Highlights
-
•
Over time, Parkinson’s disease (PD) patients declined on multiple cognitive domains.
-
•
Executive dysfunction was related to interactions between specific resting-state networks.
-
•
These interactions involved deep grey matter, frontoparietal, and attentional networks.
-
•
Destabilization of functional network interactions may influence PD progression.
Keywords: Functional MRI, Parkinson's disease, Resting-state networks, Cognition, (Dynamic) functional connectivity
Abstract
Deficits in cognitive functioning are a common yet poorly understood symptom in Parkinson’s disease (PD). Recent studies have highlighted the importance of (dynamic) interactions between resting-state networks for cognition, which remains understudied in PD. We investigated how altered (dynamic) functional interactions between brain networks relate to cognitive dysfunction in PD patients.
In this fMRI study, 50 PD patients (mean age 65.5 years ± 6.27) on dopaminergic medication were studied cross-sectionally, and of this cohort 31 PD patients were studied longitudinally. MRI imaging and neuropsychological testing was performed at two time points, with a follow-up duration of approximately three years. Functional connectivity within and between seven resting-state networks was calculated (both statically and dynamically) and correlated with four neuropsychological test scores; a combined score of (four) executive tasks, a motor perseveration, memory, and category fluency task. Cognitive dysfunction was determined based on a longitudinal sample of age-matched healthy controls (n = 13).
PD patients showed dysfunction on six out of seven cognitive tasks when compared to healthy controls. Severity of executive dysfunction was correlated with higher static and lower dynamic functional connectivity between deep gray matter regions and the frontoparietal network (DGM-FPN). Over time, declining executive function was related to increasing static DGM-FPN connectivity, together with changes of connectivity involving the dorsal attention network (amongst others with the ventral attention network). Static functional connectivity between the ventral and dorsal attention network correlated with motor perseveration.
Our findings demonstrate that in PD patients, dysfunctional communication between (i) subcortical, fronto-parietal and attention networks mostly underlies worsening of executive functioning, (ii) attention networks are involved in motor perseveration.
1. Introduction
Cognitive impairment is a common non-motor symptom of Parkinson’s disease (PD) that negatively impacts daily functioning and quality of life (Aarsland et al., 2017). Cognitive impairment in non-demented PD patients is typically dominated by attentional, visuospatial and executive deficits, with strong heterogeneity between patients (Aarsland et al., 2017). An executive syndrome in PD (deficits in cognitive flexibility, planning, working memory, and learning) is possibly related to suboptimal dopamine levels: both excesses and deficits of dopamine in the frontostriatal pathways have been associated with impairments in task performance (Kehagia et al., 2013), as was demonstrated by pharmacologica l(Gotham et al., 1988), pharmacogenomics (Cools, 2006), and neuroimaging research (Christopher et al., 2014).
Importantly, executive deficits can occur at any stage of the disease and can be progressive, but are not predictive of the development of PD dementia (Williams-Gray et al., 2009). Progressive executive and general cognitive decline in PD may reflect complex brain pathology and involvement of other non-dopaminergic neurotransmitter systems (Bassetti, 2011), indicating global network disruptions, which remain understudied.
Previous research in PD indicated disturbances of connectivity patterns of the default mode network (DMN) in relation to global cognitive dysfunction (Tessitore et al., 2012). Domain-specific cognitive dysfunction in PD has been related to other networks, such as the attention networks (dorsal attention network, DAN; ventral attention network, VAN) and the bilateral frontoparietal networks (FPN) (Baggio et al., 2014, Baggio et al., 2015). In addition, recent technological advances have allowed for the evaluation of time-varying fluctuations in functional connectivity, the so-called dynamic connectivity, for instance showing that the reconfiguration between frontoparietal and frontotemporal brain networks is related to executive functioning (Braun et al., 2015).
As such, there are strong indications that PD features extensive cortical network dysfunction in addition to the classical dopaminergic systems, possibly driven by a loss of dynamic interplay between networks. In this study, we longitudinally investigated cognitive dysfunction, in particular executive dysfunction, and functional connectivity (FC) within and between RSNs. We hypothesized that more dispersed network interactions, in addition to connections between the deep gray matter and fronto-parietal network, would be involved in the development of executive deficits as well as other cognitive domains in PD.
2. Methods
2.1. Participants
In this retrospective study, data of idiopathic PD patients and healthy controls was used, obtained in the context of a longitudinal study cohort. Exclusion criteria for PD patients were stereotactic surgery in the past and extensive white matter lesions or other abnormalities at MRI (see also (Stoffers et al., 2007) for details on recruitment and inclusion). At initial inclusion, the patients did not receive fMRI scans; all fMRI and neuro(psycho)logical data used in this manuscript was acquired at 4 (“first timepoint”) and 7 year (“second timepoint”) follow-up visits (between 2008 and 2012) in the outpatient clinic of the Amsterdam UMC, location VUmc. For an overview of the timeline of the data acquisition, see Fig. 1. All examinations were performed in the dopamine “ON” state”. All patients and healthy controls have been reported in previous analyses on this dataset in which functional connectivity differences were linked to global cognitive decline and visual hallucinations (Hepp et al., 2017, Olde Dubbelink et al., 2014), but these studies did not specifically assess (dynamic) RSN connectivity in relation to domain-specific cognitive dysfunction.
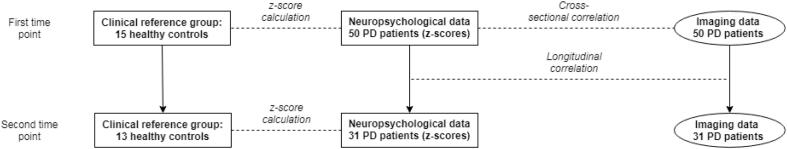
Fig. 1.
Overview of data analysis.
At the first time point, a cross-sectional correlation was performed between (dynamic) functional connectivity of resting-state networks and neuropsychological data for the Parkinson’s disease (PD) group (n = 50). The neuropsychological data of the PD patients were converted to z-scores, based on the scores of healthy controls. Next, for the 31 PD patients who had undergone a second study visit, longitudinal correlations were made between the change in functional (dynamic) connectivity and the change in neuropsychological performance (expressed as z-scores).
Unified PD Rating Scale motor ratings were obtained by a trained physician. Global cognitive functioning was assessed using the Cambridge Cognitive Examination scale. The total dose of dopaminomimetics was converted to a so-called levodopa equivalent daily dose (LEDD) using a previously described conversion rate, see (Olde Dubbelink et al., 2013) for other definitions.
All participant gave written informed consent to the research protocol, which was approved by the local medical ethical committee conform the Helsinki declaration.
2.2. Neuropsychological evaluation
Neuropsychological function was assessed using the Cambridge Neuropsychological Test Automated Battery (Eclipse 2.0, Cambridge, England). Executive tests included spatial working memory (outcome measure: strategy), stockings of Cambridge, spatial span length (partly a working memory test, hence executive function), and intra- extra dimensional set-shifting (outcome measure: number of stages completed). Memory was tested using the pattern recognition memory test, motor perseveration using the Vienna perseveration task, and verbal fluency using the one-minute category verbal fluency test (animals) from the CAMCOG. Cognitive scores were converted to z-scores based on the scores of the healthy controls at each time point as (Dutch) norm scores were not available for all tests, and in order to account for learning effects (Frank et al., 1996, Rabbitt et al., 2001). Negative z-scores represent poorer performance on that particular neuropsychological test. The z-scores were used as clinical input for the relationship with (dynamic) functional connectivity. Subjects with a z-value < -1.5 were considered to be impaired.
2.3. MRI data acquisition
3 T-MR scans (GE Signa HDXT, V15M) included a sagittal three-dimensional T1-weighted fast spoiled gradient-echo sequence (repetition time 7.8 ms, echo time 3.0 ms, inversion time 450 ms, flip angle 12, 1.0 × 0.9 × 0.9 mm voxel size, 170 slices in sagittal plane). Functional MRI included 202 volumes of axial echo-planar images, of which the first two were discarded (repetition time 1800 ms, echo time 35 ms, flip angle 90, 3.3 mm isotropic voxel size, 33 slices in axial plane). Both in the structural and functional recordings, full-brain coverage was reached. Recordings took place in an eyes-closed, resting-state condition.
2.4. Image processing
Resting-state fMRI images were pre-processed by MMS, DH, and LIB (neuroscientist and medical doctors with respectively 10, 5 and 4 years of experience) using standard FSL-5 software (FMRIB Software Library, Oxford, England; http://www.fmrib.ox.ac.uk.fsl) and custom built scripts in bash and Matlab, version 2012a (Mathworks, Natick, MA, USA). We used an independent component analysis-based strategy for Automatic Removal of Motion Artifacts (ICA-AROMA, v0.4-beta 2017, Nijmegen, the Netherlands) (Pruim et al., 2015). Cortical regions of interest (ROIs) were derived from the Brainnetome atlas (Fan et al., 2016) and deep gray matter (DGM) ROIs were derived from FIRST (part of FSL). All cortical ROIs were non-linearly registered to 3D-T1 space with inverted FNIRT parameters and multiplied with grey matter segmentation maps of SIENAX, while using FIRST ROIs for DGM areas.
Subsequently, all ROIs were combined into one atlas (225 ROIs) and this combined atlas was registered to fMRI using inverted boundary-based registration parameters and masked with a custom-made fMRI mask to remove any residual nonbrain tissue and to reduce the effect of echo planar imaging disortions. After this masking, not all atlas-based brain regions had sufficient numbers of voxels in order to be representative. We therefore included only those regions with at least 30% voxels remaining in at least 90% of the subjects (Meijer et al., 2017). On the basis of these criteria, 29 regions were excluded (bilateral orbital gyrus, nucleus accumbens, parahippocampal gyrus, and inferior temporal gyrus). The final atlas therefore segmented the fMRI sequence into 196 regions for which mean time series were obtained.
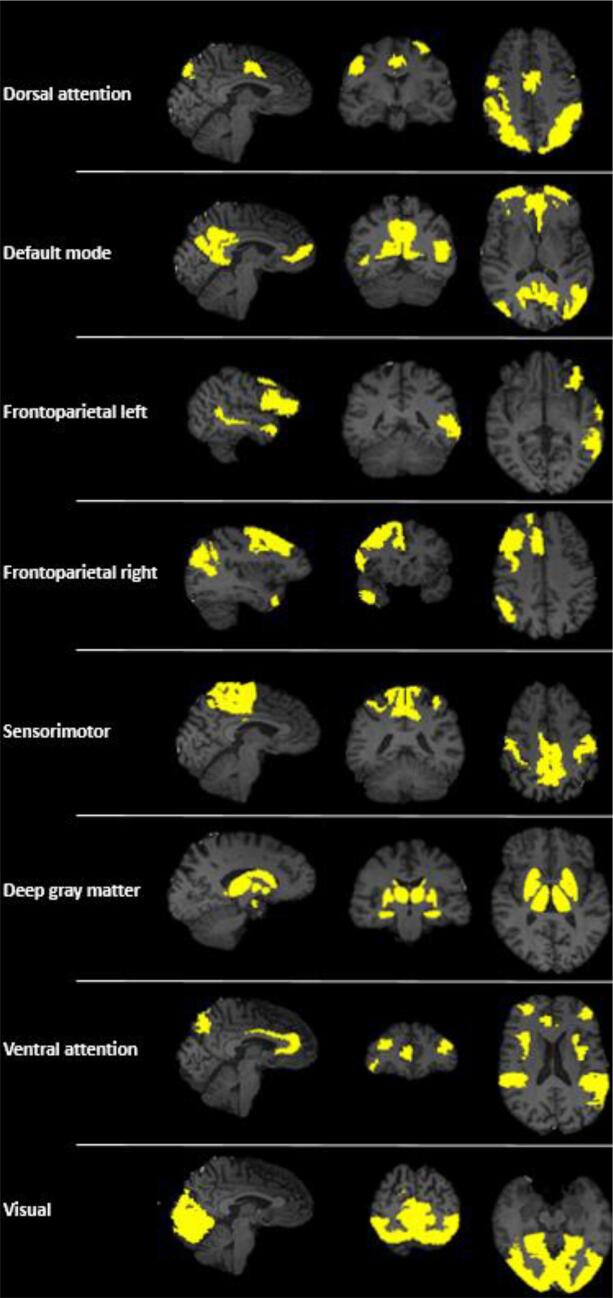
To define resting-state networks, fMRI scans were registered to standard space using aforementioned parameters, where an independent component analysis was run using the MELODIC pipeline (part of FSL). The concatenated fMRI dataset was decomposed into 30 components, which led to seven visually identified RSNs: DAN, DMN, bilateral FPN, sensorimotor network (SMN), DGM, VAN, and visual network) (Fig. 2; Supplementary Table 1; see Supplementary Table 2 for the location of the peak coordinates of each component). RSNs identified were visually inspected (by MMS, LIB) to match with previous literature (Smith et al., 2009). Each brain region was assigned to one network only, based on maximum overlap. The localization of RSNs was based on an independent component analysis of 50 PD patients (based only on the first time point of the study).
Fig. 2.
(Color) Brain regions involved in the resting-state networks (RSNs) of interest.
Sagittal, coronal and transverse views are shown. The allocation of regions of interest to RSNs can also be found in Supplementary Table 1
2.5. FC analysis
For each participant, Pearson correlation coefficients were calculated between time series of all 196 brain regions to construct connectivity matrices. Negative correlations were converted to absolute values. Next, we calculated the average connectivity of each of the seven RSNs with the rest of the brain, as well as within- and between-RSN connectivity.
2.6. Dynamic FC analysis
In addition to static analyses, Pearson correlation coefficients were calculated per window per subject, with a window length of 48.6 s (27x repetition time), resulting in 34 sliding windows, using a shift of 10 s. The choice of the window length and # windows was based on an earlier study (Engels et al., 2018). The standard deviation over time for each functional connection was calculated and normalized for the average FC across time of that connection, which resulted in the calculation of the coefficients of variation of each connection. Subsequently, within- and between-RSN dynamic FC was calculated.
Dynamic FC interactions that were significantly correlated with neuropsychological test performance were compared with null-models to assess whether the effects were due to random noise. These models were created using phase-randomization of our (Fourier-transformed) data (Prichard and Theiler, 1994). Next, we averaged dynamic FC over 50 randomization runs and compared empirical- with randomized dynamic FC values.
2.7. Statistical analysis
Statistical analyses were performed in IBM SPSS version 22 (Chicago, IL, USA), p < 0.05 was considered significant. Clinical characteristics of participants were compared using independent samples t-tests. Normality of all variables was assessed with Kolmogorov-Smirnov tests and histogram inspection. As static FC did not meet this criterion, it was transformed using an inversion transformation (1/x), resulting in normality (the sign of resulting beta values was flipped because of this transformation). Longitudinal changes in neuropsychological performance and functional connectivity were assessed using paried t-tests. To test the association between (dynamic) FC and cognition, a hierarchical linear regression using a backward elimination method was performed per cognitive outcome measure (in which averaged executive performance was treated as one outcome measure). Note that multiple functional connections were tested within the same regression model. Covariates in the regression models included age, sex, education level (dichotomized at level 3), disease duration and LEDD. Residuals were normally distributed for all regression analyses, justifying the use of parametric regression models.
To limit the number of networks analyzed, FC between one RSN and the rest of the brain was used as input for the first (cross-sectional) regression model and only those RSNs showing a main effect with the rest of the brain were explored further.
In the longitudinal regression models, longitudinal cognitive change was related to change in FC. Longitudinal FC scores were normalized by the individual mean overall FC, to exclude the possibility that global connectivity changes over time (e.g. a physiological and/or technical explanation) affected the results.
Dynamic FC values were compared to null models using paired t-tests.
3. Results
3.1. Study participants
A total of 59 patients were included at the first time point, who all had undergone imaging and neuropsychological assessments. 15 Healthy controls were included with neuropsychological assessments. Four patients had severe motion artifacts during fMRI registration and for 5 patients neuropsychological data was not available (due to incomplete cognitive evaluations), leading to an analysis of 50 patients (as opposed to 55 PD patients in previous studies on this cohort (Hepp et al., 2017, Olde Dubbelink et al., 2014)). For the second time point (~3 years later), imaging data was available for 37 patients. The reason for not partcipating in the follow-up evaluation varied and included deep brain stimulation placement, death, strong clinical worsening, and refusal to undergo an MRI scan. Of the 37 patients, six were excluded due to motion artefacts or the absence of neuropsychological data, leaving 31 PD patients with longitudinal data. In addition, 13 healthy controls had longitudinal neuropsychological measurements. Table 1 summarizes the clinical characteristics of all participants. At the first time point, PD patients did not differ from the healthy controls on age (t(63) = 0.403; p = 0.688) and sex (X2(1, 65) = 0.093; p = 0.761). Furthermore, PD patients showed lower global cognitive performance than healthy controls (t(63) = 2.62; p = 0.011). The groups studied cross-sectionally and longitudinally (both in case of the controls and PD patients) did not differ significantly with respect to age, sex, disease duration, LEDD, global cognitive function, and neuropsychological function (all seven tests were compared separately; statistics not shown).
Table 1.
Baseline and longitudinal demographic and cognitive measures of study sample.
| Cross-sectional analysis | Longitudinal analysis | ||||||
|---|---|---|---|---|---|---|---|
| PD (n = 31) | |||||||
| Controls (n = 15) | PD (n = 50) | Time point 1 | Time point 2 | ||||
| Sex (M/F) | 10/5 | 26/24 | 14/17 | ||||
| Age (years) | 64.4 (8.65, range 48–79)) | 65.5 (6.27, range 50–77) | 66.2 (5.62, range 54–77) | 69.1 (6.04, range 57–80) | |||
| ISCED (0/1/2/3/4/5/6) | 0/0/2/3/1/8/1 | 0/0/20/15/2/13/0 | 0/0/15/8/1/7/0 | ||||
| Disease duration (years) | n/a | 9.20 (3.63) | 8.87 (3.75) | 11.9 (3.75) | |||
| UPDRS-III | n/a | 26.1 (8.64) | 25.7 (8.55) | 36.4 (9.82)** | |||
| LEDD total dose | n/a | 795 (5 4 3) | 675 (4 2 4) | 964 (4 8 5) ** | |||
| Cambridge Cognitive Examination | 99.2 (1.93) | 93.3 (8.04)* | 93.8 (7.53) | 89.8 (14.71)** | |||
| Specific neuropsychological evaluation | |||||||
| Executive tests | Z < -1.5; n= | Z < -1.5; n= | Z < -1.5; n= | ||||
| Working memory | 0 (1) | −1.03 (1.48)* | 15 | −0.821 (1.26) | 6 | −2.17 (1.75)** | 19 |
| Spatial span | 0 (1) | −0.745 (0.924)* | 8 | −0.549 (0.764) | 3 | −1.11 (1.14)** | 14 |
| Spatial planning | 0 (1) | −0.481 (1.40) | 9 | −0.340 (1.07) | 3 | 0.043 (1.06) | 4 |
| Set shifting | 0 (1) | −1.83 (3.65)* | 17 | −1.368 (3.39) | 9 | −1.45 (2.92) | 9 |
| Other domains | |||||||
| Memory | 0 (1) | −0.996 (1.57)* | 14 | −0.598 (1.14) | 5 | −1.43 (2.48)** | 12 |
| Motor perseveration | 0 (1) | −1.30 (2.17)* | 16 | −1.15 (2.22) | 10 | −1.32 (1.64) | 11 |
| Fluency | 0 (1) | −0.851 (1.41)* | 15 | −0.678 (1.31) | 8 | −0.621 (0.929) | 7 |
Values are expressed as mean (SD) unless otherwise indicated.
Disease duration was calculated on the basis of the estimated onset of first motor symptoms. Education level was determined using the International Standard Classification.
PD, Parkinson’s disease; ISCED, International Standard Classification of Education; UPDRS-III, Unified Parkinson’s Disease Rating Scale motor ratings; LEDD, Levodopa Equivalent Daily Dose; Working memory, Spatial Working Memory (between errors); Spatial planning, Stockings of Cambridge; Set shifting, Intra- Extra Dimensional Set-shifting; Memory, Pattern Recognition Memory (correct responses); Motor perseveration, Vienna perseveration task redundancy; Fluency, Semantic Fluency (no. of words); n/a, non-applicable. * significant difference in cross-sectional analysis (p < 0.05); ** significant difference in longitudinal analysis (p < 0.05). Z < -1.5; number of subjects with a Z-score < -1,5 below average.
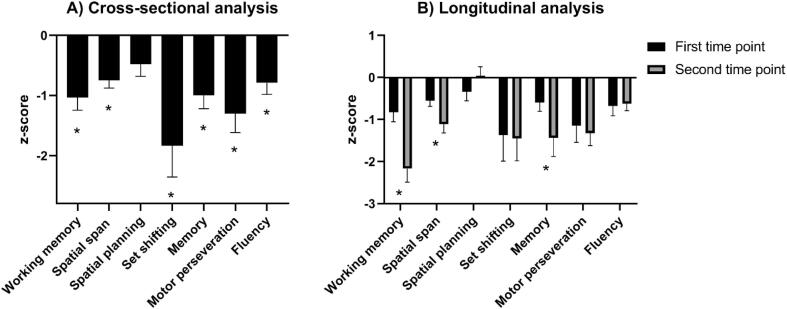
PD patients scored significantly lower than healthy controls on all but one of the specific neuropsychological tests (Stockings of Cambridge, an executive test, which was retained in the average executive z-score) (Table 1 and Fig. 3A).
Fig. 3.
Z-scores of indidivual neuropsychological tests of Parkinson’s disease patients.
-
A)Cross-sectional analysis of neuropsychological performance of 50 Parkinson’s disease (PD) patients, compared with a reference group of healthy controls (n = 15). PD patients scored significantly lower on six out of seven neuropsychological tests (*; p < 0,05).
-
B)Longitudinal analysis of neuropsychological performance of 31 PD patients. Z-scores were based on cross-sectional comparisons between PD patients and a group of healthy controls (first time point, n = 15; second time point, n = 13). Over time, performance of the PD-group significantly worsened for three out of seven tests (*; p < 0,05).
Working memory, Spatial Working Memory (between errors); Spatial planning, Stockings of Cambridge; Set shifting, Intra- Extra Dimensional Set-shifting; Memory, Pattern Recognition Memory (correct responses); Motor perseveration, Vienna perseveration task redundancy; Fluency, Semantic Fluency (no. of words).
Longitudinal assessment of clinical parameters in PD patients showed a decrease in global cognitive performance (t(30) = 2.25; p = 0.034) and in motor performance (t(30) = 5.31; p < 0.001), and an increase in LEDD (t(30) = 7.28; p < 0.001). PD patients longitudinally declined on three neuropsychological tests; spatial working memory (between errors; t(30) = 5.43; p < 0.001), spatial span length (t(30) = 2.95; p = 0.006) and pattern recognition memory test (correct responses; t(30) = 2.47; p = 0.020) (Table 1 and Fig. 3B). The raw scores of neuropsychological performance can be found in Supplementary Table 3.
3.2. Static functional connectivity
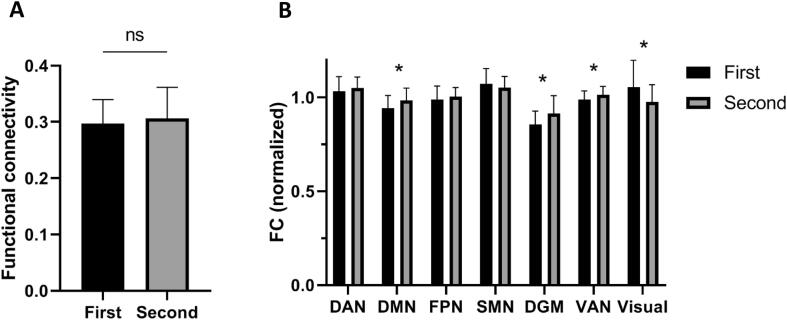
Global functional connectivity did not significantly change between baseline and follow-up (Fig. 4A; 0.270 at baseline, 0.281 at FU1 (t(30) = -0.734; p = 0.469)). However, longitudinal changes in (normalized) functional connectivity between RSNs did show significant changes, as summarized in Fig. 4B. Over time, functional connectivity with the rest of the brain significantly increased for the DMN, DGM and VAN, and significantly decreased for the visual network. No changes were observed for DAN, FPN and SMN.
Fig. 4.
Longitudinal changes in functional connectivity.
-
A)Global functional connectivity over time between the first and second time point (n = 31 Parkinson’s disease (PD) patients), which was not significantly different.
-
B)Functional connectivity (normalized) between resting-state networks and the rest of the brain. DAN-rest t(30) = 1.12 p = 0.272; DMN-rest t(30) = 2.93 p = 0.006; FPN-rest t(30) = 0.826 p = 0.415; SMN-rest t(30) = -1.37 p = 0.180; DGM-rest t(30) = 2.33 p = 0.027; VAN-rest t(30) = 3.013 p = 0.005; Visual-rest t(30) = -2.693 p = 0.011. (*; p < 0,05).
DAN, dorsal attention network; DMN, default-mode network; FPN, frontoparietal network; SMN, sensorimotor network; DGM, deep gray matter; VAN, ventral attention network.
3.3. Executive cognition and static FC
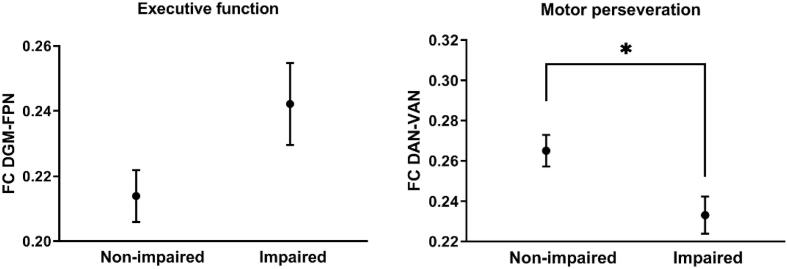
The cross-sectional regression analysis with executive tests (average of four executive function tests) as dependent variable identified FC of DGM with the rest of the brain as important, as well as DAN with the rest of the brain. The subsequent regional analysis revealed a negative correlation between executive functioning at baseline and DGM-FPN FC, indicating higher FC in patients with dysfunction (see Table 2A for statistical values). However, patients with impaired executive functioning (z-value < -1.5) did not show significantly higher FC than non-impaired patients (z-value > -1.5; Fig. 5). The DAN did not show additional cross-network correlations with executive functioning.
Table 2A.
Cross-sectional correlations between cognitive functioning and RSN functional connectivity in PD.
| Cognitive tests | R2 | RSNs involved | RSN interactions involved | Unstandardized Beta | Standardized Beta | p-value |
|---|---|---|---|---|---|---|
| Executive tests ǂ | 0.247 | |||||
| DAN-rest | 0.859 | 0.374 | 0.050 | |||
| DGM-rest | −1.035 | −0.596 | 0.006* | |||
| 0.325 | ||||||
| DAN-Visual | 0.290 | 0.239 | 0.076 | |||
| DGM-FPN | −0.561 | −0.402 | 0.004* | |||
| Visuospatial | – | – | – | – | – | – |
| Motor perseveration | 0.155 | |||||
| DAN-rest | 1.307 | 0.394 | 0.003* | |||
| 0.175 | ||||||
| DAN-VAN | 1.294 | 0.419 | 0.001* | |||
| Semantic Fluency | – | – | – | – | – | – |
Linear hierarchical regression analyses were performed with a backward elimination method per cognitive outcome measure, corrected for age, sex, education, disease duration, and LEDD. First, global functional connectivity between all individual RSNs and the rest of the brain was correlated with cognitive function (second column). Next, interactions between individual RSNs were assessed (based on the significant correlations on whole-brain interactions) and correlated with cognitive function (third column). Note that multiple functional connections were tested within the same regression model. R2 values are displayed in the second column as a single value for each separate regression model.
RSN, resting-state network; DGM, Deep Gray Matter; DAN, Dorsal Attention Network; FPN, Frontoparietal Network; PRM, Pattern Recognition Memory; VPT, Vienna Perseveration Test; VAN, Ventral Attention Network.
Average of Spatial Working Memory, Spatial Span, Stockings of Cambridge and Intra- Extra Dimensional Set-shifting. * significant correlation (p < 0.05).
Fig. 5.
Plots show mean functional connectivity (FC; non-transformed; with standard error of the mean depicted as error bars). As depicted on the left, FC between the deep gray matter (DGM) and the frontoparietal networks (FPN) was non-significantly higher for PD patients impaired (z-value < -1.5) on executive tests ((t(48) = 1.651 p = 0.106). As depicted on the right, FC between the dorsal attention network (DAN) and ventral attention network (VAN) was significantly lower for PD patients impaired (z-value < -1.5) on motor perseveration (t(48) = 2.582 p = 0.013).
Next, longitudinal changes in cognitive function were correlated with longitudinal RSN FC measurements. Over time, change in executive functioning correlated positively with DGM-DMN connectivity changes, whereas it correlated negatively with DGM-FPN and DGM-DGM connectivity changes (Table 2B). In addition, executive cognitive decline was negatively correlated with DAN-SMN FC, while it was positively correlated with DAN-VAN connectivity changes (Table 2B).
Table 2B.
Longitudinal correlations between cognitive functioning and functional connectivity between RSNs in PD.
| Cognitive tests | R2 | RSN interactions involved | Unstandardized Beta | Standardized Beta | p-value |
|---|---|---|---|---|---|
| Executive tests | 0.620 | ||||
| DAN-SMN | −12.825 | −1.312 | 0.002* | ||
| DAN-VAN | 6.545 | 0.793 | 0.015* | ||
| DAN-Visual | 2.612 | 0.474 | 0.072 | ||
| DGM-DMN | 6.171 | 0.871 | 0.007* | ||
| DGM-FPN | −3.100 | −0.591 | 0.035* | ||
| DGM-DGM | −5.742 | −0.647 | 0.020* | ||
| DGM-Visual | −1.537 | −0.354 | 0.098 | ||
| Motor perseveration | – | – | – | – | – |
Linear hierarchical regression analyses were performed with a backward elimination method per cognitive outcome measure, corrected for age, sex, education, disease duration, and LEDD. Selection of functional connectivity interactions between RSNs was based on RSNs deemed to be important based on Table 2A. Note that multiple functional connections were tested within the same regression model. R2 values are displayed in the second column as a single value for each separate regression model.
3.4. Executive cognition and dynamic FC
Only connections showing effects of static connectivity were also explored using dynamic connectivity, thus only RSN interactions concerning the DGM and the DAN. A regression analysis with executive tests as dependent variable showed a positive correlation with DGM-FPN dynamic FC and a negative correlation with DAN-VAN dynamic FC. Both interactions showed significantly higher dynamic connectivity than the null models (DGM-FPN: real data 0.616 ± 0.032, surrogate data 0.609 ± 0.036; t(48) = 3.17; p = 0.003, DAN-VAN: real data 0.593 ± 0.032, surrogate data 0.585 ± 0.033; t(48) = 2.67; p = 0.010).
No significant longitudinal correlation with dynamic RSN interactions was found.
3.5. Other cognitive domains and FC
Using a similar approach, visuospatial function, verbal fluency, and motor perseveration were investigated. In the cross-sectional analysis, visuospatial function and semantic fluency did not correlate with FC of any of the RSNs and these neuropsychological tests were excluded for further analysis. Dysfunctional motor perseveration correlated with lower FC between the DAN and the rest of the brain. In the subsequent regression analysis, dysfunctional motor perseveration correlated with lower DAN-VAN FC only (Table 2A). In accordance, DAN-VAN FC was significantly different between patients with motor perseveration (z-value < -1.5, hence impaired) and non-impaired patients (z-value > -1.5; Fig. 5).
No longitudinal correlations were observed for motor perseveration (Table 2B).
Again, only connections showing effects of static connectivity were also explored using dynamic connectivity, thus only RSN interactions concerning the DAN were correlated with motor perseveration. Dysfunctional motor perseveration correlated with higher DAN-SMN dynamic FC (Table 2C). DAN-SMN showed significantly higher dynamic connectivity than the null model (real data 0.555 ± 0.053, surrogate data 0.545 ± 0.051; t(48) = 2.76; p = 0.008). No longitudinal correlation was found between motor perseveration and dynamic RSN interactions.
Table 2C.
Cross-sectional correlations between cognitive functioning and dynamic functional connectivity between RSNs in PD.
| Cognitive tests | R2 | RSN interactions involved | Unstandardized Beta | Standardized Beta | p-value |
|---|---|---|---|---|---|
| Executive tests | 0.323 | ||||
| DAN-VAN | −18.815 | −0.471 | 0.007* | ||
| DGM-DMN | 10.399 | 0.294 | 0.098 | ||
| DGM-FPN | 14.185 | 0.383 | 0.049* | ||
| Motor perseveration | 0.140 | ||||
| DAN-FPN | −19.57 | −0.401 | 0.053 | ||
| DAN-SMN | −11.93 | −0.294 | 0.030* | ||
| DAN-DGM | 17.80 | 0.358 | 0.080 |
Linear hierarchical regression analyses were performed with a backward elimination method per cognitive outcome measure, corrected for age, sex, education, disease duration, and LEDD. Selection of functional connectivity interactions between RSNs was based on RSNs deemed to be important based on Table 2A. Note that multiple functional connections were tested within the same regression model. R2 values are displayed in the second column as a single value for each separate regression model.
4. Discussion
Our results showed that PD patients displayed deficits in almost all cognitive domains, which worsened over time. Furthermore, we found relative increases in functional connectivity with the rest of the brain for the DGM, DMN and VAN and decreases for the visual network.
Next, we observed a cross-sectional correlation between executive dysfunction and increased static, but decreased dynamic DGM-FPN FC. Longitudinally, only static FC changes were related to decline in executive function, and this included RSN-interactions in addition to DGM-FPN, especially highlighting the role of the DAN. The DAN was also related to cross-sectional deficits in motor perseveration.
We found that higher static connectivity between DGM and the FPN was related to poorer executive functioning, possibly reflecting frontostriatal dysfunction with a dopaminergic basis. However, executive dysfunction did not correlate with the LEDD (β = -0.179; p = 0.179), and DGM-FPN connectivity did not correlate with motor dysfunction (β = 0.049; p = 0.688), nor with the LEDD (β = -0.059; p = 0.666). In spite of the lack of these correlations, and the fact that we added LEDD as covariate in our analyses, in line with previous studies, we hypothesize that dopaminergic therapy could still have impacted the correlations observed, by lowering frontostriatal FC (Kwak et al., 2010) and simultaneously having beneficial effects on executive functioning (Gotham et al., 1988). Since individual patients may have different baseline levels of dopamine they may exhibit a differential sensitivity to the positive and negative effects of dopaminergic medication, which may interfere with LEDD correlations (Cools, 2006). Interestingly, increases in FC within the basal ganglia (Szewczyk-Krolikowski et al., 2014) and sensorimotor network (Esposito et al., 2013) have been observed upon a dopaminergic challenge in PD patients. Hence, it remains to be determined whether a hyperdopaminergic state, in line with the so-called ‘dopamine-overdose hypothesis’ (Cools, 2006), or a hypodopaminergic state explains the correlations observed. This knowledge would be a prerequisite for better clinical management of executive dysfunction.
Our longitudinal regression models suggest that not only FC of the DGM or FPN, but also FC of the DAN is involved in executive dysfunction. Over time, performance on executive tests correlated with FC changes not restricted to the DGM-FPN, involving a negative correlation with DAN-SMN connectivity and a positive correlation with DAN-VAN connectivity. The DAN and VAN are attention networks, involved in reorienting attention towards salient stimuli in the healthy brain (Fox et al., 2006). Previous work has shown that as PD progresses, such networks may become involved to maintain optimal performance on executive tests (Bassetti, 2011), as also suggested by studies on (healthy) aging (Reuter-Lorenz, 2002/08/30.). A recent study has confirmed that reduced FC between the DAN and insular brain regions (the latter being part of the VAN) is associated with worse performance on attention/executive tasks (Baggio et al., 2015). Furthermore, in another study cognitively impaired PD patients had reduced insular dopaminergic D2 receptors, which was associated with worse executive functioning (Christopher et al., 2014).
Apart from executive dysfunction, our study investigated several additional cognitive testss, of which only motor perseveration showed relations with FC of brain networks. Motor perseveration (when one is instructed to demonstrate random motor behavior) has been demonstrated to occur in early stages of PD (Stoffers et al., 2001) and was related to lower DAN FC, especially DAN-VAN connectivity. The generation of random motor behavior is considered to involve retention of information, suppression of habitual responses, and switching of production strategies (Nagano-Saito et al., 2008), all of which are attention demanding processes. Therefore, lower connectivity between the attention networks may play a role in perseverative tendencies such as motor perseveration. The primary cause of attentional network dysfunction remains unclear hower, but is thought to revolve around cholinergic deficits (Sarter et al., 2014).
In addition to static FC, we have added dynamic FC, which has recently been shown to be important for cognitive functioning in PD (Engels et al., 2018, Fiorenzato et al., 2019). Accumulating evidence indicates that functional connectivity between brain regions is nonstationary and alternates between periods of low and high functional coupling over time (Hutchison et al., 2013). In our study, dynamic FC was expressed as the variability (coefficient of variation) of functional connectivity over a number of time windows, in which more variability/fluctuation represents higher dynamic FC. The results of our dynamic FC analysis did not simply mirror static FC results, as demonstrated by correlations involving DAN-VAN (executive dysfunction) and DAN-SMN (Vienna perseveration task) dynamic FC. At the same time, the role of DGM-FPN dynamic FC in executive dysfunction was confirmed, although in this case higher DGM-FPN dynamics seems to be beneficial rather than disadvantageous for cognitive functioning. Together, these results support the notion that the balance of excitation and inhibition, a fundamental feature of brain network activity (Dehghani et al., 2016), may be disturbed in PD, thereby leading to dynamic FC changes. Our longitudinal analysis adds to the recent (cross-sectional) dynamic functional connectivity work in PD showing that PD patients with dementia dwell longer in segregated between-network states (Fiorenzato et al., 2019), and dynamic functional connectivity of the DMN correlated with visuospatial memory disturbances (Engels et al., 2018).
This study has several limitations. First, several subjects were lost to follow-up, leading to the possibility that only PD patients with a relatively mild disease course were included in the longitudinal analysis. However, there were no significant differences between the group studied cross-sectionally and the group studied longitudinally (sex, age, ISCED, disease duration, UPDRS-III, LEDD total dose, CAMCOG, and the specific neuropsychological tests). Moreover, clinically relevant correlations were still identified in the longitudinal analysis. Second, the patients were in the dopamine “ON” state during the MRI acquisition, which at the time was decided to minimize head motion and thus motion-related artefacts. This may have influenced resting-state functional connectivity, but the clinical assessments were also in the dopamine “ON” state. Therefore, our conclusions only apply to PD patients in the dopamine “ON” state. Third, we chose to study only those RSNs showing a main effect with the rest of the brain in the first cross-sectional analysis (DGM and DAN). We are aware that using this approach, we may have missed relevant interactions between RSNs. However, we think this study did not have sufficient statistical power to study the multitude of all RSN interactions. Future studies with larger sample sizes may be able to identify additional connections relevant for cognitive decline in PD. Importantly, the method we have used allowed us to find an a-priori hypothesized result (link between executive functioning and DGM-FPN FC). Fourth, as dynamic FC has only recently been introduced, its biological correlate remains to be established. Therefore, assessing whether observed time-varying fluctuations in FC are due to statistical uncertainty/noise or reflect true changes in FC is important (Hindriks et al., 2016). Therefore, in our study we have confirmed that the observed fluctuations in FC (of relevant interactions) significantly deviated from surrogate data/null models. Fifth, spatial span length is sometimes considered to be a visuospatial rather than a working memory test. In our study, by reasoning from the latter, working memory was included as part of the executive functioning spectrum that we aimed to measure. Future research is necessary to make well-grounded choices for the allocation of neuropsychological tests into specific domains.
In summary, we demonstrated that executive dysfunctioning in PD on dopaminergic medication is associated with higher static, but lower dynamic, FC between deep gray matter areas and the FPN. Over time, worsening executive function was associated with further connectivity changes between RSNs not restricted to the DGM-FPN, centered around attention networks. In addition, attentional network changes were also implicated in motor perseveration. These findings suggest that the pathophysiological mechanisms of executive dysfunction are not merely driven by dopaminergic mechanisms, but also by attention network effects.
CRediT authorship contribution statement
Lennard I. Boon: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Dagmar H. Hepp: Conceptualization, Data curation, Formal analysis, Writing - review & editing. Linda Douw: Writing - review & editing. Noëlle Geenen: Formal analysis, Writing - review & editing. Tommy A.A. Broeders: Formal analysis, Writing - review & editing. Jeroen J.G. Geurts: Writing - review & editing. Henk W. Berendse: Conceptualization, Data curation, Writing - review & editing. Menno M. Schoonheim: Conceptualization, Data curation, Formal analysis, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank all patients and control subjects for their participation. We thank K.T.E. Olde Dubbelink MD PhD for the collection of data and her help in preprocessing of the imaging data.
Funding
The original study on which the present analysis relied was supported by the Dutch Parkinson Foundation (Parkinson Vereniging).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102468.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aarsland D., Creese B., Politis M., Chaudhuri K.R., Ffytche D.H., Weintraub D. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017;13(4):217–231. doi: 10.1038/nrneurol.2017.27. PubMed PMID: 28257128; PubMed Central PMCID: PMCPMC5643027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia A.A., Barker R.A., Robbins T.W. Cognitive impairment in Parkinson's disease: the dual syndrome hypothesis. Neuro-degenerative Dis. 2013;11(2):79–92. doi: 10.1159/000341998. PubMed PMID: 23038420; PubMed Central PMCID: PMCPMC5079071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham A.M., Brown R.G., Marsden C.D. 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. Epub 1988/04/01 PubMed PMID: 3378138. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience Biobehav. Rev. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.02. PubMed PMID: 15935475. [DOI] [PubMed] [Google Scholar]
- Christopher L., Marras C., Duff-Canning S., Koshimori Y., Chen R., Boileau I. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson's disease with mild cognitive impairment. Brain. 2014;137(Pt 2):565–575. doi: 10.1093/brain/awt337. PubMed PMID: 24334314; PubMed Central PMCID: PMCPMC4454524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray C.H., Evans J.R., Goris A., Foltynie T., Ban M., Robbins T.W. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. PubMed PMID: 19812213. [DOI] [PubMed] [Google Scholar]
- Bassetti C.L. Nonmotor disturbances in Parkinson's disease. Neuro-degenerative Dis. 2011;8(3):95–108. doi: 10.1159/000316613. PubMed PMID: 21196687. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Vitale C., Santangelo G., Amboni M., Russo A. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. PubMed PMID: 23100395. [DOI] [PubMed] [Google Scholar]
- Baggio H.C., Sala-Llonch R., Segura B., Marti M.J., Valldeoriola F., Compta Y. Functional brain networks and cognitive deficits in Parkinson's disease. Hum Brain Mapp. 2014;35(9):4620–4634. doi: 10.1002/hbm.2249. PubMed PMID: 24639411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio H.C., Segura B., Sala-Llonch R., Marti M.J., Valldeoriola F., Compta Y. Cognitive impairment and resting-state network connectivity in Parkinson's disease. Hum Brain Mapp. 2015;36(1):199–212. doi: 10.1002/hbm.22622. PubMed PMID: 25164875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U., Schafer A., Walter H., Erk S., Romanczuk-Seiferth N., Haddad L. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. U S A. 2015;112(37):11678–11683. doi: 10.1073/pnas.1422487112. PubMed PMID: 26324898; PubMed Central PMCID: PMCPMC4577153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D., Bosboom J.L., Deijen J.B., Wolters E.C., Berendse H.W., Stam C.J. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130(Pt 7):1847–1860. doi: 10.1093/brain/awm034. awm034 [pii];10.1093/brain/awm034 [doi] [DOI] [PubMed] [Google Scholar]
- Hepp D.H., Foncke E.M.J., Olde Dubbelink K.T.E., van de Berg W.D.J., Berendse H.W., Schoonheim M.M. Loss of Functional Connectivity in Patients with Parkinson Disease and Visual Hallucinations. Radiology. 2017;285(3):896–903. doi: 10.1148/radiol.2017170438. PubMed PMID: 28952907. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T., Schoonheim M.M., Deijen J.B., Twisk J.W., Barkhof F., Berendse H.W. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83(22):2046–2053. doi: 10.1212/wnl.0000000000001020. PubMed PMID: 25355821. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T., Stoffers D., Deijen J.B., Twisk J.W., Stam C.J., Berendse H.W. Cognitive decline in Parkinson's disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2013;34(2):408–418. doi: 10.1016/j.neurobiolaging.2012.02.029. S0197-4580(12)00178-9 [pii];10.1016/j.neurobiolaging.2012.02.029 [doi] [DOI] [PubMed] [Google Scholar]
- Frank R., Wiederholt W.C., Kritz-Silverstein D.K., Salmon D.P., Barrett-Connor E. Effects of sequential neuropsychological testing of an elderly community-based sample. Neuroepidemiology. 1996;15(5):257–268. doi: 10.1159/000109915. PubMed PMID: 8878078. [DOI] [PubMed] [Google Scholar]
- Rabbitt P., Diggle P., Smith D., Holland F., Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39(5):532–543. doi: 10.1016/s0028-3932(00)00099-3. PubMed PMID: 11254936. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.-F.-I.-C.-A.-A.-R.-O.-M.-A. A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;Epub 2015/03/17(112):267–277. doi: 10.1016/j.neuroimage.2015.02.064. PubMed PMID: 25770991. [DOI] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. PubMed PMID: 27230218; PubMed Central PMCID: PMCPMC4961028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer K.A., Eijlers A.J.C., Douw L., Uitdehaag B.M.J., Barkhof F., Geurts J.J.G. Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology. 2017;88(22):2107–2714. doi: 10.1212/wnl.0000000000003982. PubMed PMID: 28468841. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. PubMed PMID: 19620724; PubMed Central PMCID: PMCPMC2722273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels G., Vlaar A., McCoy B., Scherder E., Douw L. Dynamic Functional Connectivity and Symptoms of Parkinson's Disease: A Resting-State fMRI Study. Front.Aging Neurosci. 2018:10–388. doi: 10.3389/fnagi.2018.00388. PubMed PMID: 30532703; PubMed Central PMCID: PMCPMC6266764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard D., Theiler J. Generating surrogate data for time series with several simultaneously measured variables. Physical Review Letters. 1994;73(7):951–954. doi: 10.1103/PhysRevLett.73.951. [DOI] [PubMed] [Google Scholar]
- Kwak Y., Peltier S., Bohnen N.I., Muller M.L., Dayalu P., Seidler R.D. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 2010:4–143. doi: 10.3389/fnsys.2010.00143. PubMed PMID: 21206528; PubMed Central PMCID: PMCPMC3009475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk-Krolikowski K., Menke R.A., Rolinski M., Duff E., Salimi-Khorshidi G., Filippini N. Functional connectivity in the basal ganglia network differentiates PD patients from controls. Neurology. 2014;83(3):208–214. doi: 10.1212/wnl.0000000000000592. PubMed PMID: 24920856; PubMed Central PMCID: PMCPMC4117363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Tessitore A., Giordano A., De Micco R., Paccone A., Conforti R. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain. 2013;136(Pt 3):710–725. doi: 10.1093/brain/awt007. PubMed PMID: 23423673. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. PubMed PMID: 16788060; PubMed Central PMCID: PMCPMC1480402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends in cognitive sciences. 2002;6(9):394. Epub. 2002/08/30. doi: 10.1016/s1364-6613(02)01957-5. PubMed PMID: 12200182. [DOI] [PubMed] [Google Scholar]
- Stoffers D., Berendse H.W., Deijen J.B., Wolters E.C. Motor perseveration is an early sign of Parkinson's disease. Neurology. 2001;57(11):2111–2113. doi: 10.1212/wnl.57.11.2111. Epub 2001/12/12 PubMed PMID: 11739836. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Leyton M., Monchi O., Goldberg Y.K., He Y., Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28(14):3697–3706. doi: 10.1523/jneurosci.3921-07.2008. PubMed PMID: 18385328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Albin R.L., Kucinski A., Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014;257:120–129. doi: 10.1016/j.expneurol.2014.04.032. PubMed PMID: 24805070; PubMed Central PMCID: PMCPMC4348073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenzato E., Strafella A.P., Kim J., Schifano R., Weis L., Antonini A. Dynamic functional connectivity changes associated with dementia in Parkinson's disease. Brain. 2019 doi: 10.1093/brain/awz192. PubMed PMID: 31280293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., Bandettini P.A., Calhoun V.D., Corbetta M. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. PubMed PMID: 23707587; PubMed Central PMCID: PMCPMC3807588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N., Peyrache A., Telenczuk B., Le Van Quyen M., Halgren E., Cash S.S. Dynamic Balance of Excitation and Inhibition in Human and Monkey Neocortex. Sci Rep. 2016;6:23176. doi: 10.1038/srep23176. PubMed PMID: 26980663; PubMed Central PMCID: PMCPMC4793223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R., Adhikari M.H., Murayama Y., Ganzetti M., Mantini D., Logothetis N.K. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage. 2016;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. PubMed PMID: 26631813; PubMed Central PMCID: PMCPMC4758830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.