (Eagle et al., 2015; Dawson et al., 2020).
Data from modern ectothermic species, such as alligators, show that with increasing body mass, the average body temperature increases whereas, at the same time, the fluctuation in the temperature decreases (Seebacher, 2003). Gillooly et al. (2006) reported that at an environment of 25 °C (mean annual temperature), body temperature of crocodiles increased from 25 °C to 30 °C with increasing body weight from ~30 kg to 1000 kg. Based on these data, a developmental growth trajectory model was proposed by these authors, which suggested that dinosaur body temperature increases with body mass, from roughly 26 °C at 10 kg to 41 °C at 15,000 kg. The model data showed that larger species of dinosaurs had higher body temperature maintained through thermal inertia (picturesquely called gigantothermy) whereas body temperatures of smaller dinosaurs were close to the environmental temperatures. This would imply that smaller dinosaurs were similar to the modern reptiles. However, this issue remains subject of an ongoing scientific debate (Griebeler, 2013).
Stable carbon and oxygen isotopic compositions of biologically precipitated apatite in fossil bone, teeth, scales and eggshells give information on the diet, behaviour, and physiology of the species (Eagle et al., 2015; Sarkar et al., 1991; Laskar, 2017; Löffler et al., 2019). The oxygen isotope ratio of biogenic carbonates, in particular, is often used for delineating the body temperature, but it requires knowledge of the isotopic composition of internal body water medium (which can only be inferred indirectly, like from fossil bones; Kohn and Law, 2006). Clumped isotope analysis in eggshell carbonates provides an alternative way of constraining the carbonate precipitation temperature inside the oviduct of the egg laying (oviparous) animal if the process occurs under thermodynamic equilibrium (Eagle et al., 2011). It is known that the abundance of heavy isotope substituted isotopologues is thermodynamically favoured due to the zero-point energy effect (Bigeleisen, 1965). The effect is further enhanced when two heavy isotopes are involved in the same molecule. For example, in carbonate minerals, this is reflected by a higher propensity of bonds formed by 13C and 18O (popularly known as clumping; see Eiler, 2007). The enhancement depends on the temperature but not on the bulk abundances of the isotopes taking part in the carbonate forming reaction (Ghosh et al., 2006). In practice, clumped isotope thermometry needs calibration of the enhancement as a function of temperature. The higher propensity of 13C-18O type carbonate species (relative to the statistical abundance) can be measured by analysing the CO2 gas derived by acid digestion of the carbonate. This is possible because the relative abundance information (i.e., the 13C-18O clumping) is retained despite the fact that only two of the three oxygen atoms in CaCO3 are incorporated in the evolved CO2 (Guo, 2008). The enhancement is quantitatively estimated by the observed increase in the mass 47 signal (~97% comes from 16O-13C-18O) of the sample CO2 relative to an isotopically scrambled CO2. An isotopically scrambled CO2 is one in which all the isotopes are randomly distributed, i.e., it has no preference towards a particular bond, e.g., 13C-18O. Isotopic scrambling is achieved by heating a CO2 gas at 1000 °C for several hours, which randomizes the isotope distribution within the isotopologues (Ghosh et al., 2006).
In the present study, we apply the clumped isotope thermometer to several fossilized eggshell samples of two species of Indian dinosaurs to estimate their body temperatures. The Indian landmass belongs to the Asian Plate and during the Late Cretaceous the sampling region was located in the southern hemisphere at a latitude of ~22 oS and had mean air temperature of about 25 °C (Chen et al., 2013; Ghosh et al., 2016). We conjectured that a comparison of the eggshell temperatures with the late Cretaceous environmental temperature could offer valuable constraints on the models of metabolism of dinosaurs. We also wanted to investigate the correlation of the body temperature with the body mass to improve our understanding of the nature of the dinosaurian thermoregulation.
2. Materials and methods
2.1. Dinosaur nesting sites and their geologic settings
During the past few decades, a number of nest sites with well-preserved sauropod and theropod eggs of diverse morphotypes have been discovered in the Late Cretaceous sediments constituting the Lameta Formation in Western and Central Indian states of Gujarat (Mohabey 1984, 1998; Srivastava et al., 1986; Mohabey and Mathur, 1998), Madhya Pradesh (Vianey-Liaud et al., 1988; Sahni and Tripathi, 1990; Mohabey, 1996b) and Maharashtra (Mohabey 1996a). Resting over rocks of the Precambrian and Gondwana Supergroup, the Lameta Formation occurs as a series of horizontal sedimentary shelves below the Deccan traps. There are evidences that the Lameta sedimentation started a long time before the Deccan eruptions in Late Cretaceous and continued till Cretaceous–Paleogene boundary. The geological set-up of the fossil bearing sediments and field setting of dinosaur nests have been discussed by many researchers (Mohabey, 1984, 1998, 2001, 2005; Tandon et al., 1995). Briefly, the Lameta Formation comprises a group of pedogenically modified sediments deposited under an alluvial-limnic environment (Mohabey and Udhoji, 1993; Tandon et al., 1995; Mohabey, 1996a, 1996b). The overbank sediments are covered by very finely laminated lacustrine clays interbedded with limestone and calcareous mudstone. The infratrappean Lameta Beds are not readily distinguishable from the intertrappean sedimentary successions (Mohabey and Udhoji, 1990). The fossil eggshells are found in both types of sediments. According to Tandon et al. (1995), some of the sauropod nesting sites in central India occur in the sandy carbonate lithofacies of the Lameta formation and post-date the initial eruptive activity in the region. The Deccan basalts started their eruption from 66.4 and continued untill 65.4 Ma with pauses between the eruptive fluxes (each lasting for about 50000 years; see Figure 4 of Sprain et al., 2019) during which the intertrappean sediments were deposited. Bajpai et al. (1990) reported eggshell fragments belonging to sauropod and ornithoid species in sediments occurring between two flows near the bottom part of the Deccan basalts in Kutch, Gujarat. This indicates that dinosaurs started to inhabit this area during the end phase of the Lameta sedimentation and continued to do so till the initial eruptive phases of the Deccan basalts (see also Vianey-Liaud et al., 1988; Bajpai et al., 1983; Sriniwasan, 1996; Mohabey, 2001).
The fossil eggshells were found in the calcretised channel-related sandstones, suggesting that these animals preferred to nest and bury their eggs in the soft sands of the river banks. Pteridophytic-angiospermic plants have been found in the Lameta sediments deposited in lakes and ponds in the flood-plain areas (Samant and Mohabey, 2014). Coprolites (dung mass) rich in comminute soft plant tissues were found in large concentrations as lag deposits associated with the skeletal remains of titanosaurid (Isisaurus) sauropods (Mohabey, 2001, 2005; Mohabey and Samant, 2003). Available evidences show that during the Maastrichtian, this region was an ideal habitat for both sauropod and theropod dinosaurs and the fossil eggshells and bones provide signature of their breeding and nesting behaviour.
2.2. Eggshell samples
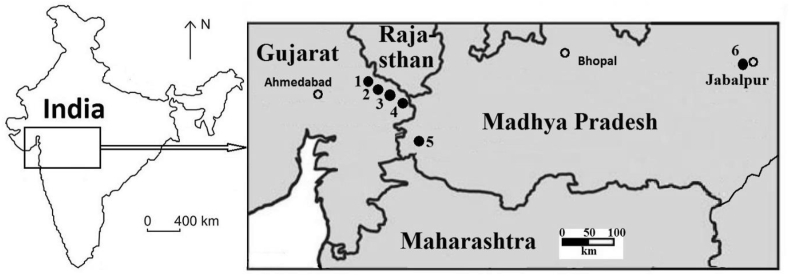
The eggshell samples were collected from egg clutches in different nesting sites belonging to six widely separated localities of the states of Gujarat and Madhya Pradesh, India (Figure 1). The sampled eggshells are parataxonomically assigned to oogenus Megaloolithus and oogenus Ellipsoolithus of the oofamily Elongatoolithdae (Mikhailov, 1991; Mohabey, 1998, 2001) and taxonomically belong to sauropods and theropods (Zhao, 1975; Erben et al., 1979; Hirsch and Packard, 1987; Mikhailov, 1991; Chiappe et al., 1998; Loyal et al., 1999; Mohabey, 2005). The taxonomic revision of Late Cretaceous (Maastrichtian) Indian dinosaurs has clarified that only Titansauriforme sauropods (Isisaurus, Jainosaurus) and Abelisaurid thereopods (large sized Indosaurus, Indosuchus, Rajasaurus, Rahiolisaurus and small sized Noasaurids Laevisuchus) were present in the Indian subcontinent (Wilson et al., 2003; Wilson and Mohabey, 2006; Novas et al., 2010; Mohabey and Samant, 2013). Some photographs of complete eggshells and their occurrence in clutches were given in one of our earlier publications (Sarkar et al., 1991). From the pattern of layout in the present case, it is inferred that the eggs under study were laid by one individual in one session. It is known that female dinosaurs laid anywhere from a handful (3–5) to a whole clutch (15–20) of eggs at a single sitting, depending on the genus and species (https://www.thoughtco.com/facts-about-dinosaur-eggs-1092047). More details about the geological settings, mode of occurrence of the eggshells and their distribution pattern, sample collection, and preservation have been discussed elsewhere (Mohabey, 1984, 1998, 2005). Despite their fragility, the excellent preservation of the eggshells in many places is believed to be due to the safety capping provided by the hard Deccan basalts (A. Sahni, personal communication).
Figure 1.
Locations (marked by filled circles) of Late Cretaceous dinosaur bearing Lameta Formations in western and central India in the state of Gujarat and Madhya Pradesh. The locations are: 1. Lavariya Muwada. 2. Rahioli. 3 Khempur. 4. Dohad. 5. Padali. 6. Lameta Ghat.
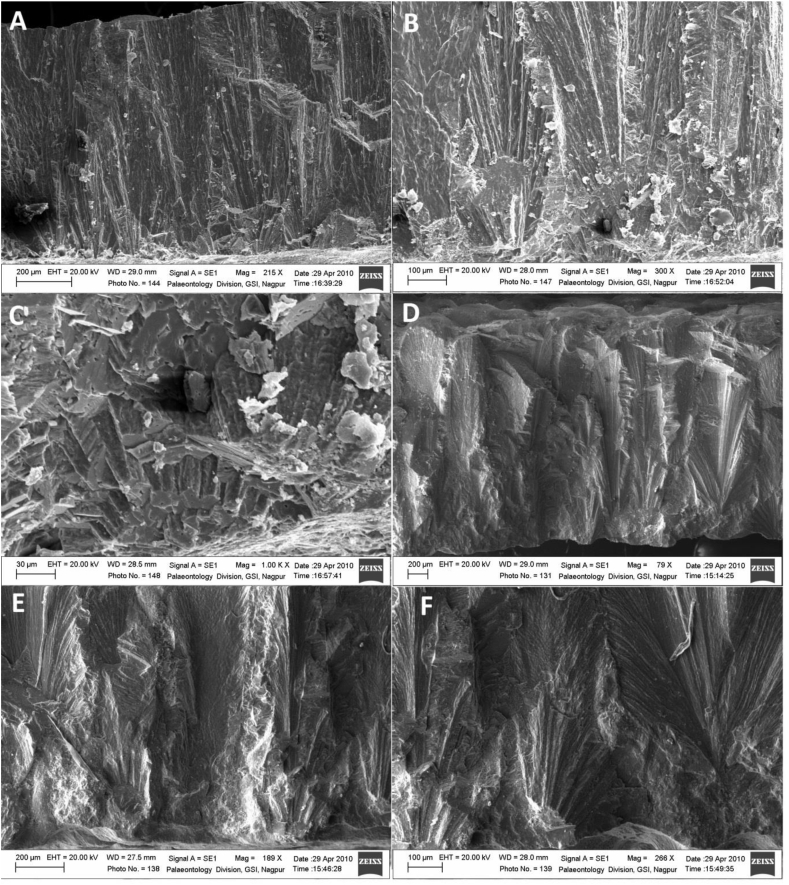
In Figure 2, we present a few high-resolution scanning electron microscope (SEM) photos of thin sections of the eggshells to demonstrate that the samples under study are indeed pure carbonate phase of the eggshells with all their microstructure preserved. For example, the basal mammillary zone and the upper spongy zone seen in Figure 2A and B prove that despite their occurrence long time ago (~65 Ma) the shells remained well preserved without any alteration. The shell fragments were strongly cemented with the host sedimentary minerals and we confirmed that there was no isotopic exchange between the two (see section on Diagenetic alteration).
Figure 2.
Scanning electron microscope images of thin sections from selected eggshells showing microstructures of the shell units. A–C: Radial section of Ellipsoolithus khedaensis egshells belonging most likely to abelisaurid theropods. A: Distinct two layered structure showing a basal mammillary zone and an upper spongy zone. B: Same as A, closer view. C: Distinct calcite spherulite structure of the mammillae. D–F: Radial sections Tubospherulithic Megaloolithus rahioliensis eggshells belonging most likely to titanosaurid sauropods. D. Discrete shell units showing spherulite structure of basal mammillae and radiating calcite crystals. E–F: calcite crystal radiating from the mammillae and fanning to the top of the shell units.
A few researchers have shown that the clumped isotope thermometry can be used to estimate the body temperature of oviparous animals using the eggshell carbonates (e.g., Wacker et al., 2014; Eagle et al., 2015; Dawson et al., 2020). To confirm the applicability of this thermometer to infer the oviduct temperature of egg laying animals, we analysed eggshells of modern birds and reptiles including snakes and crocodiles collected from two Taiwan zoos. Our choice was limited to species whose eggshells were easily available from the zoo authorities. The chicken and bird (Koyal) egg samples from a few local stores in Taipei were collected during the period November 2013 to September 2014. Eggshell samples of birds and reptiles from Taipei zoo and Madou Crocodile King Zoo (a private zoo in Tainan, Taiwan) were collected in two batches in August 2014 and October 2014, respectively. The animals inside the Taipei zoo were kept in air-conditioned environment maintained at ~25 °C from evening 18:00 h to morning 8:00 h and in natural conditions (temperature varying from 10 to 35 °C) during the rest of the day. In the Madou Crocodile King Zoo, the animals were kept always in natural conditions. It must be noted that the animals in both the zoos have access to ponds, shades, and caves. Therefore, the body temperatures of reptiles could be significantly different from those of the environment due to behavioural thermoregulation (discussed later). The air temperature in Tainan varies from 10 to 35 °C during the year; in October 2014, the average (±1 σ stdandard deviation) value was 26 ± 3 °C.
The modern eggshell samples were collected either from local markets or from the officials of the zoos and they were not living eggs hosting babies, but mostly dead eggshells. We checked and ensured that there was no need for local ethical committee permission for this study.
2.3. CO2 extraction, analytical protocol and temperature reconstruction
All eggshell samples (both modern and fossil) were cleaned by ethanol in an ultrasonic bath for ~10 min to remove the dust particles sticking on their surfaces. Eggshell pieces of dinosaurs were cut perpendicular to the exterior surface to expose the shell structure in cross-section under the microscope. The shell pieces were then cleaned with deionized water, dried and treated with 10 % H2O2 for ~24 h to decompose any organic matter, washed again with deionized water and dried at 70 °C for 24 h. Powder samples were drilled out from the central part of the cross-section using a hand-drill with a diamond drill-bit of size 0.5 mm. Pieces of modern eggshells were also treated with 10 % H2O2 and powdered using an agate mortar. For CO2 extraction from carbonates, we used the conventional acid digestion technique. 40–70 mg of powder was reacted with 104 % orthophosphoric acid for ~12 h at 25 °C using McCrea-type reaction vessel kept in a temperature controlled water bath. The sample used for reaction was about 4–7 times more than required (~10 mg) to avoid any possible bias due to inhomogeneity. For snake eggs, the amount used for each sample was more than 300 mg as the carbonate content was quite low. The CO2 (along with trace amount of water) produced was cryogenically trapped in a glass U-tube immersed in liquid nitrogen. Next, the trap was warmed to -77 °C using a dry ice-acetone slush, which releases the CO2 but retains the frozen water in the trap. The CO2 was then collected in a small glass bottle (volume ~2 cc) and purified using gas chromatography (Laskar et al., 2016a) to remove traces of acid, water, hydrocarbons and halocarbons which might interfere with the mass spectrometric analysis. Except for a couple of modern eggshell samples, all samples were measured in replicates (2–4 times) to ensure the reliability of the data and estimate the analytical uncertainty.
We also analysed international carbonate standard IAEA NBS-19 for checking the reproducibility and accuracy of measurements (Table 1). The analytical procedure for NBS-19 was the same as that used for the eggshell samples except that the standard did not undergo the ultra-sonication and H2O2 treatment. We checked for the effect of H2O2 treatment on isotopic compositions using a few marble samples but did not see any significant change in the carbon and oxygen isotope ratios as well as in the clumped isotopes (Laskar et al., 2016a). Our data agree with Zhang et al. (2020) who used biogenic carbonates for the same purpose.
Table 1.
Stable isotopic compositions including clumped isotope data of IAEA NBS-19 for reproducibility test. These samples were analysed during 2013–2014. The working gas (WG) used was AS-2 with δ13 C = -32.54 ‰ and δ18 O = -4.67 ‰ (in VPDB scale).
| Sl. No. | δ13 C (‰) (VPDB) | δ18 O (‰) (VPDB) | δ47 (WG) (‰) | Std. Err. | Δ47 (‰) (ARF) |
Std. Err |
|---|---|---|---|---|---|---|
| 1 | 2.02 | -2.21 | 35.22 | 0.01 | 0.382 | 0.010 |
| 2 | 2.02 | -2.11 | 35.54 | 0.02 | 0.394 | 0.012 |
| 3 | 2.02 | -2.19 | 35.28 | 0.01 | 0.416 | 0.010 |
| 4 | 2.01 | -2.28 | 35.15 | 0.01 | 0.408 | 0.011 |
| 5 | 2.00 | -2.27 | 35.24 | 0.02 | 0.388 | 0.016 |
| 6 | 2.00 | -2.16 | 35.27 | 0.02 | 0.370 | 0.013 |
| 7 | 2.02 | -2.27 | 35.21 | 0.01 | 0.398 | 0.009 |
| 8 | 2.02 | -2.20 | 36.48 | 0.01 | 0.363 | 0.008 |
| 9 | 2.01 | -2.20 | 36.56 | 0.02 | 0.392 | 0.006 |
| 10 | 2.01 | -2.15 | 36.46 | 0.01 | 0.399 | 0.012 |
| 11 | 2.01 | -2.20 | 36.57 | 0.01 | 0.393 | 0.010 |
| 12 | 2.02 | -2.21 | 36.32 | 0.01 | 0.387 | 0.009 |
| 13 | 2.01 | -2.18 | 36.43 | 0.01 | 0.368 | 0.014 |
| 14 | 2.01 | -2.16 | 35.81 | 0.01 | 0.379 | 0.010 |
| 15 | 2.00 | -2.18 | 35.76 | 0.01 | 0.387 | 0.006 |
| Average | 2.01 | -2.20 | 35.82 | 0.388 | ||
| Std. Dev. | 0.01 | 0.05 | 0.58 | 0.014 |
Isotopic measurements were carried out using a MAT 253 stable isotope ratio mass spectrometer equipped to measure masses 44–49 in CO2, at the Institute of Earth Sciences, Academia Sinica, Taiwan. The mass spectrometric ratios were converted to standard delta notation (e.g., δ18O=(18Rsa/18Rst-1)) where 18R is the isotope ratio 18O/16O, and subscripts sa and st stand for sample and standard, respectively. All δ13C and δ18O values are expressed relative to VPDB in per mil (‰). Measurements were carried out against the Academia Sinica working reference (called AS-2; Laskar et al., 2018), a high purity commercial CO2 with δ13C = -32.54 ‰ and δ18O = -4.67 ‰, respectively (procured from Air Products and Chemicals, Inc., Taiwan). Δ47 values were calculated using the following relation
| (1) |
where R13 and R18 (ratios 13C/12C and 18O/16O) were obtained by measuring the masses 44, 45 and 46 of the CO2 sample and R17 was calculated by assuming a mass dependent relation with R18 with a slope of 0.5164. The ‘Gonfiantini’ parameters i.e., R13(VPDB) = 0.0112373 (determined from NBS-19) R17(VSMOW) = 0.0003799 and R18(VSMOW) = 0.0020052 (IAEA, 2006), were used for the Δ47 calculations. In the first step, the Δ47 values were expressed relative to a CO2 whose isotopes are randomly distributed, that is a CO2 gas with Δ47 = 0 ‰ (isotopically scrambled CO2). Practically, this was achieved by heating an aliquot of the sample CO2 at 1000 °C for more than two hours. Both the sample CO2 and the scrambled CO2 were measured against the same working reference (AS-2) and the sample Δ47 values were expressed relative to the scrambled CO2. Measurements were made with a stable ~12 V signal at mass 44, with peak centring, background scanning, and pressure-balancing before each acquisition. Each sample was analysed for 10 acquisitions with 10 cycles in each acquisition using an integration time of 8 s (more details are given in Laskar et al., 2016a; 2019). In the second step, the measured Δ47 values, calculated using Eq. (1) were converted to the absolute scale following Dennis et al. (2011), described below.
Clumped isotope measurements are often affected by nonlinearity in the mass spectrometer, which leads to significant differences between the measured and the true R47 values. This can be corrected empirically by establishing a relation between the values of δ47 and Δ47 using a set of isotopically altered CO2 gas samples having different δ47 values but all equilibrated at a given temperature. Ideally, the Δ47 values should depend only on the temperature and the curve should be flat but in routine analysis this is not usually observed. We evaluated this empirical correction by a separate set of experiments. The δ47 value was varied by equilibrating (at 17 °C and 32 °C) two aliquots of CO2 (taken from the AS-2 cylinder) with three samples of waters having δ18O values varying from -109 ‰ to +22 ‰ (relative to VSMOW). A third set of experiments was done using scrambled CO2 at 1000 °C. We find that the Δ47 is linearly related with δ47 (Figure 3) with a slope of -0.0017 (for all the three cases). By definition, the corrected clumped isotope ratio of a sample CO2 is the Δ47 value corresponding to δ47 = 0 (Dennis et al., 2011). That is, the corrected value is obtained by applying the relation Δ47-0 = Δ47(ms) -0.0017 × δ47, where Δ47-0 is the corrected Δ47 value and Δ47(ms) is the Δ47 value obtained from the mass spectrometer. This corrected value is then expressed in the Absolute Reference Frame (ARF) using an Empirical Transfer Function. Empirical Transfer Function is a relation between the observed Δ47 values at different temperatures and those predicted theoretically at those temperatures (Wang et al., 2004). The Empirical Transfer Function used in the present study is given by: Δ47-ARF = 1.0996Δ47-0 + 0.9145. Details of the conversion procedure to obtain the transfer function is discussed elsewhere (Laskar and Liang, 2016; Laskar et al., 2019).
Figure 3.
Cross plot of Δ47 against δ47 of Equilibrated Gas (EG) relative to the Working Gas WG (in ‰) for CO2 equilibrated with water at 17 °C (filled circle) and 32 °C (star) and scrambled by heating at 1000 °C (triangle). The true Δ47 values of the samples were obtained by calculating the Δ47 values corresponding to δ47 = 0, which were then converted to the Absolute Reference Frame using the Empirical Transfer Function as discussed in the text.
In the analysis of mass spectrometric signal, a background correction is required where the net peak voltage is obtained by subtracting the baseline voltage just before the appearance of the peak. It is occasionally observed that there is a shift in the baseline signal in the sensitive collectors (m/z 47, 48 and 49) when a CO2 sample is introduced into the mass spectrometer. This may cause significant change in the final Δ47 (He et al., 2012; Daëron et al., 2016). In our case, the effect due to pressure induced baseline change at mass 47 was monitored with and without CO2 in the mass spectrometer. Without CO2 in the source, the typical baseline signal for mass 47 was ~5 mV which reduced to -7 mV and -0.5 mV, respectively, before and after the appearance of the mass 44 signal of ~12000 mV. Thus the drop in the baseline for mass 47, on introduction of CO2, was only about 0.5 % of the normal signal for mass 47 (~1900 mV). Coupled with the fact that the dependence of Δ47 on δ47 is small (Figure 3), no pressure baseline correction was necessary in our case.
As mentioned above, correction for nonlinearity in the mass spectrometer and procedure for conversion of the raw Δ47 numbers to values in absolute scale are discussed in detail in Laskar et al. (2016b). The analytical uncertainty in the Δ47 value is ±0.014 ‰ (1 σ std. dev.) as determined based on replicate analysis (n = 15) of IAEA NBS-19 (Table 1). Coupled with the analysis of masses 44 to 47, routine analysis of masses 48 and 49 was done to monitor the degree of interference from sample impurity on Δ47 (Ghosh et al., 2006). This is because presence of contaminants causes systematic changes in the sensitive ratios of Δ48 and Δ49. Carbonate formation temperature was estimated using the Δ47 values of CO2 and using a temperature-Δ47 relationship obtained by Kelson et al. (2017) using a large variety of synthetic carbonates. We assume that this value represents the oviduct temperature where the egg forms in equilibrium with the body fluids. For comparison, we also estimated temperatures from another commonly used relationship given by Ghosh et al. (2006). However, the final interpretation is based on the values obtained using the Kelson et al. (2017) relationship given below:
| Δ47 = 0.0407 × (106/T2) + 0.242 | (2) |
3. Results
3.1. Body temperatures of modern birds and reptiles
Our first aim was to establish the feasibility of Δ47 thermometry using modern eggshells following the analytical protocol discussed above. The body temperatures estimated from the Δ47 values of the modern birds and reptiles using Eq. (2) are presented in Table 2 along with the expected body temperatures. For birds, the expected body temperatures are taken from the literature while for reptiles they are assumed to be the same as the ambient air temperatures. For the 13 species of birds analysed here, the derived temperatures lie between 36 °C and 51 °C with an average of 42 °C, very similar to the average body temperature of birds (41 °C) known from earlier studies. However, a significant fluctuation is observed among different individuals of the same species. Clarke and Rothery (2008) studied the body temperature of 486 species of birds from 27 orders and 85 families and found that the values vary from 38 °C to 43.4 °C for body masses between 0.002 kg (Lophornis magnificus) to 104 kg (Struthio camelus). They did not find any statistically significant correlation between the body mass and the body temperature. Prinzinger et al. (1991) studied 26 bird orders and found three different ranges of body temperatures, 38.5 ± 1.0 °C (202 species), 41.0 ± 1.3 °C (724 species), and 43.9 ± 1.0 °C (74 species) that correspond to the resting phase, active phase and hyperactive phase, respectively. It is clear that depending on the intensity of activity, the body temperature fluctuates significantly even within the same species, a feature described as behavioural thermoregulation.
Table 2.
Stable isotope ratios (in ‰ relative to VPDB), δ47 rel. to Working Gas and Δ47 values (in Absolute Reference Frame ARF) in eggshell carbonates of modern birds and reptiles and estimated body temperatures using Kelson et al. (2017) calibration (Eq. 1 in the text).
| Name of species (n#) |
δ13 C ± 1 SE∗ (‰) VPDB |
δ18 O ± 1 SE∗ (‰) VPDB |
δ47 (‰) (WG) |
Δ47 ± 1 SE∗ (‰) (ARF) |
Temp. ± 1 SE∗ (oC) |
Expected Temp. range (oC)1,2 |
|---|---|---|---|---|---|---|
| Modern birds | ||||||
| From Madou Crocodiles King Zoo | ||||||
| Chicken (Gallus gallus domesticus) white egg (2) | -1.55 ± 0.02 | -5.12 ± 0.01 | 30.16 ± 0.04 | 0.630 ± 0.002 | 51 ± 1 | 38–44 |
| Duck (Anas platyrhynchos) (2) | -2.02 ± 0.10 | -5.68 ± 0.08 | 29.00 ± 0.20 | 0.654 ± 0.008 | 41 ± 3 | |
| Goose (Anser cygnoides) (3) | -0.42 ± 0.01 | -6.54 ± 0.01 | 29.66 ± 0.23 | 0.637 ± 0.008 | 48 ± 3 | |
| Mallard (Anas platyrhynchos) (3) | -1.95 ± 0.02 | -7.30 ± 0.01 | 27.37 ± 0.06 | 0.652 ± 0.002 | 42 ± 1 | |
| From local market, Taipei, Taiwan | ||||||
| Chicken (Gallus gallus domesticus) white egg (1) | -1.80 ± 0.007 | -7.65 ± 0.01 | 27.05 ± 0.01 | 0.649 ± 0.007 | 43 ± 3 | 38–44 |
| Chicken (Gallus gallus domesticus) brown egg (1) | 0.79 ± 0.01 | -5.21 ± 0.02 | 32.39 ± 0.02 | 0.636 ± 0.010 | 48 ± 4 | |
| Koyal Bird (Eudynamys scolopaceus) (3) | -1.72 ± 0.51 | -4.93 ± 0.87 | 29.89 ± 0.65 | 0.651 ± 0.001 | 42 ± 1 | |
| From Taipei Zoo | ||||||
| Great argus (Argusianus argus) (2) | -3.33 ± 0.24 | -2.12 ± 0.24 | 31.12 ± 0.81 | 0.650 ± 0.007 | 43 ± 3 | 38–44 |
| Mallard (Anas platyrhynchos) (2) | -6.10 ± 0.12 | -5.46 ± 0.11 | 25.34 ± 0.01 | 0.640 ± 0.007 | 47 ± 3 | |
| Javan myna (Acridotheres javanicus) (2) | -15.46 ± 0.11 | -3.28 ± 0.11 | 18.16 ± 0.07 | 0.659 ± 0.021 | 40 ± 8 | |
| Great curassow (Crax rubra) (2) | -4.07 ± 0.12 | -4.91 ± 0.11 | 27.27 ± 0.34 | 0.668 ± 0.005 | 36 ± 2 | |
| Common peafowl (Pavo cristatus) (2) | -7.59 ± 0.12 | -5.05 ± 0.10 | 24.38 ± 0.04 | 0.651 ± 0.004 | 42 ± 2 | |
| African penguin (Spheniscus demersus) (2) | -11.33 ± 0.02 | -5.72 ± 0.07 | 19.73 ± 0.05 | 0.665 ± 0.018 | 37 ± 7 | |
| King penguin (Aptenodytes petagoniscus) (2) | -14.90 ± 0.01 | -5.62 ± 0.01 | 16.51 ± 0.02 | 0.660 ± 0.004 | 39 ± 2 | |
| Swinho Pheasant (Lophura swinhoii) (3) | -2.28 ± 0.20 | -2.65 ± 0.07 | 31.75 ± 0.28 | 0.660 ± 0.006 | 39 ± 2 | |
| Modern reptiles | ||||||
| From Madou Crocodiles King Zoo | ||||||
| Spectacled Ciaman (Caiman crocodilus) (2) | -18.42 ± 0.02 | -7.68 ± 0.18 | 10.74 ± 0.06 | 0.716 ± 0.002 | 20 ± 1 | 26 ± 3$ |
| Saltwater crocodile (Crocodylus porosus) (1) | -14.10 ± 0.01 | -6.58 ± 0.01 | 16.35 ± 0.03 | 0.716 ± 0.011 | 20 ± 3 | |
| False gharial (Tomistoma schlegelii) (2) | -6.69 ± 0.42 | -6.34 ± 0.16 | 14.35 ± 0.02 | 0.714 ± 0.001 | 21 ± 1 | |
| Asiatic softshell turtle (Amyda cartilaginea) (2) | -11.71 ± 1.11 | -9.78 ± 1.41 | 17.37 ± 0.11 | 0.701 ± 0.002 | 25 ± 1 | |
| Chinese stripped necked turtle (Ocadia sinensis) (2) | -10.59 ± 1.84 | -4.75 ± 1.48 | 17.00 ± 0.01 | 0.737 ± 0.004 | 14 ± 1 | |
| Florida softshell turtle (Apalone ferox) (2) | -13.78 ± 1.57 | -8.03 ± 1.19 | 15.48 ± 0.03 | 0.674 ± 0.003 | 34 ± 1 | |
| African spurred tortoise (Geochelone sulcata) (2) | -11.91 ± 0.38 | -4.75 ± 1.49 | 19.43 ± 0.09 | 0.714 ± 0.012 | 21 ± 4 | |
| Redfooted tortoise (Chelonoidis carbonaria) (2) | -9.86 ± 0.36 | -5.52 ± 0.01 | 23.23 ± 0.01 | 0.714 ± 0.013 | 21 ± 4 | |
| Indian python (Python molurus) (2) | -9.79 ± 0.65 | -3.08 ± 0.88 | 26.40 ± 0.11 | 0.801 ± 0.001 | BR | |
| Corn snake (Pantherophis guttatus guttatus) (1) | -12.42 ± 0.01 | -4.61 ± 0.02 | 19.72 ± 0.02 | 0.796 ± 0.021 | BR | |
| Ball Python (Ophiophagus Hannah) (1) | -10.72 ± 0.01 | -4.35 ± 0.01 | 21.68 ± 0.02 | 0.786 ± 0.012 | BR | |
| From Taipei Zoo | ||||||
| Yellow pond turtle (Mauremys mutica mutica) (3) | -11.26 ± 0.19 | -3.46 ± 0.14 | 22.20 ± 0.24 | 0.683 ± 0.004 | 31 ± 2 | 27 ± 3$ |
| Elongated tortoise (Indotestudo Elongata) (2) | -13.85 ± 0.48 | -3.78 ± 0.54 | 19.48 ± 0.07 | 0.700 ± 0.006 | 25 ± 2 | |
| Burmese star tortoise (Geochelone Platynota) (2) | -12.34 ± 0.16 | -5.34 ± 0.45 | 19.36 ± 0.21 | 0.705 ± 0.005 | 23 ± 2 | |
| Redfooted tortoise (Geochelone carbonaria) (2) | -13.32 ± 1.80 | -5.91 ± 0.64 | 17.80 ± 0.26 | 0.705 ± 0.003 | 24 ± 1 | |
| Sudan plated lizard (Gerrhosaurus major) (2) | -16.40 ± 0.11 | -4.66 ± 0.11 | 15.56 ± 0.21 | 0.699 ± 0.012 | 25 ± 4 | |
#Number of analysis; ∗Standard error by compounding all sources of error.
2Clarke and Rothery (2008); BR: beyond the range of calibration.
$Average temperature of October, 2014 of Tainan and July–August, 2014 of Taipei.
For reptile samples from the Madou Crocodile King Zoo, temperatures estimated from the Δ47 values should be compared to the environmental temperature, i.e., the mean October temperature during 2014 (26 ± 3 °C) in the Tainan region. For Taipei zoo, we take 27 °C as the environmental temperature (the mean of night-time temperature of 25 °C and day-time temperature of 29 °C) for comparison. Our estimate, based on analysis of the eggshells of sixteen species of reptiles, ranges between 20 °C and 34 °C (excluding one outlier and the snake samples). The average value and standard deviation are 24 and 4 °C, respectively. The average value is slightly less than the mean environmental temperature (~27 °C) mentioned above. It is known that the variation in the body temperature of reptiles (Table 2) is controlled by two factors, their size and environmental temperature. Since they lack any active metabolic control, these animals absorb the available environmental heat and regulate their behaviour accordingly to maintain a preferred body temperature (Raske et al., 2012). On the other hand, the estimated body temperatures of birds (42 ± 4 °C) are much higher than the environmental temperature (~27 °C) as expected from their endothermic nature.
To show how well the estimated temperatures from Δ47 values of the biogenic carbonates agree with the expected values inferred from inorganic carbonates precipitated at known temperatures, we compare the eggshell Δ47 values with the available synthetic carbonate data (Figure 4). A plot of the Δ47 values of artificially synthesized carbonates as a function of 1/T2 (following the Kelson et al. (2017) relation given above) is shown in Figure 4A. We also show a second plot following Ghosh et al. (2006) calibration for comparison with the earlier publications. In the same figure, we plot the measured Δ47 values of the birds and reptiles (Table 2) along with their expected body temperatures. The latter values are taken from the Clarke and Rothery (2008) compilation (for birds) while for the reptiles, the mean air temperature of the sampling month is assumed to represent the body temperature. Despite the large uncertainty introduced by analytical errors and sample heterogeneity, the Δ47 -1/T2 points scatter around the two calibration lines (or expected lines) quite well. This indicates that the assumption of thermodynamic equilibrium during the biogenic carbonate precipitation within the body is reasonably valid. However, the points do show some deviations from the lines, especially for the reptiles. The deviations could be due to one or more of the following reasons: (1) behavioural thermoregulation causing some deviation in the body temperature of the reptiles from the environmental temperature of the region; (2) applicability of the Δ47 versus temperature calibration lines (discussed later), and (3) vital effects i.e., deviation from equilibrium fractionation. Notwithstanding these issues, we note that, even if the exact temperatures are not recovered for each species, there is a clear distinction between the endotherms and the ectotherms in terms of Δ47 values (see Figure 4B). This is discussed later with more detailed statistics.
Figure 4.
(A) Plot between Δ47 and 1/T2 for modern birds and reptiles along with the calibrations made using synthetic carbonates precipitated at known temperatures (Kelson et al., 2017; Ghosh et al., 2006). For the modern birds, the average of actual body temperatures available from the literature (Clarke and Rothery, 2008) are used (temperatures on the top axis). For reptiles, the average air temperature during October 2014 (~26 °C for Madou Crocodile King Zoo) and 27 °C for Taipei Zoo are used. The cross indicates error bars where the horizontal bar shows ±1 σ standard deviation in the Δ47 values and the vertical bar shows the range of body temperature (for birds) or the environmental temperature (for reptiles). (B) Temperature as a function of Δ47 values for modern birds, reptiles and Cretaceous dinosaurs. Temperatures were estimated from the Δ47 values using the calibration based on Kelson et al. (2017) (see Eq. (1) in the text). 1 standard errors associated with the measured Δ47 values and corresponding estimated temperatures are shown as a cross at the bottom right of the plots. The error gives total uncertainty calculated from the uncertainties in calibration and sample Δ47 values.
We note that the temperature estimates for the four snake species lie beyond the lower range of the Kelson et al. (2017) calibration. This could be due to an analytical artefact caused by the low carbonate content of the snake eggshells. In the case of some species such as the California King Snake the carbonate amount was too low. We had to collect 300–500 mg of eggshell samples for these species to get 20–30 μmole of CO2. Therefore, some contamination effect in these eggshells cannot be ruled out.
We conclude that, barring the snakes, clumped thermometry for eggshells of modern species is feasible and could be improved to obtain better temperature estimates with appropriate calibration. The clustering of the Δ47 values into two distinct groups gives confidence for obtaining the body temperatures of extinct dinosaurs using their fossilized eggshells.
3.2. Diagenetic alteration
To infer the temperature from Δ47 values, it is important to ensure that the fossilized material preserves the primary isotopic signature. It is known that alteration of 13C-18O bond ordering is possible through dissolution/re-precipitation reactions associated with the intrusion of a secondary carbonate solution (Eagle et al., 2015; Dennis and Schrag, 2010). However, such modifications, known as diagenetic alterations, normally occur at temperatures higher than ~250 °C (Dennis and Schrag, 2010) and destroy the typical shell crystalline structure. Scanning electron microscope (SEM) photograph of a few of our eggshell fragments (Figure 2) reveal that the primary shell structure has remained unchanged. The original microstructure is characterized by unaltered discrete to fused shell units with well-preserved mammillae of prismatic and spongy layers. The radiating lines indicate well-preserved radial growth of shell calcite. The SEM picture of Sarkar et al. (1991) of eggshells from the same locality also showed microstructure of arched incremental lines in spherolith (albeit at lower resolution) and how they increase in size with the shell growth. It is known that unlike birds who lay a single egg every day in any season, all contemporary reptiles including crocodiles, turtles, snakes and lizards lay all of their eggs arranged in a clutch in a single session which happens generally during the summer. Among the samples analysed here, a set of eggs came from a single nest in the limestone; all of them have similar morphology, micro- and histo-structure as expected for eggs from a single individual ruling out microscopic alterations (Mohabey, 1998).
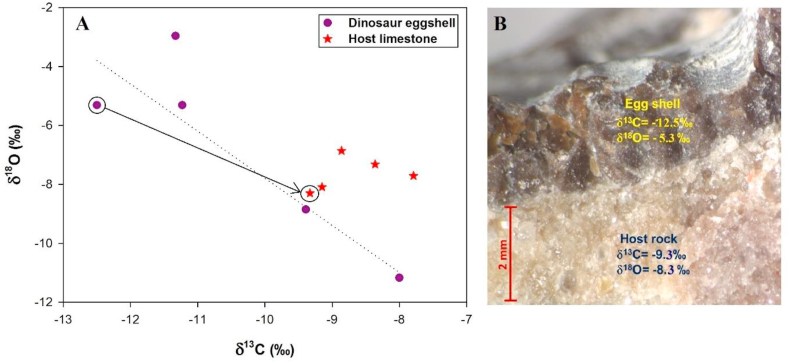
Additional evidence regarding the absence of significant diagenetic changes in shells from this region is provided by the stable isotopic compositions and MgO content (Sarkar et al., 1991). The δ18O values of the eggshell carbonates differ significantly from those of the surrounding limestone and the MgO content in eggshells is about six times less than that of the host limestones (Sarkar et al., 1991). We also argue that unaltered primary eggshells should be different in isotopic composition from coexisting carbonates of lacustrine origin. The δ13C and δ18O values of some of the eggshells and attached cement carbonates are presented in Table 3 and Figure 5. Large differences in the isotopic compositions of the eggshell and cement carbonates indicate that significant diagenetic alteration could not have taken place as alteration would cause homogenization of δ13C and δ18O values. For example, the differences in δ13C and δ18O values between the eggshell and host rock for Khempur samples are quite large at 3.2 and 3.0 ‰ respectively (Figure 5). On the basis of these evidences, we believe that diagenetic alteration in the isotopic composition associated with burial could be ruled out for most of the eggshells of this region.
Table 3.
Bulk isotope ratios of eggshells and their host limestone's for some selected samples showing significant difference in values (in ‰ relative to VPDB).
| Location | Sample | δ13 C (VPDB) | δ18 O (VPDB) |
|---|---|---|---|
| SN-1 Rahioli clutch 1 |
Eggshell | -9.39 | -8.85 |
| Host limestone | -8.86 | -6.86 | |
| SN-3 Rahioli clutch 2 |
Eggshell | -8.00 | -11.17 |
| Host limestone | -9.15 | -8.09 | |
| SN-4 Khempur |
Eggshell | -12.50 | -5.31 |
| Host limestone | -9.33 | -8.30 | |
| SN-5 Lameta ghat |
Eggshell | -11.23 | -5.31 |
| Host limestone | -7.79 | -7.71 | |
| SN-7 Dohad |
Eggshell | -11.33 | -2.96 |
| Host limestone | -8.36 | -7.32 |
Figure 5.
(A) Cross plot of δ18O-δ13C values to show that the host limestone samples plot away from the correlation line of eggshell carbonate points suggesting insignificant diagenetic alterations. The arrow shows the difference in isotopic values between the eggshell and the host limestone for the Khempur sample. (B) Cementation of eggshell with host limestone along with δ13 C and δ18 O values in the two phases of the Khempur sample.
3.3. Dinosaur body temperatures
The estimated body temperatures of the four species of sauropods from five locations lie in the range of 29 °C–47 °C (Table 4). Two sauropod eggshell samples (SN-1, SN-2), taken from two different eggs of the same clutch in the Rahioli area, yielded quite different temperatures of 34 and 46 °C, respectively. One eggshell sample (SN-3) of the same species from the same nest-site but from a different clutch gave a temperature of 44 °C. Samples from Khempur (SN-4), Lameta Ghat (SN-5) and Padali (SN-6) gave consistently low temperatures of 32 °C, 29 °C, and 29 °C, respectively. In contrast, two samples from Dohad (SN-7 and SN-8) gave higher values of 37 °C and 47 °C. It is surprising to see that eggs from the same clutch can yield quite different values. This variation in the estimated body temperature within the same clutch could possibly be due to some vital effect causing departure from isotopic equilibrium inside the oviduct during the individual egg formation. The theropod eggshells (SN-9 and SN-10) from two clutches at Lavariya Muwada yielded similar body temperatures of 37 °C and 40 °C. They are considered similar because the error in the estimated temperature due to a combination of the analytical errors and the uncertainty in the calibration curve is ~3 °C (1 σ).
Table 4.
Stable isotope ratios including Δ47 values of dinosaur eggshell carbonates obtained from six localities in India and estimated body temperatures using Kelson et al. calibration (Eq. (1) in text). Isotopic ratios are in ‰ relative to VPDB. WG = Working Gas; ARF = Absolute Reference Frame.
| Sample No. | Name of species (n#) (ref. 29) | Locality | Taxonomic affinity | δ13 C ± 1σ∗ | δ18 O ± 1σ∗ | δ47 ± 1σ∗(‰) (WG)$ | Δ47 ± 1 σ∗ in ‰ ARF | Temp ± 1σ∗ (oC) |
|---|---|---|---|---|---|---|---|---|
| SN-1 | Megaloolithus rahioliensis (2) | Rahioli clutch 1 | Sauropod (Titanosuriforme) | -9.39 ± 0.01 | -8.85 ± 0.17 | 18.57 ± 0.47 | 0.674 ± 0.009 | 34 ± 3 |
| SN-2 | Megaloolithus rahioliensis (3) | Rahioli Clutch 1 | Sauropod (Titanosuriforme) | -9.26 ± 0.02 | -8.64 ± 0.15 | 18.80 ± 0.19 | 0.641 ± 0.008 | 46 ± 3 |
| SN-3 | Megaloolithus rahioliensis (2) | Rahioli Clutch 2 | Sauropod (Titanosuriforme) | -8.00 ± 0.10 | -11.17 ± 0.02 | 17.28 ± 0.17 | 0.647 ± 0.002 | 44 ± 1 |
| SN-4 | Megaloolithus khempurensis(3) | Khempur | Sauropod (Titanosuriforme) | -12.50 ± 0.02 | -5.31 ± 0.13 | 19.12 ± 0.30 | 0.678 ± 0.020 | 32 ± 7 |
| SN-5 | Megaloolithus sp. (3) | Lameta Ghat (Jabalpur) | Sauropod (Titanosuriforme) | -11.23 ± 0.12 | -5.31 ± 0.12 | 21.30 ± 1.13 | 0.689 ± 0.021 | 29 ± 7 |
| SN-6 | Mealoolithus megadermus (3) | Padali (Bagh) | Sauropod (Titanosuriforme) | -10.38 ± 0.12 | -8.81 ± 0.32 | 17.86 ± 0.52 | 0.688 ± 0.020 | 29 ± 7 |
| SN-7 | Megalooltus megadermus (2) | Dohad | Sauropod (Titanosuriforme) | -11.33 ± 0.01 | -2.96 ± 0.13 | 23.02 ± 0.53 | 0.665 ± 0.001 | 37 ± 1 |
| SN-8 | Megaloolitus megadermus (4) | Dohad | Sauropod (Titanosuriforme) | -11.33 ± 0.04 | -3.19 ± 0.36 | 22.87 ± 1.13 | 0.640 ± 0.019 | 47 ± 8 |
| SN-9 | Ellipsoolithus khedaensis (4) | Lavariya Muwada Clutch 1 | Theropod (Abelisaurid) | -11.31 ± 0.10 | -7.03 ± 0.20 | 18.12 ± 0.42 | 0.659 ± 0.004 | 40 ± 2 |
| SN-10 | Ellipsoolithus khedaensis (3) | Lavariya Muwada Clutch 2 | Theropod (Abelisaurid) | -11.18 ± 0.22 | -7.20 ± 0.12 | 17.96 ± 0.32 | 0.666 ± 0.007 | 37 ± 3 |
#Number of analysis.
∗Standard deviation.
$Larger standard deviation is partly due to use of different aliquots of AS-2 (WG) used at different times. As the aliquots were calibrated independently, the variation is not reflected in the conventional isotope ratios and the clumped isotope ratios.
4. Discussion
Overall, the clumped isotope thermometry of the sampled eggshells from localities in the western and central part of India yields body temperatures for the dinosaurs in the range of 29–47 °C and 37–40 °C for the sauropod and theropod species, respectively. It is clear that the average of the estimated body temperatures is close to that of the modern mammals. However, there is a large fluctuation, which probably indicates variable thermoregulation between and among the different species, a conclusion consistent with a recent study of dinosaur eggshells (Eagle et al., 2015; Dawson et al., 2020). It is also possible that some of the dinosaur species regulated their body temperatures to some extent by altered behaviour, while some others could maintain theirs like modern endotherms (Dawson et al., 2020). Two more factors that might control the variation are intermediate metabolism (Clarke, 2013) and mesothermic property (Grady et al., 2014). In addition, some fluctuations could also be due to minor diagenetic alterations in some of the samples. Figure 6 shows box and whisker plots and bootstrap distribution of the Δ47 values of the eggshells of modern birds and reptiles and extinct dinosaurs. A large separation between the modern birds and reptiles indicate that their eggshell carbonate Δ47 values are controlled by their body temperatures. The intermediate Δ47 values of the dinosaurs and a relatively larger variation are an indicator of the complex and multiple controlling factors discussed above. The major constraint here is the number of samples available for analysis. Their limited number does not allow us to reach robust conclusions regarding the causes of variation in body temperatures.
Figure 6.
Box-and-whisker plots (top) and bootstrap probability density distributions with kernel smoothing (bottom) of the mean Δ47 values (see Tables 2 and 3 for the values). The vertical lines inside the boxes represent the mean value and the box limits are the upper and lower quartiles of each group; the whiskers are the 5th and 95th percentiles. The separation of the dinosaur group from Birds (endothermic) and Reptiles (ectothermic) indicates variable thermoregulation.
We also examined whether the dinosaur body temperature was influenced in any way by the ambient temperature due to an ectothermic biochemistry. As mentioned, the mean annual temperature in the sampling region during the Late Cretaceous was ~25 °C, which is much lower than the observed theropod body temperatures (see Section 4.1). This goes against a typical ectothermic behaviour. In contrast, it is possible that the lower body temperature (~29 °C) of some sauropod dinosaurs reflects merely the environmental temperature, similar to the modern ectothermic animals.
An important factor controlling the body temperatures of modern animals is behavioural thermoregulation. This property confounds the dichotomous categorization into ectothermic and endothermic animals. Depending on the environmental conditions, the animals often change their behavioural, anatomical and physiological conditions to avoid over-heating or excess heat loss to the surroundings (Raske et al., 2012). For example, in cold conditions, endotherms may decrease the body exposure to minimize heat loss and vice versa. Similarly, ectotherms may try to increase or decrease heat exchange with the environments depending on the situation. For example, a temperature variation of ~5 °C in the same species of birds but living under different conditions has been reported by Prinzinger et al. (1991). The assumption that the body temperatures of reptiles in the summer is the same as the surroundings is probably an oversimplification as they may try to regulate their body temperatures during extreme conditions. For example, at the peak summer temperature of Taiwan (~35 °C), endotherms would adopt measures such as resting under relatively cold water to maintain body temperature. Thus, an assumed mean environmental temperature may not correctly represent the actual body temperature. It is possible that dinosaurs adopted some kind of behavioural thermoregulation that lead to a discrepancy between the estimated and expected body temperatures.
Some difference in the temperature estimates using Kelson et al. (2017) and Ghosh et al. (2006) calibrations is observed (Tables 2 and 4). The two calibrations match at ~25 °C but due to difference in the slopes the estimated temperatures diverge on both sides of 25 °C. In the present study, we preferred to use the Kelson et al. (2017) relationship, because it was derived by using diverse types of carbonate samples. Like all other clumped isotope applications, a calibration curve with simultaneously measured body temperatures and Δ47 values of modern eggshell carbonates would be the most appropriate to use in the future. It is also important to investigate if there is any difference between the calibrations for endothermic and ectothermic species.
4.1. Body temperature versus body mass
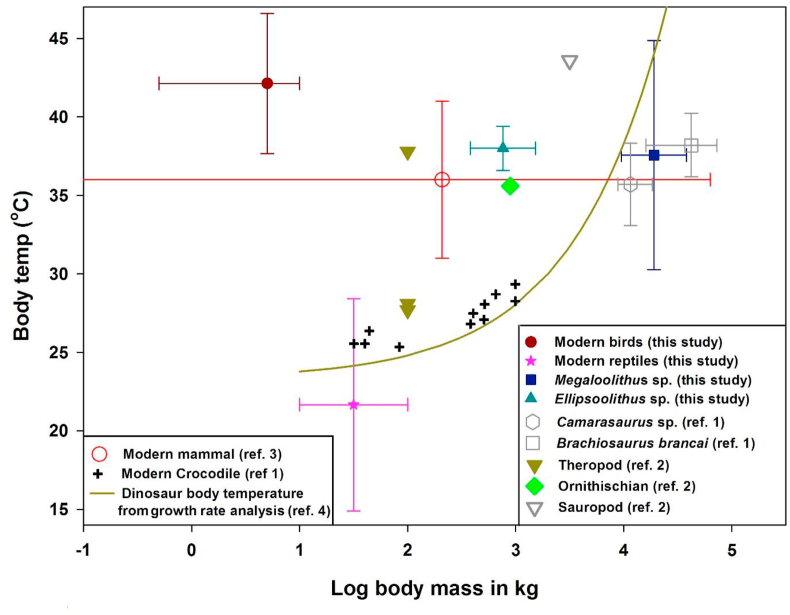
For Indian dinosaurs, no bone skeletons (partial or full), embryonic remains or hatched babies have been recovered so far, and hence no direct estimate of body size or mass is available. However, circumstantial evidences suggest the presence of titanosaurids (Jainosaurus, Isisaurus) and abelisaurid theropods (Large theropods: Rajasaurus, Rahiolisaurus, Indosuchus, Indosaurus and small theropods: Laevisuchus/Noavasaurid) in India during the Late Cretaceous. The eggshells studied in the present work were recovered from sediments known to have been deposited during the magnetochron C30N of the Maastrichtian era (Courtillot et al., 1988). Remains of Rajasaurus and bones of a titanosauriforme have been found in these egg-bearing sediments. In addition, Isisaurus remains from 30N sediments of Nand-Dongargaon basin and Jainosaurus remains from the younger Maastrichtian C29R sediments (Samant and Mohabey, 2014) have been discovered. Through this association we classify our sauropods as of Isisaurus and theropods as of Rajasaurus with tentative body masses of ~20000 kg and ~800 kg, respectively (Benson et al., 2014) (Michael D. D'Emic, personal communication). An uncertainty of a factor two in these estimates is possible based on an analogy with the body mass range of modern reptiles. As mentioned before, body temperatures of ectothermic animals follow the environmental temperature and also depend on the body mass (Seebacher, 2003). The present study shows that the dinosaurs discussed herein do not present such a trend. For example, the two theropod species with tentative body masses of ~800 kg had their body temperatures significantly higher than the environmental temperature. On the other hand, among the gigantic sauropod species, a few had body temperatures slightly higher than the environmental temperature but distinctly lower than that expected for the high body mass. This suggests that their body mass cannot be taken as an indicator of thermal control mechanism and possibly they were endowed with a capacity of variable thermoregulation.
The temperature estimations by clumped isotope thermometry may have some bias based on our data for modern birds and reptiles. Body temperatures of all the species measured herein along with literature data for modern mammals (Clarke and Rothery, 2008) and model predicted body temperatures of reptiles with different body masses are shown in Figure 7. The model predicts a body temperature of ~41 °C for sauropods at a body mass of 15000 kg (Gillooly et al., 2006) which is similar to the highest temperature estimated in the present study (within 2 σ uncertainty). Such a high temperature could be due to inertial homeothermy or gigantohthermy proposed by earlier workers (Gillooly et al., 2006). We also obtained a temperature as low as 29 °C, for the same sauropod species, which is similar to the modern reptiles. The observations suggest that these fluctuations in the body temperatures, especially relatively higher body temperatures for smaller theropod species and lower than expected body temperatures for a few of the giant animals (suggesting partly ectothermic nature) point to variable thermoregulation in these dinosaurs. Comparison of the dinosaur's body temperatures with data from the studies by Eagle et al. (2013) and Dawson et al. (2020) shown in Figure 7 makes it clear that overall the ranges of temperatures among the studies agree well except for a small number of species analysed by Dawson et al. (2020) from Wann's Hill, Canada. It is possible that some of the dinosaur species were ectothermic while others had intermediate or even endothermic metabolism which demands further investigation with more samples from wider geographical distributions.
Figure 7.
Inferred body temperatures of various egg-laying species as a function of body mass. Body mass represents the size at which the maximum growth occurs (about half of the adult size). The body masses for the sauropod and theropod species sampled here are ~20000 kg and ~800 kg respectively (see text for details). Vertical bar represents 1 σ (standard deviation) derived from the Δ47 values. The horizontal bar represents the range of adult body mass. Body temperatures obtained from the measured Δ47 values of the selected modern birds and reptiles along with 1 σ standard error are shown. Modern crocodile and modern mammal data are taken from Eagle at al. (2011; ref 1) and Clarke and Rothery (2008; ref. 3) respectively. Body temperatures of dinosaurs derived from the carbonate Δ47 values obtained from two other studies (Eagle at al. 2011; ref. 1 and Dawson et al., 2020; ref. 2) are plotted for comparison. The curved line represents an empirical model in which the body temperatures are scaled with body mass using fossil dinosaurs (Gillooly et al., 2006; ref. 4).
5. Summary
Body temperatures (oviduct temperature) of egg-laying animals were estimated using clumped isotope thermometry of their eggshells (composed of carbonate minerals). The estimated temperatures in modern eggshells match the body temperature of the animals reasonably well except for minor positive bias for some birds (up to 7 °C too warm), and possible negative bias for some reptiles (down to 8 °C too cold). The success of this exercise allowed us to apply this method to obtain body temperatures of Late Cretaceous Indian dinosaurs using fossil eggshells. The derived body temperatures of dinosaurs range from 29 °C to 47 °C for sauropods and 37 °C–39 °C for theropods when the environmental temperature in Cretaceous central India was about 25 °C. The large range of body temperatures and particularly the variation within the same species indicate variable thermoregulation including some amount of behavioural effect. In addition, the anomalous presence of low body mass but high body temperature theropods and high body mass but low body temperature sauropods can only be explained with the variable thermoregulation hypothesis.
Declarations
Author contribution statement
A. H. Laskar, S. K. Bhattacharya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
D. Mohabey: Analyzed and interpreted the data; Wrote the paper.
M. -C. Liang: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
M.-C. Liang was supported by the Ministry of Science and Technology, Taiwan (101-2628-M-001-001-MY4, 105-2111-M-001-006-MY3, 105-2119-M002-001, and 108-2111-M-001-011-MY3) and Academia Sinica (Career Development Award). A. H. Laskar and S. K. Bhattacharya were supported by the Ministry of Science and Technology, Taiwan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the authorities of Taipei Zoo (in Taipei) and Madou Crocodiles King Zoo (in Tainan) for providing eggshells of birds and reptiles. We thank Michael D'Emic for providing the body weight estimates of the dinosaur species. We thank Shraddha Band for help on bulk isotopic measurements of the host rocks.
Contributor Information
Amzad H. Laskar, Email: amzad@prl.res.in.
Mao-Chang Liang, Email: mcl@gate.sinica.edu.tw.
References
- Amiot R., Lécuyer C., Buffetaut E., Escarguel G., Fluteau F., Martineau F. Oxygen isotopes from biogenic apatites suggest widespread endothermy in Cretaceous dinosaurs: Earth Planet. Sci. Lett. 2006;246:41–54. [Google Scholar]
- Bajpai S., Sahni A., Sriniwsan S. Ornithoid eggshells from Deccan intertrappean bed near Anjar (Kachchh), western India. Curr. Sci. 1983;64:42–45. [Google Scholar]
- Bajpai S., Sahni A., Jolly A., Srinivasan S. Kachchh Intertrappean biotas: affinities and correlation. In: Sahni A., Jolly A., editors. Cretaceous Event Stratigraphy and the Correlation of the Indian Non-marine Strata, Chandigarh. 1990. pp. 101–105. [Google Scholar]
- Bakker R.T. Anatomical and ecological evidence of endothermy in dinosaurs. Nature. 1972;238:81–85. [Google Scholar]
- Barrick R.E., Showers W.J., Fischer A.G. Comparison of the thermoregulation of four ornithischian dinosaurs and a varanid lizard from the Cretaceous two medicine formation: evidence from oxygen isotopes. Palaios. 1996;11:295–305. [Google Scholar]
- Barrick R.E., Stoskopf M.K., Showers W.J. Oxygen isotopes in dinosaur bone. In: Farlow J.O., Brett-Surman M.K., editors. The Complete dinosaur. Indiana University Press; Bloomington: 1997. pp. 474–490. [Google Scholar]
- Benson R.B.J., Campione N.E., Carrano M.T., Mannion P.D., Sullivan C. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 2014;12(5) doi: 10.1371/journal.pbio.1001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigeleisen J. Chemistry of isotopes. Science. 1965;147:463–471. doi: 10.1126/science.147.3657.463. [DOI] [PubMed] [Google Scholar]
- Chen J., Zhao P., Wang C., Huang Y., Cao K. Modeling East Asian climate and impacts of atmospheric CO2 concentration during the late cretaceous (66 Ma) Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013;385:190–201. [Google Scholar]
- Chiappe I.M., Rodolpho A.C., Dinus L., Jackson F., Chinasamy A., Marllyn F. Sauropod dinosaur embryos from the late cretaceous of patagonia. Nature. 1998;396:258–261. [Google Scholar]
- Chinsamy A. Bone histology and growth trajectory of theprosauropod dinosaur Massopondylus carinatus Owen. Mod. Geol. 1993;18:319–329. [Google Scholar]
- Clarke A., Rothery P. Scaling of body temperatures in mammals and birds. Funct. Ecol. 2008;22:58–67. [Google Scholar]
- Clarke A. Dinosaur energetics: setting the bounds on feasible physiologies and ecologies. Am. Nat. 2013;182(3):283–297. doi: 10.1086/671259. [DOI] [PubMed] [Google Scholar]
- Courtillot V., Feraud G., Maluski H., Vandamme D., Moreau M.G., Besse J. Deccan flood basalts and the Cretaceous/Tertiary boundary. Nature. 1988;333:843–846. [Google Scholar]
- de Ricqlès A.J. Evolution of endothermy: histological evidence. Evol. Theor. 1974;1:51. [Google Scholar]
- Daëron M., Blamart D., Peral M., Affek H. Absolute isotopic abundance ratios and the accuracy of Δ47 measurements. Chem. Geol. 2016;441:83–96. [Google Scholar]
- Dawson R.R., Field D.J., Hull P.M., Zelenitsky D.K., Therrien F., Affek H.P. Eggshell geochemistry reveals ancestral metabolic thermoregulation in Dinosauria. Sci. Adv. 2020;6(7) doi: 10.1126/sciadv.aax9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis K.J., Affek H.P., Passey B.H., Schrag D.P., Eiler J.M. Defining an absolute reference frame for ‘clumped’ isotope studies of CO2. Geochem. Cosmochim. Acta. 2011;75:7117–7131. [Google Scholar]
- Dennis K.J., Schrag D.P. Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochem. Cosmochim. Acta. 2010;74:4110. [Google Scholar]
- Eagle R.A., Schauble E.A., Tripati A.K., Tütken T., Hulbert R.C., Eiler J.M. Body temperatures of modern and extinct vertebrates from 13C-18O bond abundances in bioapatite: Proc. Nat. Acad. Sci. 2010;107(23):10377–10382. doi: 10.1073/pnas.0911115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle R.A., Eiler J.M., Tripati A., Ries J.B., Freitas P.S., Hiebenthal C., Wanamaker A.D., Taviani M., Elliot M., Marenssi S., Nakamura K., Ramirej P., Roy K. The influence of temperature and seawater carbonate saturation state on 13C–18O bond ordering in bivalve mollusks. Biogeosciences. 2013;10:4591–4606. [Google Scholar]
- Eagle R.A., Tütken T., Martin T.S., Tripati A.K., Fricke H.C., Connely M., Cifelli R.L., Eiler J.M. Dinosaur body temperatures determined from isotopic (13C-18O) ordering in fossil biominerals. Science. 2011;333:443–445. doi: 10.1126/science.1206196. [DOI] [PubMed] [Google Scholar]
- Eagle R.A., Enriquez M., Grellet-Tinner G., Pérez-Huerta A., Hu D., Tütken T., Montanari S., Loyd S.J., Ramirez P., Tripati A.K., Kohn M.J., Cerling T.E., Chiappe L.M., amd Eiler J.M. Isotopic ordering in eggshells reflects body temperatures and suggests differing thermophysiology in two Cretaceous dinosaurs. Nat. Commun. 2015;6:8296. doi: 10.1038/ncomms9296. [DOI] [PubMed] [Google Scholar]
- Eiler J.M. “Clumped-isotope” geochemistry—the study of naturally-occurring, multiply-substituted isotopologues. Earth Planet Sci. Lett. 2007;262(3–4):309–327. [Google Scholar]
- Erben H.K., Hoefs J., Wedepohl K.H. Palaeobiologicand isotope studies of eshells from a declining dinosaur species. Palaeoiology. 1979;5(4):380–414. [Google Scholar]
- Erickson G.M. Assessing dinosaur growth patterns: a microscopic revolution. Trends Ecol. Evol. 2005;20:677. doi: 10.1016/j.tree.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Farlow J.O. Dinosaur energetics and thermal biology. In: Weishampel D.B., Dodson P., Osmólska H., editors. The Dinosauria. first ed. University of California Press; Berkeley: 1990. pp. 43–55. [Google Scholar]
- Fricke H.C., Rogers R.R. Multiple taxon–multiple locality approach to providing oxygen isotope evidence for warm-blooded theropod dinosaurs. Geology. 2000;28:799. [Google Scholar]
- Ghosh P., Adkins J., Affek H.P., Balta B., Guo W., Schauble E., Schrag D., Eiler J.M. 13C–18O bonds in carbonate minerals: a new kind of paleothermometer. Geochim. Cosmochim. Acta. 2006;70:1439–1456. [Google Scholar]
- Ghosh P., Vasiliev M.V., Ghosh P., Sarkar S., Ghosh S., Yamada K., Ueno Y., Yoshida N., Poulsen C.J. Tracking the migration of the Indian continent using the carbonate clumped isotope technique on Phanerozoic soil carbonates. Sci. Rep. 2016;6:22187. doi: 10.1038/srep22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebeler E.M. Body temperatures in dinosaurs: what can growth curves Tell Us. PLoSONE. 2013;8(10) doi: 10.1371/journal.pone.0074317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly J.F., Allen A.P., Charnov E.L. Dinosaur fossils predict body temperatures. PLoS Biol. 2006;4(8):1467–1469. doi: 10.1371/journal.pbio.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady J.M., Enquist B.J., Robinson E.D., Wright N.A., Smith F.A. Evidence for mesothermy in dinosaurs. Science. 2014;344:1268–1272. doi: 10.1126/science.1253143. [DOI] [PubMed] [Google Scholar]
- Guo W. California Institute of Technology; California: 2008. Carbonate Clumped Isotope Thermometry: Application to Carbonaceous Chondrites and Effects of Kinetic Isotope Fractionation: Pasadena. Ph.D. thesis. [Google Scholar]
- Hirsch K.F., Packard M. Review of fossil eggs and their shell structure. Scanning Microsc. 1987;1(1):383–400. [Google Scholar]
- He B., Olack G.A., Colman A.S. Pressure baseline correction and high-precision CO2 clumped-isotope (Δ47) measurements in bellows and micro-volume modes. Rapid Commun. Mass Spectrom. 2012;26(24):2837–2853. doi: 10.1002/rcm.6436. [DOI] [PubMed] [Google Scholar]
- IAEA . 2006. Reference Sheet for International Measurement Standards, Vienna, Austria. [Google Scholar]
- Kelson J.R., Huntington K.W., Schauer A.J., Saenger C., Lechler A.R. Toward a universal carbonate clumped isotope calibration: diverse synthesis and preparatory methods suggest a single temperature relationship. Geochem. Cosmochim. Acta. 2017;197:104–131. [Google Scholar]
- Klein N., Sander P.M. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology. 2008;34:247–263. [Google Scholar]
- Kohn M.J., Law M. Stable isotope chemistry of fossil bone as a new paleoclimate indicator. Geochem. Cosmochim. Acta. 2006;70(4):931–946. [Google Scholar]
- Laskar A.H., Yui T.F., Liang M.C. Clumped isotope composition of marbles from the backbone range of Taiwan. Terra. Nova. 2016;28(4):265–270. [Google Scholar]
- Laskar A.H., Liang M.C. Clumped isotopes in near surface atmospheric CO2 over land, coast and ocean in Taiwan and its vicinity. Biogeosciences. 2016;13:5297–5314. [Google Scholar]
- Laskar A.H., Mahata S., Liang M.C. Identification of anthropogenic CO2 using triple oxygen and clumped isotopes. Environ. Sci. Technol. 2016;50(21):11806–11814. doi: 10.1021/acs.est.6b02989. [DOI] [PubMed] [Google Scholar]
- Laskar A.H., Lin L.C., Jiang X., Liang M.C. Distribution of CO2 in western Pacific, studied using isotope data made in Taiwan, OCO-2 satellite retrievals, and Carbon Tracker products. AGU Earth Space Sci. 2018;5(11):827–842. doi: 10.1029/2018EA000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskar A.H., Mahata S., Bhattacharya S.K., Liang M.C. Triple oxygen and clumped isotope compositions of CO2 in the middle troposphere. Earth Space Sci. 2019;6(7):1205–1219. [Google Scholar]
- Laskar A.H. Conventional and clumped isotopes in ecological research. Ecol. Environ. Sci. 2017;2(4):148–156. [Google Scholar]
- Löffler N., Böttcher M.E., Fiebig J., Tütken T., Mulch A. Applying the clumped isotope paleothermometer to teeth from T. rex and C. megalodon. Goldschmidt Abstr. 2017:2442. [Google Scholar]
- Löffler N., Fiebig J., Mulch A., Tütken T., Schmidt B.C., Bajnai D., Conrad A.C., Wacker U., Böttcher M.E. Refining the temperature dependence of the oxygen and clumped isotopic compositions of structurally bound carbonate in apatite. Geochem. Cosmochim. Acta. 2019;253:19–38. [Google Scholar]
- Loyal R.S., Mohabey D.M., Khosla A., Sahni A. Status and palaeobiology of the Late Cretaceous Indian theropods with description of new theropod eggshell oogenus and oospecies Ellipsoolithus khedaensis from the Lameta Formation, district Kheda, Gujarat, western India. In: Perez-Moreno B.P., Holz T., Sanz Z.L., Mortella J., editors. Aspects of Theropod Biology. GAIA; Lisbon, Portugal: 1999. pp. 379–387. [Google Scholar]
- Mikhailov K.E. Classification of eggshells of amniotic vertebrates. Acta Palaeontographicao Polonica. 1991;36:193–238. [Google Scholar]
- Mohabey D.M., Mathur U.B. Upper Cretaceous dinosaur eggs from new localities of Gujarat. J. Geol. Soc. India. 1998;33:32–37. [Google Scholar]
- Mohabey D.M., Samant B. Deccan continental flood basalt eruptions terminated Indian dinosaurs before the cretaceous-palaeoene boundary. Geol. Soc. India. 2013;1:260–267. [Google Scholar]
- Mohabey D.M., Samant B. Floral remains from Late Cretaceous faecal mass of Sauropods from central India: implications to their diet and habitat. Gondwana Geol. Mag. 2003;6:225–238. [Google Scholar]
- Mohabey D.M., Udhoji S.G. Palaeoenvironment interpretation of Lameta formation (late cretaceous) of Nand area, Nagpur district Maharashtra. Gondwana Geol. Mag. 1993;4:13–22. [Google Scholar]
- Mohabey D.M., Udhoji S.G. Symp. Workshop IGCP- 216 and 245, Chandigarh. 1990. Fossils occurrences and sedimentation of Lameta formation of Nand area, Maharashtra: Palaeoenvironmental, palaeoecological and taphonomical implications; pp. 30–32. [Google Scholar]
- Mohabey D.M. Study of dinosaurian eggs from Infra- trappean Limestone in Kheda district, Gujarat. J. Geol. Soc. India. 1984;25:329–337. [Google Scholar]
- Mohabey D.M. Depositional environment of Lameta formation (late cretaceous) of nand-dongargaon basin, Maharashtra: the fossil and lithofacies evidences. Mem. Geol. Soc. India. 1996;37:363–386. [Google Scholar]
- Mohabey D.M. A new oospecies, Megaloolithus matleyi, from the Lameta Formation (Upper Cretaceous) of Chandrapur district, Maharashtra, India, and general remarks on the palaeoenvironment and nesting behaviour of dinosaurs. Cretac. Res. 1996;17:183–196. [Google Scholar]
- Mohabey D.M. Indian dinosaur eggs: a review. J. Geol. Soc. India. 2001;58:479–508. [Google Scholar]
- Mohabey D.M. Late Cretaceous (Maastrichtian) nests, eggs, and dung mass (Coprolites) of sauropods (Titanosaurs) from India. In: Tidwell V., Carpenter K., editors. Thunder- Lizards. Indiana University Press; New York: 2005. pp. 466–489. [Google Scholar]
- Mohabey D.M. Systematics of Indian Upper Cretaceous dinosaur and Chelonian eggshells. J. Vertebr. Paleontol. 1998;18(2):348–362. [Google Scholar]
- Novas F.E., Chatterjee S., Rudra D.K., Dutta P.M. Rahiolisaurus gujaratensis, n. gen. n. sp., a new theropod from the Late Cretaceous of India. In: Bandyopadhya S., editor. Lecture Notes in Earth Sciences, 132 New Aspects of Mesozoic Biodiversity. Springer-Verlag Berlin; Heidelberg: 2010. [Google Scholar]
- O'Conner M.P., Dodson P. Biophysical constraints on the thermal ecology of dinosaurs. Paleobiology. 1999;25:341–368. [Google Scholar]
- Ostrom J.H. The evidence for endothermy in dinosaurs. In: Thomas R.D.K., Olson E.C., editors. A Cold Look at the Warm-Blooded Dinosaurs. Bolder; Westview: 1980. pp. 15–54. [Google Scholar]
- Pontzer H., Allen V., Hutchinson J.R. Biomechanics of running indicates endothermy in Bipedal dinosaurs. PloS One. 2009;4 doi: 10.1371/journal.pone.0007783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzinger R., Prebmar A., Schleucher E. Body temperature in birds: Comp. Btochem. Physiol. 1991;99A(4):499–506. [Google Scholar]
- Raske D.V.M., Lewbart G.A., Dombrowski D.S., Hale P., Correa M., Christian L.S. Body temperatures of selected amphibian and reptile. J. Zoo Wildl. Med. 2012;43(3):517–521. doi: 10.1638/2011-0244R.1. [DOI] [PubMed] [Google Scholar]
- Ruben J.A., Leitsch A., Hillenius W.J., Geist N. New insights into the metabolic physiology of dinosaurs. In: Farlow J.O., Brett-Surman M.K., editors. The Complete Dinosaur. Indiana Univ Press; Bloomington: 1997. pp. 505–518. [Google Scholar]
- Russell L.S. Body temperature of dinosaurs and its relationships to their extinction. J. Palaeontol. 1965;39:497–501. [Google Scholar]
- Sahni A., Tripathi A. Cretaceous Event Stratigraphy and the Correlation of Indian Non-marine Strata; International Geological Correlation Programme 216 and 245. Symposium cum Workshop; Chandigarh: 1990. Age implications of the Jabalpur Lameta formation and Inter-trappean biotas; pp. 35–37. [Google Scholar]
- Samant B., Mohabey D.M. Deccan volcanic eruptions and their impact on flora: palynological evidence. J. Geol. Soc. India. 2014;505:171–191. [Google Scholar]
- Sander P.M., Christian A., Clauss M., Fechner R., Gee C.T., Griebeler E.M., Gunga H.C., Hummel J.R., Mallison H., Perry S.F., Preuschoft H., Rauhut O.W.M., Remes K., Tütken T., Wings O., Witzel U. Biology of the sauropod dinosaurs: the evolution of gigantism. Biol. Rev. 2011;86(1):117–155. doi: 10.1111/j.1469-185X.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Bhattacharya S.K., Mohabey D.M. Stable-isotope analyses of dinosaur eggshells: paleoenvironmental implications. Geology. 1991;19:1068–1071. [Google Scholar]
- Seebacher F. Dinosaur body temperatures: the occurrence of endothermy and ectothermy. Paleobiology. 2003;29:105–122. [Google Scholar]
- Spotila J.R. Constraints of body size and environment on the temperature regulation of dinosaurs. In: Thomas R.D.K., Olson E.C., editors. A Cold Look at the Warm-Blooded Dinosaurs. Bolder; Westview: 1980. pp. 233–252. [Google Scholar]
- Sprain C.J., Renne P.R., Vanderkluysen L., Pande K., Self S., Mittal T. The eruptive tempo of Deccan volcanism in relation to the Cretaceous-Paleogene boundary. Science. 2019;363:866–870. doi: 10.1126/science.aav1446. [DOI] [PubMed] [Google Scholar]
- Sriniwasan S. Late Cretaceous eggshells from the Deccan volcano-sefimentary sequences of central India. Mem. Geol. Soc. India. 1996;37:219–233. [Google Scholar]
- Srivastava S., Mohabey D.M., Sahni A., Pant S.C. Upper Cretaceous dinosaur egg clutches from Kheda District, Gujarat, India: their distribution, shell structure and palaeoecology. Palaeontogr. A. 1986;193:219–233. [Google Scholar]
- Tandon S.K., Sood A., Andrews J.E., Dennis P.F. Palaeoenvironments of the dinosaur bearing Lameta beds (Maastrichtian), Narmada valley, central India: palaeogeoraphy, palaeoclimatoloy. Palaeoecology. 1995;117:153–184. [Google Scholar]
- Vianey-Liaud M., Jain S.L., Sahni A. Dinosaur eggshells (Saurischia) from the late cretaceous intertrappean and Lameta formations (Deccan, India) J. Vertebr. Paleontol. 1988;7(4):408–424. [Google Scholar]
- Wacker U., Fiebig J., Tödter J., Schöne B.R., Bahr A., Friedrich O., Tütken T., Gischler E., Joachimski M.M. Empirical calibration of the clumped isotope paleothermometer using calcites of various origins. Geochem. Cosmochim. Acta. 2014;141:127–144. [Google Scholar]
- Wang Zhengrong, Schauble Edwin A., Eiler John M. Equilibrium thermodynamics of multiply substituted isotopologues of molecular gases. Geochimica et Cosmochimica Acta. 2004;68(23):4779–4797. [Google Scholar]
- Werner J., Griebeler E.M. Allometries of maximum growth rate versus body mass at maximum growth indicate that non-avian dinosaurs had growth rates typical of fast growing ectothermic sauropsids. PloS One. 2014;9 doi: 10.1371/journal.pone.0088834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J., Mohabey D.M. A titanosauriform (Dinosauria: sauropaoda) axis from the Lameta formation (upper cretaceous: maastrichtian) of Nand, Central India. J. Vertebr. Paleontol. 2006;26(2):471–479. [Google Scholar]
- Wilson J.A., Sereno P.C., Srivastava S., Bhatt D.K., Khosla A., Sahni A. A new abelisaurid (Dinosauria, Theropoda) from the Lameta formation (Cretaceous, Maastrichtian) of India. Contrib. Mus. Paleontol. Univ. Mich. 2003;31:1–32. [Google Scholar]
- Woodward H.N., Lehman T.M. Bone histology and microanatomy of Alamosaurus sanjuanensis (Sauropoda: Titanosauria) from the Maastrichtian Javelina Formation, Texas, U.S.A. J. Vertebr. Paleontol. 2009;29:807–821. [Google Scholar]
- Zhang N., Lin M., Yamada K., Kano A., Liu Q., Yoshida N., Matsumoto R. The effect of H2O2 treatment on stable isotope analysis (δ13C, δ18O and Δ47) of various carbonate minerals. Chem. Geol. 2020;532:119352. [Google Scholar]
- Zhao Z. The microctructure of dinosaur egg, Nanhsiung, Kwngtung. Vertebr. Pal Asiat. 1975;13:304–309. [Google Scholar]