Abstract
Neural stem cells (NSCs) actively proliferate and generate neurons and glial cells (active state) in the embryonic brain, whereas they are mostly dormant (quiescent state) in the adult brain. The expression dynamics of Hes1 are different between active and quiescent NSCs. In active NSCs, Hes1 expression oscillates and periodically represses the expression of proneural genes such as Ascl1, thereby driving their oscillations. By contrast, in quiescent NSCs, Hes1 oscillations maintain expression at higher levels even at trough phases (thus continuous), thereby continuously suppressing proneural gene expression. High levels of Hes1 expression and the resultant suppression of Ascl1 promote the quiescent state of NSCs, whereas oscillatory Hes1 expression and the resultant oscillatory Ascl1 expression regulate their active state. Furthermore, in other developmental contexts, high, continuous Hes1 expression induces astrocyte differentiation or the formation of boundaries, which function as signaling centers. Thus, the expression dynamics of Hes1 are a key regulatory mechanism generating and maintaining various cell types in the nervous system.
Keywords: active state, bHLH factor, boundary cell, neural stem cell, oscillation, quiescent state
1. Introduction
Many forms of biological activity are continuous, and their amplitude and duration represent important information for cellular events. For example, Shh signaling specifies progenitor cell identity, such as V3 interneuron or motor neuron progenitors in the spinal cord, in amplitude- and duration-dependent manners: higher amplitude or longer duration of Shh signaling induces V3 interneurons, whereas lower amplitude or shorter duration of Shh induces motor neurons.1) However, recent studies revealed that many other forms of biological activity are pulsatile or oscillatory, and that not only their amplitude and duration but also their frequency and phase convey essential information for cellular events.2–4) In some cases, the same factors can cause different outcomes depending on whether they exhibit pulsatile or continuous activity. For example, luteinizing hormone-releasing hormone (LHRH) is secreted in a pulsatile manner and activates estrogen and testosterone secretion.5) However, when LHRH is administered continuously, its receptor is rapidly desensitized, losing the response to LHRH and suppressing estrogen and testosterone secretion.5) Thus, pulsatile LHRH functions as an activator, but continuous LHRH functions as an inhibitor for estrogen and testosterone formation.
Another example is the somite segmentation clock gene Hes7. Somites are metameric structures, which later give rise to the vertebrae, ribs, skeletal muscles, and subcutaneous tissues. Somites repeatedly form by segmentation of the anterior parts of the presomitic mesoderm, which is located in the caudal part of an embryo. Hes7 expression oscillates in a synchronous manner in the presomitic mesoderm, and each cycle of Hes7 oscillation leads to the segmentation of a pair of somites.6–8) When Hes7 expression becomes sustained, all somites and their derivatives are severely fused.9) Thus, oscillatory Hes7 expression leads to periodic somite segmentation, but sustained Hes7 expression results in severe somite fusion. Accumulating evidence suggests that oscillatory or sustained gene expression dynamics exhibit different activities in various biological events.
In this review, we discuss the significance and mechanisms of gene expression dynamics in tissue stem cells, particularly focusing on neural stem cells (NSCs). A Hes7-related gene, Hes1, causes various outcomes in NSCs, depending on its oscillatory or sustained expression.
2. NSC regulation by bHLH factors
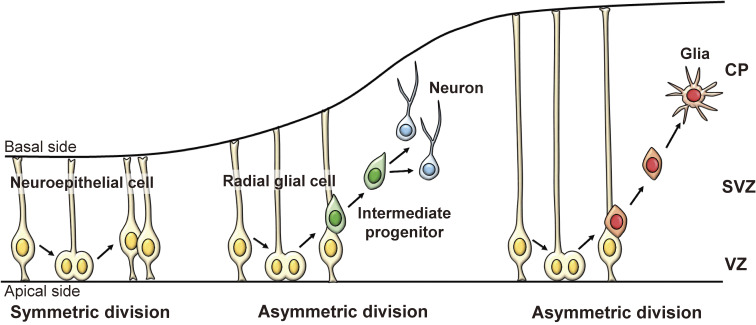
In the developing nervous system, neuroepithelial cells, which constitute the wall of the neural tube, proliferate actively (Fig. 1). As development proceeds, neuroepithelial cells elongate gradually and become radial glial cells that retain their cell bodies in the innermost neural tube layer, called the ventricular zone (VZ), and extend their processes, called radial fibers, to the external surface of the neural tube (Fig. 1). Neuroepithelial cells undergo symmetric cell division, in which each neuroepithelial cell produces two neuroepithelial cells, whereas radial glial cells undergo asymmetric cell division, in which each radial glial cell produces two distinct cell types, i.e., one radial glial cell and one intermediate progenitor (INP) (Fig. 1).10,11) INPs migrate outside of the VZ into the outer layer called the subventricular zone (SVZ), where they further divide a few more times and differentiate into mature neurons. After producing neurons, radial glial cells finally differentiate into glial cells (oligodendrocytes and astrocytes) (Fig. 1), although some of them are maintained as NSCs in the postnatal and adult brain. Neuroepithelial cells and radial glial cells are collectively called embryonic NSCs.
Figure 1.
Differentiation of NSCs in the embryonic cortex. Neuroepithelial cells repeatedly undergo self-renewal by symmetric division. As development proceeds, neuroepithelial cells elongate to become radial glial cells, which have cell bodies in the inner region (ventricular zone, VZ) of the neural tube and long processes (radial fibers) that reach the outer surface (Basal side). Radial glial cells give rise to intermediate progenitors or neurons. Each intermediate progenitor migrates into the subventricular zone (SVZ) and produces neurons. Neurons further migrate into the basal side and form the cortical plate (CP). After producing neurons, radial glial cells differentiate into glia (oligodendrocytes and astrocytes). Neuroepithelial cells and radial glial cells are both considered embryonic NSCs.
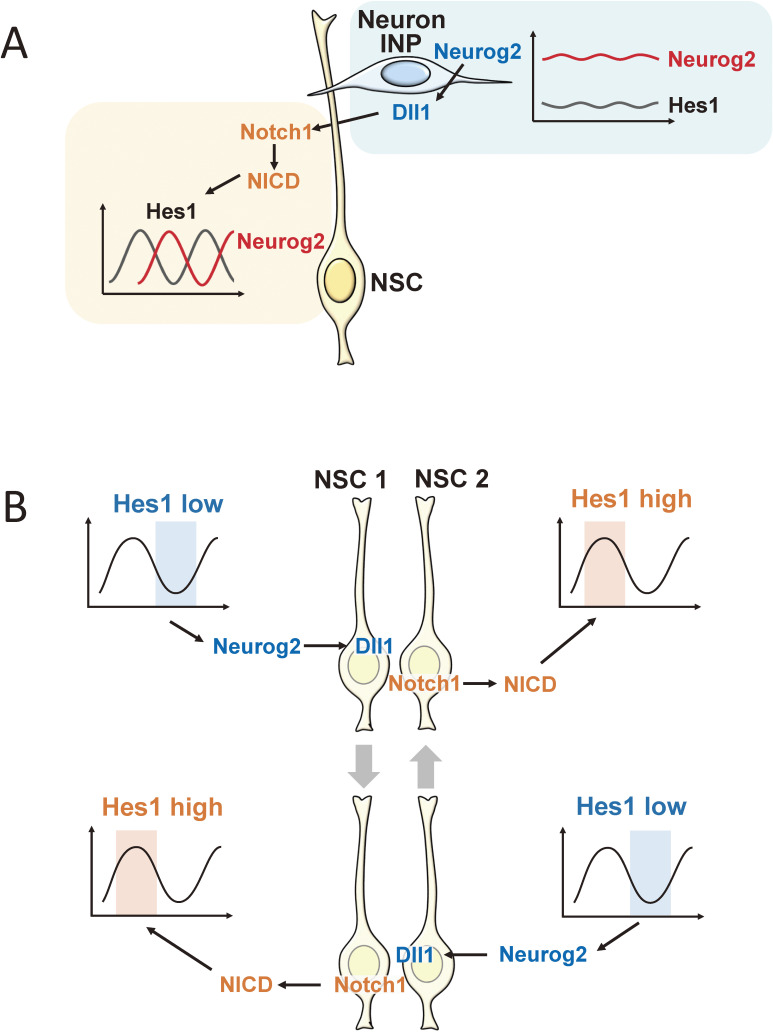
The maintenance of NSCs and their differentiation into neurons are antagonistically controlled by basic helix-loop-helix (bHLH) transcriptional activators and repressors.12–14) bHLH transcriptional activators include proneural factors such as Ascl1 and Neurog2, and bHLH transcriptional repressors include Notch signaling effectors such as Hes1 and Hes5. Ascl1 and Neurog2 up-regulate genes involved in neuronal differentiation, inducing the formation of INPs and neurons. By contrast, Hes1 and Hes5 repress Ascl1 and Neurog2 expression and thereby inhibit neuronal differentiation, leading to the maintenance of NSCs. The inactivation of Hes1 and Hes5 up-regulates Ascl1 and Neurog2 expression, accelerates neurogenesis, and prematurely depletes NSCs in the developing nervous system.15,16) Thus, antagonistic regulation between bHLH transcriptional activators and repressors is essential for normal neural development. Of note, Ascl1 and Neurog2 up-regulate ligands of Notch signaling such as Delta-like 1 (Dll1), which activate Notch signaling in neighboring cells (Fig. 2A). Upon activation of Notch signaling, Notch intracellular domain (NICD), an active form of Notch signaling, is formed and induces Hes1 and Hes5 expression, thereby inhibiting neuronal differentiation (Fig. 2A). These results indicate that Ascl1- or Neurog2-expressing differentiating INPs and neurons activate Notch signaling in neighboring cells, which are inhibited from undergoing neuronal differentiation, a process called lateral inhibition.17,18) Thus, Notch signaling is essential for the maintenance of NSCs in the developing nervous system.
Figure 2.
Dynamic control of Notch signaling. (A) In INPs and neurons, proneural factors induce Dll1 expression, which activates Notch signaling in neighboring cells. The activation of Notch signaling releases the Notch intracellular domain (NICD), which induces Hes1 and Hes5 expression. This process is called lateral inhibition. In NSCs, Hes1 and Hes5 expression autonomously oscillates, driving the oscillatory expression of proneural genes such as Neurog2. (B) Before INPs and neurons are generated, the oscillatory expression of Dll1 enables the mutual activation of Notch signaling between neighboring NSCs, suggesting that oscillatory expression is beneficial for the maintenance of a group of undifferentiated cells.
3. NSC regulation by oscillatory gene expression
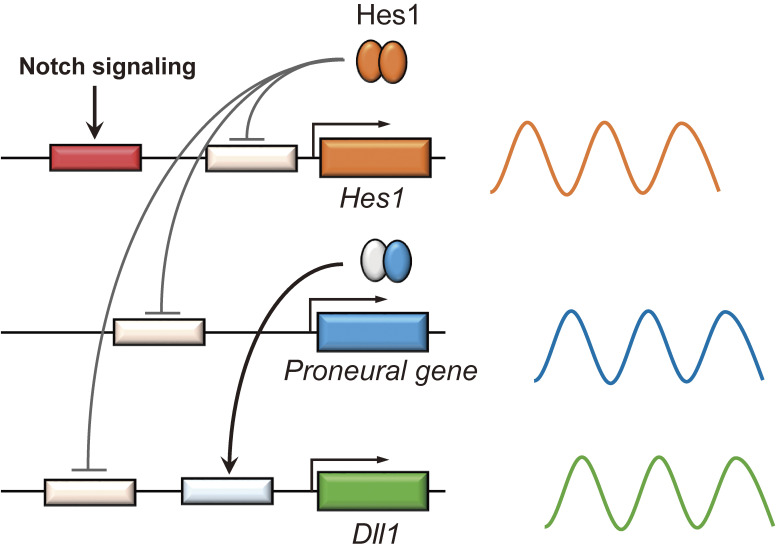
The above observations indicate that INPs and neurons play an important role in maintaining NSCs by Dll1 expression; however, this raises the question of how NSCs are maintained before INPs and neurons are generated. Accumulating evidence suggests that Notch signaling regulation is not as simple as a one-way pathway from differentiating INPs and neurons to neighboring NSCs. Ascl1, Neurog2, and Dll1 expression is not unique to differentiating INPs and neurons, but occurs in NSCs in a salt-and-pepper pattern (i.e., a mixture of positive and negative cells) in the early stages of neural development, particularly before INPs and neurons are generated. These findings indicate that in addition to Hes1 and Hes5, Ascl1, Neurog2, and Dll1 are expressed by subsets of NSCs, raising the possibility that NSCs mutually activate Notch signaling. Time-lapse imaging analysis revealed that Hes1 and Hes5 expression oscillates with a 2–3-h periodicity in NSCs.19,20) Hes1 and Hes5 can repress their own expression by binding directly to their own promoters (negative feedback), leading to their down-regulation (Fig. 3).21) However, when these factors disappear, negative feedback is cancelled, allowing the next round of expression. Thus, Hes1 and Hes5 expression oscillates autonomously by negative feedback in NSCs (Fig. 3).21) Hes1 and Hes5 oscillations then periodically repress Ascl1, Neurog2, and Dll1 expression, which also oscillates in NSCs (Fig. 3). On the basis of this observation, the current view of Notch signaling during the early stages of development is as follows (Fig. 2B).18,19) When Hes1 and Hes5 expression is low in certain cells, Ascl1 and Neurog2 expression increases, inducing Dll1 expression, and high Dll1 levels activate Notch signaling in neighboring cells, which express high levels of Hes1 and Hes5. However, because of oscillatory expression, Hes1 and Hes5 expression is suppressed 1–1.5 h later in the latter cells, resulting in high Ascl1, Neurog2, and Dll1 expression, which activates Notch signaling in the former cells (Fig. 2B). Thus, according to these oscillations, NSCs can mutually activate Notch signaling, inhibiting neuronal differentiation of each other and maintaining a group of cells in an undifferentiated state. This two-way regulation is important in the early stages of development before INPs and neurons are generated. As development proceeds, this two-way regulation disappears, and Dll1 signals are produced only by differentiating INPs and neurons.
Figure 3.
Dynamic gene expression in active NSCs. Notch signaling activates Hes1 expression, which represses its own expression. From this negative feedback, Hes1 expression oscillates autonomously with a 2–3-h periodicity. Hes1 oscillations periodically repress the expression of proneural genes and Dll1. As a result, these genes are also expressed in an oscillatory manner. Adapted from Ref. 51.
The oscillations observed in the developing nervous system are anti-phase or out-of-phase between neighboring NSCs, unlike the in-phase oscillations of Hes7 in the presomitic mesoderm.22,23) However, the significance of anti-phase or out-of-phase oscillations is not known. NSCs are required to produce diverse cell types during neural development, and anti-phase or out-of-phase oscillations may be useful for the generation of the diverse responses of NSCs. For example, Hes1-low cells can respond to certain signals, but Hes1-high cells cannot. Further analysis is required to test this hypothesis.
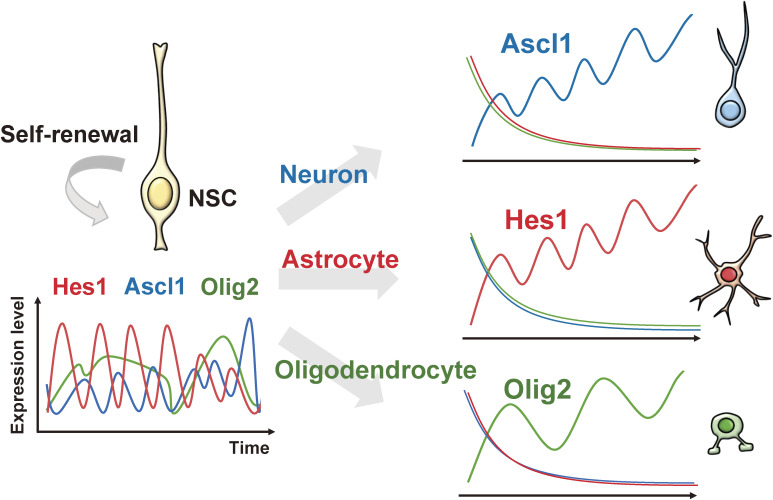
Oscillatory expression itself seems to be very important for the activity of NSCs. Hes1 expression oscillates in proliferating NSCs, but is higher even during the trough phase (thus continuous) in differentiating astrocytes (Fig. 4).20) Similarly, the proneural gene Ascl1 and the oligodendrocyte determination gene Olig2 are expressed in an oscillatory manner depending on Hes1 oscillations in proliferating NSCs, but become sustained and high in differentiating neurons and oligodendrocytes, respectively (Fig. 4).20) Ascl1, Hes1, and Olig2 play important roles in the proliferation of NSCs, in addition to their respective neurogenic, astrogenic, and oligodendrogenic functions.20,24,25) Thus, each factor has opposing functions, i.e., maintaining NSCs and promoting the differentiation of specific cell types, but it was unknown how these factors regulate their opposing functions. The observation that these factors exhibit different expression dynamics (oscillatory or sustained) between NSCs and differentiating cells suggested a hypothesis that such different expression dynamics are responsible for the regulation of their opposing functions. Indeed, optogenetic analysis, which can induce pulsatile or sustained expression in cultured NSCs by changing light illumination patterns, showed that Ascl1 induces neuronal differentiation when its expression is sustained, but activates NSC proliferation when its expression is oscillatory.20) These observations suggest that cell fate determination factors such as Ascl1, Hes1, and Olig2 exhibit opposing functions depending on their expression dynamics.
Figure 4.
Expression dynamics of bHLH factors in multipotency and cell fate choice. The proneural gene Ascl1, the astrogenic gene Hes1, and the oligodendrogenic gene Olig2 are expressed in an oscillatory manner in proliferating NSCs, but become sustained and high in differentiating neurons, astrocytes, and oligodendrocytes, respectively. Adapted from Ref. 14.
4. In vivo significance of oscillatory expression in NSCs
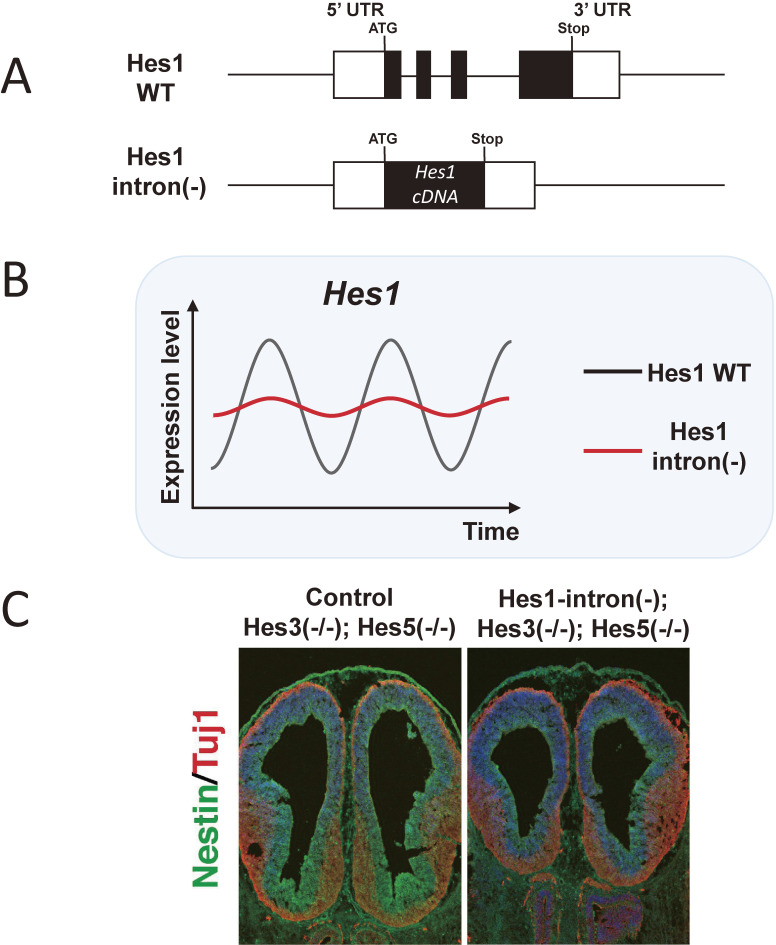
The significance of oscillatory expression in neural development in vivo has been analyzed in a number of studies. According to mathematical modeling, the time delay required for Dll1-Notch signaling transmission between cells is important for oscillatory dynamics.22) With appropriate time delays, neighboring cells exhibit in-phase oscillations, similar to Hes7 oscillations in the presomitic mesoderm, or out-of-phase oscillations, as observed in NSCs. However, when the time delays are shortened or elongated, both in-phase and out-of-phase oscillations are dampened, and in the most extreme cases, expression becomes steady, a condition known as amplitude/oscillation death.26) To dampen the oscillations, the time delay required for Dll1-Notch signaling transmission between cells was changed by shortening the Dll1 gene by deleting all introns (type-1 mutant) or elongating it by inserting an extra sequence (type-2 mutant).22) The shortened and elongated Dll1 genes exhibit accelerated and delayed expression, respectively. As a result, the time delay in Dll1-Notch signaling transmission is shortened and elongated in type-1 and type-2 Dll1 mutants, respectively. Notably, both types of Dll1 mutant mice exhibit dampened Hes1 oscillations in NSCs and dampened Hes7 oscillations in the presomitic mesoderm.22) Dampened Hes1 oscillations inhibit the proliferation of NSCs, accelerating neuronal differentiation, while dampened Hes7 oscillations lead to severe fusion of somites and their derivatives, such as vertebrae and ribs.22) Another approach was to shorten the time delay required for negative feedback. According to mathematical modeling, oscillatory expression critically depends on the proper time delay in negative feedback, i.e., accelerated negative feedback dampens oscillations, leading to steady expression.9) Intronic delay, which includes the transcription of intronic sequences and the removal of introns by splicing, constitutes a major part of the time delay required for negative feedback. Deletion of all introns from the Hes7 locus accelerates negative feedback, leading to steady Hes7 expression and severe somite fusion.9) Similarly, all introns were removed from the Hes1 locus (Hes1-intron(−)) to dampen Hes1 oscillations (Fig. 5A).27) In such mutant mice, Hes1 oscillations in NSCs are dampened (Fig. 5B), and NSC proliferation decreases slightly.27) The phenotype is rather mild, probably because Hes1 oscillations were not abolished completely, i.e., Hes1 expression still oscillated in the mutant, although the amplitude was smaller. Another reason is that the functions of Hes1 can be compensated for by Hes1-related genes, such as Hes3 and Hes5. Indeed, although Hes3-null;Hes5-null mice are almost normal, the introduction of the Hes1-intron(−) mutation significantly inhibits NSC proliferation and accelerates neurogenesis, leading to microcephaly (Fig. 5C).27) These studies clearly indicated that oscillatory expression in NSCs is important for normal brain morphogenesis in vivo.
Figure 5.
Reduced NSC proliferation by dampened Hes1 oscillations. (A) Schematic structures of the wild-type and Hes1-intron(−) Hes1 locus. (B) Hes1 expression dynamics in NSCs. Hes1 oscillations are dampened in Hes1-intron(−)-mutant NSCs. (C) The telencephalon of a control Hes3(−/−);Hes5(−/−) mouse and a Hes1-intron(−);Hes3(−/−);Hes5(−/−) mouse. Immunohistochemical analysis shows Nestin+ NSCs and Tuj1+ neurons. Adapted from Ref. 27.
Another example for the significance of expression dynamics is observed in boundary regions such as the isthmus, which separates the midbrain and the hindbrain, and the roof plate and floor plate, which separate the right and left halves of the neural tube.28–31) In these regions, cells neither proliferate nor produce neurons, unlike proliferating NSCs. In addition to separating regions, boundary cells function as signaling centers to specify their neighboring regions, e.g., the isthmus secretes Fgf8, whereas the roof plate and floor plate secrete Wnt and Shh, respectively.28–31) Notably, boundary cells express Hes1 at high levels in a sustained manner and do not express proneural genes, suggesting that sustained Hes1 expression contributes to the non-proliferative and non-neurogenic properties of boundary cells.32) The inactivation of Hes1 and Hes1-related genes leads to the ectopic expression of proneural genes and down-regulation of signaling molecules in the boundary regions, whereas the induction of sustained Hes1 expression represses proneural gene expression and inhibits NSC proliferation.32) Thus, high and sustained Hes1 expression and the resultant suppression of proneural genes are important for the non-proliferative and non-neurogenic properties of boundary cells. However, it remains to be determined how sustained Hes1 expression differentially controls astrocyte differentiation and boundary cell formation.
5. Transition from oscillatory to sustained gene expression
When NSCs start neuronal differentiation, the expression of Hes1 changes from oscillatory to repressed, whereas Ascl1 expression changes from oscillatory to sustained.19,20) How the transition from oscillatory to repressed Hes1 expression is controlled remains to be analyzed. Because Hes1 expression is regulated by Notch signaling, one possibility is that Notch signaling becomes inactive when neuronal differentiation starts. Treatment with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester, which inhibits Notch signaling, down-regulates Hes1 expression and up-regulates Ascl1 expression in NSCs, leading to neuronal differentiation.20) The Notch ligand Dll1 is mainly expressed by INPs in the SVZ when neurogenesis occurs. During this stage, NSCs undergo asymmetric cell division at the apical surface (the innermost region of the neural tube), and each cycle of cell division produces one NSC and one INP (Fig. 1). NSCs retain radial fibers, which can receive Dll1 signals from INPs in the SVZ (Fig. 2A), whereas newly formed INPs, which are present near the apical surface and separate from the SVZ, do not carry radial fibers and, therefore, do not receive Dll1 signals from INPs in the SVZ. Indeed, Notch signaling is active in NSCs but inactive in INPs.33) These observations suggest that asymmetric cell division automatically generates a pair of cells, one with active Notch signaling and the other with inactive Notch signaling.
Time-lapse imaging analysis of Hes1 expression suggested that the situation is not that simple. At several hours before asymmetric cell division begins, Hes1 expression disappears, whereas proneural gene expression is up-regulated in a sustained manner, raising the possibility that even when Notch signaling is still active, Hes1 expression is repressed before asymmetric cell division.20) One possible hypothesis is that a repressor of Hes1 gradually accumulates in NSCs before asymmetric cell division. Hes1 oscillations drive proneural gene oscillations in NSCs. Many downstream genes of proneural genes are expressed only after neuronal differentiation starts, and are not expressed in NSCs. However, some downstream genes such as Dll1 are expressed under the control of proneural genes in NSCs. Dll1 expression oscillates in NSCs because Dll1 protein is unstable,19,22) but if protein products are stable, proneural gene oscillations should lead to the gradual accumulation of such proteins. It is possible that such proteins may repress Hes1 expression when their levels reach a certain threshold. Further analyses are required to test this hypothesis.
6. High and sustained Hes1 expression in quiescent NSCs in the adult brain
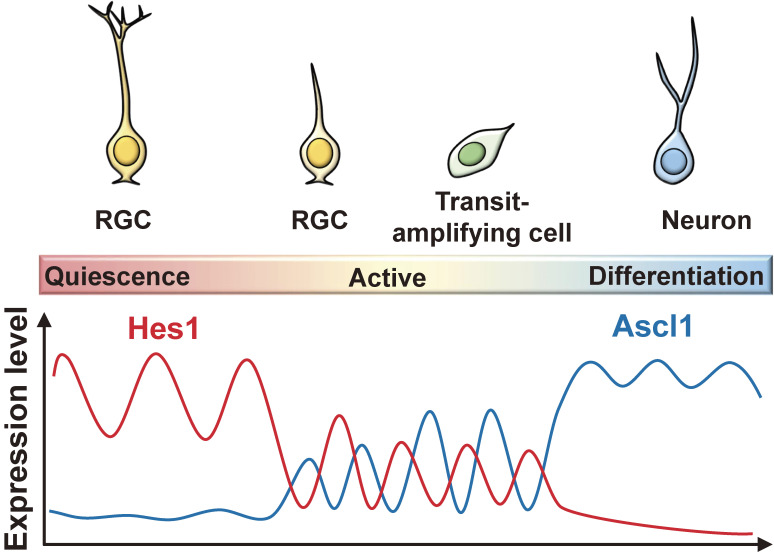
In the adult mouse brain, NSCs are present in two regions, the subgranular zone of the hippocampal dentate gyrus and the SVZ of the lateral ventricles.34–36) These adult NSCs, which have a radial glial cell morphology, are mostly quiescent/dormant, and only occasionally become activated and divide to produce transit-amplifying cells (Fig. 6). Transit-amplifying cells divide a few times and soon differentiate into neurons that integrate into the preexisting neural circuits. Thus, the characteristics of NSCs are totally different between the embryonic active and adult quiescent states.
Figure 6.
Expression dynamics of Hes1 and Ascl1 in quiescent and active NSCs, transit-amplifying cells, and differentiating neurons. RGC, radial glia-like NSC. Adapted from Ref. 38.
Notch signaling plays an essential role in maintaining active NSCs in the developing nervous system. The inactivation of the Notch signaling effector genes Hes1 and Hes1-related genes up-regulates the expression of proneural genes such as Ascl1 and Neurog2, accelerates neurogenesis, and prematurely depletes NSCs from the developing nervous system.16) Similarly, the inactivation of the Notch mediator Rbpj causes the same defects.37) Thus, the Notch-Rbpj-Hes1 pathway appears to play an essential role in maintaining active NSCs. Of note, Notch signaling is also important for maintaining quiescent NSCs in the adult brain. The inactivation of Hes1 and Hes1-related genes up-regulates Ascl1 expression, accelerates neurogenesis, and prematurely depletes quiescent NSCs from the adult brain.38) Furthermore, similar defects in adult neurogenesis occur in the absence of Rbpj,37) indicating that the Notch-Rbpj-Hes1 pathway plays an essential role in maintaining quiescent NSCs in the adult brain. Thus, Notch signaling regulates the maintenance of embryonic active and adult quiescent NSCs.
The next question is how Notch signaling leads to the active and quiescent states in embryonic and adult brains. Our recent data suggest that the dynamics of Hes1 expression are involved in these different states. The proneural gene Ascl1 plays a critical role in the activation of quiescent NSCs and subsequent formation of neuroblasts in the adult brain.39) Ascl1 is expressed at low levels in some activated NSCs and at high levels in transit-amplifying cells.39–41) Furthermore, live-imaging analysis showed that Ascl1-expressing NSCs exclusively generate neurons in the adult mouse hippocampus.42) By contrast, in the absence of Ascl1, all NSCs remain quiescent, indicating that Ascl1 is absolutely required for the activation of quiescent NSCs.39) Live-imaging analysis of the adult mouse brain demonstrated that Hes1 expression is oscillatory in quiescent NSCs, although the peaks and troughs are higher than those in active NSCs, causing Ascl1 expression to be suppressed continuously.38) The inactivation of the Notch-Rbpj-Hes1 pathway up-regulates Ascl1 expression, activates NSCs, and transiently enhances neurogenesis, but NSCs are soon depleted, ending neurogenesis prematurely.37,38) Conversely, the induction of sustained Hes1 expression represses Ascl1 expression, inhibits neurogenesis, and maintains quiescent NSCs in the adult brain.38) These results indicate that high levels of Hes1 and the resultant suppression of Ascl1 promote quiescence in NSCs in the adult brain. Indeed, the induction of Ascl1 oscillations efficiently activates NSCs to produce new neurons in the adult brain.38)
The mechanism by which high levels of Hes1 are maintained in quiescent NSCs remains to be determined. It was shown that bone morphogenetic protein signaling is important for maintaining quiescent NSCs in the adult brain.43) Bone morphogenetic protein signaling induces the expression of Id1, and Id1 is highly expressed by quiescent NSCs in the adult brain.44) Notably, Id1 interacts with Hes1 and inhibits Hes1 negative feedback, thereby up-regulating Hes1 expression, although Id1 cannot inhibit Hes1 from repressing proneural gene expression.45) It was also reported that Notch2 induces Id4 expression in quiescent NSCs.46) These findings suggest that Id1 and Id4 may be responsible for the high levels of Hes1 expression, thereby suppressing Ascl1 and maintaining quiescent NSCs in the adult brain.46,47)
Why Hes1 oscillations promote cell proliferation and why sustained Hes1 expression leads to quiescence still remain to be analyzed. Sustained Hes1 expression inhibits the proliferation of not only NSCs but also other cell types, such as muscle and hematopoietic stem cells, suggesting that this inhibitory activity of Hes1 on proliferation is rather universal in many cell types.48) Hes1 is also highly expressed by human fibroblasts when they enter quiescence due to serum deprivation or contact inhibition.49) These quiescent fibroblasts lose their ability to reenter the cell cycle and become senescent when Hes1 is knocked down, whereas high, sustained Hes1 expression is sufficient to prevent these cells from becoming senescent.49) By contrast, Hes1 expression oscillates in proliferating muscle progenitors and pancreatic progenitors.48,50) Thus, high levels of Hes1 expression are a general feature of quiescence, whereas Hes1 oscillations are a general feature of an active state.51) One hypothesis is that the expression of genes involved in cell cycle progression is well maintained when Hes1 expression is oscillatory but is totally suppressed when Hes1 expression is high and sustained. Further analyses are required to characterize the relationship between Hes1 oscillations and cell cycle progression.
7. Conclusions
Hes1 oscillations drive the oscillatory expression of the proneural gene Ascl1, leading to an active state, whereas high and sustained Hes1 expression suppresses Ascl1 expression, leading to quiescence. In other developmental contexts, high and sustained Hes1 expression is associated with boundary cell formation and astrocyte differentiation. Therefore, the dynamics of Hes1 expression are a key regulatory mechanism for generating various types of cells in the nervous system. Further understanding of the significance of gene expression dynamics will be useful for the manipulation of NSCs and the development of regenerative medicine in the future.
Acknowledgements
This work was supported by Core Research for Evolutional Science and Technology (CREST) (JPMJCR12W2, R.K.), Grant-in-Aid for Scientific Research on Innovative Areas (16H06480, R.K.) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, AMED-CREST (19gm1110002h0003, R.K.) from Japan Agency for Medical Research and Development, and Scientific Research (C) (18K06254 to H.S.) and Research Fellowship for Young Scientists (17J02922 to S.O.) from Japan Society for the Promotion of Science.
Abbreviations
- bHLH
basic helix-loop-helix
- CP
cortical plate
- Dll1
Delta-like 1
- INP
intermediate progenitor
- LHRH
luteinizing hormone-releasing hormone
- NICD
Notch intracellular domain
- NSC
neural stem cell
- SVZ
subventricular zone
- VZ
ventricular zone
Biographies
Profile
Ryoichiro Kageyama was born in Osaka in 1957 and graduated from Kyoto University School of Medicine in 1982. He received his Ph.D. degree from Kyoto University in 1986 and worked as a postdoctoral fellow at the National Cancer Institute in the U.S.A. between 1986 and 1989. He became a Professor at the Institute for Virus Research, Kyoto University, in 1997, and was appointed Director of the same institute from 2006 to 2010. He also served as Vice Director of the Institute for Integrated Cell-Material Sciences, Kyoto University, from 2013 to 2019. His current research involves studies on gene expression dynamics during cellular proliferation and differentiation. His group has developed time-lapse imaging and light-controlled gene expression systems to analyze the significance of gene expression dynamic in neural development and somite formation. He is also interested in the control mechanism of adult neurogenesis and characterizing its roles in brain functions.
Shohei Ochi was born in Mie prefecture in 1989. He earned his B.S. degree in Pharmaceutical Science from Tokyo University of Science in 2013. He received his M.S. degree in Medical Science in 2015 and Ph.D. degree in Medicine in 2020 from Kyoto University where he was provided JSPS Research Fellowship for Young Scientists in 2018. He moved to Sendai in 2020 and worked as research assistant for the Neuro Global International Joint Graduate Program in the Department of Developmental Neuroscience, United Centers for Advanced Research and Translational Medicine (ART), at Tohoku University Graduate School of Medicine where he is investigating the molecular mechanism of sexual differentiation in the embryonic murine cortex.
Risa Sueda was born in Yamaguchi in 1993 and graduated from the University of Hyogo School of Science in 2016. She received her M.S. degree in Life Science from Kyoto University and is currently a doctoral student in the laboratory of Prof. Kageyama. She has been granted a JSPS Research Fellowship for Young Scientists and is working on a project that aims to activate adult neurogenesis.
Hiromi Shimojo was born in Fukushima Prefecture in 1979 and graduated and received her D.V.M. from Nippon Veterinary and Life Science University in 2004. She received her Ph.D. in Medicine from Kyoto University in 2008. Thereafter, she began her postdoctoral training at the Institute for Virus Research in Kyoto University. From 2013, she worked at Institute for Integrated Cell-Material Sciences in Kyoto University as a program-specific research center assistant professor. From 2017, she worked at the Institute for Frontier Life and Medical Sciences in Kyoto University as a program-specific assistant professor. From 2020, She moved to the Graduate School of Frontier Biosciences in Osaka University and is currently working as an assistant professor. Her research interests are on the significance of various dynamics in gene expression and signal transmission during mammalian development.
References
- 1).Dessaud E., Yang L.L., Hill K., Cox B., Ulloa F., Ribeiro A., et al. (2007) Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717–720. [DOI] [PubMed] [Google Scholar]
- 2).Levine J.H., Lin Y., Elowitz M.B. (2013) Functional roles of pulsing in genetic circuits. Science 342, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Purvis J.E., Lahav G. (2013) Encoding and decoding cellular information through signaling dynamics. Cell 152, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Isomura A., Kageyama R. (2014) Ultradian oscillators: Rhythms and cell fate decisions. Development 141, 3627–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Matsumoto A.M., Gross K.M., Bremner W.J. (1991) The physiological significance of pulsatile LHRH secretion in man: Gonadotrophin responses to physiological doses of pulsatile versus continuous LHRH administration. Int. J. Androl. 14, 23–32. [DOI] [PubMed] [Google Scholar]
- 6).Bessho Y., Sakata R., Komatsu S., Shiota K., Yamada S., Kageyama R. (2001) Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 15, 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Hubaud A., Pourquié O. (2014) Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 15, 709–721. [DOI] [PubMed] [Google Scholar]
- 8).Oates A.C., Morelli L.G., Ares S. (2012) Patterning embryos with oscillations: Structure, function and dynamics of the vertebrate segmentation clock. Development 139, 625–639. [DOI] [PubMed] [Google Scholar]
- 9).Takashima Y., Ohtsuka T., González A., Miyachi H., Kageyama R. (2011) Intronic delay is essential for oscillatory expression in the segmentation clock. Proc. Natl. Acad. Sci. U.S.A. 108, 3300–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Fujita S. (2003) The discovery of the matrix cell, the identification of the multipotent neural stem cell and the development of the central nervous system. Cell Struct. Funct. 28, 205–228. [DOI] [PubMed] [Google Scholar]
- 11).Götz M., Huttner W.B. (2005) The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788. [DOI] [PubMed] [Google Scholar]
- 12).Bertrand N., Castro D.S., Guillemot F. (2002) Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530. [DOI] [PubMed] [Google Scholar]
- 13).Ross S.E., Greenberg M.E., Stiles C.D. (2003) Basic helix-loop-helix factors in cortical development. Neuron 39, 13–25. [DOI] [PubMed] [Google Scholar]
- 14).Imayoshi I., Kageyama R. (2014) bHLH factors in self-renewal, multipotency, and fate choice of neural progenitor cells. Neuron 82, 9–23. [DOI] [PubMed] [Google Scholar]
- 15).Ohtsuka T., Ishibashi M., Gradwohl G., Nakanishi S., Guillemot F., Kageyama R. (1999) Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO J. 18, 2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Hatakeyama J., Bessho Y., Katoh K., Ookawara S., Fujioka M., Guillemot F., et al. (2004) Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131, 5539–5550. [DOI] [PubMed] [Google Scholar]
- 17).Artavanis-Tsakonas S., Rand M.D., Lake R.J. (1999) Notch signaling: Cell fate control and signal integration in development. Science 284, 770–776. [DOI] [PubMed] [Google Scholar]
- 18).Kageyama R., Ohtsuka T., Shimojo H., Imayoshi I. (2008) Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 11, 1247–1251. [DOI] [PubMed] [Google Scholar]
- 19).Shimojo H., Ohtsuka T., Kageyama R. (2008) Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64. [DOI] [PubMed] [Google Scholar]
- 20).Imayoshi I., Isomura A., Harima Y., Kawaguchi K., Kori H., Miyachi H., et al. (2013) Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342, 1203–1208. [DOI] [PubMed] [Google Scholar]
- 21).Hirata H., Yoshiura S., Ohtsuka T., Bessho Y., Harada T., Yoshikawa K., et al. (2002) Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 298, 840–843. [DOI] [PubMed] [Google Scholar]
- 22).Shimojo H., Isomura A., Ohtsuka T., Kori H., Miyachi H., Kageyama R. (2016) Oscillatory control of Delta-like1 in cell interactions regulates dynamic gene expression and tissue morphogenesis. Genes Dev. 30, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Yoshioka-Kobayashi K., Matsumiya M., Niino Y., Isomura A., Kori H., Miyawaki A., et al. (2020) Control of coupling delay for synchronized oscillations in the segmentation clock. Nature 580, 119–123. [DOI] [PubMed] [Google Scholar]
- 24).Castro D.S., Martynoga B., Parras C., Ramesh V., Pacary E., Johnston C., et al. (2011) A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25, 930–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Sun Y., Meijer D.H., Alberta J.A., Mehta S., Kane M.F., Tien A.C., et al. (2011) Phosphorylation state of Olig2 regulates proliferation of neural progenitors. Neuron 69, 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Ramana Reddy D.V., Sen A., Johnston G.L. (1998) Experimental evidence of time delay induced death in coupled limit cycle oscillators. Phys. Rev. Lett. 80, 5109–5112. [DOI] [PubMed] [Google Scholar]
- 27).Ochi S., Imaizumi Y., Shimojo H., Miyachi H., Kageyama R. (2020) Oscillatory expression of Hes1 cell proliferation and neuronal differentiation in the embryonic brain. Development 147, dev182204. [DOI] [PubMed] [Google Scholar]
- 28).Lee K.J., Jessell T.M. (1999) The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 22, 261–294. [DOI] [PubMed] [Google Scholar]
- 29).Liu A., Joyner A.L. (2001) Early anterior/posterior patterning of the midbrain and cerebellum. Annu. Rev. Neurosci. 24, 869–896. [DOI] [PubMed] [Google Scholar]
- 30).Kiecker C., Lumsden A. (2005) Compartments and their boundaries in vertebrate brain development. Nat. Rev. Neurosci. 6, 553–564. [DOI] [PubMed] [Google Scholar]
- 31).Placzek M., Briscoe J. (2005) The floor plate: Multiple cells, multiple signals. Nat. Rev. Neurosci. 6, 230–240. [DOI] [PubMed] [Google Scholar]
- 32).Baek J.H., Hatakeyama J., Sakamoto S., Ohtsuka T., Kageyama R. (2006) Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development 133, 2467–2476. [DOI] [PubMed] [Google Scholar]
- 33).Mizutani K., Yoon K., Dang L., Tokunaga A., Gaiano N. (2007) Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355. [DOI] [PubMed] [Google Scholar]
- 34).Doetsch F. (2003) The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134. [DOI] [PubMed] [Google Scholar]
- 35).Kriegstein A., Alvarez-Buylla A. (2009) The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., et al. (2008) Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161. [DOI] [PubMed] [Google Scholar]
- 37).Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. (2010) Essential roles of Notch signaling in maintenance of neural stem cells in the developing and adult brains. J. Neurosci. 30, 3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Sueda R., Imayoshi I., Harima Y., Kageyama R. (2019) High Hes1 expression and resultant Ascl1 suppression regulate quiescent versus active neural stem cells in the adult mouse brain. Genes Dev. 33, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Andersen J., Urbán N., Achimastou A., Ito A., Simic M., Ullom K., et al. (2014) A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Kim E.J., Ables J.L., Dickel L.K., Eisch A.J., Johnson J.E. (2011) Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 6, e18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Pastrana E., Cheng L.-C., Doetsch F. (2009) Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl. Acad. Sci. U.S.A. 106, 6387–6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Pilz G.-A., Bottes S., Betizeau M., Jörg D.J., Carta S., Simons B.D., et al. (2018) Live imaging of neurogenesis in the adult mouse hippocampus. Science 359, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Mira H., Andreu Z., Suh H., Lie D.C., Jessberger S., Consiglio A., et al. (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89. [DOI] [PubMed] [Google Scholar]
- 44).Nam H., Benezra R. (2009) High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 5, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Bai G., Sheng N., Bian W., Xie Z., Yokota Y., Benezra R., et al. (2007) Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev. Cell 13, 283–297. [DOI] [PubMed] [Google Scholar]
- 46).Zhang R., Boareto M., Engler A., Louvi A., Giachino C., Iber D., et al. (2019) Id4 downstream of Notch2 maintains neural stem cell quiescence in the adult hippocampus. Cell Rep. 28, 1485–1498.e6. [DOI] [PubMed] [Google Scholar]
- 47).Blomfield I.M., Rocamonde B., Masdeu M.D.M., Mulugeta E., Vaga S., van den Berg D.L., et al. (2019) Id4 promotes the elimination of the pro-activation factor Ascl1 to maintain quiescence of adult hippocampal stem cells. eLife 8, e48561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Lahmann I., Bröhl D., Zyrianova T., Isomura A., Czajkowski M.T., Kapoor V., et al. (2019) Oscillations of Hes1 and MyoD proteins regulate the maintenance of activated muscle stem cells. Genes Dev. 33, 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Sang L., Coller H.A., Roberts J.M. (2008) Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 321, 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Seymour P.A., Collin C.A., Egeskov-Madsen A.R., Jørgensen M.C., Shimojo H., Imayoshi I., et al. (2020) Jag1 modulates an oscillatory Dll1-Notch-Hes1 signaling module to coordinate growth and fate of pancreatic progenitors. Dev. Cell 52, 731–747. [DOI] [PubMed] [Google Scholar]
- 51).Sueda R., Kageyama R. (2020) Regulation of active and quiescent somatic stem cells by Notch signaling. Dev. Growth Differ. 62, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]