Abstract
The dawn of commercial bioprinting is rapidly advancing the tissue engineering field. In the past few years, new bioprinting approaches as well as novel bioinks formulations have emerged, enabling biological research groups to demonstrate the use of such technology to fabricate functional and relevant tissue models. In recent years, several companies have launched bioprinters pushing for early adoption and democratisation of bioprinting. This article reviews the progress in commercial bioprinting since the inception, with a particular focus on the comparison of different available printing technologies and important features of the individual technologies as well as various existing applications. Various challenges and potential design considerations for next generations of bioprinters are also discussed.
Keywords: Bioprinter, bioprinting, bioinks, 3D printing, rapid prototyping, biofabrication, tissue engineering
1. Introduction to 3D Bioprinting
1.1 Definition
The advent of 3D bioprinting has opened exciting new possibilities for tissue engineering, reconstruction and in vitro drug testing studies[1,2]. This additive manufacturing-based (AM) biotechnology utilises bioinks[3] along with living cells (cell aggregates or tissue spheroids), to spatially construct 3D functional structures without pre-fabricated scaffolds. Before the more generic term of bioprinting, organ printing was first coined in 1999 and was defined as computer-aided, jetbased 3D tissue-engineering of living human organs[4]. Since then, a host of other printing technologies have been developed as well as new bioink formulations[5,6]. Lately, bioprinting is defined as “computer-aided transfer processes for patterning and assembly of living and nonliving materials with a prescribed 2D or 3D organization to produce bio-engineered structures serving in regenerative medicine, pharmacokinetics, and basic cell biology studies[7]. The sole purpose of bioprinting is to engineer a fully integrated and functionally restored biological environment[8] which could be achieved either through scaffold printing and subsequent cell seeding or direct cell printing. The scaffold-based fabrication approach involves mimicking of the extracellular matrix (ECM) by printing a temporary and biodegradable supporting structure[9]. Incorporation of live cells into the bioink increases/magnifies the complexity involved in printing but promises a more homogeneous distribution of cells as compared to dropwise seeding over a 3D printed scaffold such that the resulting three-dimensional cellular constructs better mimic complex biological functionalities found in native tissues and organs.
1.2 Market, Research and Patent Landscape
Bioprinting is a revolutionary tissue engineering (TE) strategy that holds immense potential as a manufacturing platform for fabrication of in vitro tissue. A market research report by BCC Research pegged the global bioprinting at US$ 263.8 million in 2015. It forecasted the market to reach US$ 295 million in 2016 and shoot to $1.8 billion by 2021. This growth is calculated at a compound annual growth rate (CAGR) of 43.9% from 2016 to 2021[10]. Another report valued the global 3D bioprinting market at US$ 682 million in 2016 with significant future increases[11]. It forecasted the market to reach US$ 2.6 billion by 2024. The market growth is expected to be driven by new printing technologies as well as the expansion of new applications beyond the medical field. However, medical applications (including toxicity screening, organ transplants) are expected to retain the largest growth at 30%[11].
Bioprinting as a field has been very technology intensive. John Hornick and Kai Rajan evaluated the 3D bioprinting patent landscape and found, as of June 2016, 950 patents and pending applications filed by more than a hundred companies[12]. This diverse list included both small enterprises and MNCs based in various geographical locations, suggesting a strong global interest and drive. In terms of the patent portfolio, the leaderboard was occupied by Organovo followed by Koninklijke Philips and Wake Forest University respectively. A very detailed scientometric and patentometric analyses of the field of 3D bioprinting was carried out by Rodriguez- Salvador et al.[13] using competitive technology intelligence methodology. The countries currently leading the patent race are China and USA while Organovo and Tsinghua University came out as the leading institutions (Figure 1). The patent analysis from the year Jan 2000 – July 2016 revealed at least 345 patent families (PFs). The overall proportion of patent applications–patents granted– inactive patents stood at 70%–17%–13%. Biomaterials and Biofabrication are the most popular scientific journals for publishing. The four major knowledge clusters [based on International Patent Classification (IPC)] identified are: tissue engineering (163 PFs), tissue or organ (67), polylactic acid (46) and 3D printer (45). Eight key drivers identified by the experts in the field are: (a) tissue engineering, (b) 3D bioprinting system, (c) bioinks, (d) fibres and scaffolds, (e) human body models, (f) regenerative medicine, (g) pharmaceutical research and (h) vascularization. The linkage between those knowledge clusters and the key drivers have been demonstrated. As of now, companies within the bioprinting industry can be broadly categorized into three categories–companies selling only commercial systems, companies providing the bioprinted tissue and companies providing bioprinting system as a service.
Figure 1.
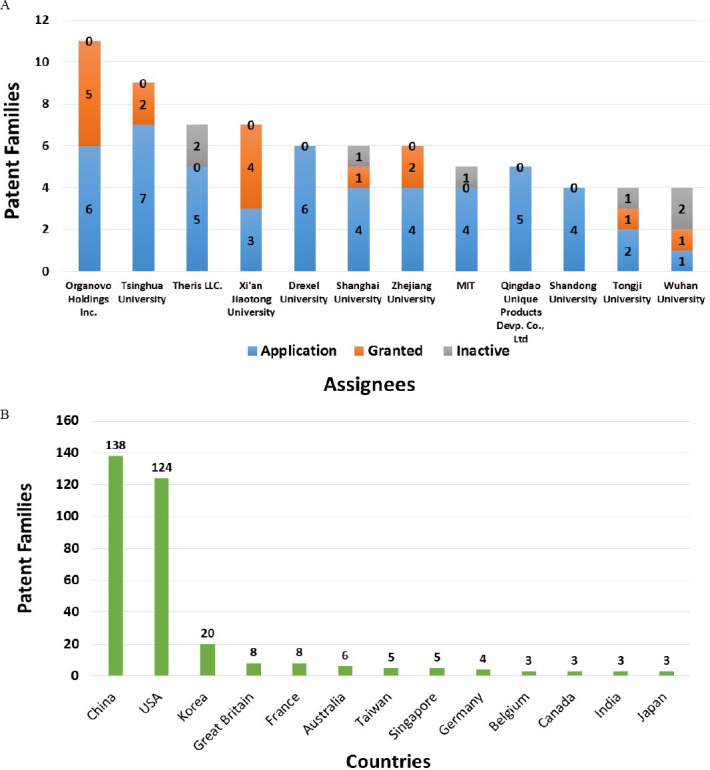
Distribution of 3D bioprinting patents (Adapted from[13]): (A) The top 10 assignee institutions as per the total number of patent families from 1st Jan 2000 till 1st July 2016. Three institutions are tied for the tenth position; (B) The top 10 most countries in which priority filing was done for the first patent of a patent family. Four countries are tied for the tenth position.
1.3 Common 3D Bioprinting Technologies
Almost all the current bioprinters make use of the traditional material deposition techniques (extrusion or ink-jet) or modern optics-based/light-based (laserassisted or stereolithography) for printing (Table 1).
Table 1.
Comparison of bioprinting approaches
| Parameters | Bioprinting Approaches | |||
|---|---|---|---|---|
| Microextrusion | Inkjet | Laser-assisted | Stereolithography | |
| Material viscosity | 30 to > 6 × 107 mPa/s | 3.5 × 12 mPa/s | 1 × 300 mPa/s | No limitation |
| Crosslinking strategy | Photocuring, thermal, chemical | Photocuring, chemical | Photocuring, chemical | Photocuring, chemical also |
| cell viability | 40% × 80% | > 85% | > 95% | > 85% |
| Cell density | High | Low | Medium | Medium |
| Printing speed | Slow | Fast | Moderate | Fast |
| Printing resolution | Medium | High | High | High |
| cost of printer | Medium | Low | High | Low |
1.3.1 Extrusion Bioprinting
The extrusion-based approach is the most common technology implemented by the majority of commercial 3D bioprinters primarily due to cheaper assembly and operational costs. The technique facilitates extrusion of cylindrical filaments of bioink, employing either a pneumatic (air pressure), mechanical (piston) or solenoid (electrical pulses) control (Figure 2A)[14]. Extrusionbased bioprinters enjoy clear advantages such as greater deposition and printing speed, but their higher throughput comes at the expense of lower resolution as compared to the other technologies[15].
Figure 2.
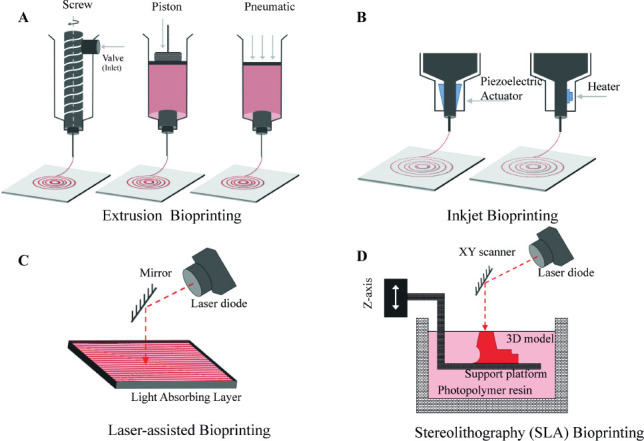
Schematics of various types of bioprinting technologies: (A) Extrusion bioprinting; (B) Inkjet bioprinting; (C) Laser-assisted bioprinting and; (D) SLA bioprinting
1.3.2 Inkjet Bioprinting
Inkjet bioprinting is a concept directly borrowed from the conventional paper printing. The printing setup includes a reservoir of ink, where an acoustic wave is produced to dispense a polymeric solution through the nozzle (Figure 2B). This acoustic wave can either be generated through air bubbles or piezoelectric actuator[16]. Since the technique requires a low-viscous bioink for successful deposition, an additional crosslinking step is accompanied in most of the cases[15].
1.3.3 Laser-assisted Bioprinting
Laser-assisted bioprinting is an emerging technology that allows bioprinting of cells and hydrogels with a celllevel resolution (Figure 2C). The technique has shown great potential towards the realisation of scaffold-free 3D cell systems[17]. It could be classified into two broad categories:
Laser-guided direct cell printing: Manipulating the difference in refractive index of the cells and suspending solution, the laser traps and guides these cells in the desired direction[18].
Laser-induced direct cell printing: The laser beam is focussed on the aqueous bioink via a laser-energy absorbing membrane. This phenomenon generates a vapour bubble, resulting in a mechanical pressure enough to extrude the bioink on the substrate[19].
1.3.4 Stereolithography Bioprinting
The basic principle of Stereolithography is selective crosslinking of a photosensitive biomaterial using a light source, which propagates into the printing solution, thereby activating the photoinitiator and crosslinking the polymeric chains together (Figure 2D)[20]. Several motors are coupled together to facilitate the movement of this light source along X-Y plane, whereas that of the printing support in the Z axis. This arrangement ensures a conventional layer-by-layer fabrication of the desired three-dimensional structure[21].
1.4 Applications of 3D Bioprinting
Bioprinting enables a wide variety of applications, encompassing many industry sectors.
1.4.1 Regenerative Medicine (Engineered organs for transplantation)
The holy grail of tissue engineering is to achieve a fully functional organ which can be transplanted into a patient. However, there are many complex challenges[2,22] which are hindering fabrication of functional organs: (a) vascularization to the extent of capillaries (b) integrating all the various cell types to achieve complex organotypic biology (c) a stable structural and mechanical integrity (d) innervation (e) maturation of printed construct inside a bioreactor and long-term stable functions.
The bioprinted skin models[23–25] mostly involved two cell types, fibroblasts and keratinocytes and did not take into consideration melanocytes, the pigment-producing cells until Ng et al.[26] demonstrated 3D in vitro pigmented human skin constructs by incorporating melanocytes via a two-step drop-on-demand bioprinting strategy. Solid organs are very challenging to print because they require innervation, vascularization, and have dense biomass[2]. However, many attempts have been made for bioprinting solid tissues such as bone[27], cartilage[28], tendon[29], lung[30], liver[31,32], cardiac[33,34] and neural[35] etc. Kang et al.[36] using their specially customised integrated tissueorgan printer (ITOP) demonstrated that it is possible to print centimetre scale tissue/organ constructs of the mandible and calvarial bone, cartilage and skeletal muscle. Detailed list of bioprinted tissues and organs have been covered elsewhere[37].
1.4.2 Drug Discovery and Drug Development
Bioprinting can advance pharmaceutics under four different broad categories[38]: (a) Drug delivery (b) Drug screening (c) Microarrays and high-throughput screenings and (d) Absorption, distribution, metabolism and excretion (ADME) assays. Compared to other in vitro models, 3D printed human tissues have better spatial control of cells, in vivo-like tissue microarchitecture, scalability, easier handling, co-culture capability, cell-cell and cell-matrix interactions and low risk of cross-contaminations[2]. Bioprinting potentially can also ensure controlled delivery of growth factors and possibly genes, an important consideration for longer tissue cultivation[39]. Additionally, these printed constructs should be open to complicated phenotypic assays used inside a person including biochemistry, histology and various ‘omics’ methods[40]. The coprinting technology of multiple materials facilitates even closer replication of cellular microenvironment, opening novel approaches for drug screening. To date, simplified in vitro models of liver[31,32,41,42] and kidney[43] have been successfully fabricated using bioprinting.
1.4.3 Disease Modelling
The premise of modelling disease in vitro is to get the diseased model as similar to that in the actual human body and bioprinting can essentially advance this research. One example is the cancer metastasis process; the mechanical properties (such as stiffness) and composition of a tumour microenvironment often undergo quick changes[44,45], making it challenging to diagnose and treat. Bioprinting can enable the fabrication of various structural aspects of a tumour thereby creating a realistic tumour microenvironment with heterogeneous cells and orders of complexity complete with vasculature[40,45]. To date, bioprinting has been employed to create blood vessels of different diameters to study cancer cell migration[46] as well as uniform tumour spheroids with hollow necrotic cores[47]. The cardiovascular disorder is another prevalent modern life-style dependent disease which can be modelled using bioprinting[48].
1.4.4 Bio-hybrid Robotics
In recent times, there has been a gradual shift towards soft robotics from the conventional one[49]. Researchers have been cautiously inching towards secundum naturam (according to nature) for a better understanding of the intricate organization in living beings, and their subsequent biomimicry[4]. This interest germinates from the desire to tackle more complex, unpredictable environments, which demands higher order of mechanical intelligence. Hybrid bio-bots are defined as an intelligent assembly of highly deformable materials that can be activated by an external stimulus to generate desired motions[50,51]. Bashir et al.[52] first conceptualised the notion of 3D printed “bio-bots” by introducing a class of miniaturized walking biological machines, powered by beating cardiac cells. Through precise patterning of cell-laden hydrogels, it is possible to “build” robots that are powered by cells.
1.4.5 Other Emerging Applications
Another upcoming application for bioprinting lies in the consumer product domain. AM has recently emerged as a promising strategy for designing food materials with complex geometry, detailed patterning, and customized nutritional value[53]. One such revolutionary approach has been the production of tissue engineered meat products through drop-on-demand deposition. Researchers at the University of Missouri–Columbia have manipulated AM-based tissue engineering for the development of comestible food products as an excellent source of protein[54]. Their patents have primarily focused on initiating an alternate technology to meet the growing demand for consumable meat, which so far has been exclusively catered by the overburdened livestock industry[54,55].
In an interesting study, Schroeder et al.[56] made a softer artificial electric organ (power source) by getting inspired from an electric eel. The construct was made up of gradients of ions sandwiched between tiny polyacrylamide hydrogel compartments which were bounded by a repeating sequence of cation- and anionselective hydrogel membranes. This electric organ is soft, transparent and flexible in comparison to traditional batteries. In future, such artificial electric organs could potentially power advanced implant materials such as pacemakers and implantable sensors etc.
Green bioprinting[57] is defined as an AM approach which involves processing of cells from the plant kingdom for studying secondary metabolites production and monitoring methods. Plant bioprinting can also potentially revolutionize many applications like, production of plant-based materials from printed tissues, chimeric grafting, in situ/in vivo bioprinting for repairing tissues and printing designer plant-based food[58].
2. Commercialization of 3D Bioprinting Technology
The expanded applications of bioprinting have driven the development of bioprinting processes as well as platforms over the last few years (Figure 3). Many new companies have emerged to exploit this upcoming industry, and there are three key areas/business models here: (a) directly selling the bioprinters, (b) providing contractual bioprinting services and (c) directly entering with a partnership with a client who has a tissue/organ model of interest. All these companies are based on their unique value proposition of providing bioprinting services or partnerships for manufacturing of functional tissues. In the following section 2.2, we have highlighted all the bioprinting companies (in alphabetical order) which are currently in operation to our knowledge. The detailed specifications of printer dimensions, nozzle diameter, build volume, resolution of printing, materials that can be printed, additional interesting features have been duly summarized in a table (Table 2). Highresolution images of these printers have been included in Figures 4–6 (courtesy of the companies). We have also included a chronological order of important events/ technological advancements in the field of bioprinting (Figure 7) which shows the tremendous progress made just under two decades. Lastly, we have also summarized the current challenges and future innovations which could be integrated with a bioprinter. We hope this review will benefit uninitiated researchers who want to venture into the exciting and revolutionary world of bioprinting as well as those experienced researchers who may not be aware of the sheer number of the companies in bioprinting.
Figure 3.
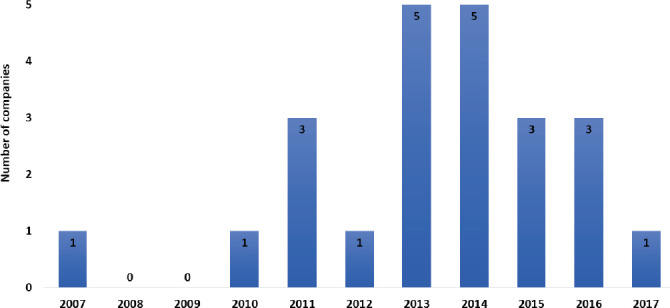
Growth in commercial bioprinting companies over the years
Table 2.
Summary table of commercially available bioprinters
(* IPF: Individually Pore Filling; IF: Injection Filling; FDM: Fused Deposition Modelling; NA: Not available; *: Information unavailable, # Unless additional materials are highlighted, all extrusion-based bioprinters are at least capable of printing hydrogels)
| company | Model | Technology and Materials # | Nozzle Diameter (μm) | Build Volume (mm) | Resolution μm) | Number of printing heads available | Temperature Control | |
|---|---|---|---|---|---|---|---|---|
| X/Y | Z | |||||||
| 3Dynamic Systems | Alpha | Extrusion | * | 150 × 150 × 60 | ±75 (Accuracy) | 1 | * | |
| Omega | 210 × 140 × 60 | ±50 (Accuracy) | 2 | * | ||||
| Advanced Life Sciences | BioAssemblyBot | Extrusion | * | 250 × 300 × 250 | * | 8 Interchangeable tools | Tool: 5–110 °C Bed: 5–110 °C |
|
| Biobot | * | 190 × 100 (Cylinder) | 10 (Linear) | 10 (Rotary) | 5 | NA | ||
| Rotational stage | ||||||||
| Aether | Aether 1 | Extrusion, Droplet jetting, FDM | 50 | 315 × 229 × 132 | 1.055 | 0.00043 | 10+ Pneumatic: 8 FDM: 2 | Anodized aluminum heated syringe mount and stage |
| Allevi | Allevi 1 | Extrusion (hydrogel and thermopolymer) | 150 | 90 × 130 × 60 | 10 | 10 | 1 | Nozzle: 4–200 °C Bed: NA |
| Allevi 2 | Extrusion | 150 | 90 × 90 × 90 | 5.5 | 5 | 2 | Nozzle: RT–160 °C Bed: NA | |
| Allevi 6 | Extrusion Thermoplastics, hydrogels | * | * | 1 | 1 | 6 | Nozzle: 4–200 °C Bed: RT–120 °C | |
| Bio3D Technologies | Explorer | Extrusion | * | * | 5 | 5 | Up to 4 | * |
| CELLINK | INKREDIBLE+ | Extrusion | 100 | 130 × 80 × 100 | 10 | 10 | 2 | Nozzle: Heating Bed: NA |
| BIO X | Extrusion, Inkjet, FDM | * | 130 × 90 × 70 | 1 | 1 | 3 Heated pneumatic: 2 Piston-driven: | Nozzle: 4–130 °C Bed: 4–60 °C 1 | |
| Cyfuse biomedical | Regenova | Kenzan method | 170 (Needle) | 10 × 20 × 18 (9 × 9) 20 × 30 × 18 (26 × 26) | - | Up to 2 types of spheroids | NA | |
| Envision TEC | Bioplotter Starter | 1 | 1 | 2 | Nozzle: 30–250 °C | |||
| 100 | 150 × 150 × 140 | Only high temperature | Bed: NA | |||||
| Bioplotter Developer | Extrusion Hydrogel, thermoplastic, ceramic, metal paste | 100 | 150 × 150 × 140 | 1 | 1 | 3 High and low | Nozzle: 0–250 °C Bed: Available | |
| Bioplotter Manufacturer | 100 | 150 × 150 × 140 | 1 | 1 | 5 High and low |
Nozzle: 0 – 250°C Bed: -10 – 80°C |
||
| GeSiM | BioScaffolder 3.1 | Extrusion, Inkjet, Melt electrospinning | * | 310 × 200 (Area) | 2 | 10 | 3 | Nozzle: * - 120°C Bed: NA |
| nScrypt | 3Dn 300 TE series | Extrusion | * | 300 × 300 × 150 | 0.01 | 0.5 | 4 | * |
| ± 5 (Accuracy) | ||||||||
| Pensees | VitarixTM | Extrusion | 200 | 100 × 60 (Area) | ±10 (Accuracy) | 2 | NA | |
| REGEMAT 3D | V1 | IPF, IF, FDM * | 100 | 150 × 150 × 110 | 150 | 0.4 | FDM: 2 IPF/IF: up to 8 | * |
| regenHU | 3DDiscovery™ Bench-Top | Extrusion, Inkjet Hydrogel, thermopolymer | * | 130 × 90 × 60 | 5 | 5 | Up to 9 technologies | Nozzle: 0–80 °C Bed: 0–80 °C |
| Biofactory™ | Extrusion, Inkjet Hydrogel, thermopolymer | * | 60 × 60 × 60 | 5 | 5 | Up to 8 | Nozzle: 5–80 °C Bed: 5–80 °C |
|
| Regenovo | Bio-Architect Lite | Extrusion | * | 160 × 160 × 150 | 10 | 10 | NA | Nozzle: RT–300 °C Bed: NA |
| ± 20 (Accuracy) | ||||||||
| Bio-Architect Pro | Extrusion | * | 160 × 160 × 150 | 1 | 1 | NA | Nozzle: -5-260 °C Bed: -5 °C-RT | |
| ± 10 (Accuracy) | ||||||||
| Bio-Architect WS | Extrusion | * | 170 × 170 × 150 | 1 | 1 | NA | Nozzle: -10 - 260 °C Bed: -5 °C-RT |
|
| ± 10 (Accuracy) | ||||||||
| ROKIT | INVIVO | Bio-dispenser, FDM | Hydrogel: 50 FDM: 200 | 100 × 100 × 90 | Hydrogel: 50 FDM: 200 |
2 | Nozzle: -10 – 80 °C Bed: -4 – 80 °C |
|
| Se3D | r3bEL MINI | Extrusion | 100 | 130 × 120 (Area) | 10 | * | 1 | NA |
| r3bEL X | Extrusion (Hydrogel and thermopolymer) | Extrusion: 100 FDM: 300 |
200 × 200 (Area) | 10 | 100 | 2 Interchangeable tools | Only for FDM 25 – 260 °C | |
| Seraph Robotics | Scientist™ | Piston-driven or pneumatic extrusion, Traditional FDM | * | * | * | Up to 4 | Nozzle: -3.6 – 80 °C Bed: -3.6 – 150 °C | |
| SunP Biotech | ALPHA-BP11 | Extrusion | 100 | * | * | 2 - 4 | Nozzle: RT - 80 °C Bed: -30 °C - RT |
|
| ALPHA-CPT1 | Electro-mechanical | 100 | * | * | 2 – 4 | Nozzle: RT - 37 °C Bed: NA | ||
| ALPHA-CPD1 | Extrusion | 50 | 170 × 285 × 70 | 5 | 5 | 1 – 3 | Nozzle: RT - 37 °C Bed: NA | |
| ALPHA-CPM1 | Extrusion | 100 | 120 × 160 × 50 | 5 | 5 | 1 – 2 | NA | |
Figure 4.
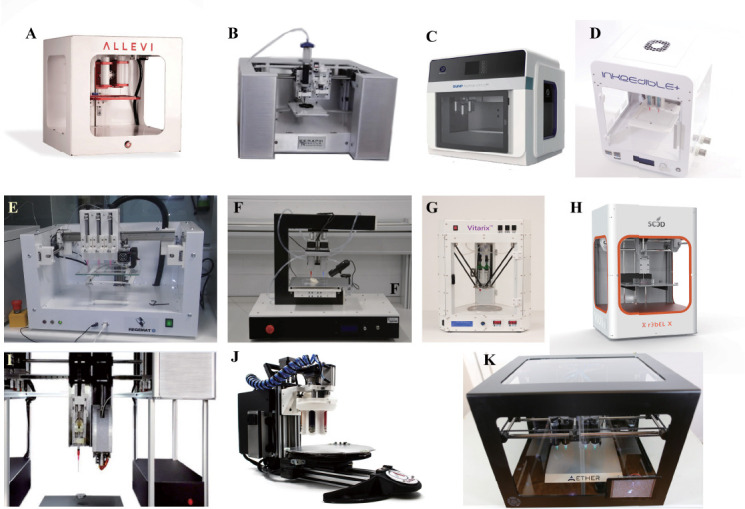
Portable Bioprinters: (A) Allevi 2 (courtesy of Allevi, Philadelphia, US) (B) Scientist™ (courtesy of Seraph Robotics, US) (C) CPD1 (courtesy of SunP Biotech International, NJ, US) (D) INKREDIBLE + (courtesy of CELLINK, Sweden) (E) REGEMAT 3D V1 (courtesy of Regemat 3D, Spain) (F) 3Dynamic Alpha (courtesy of 3Dyanmic Systems, UK) (G) Vitarix (courtesy of Pensees, Republic of Korea) (H) r3bel (courtesy of Se3D, Santa Clara, US) (I) SYN^ (courtesy of Bio3D, Singapore) (J) BIOBOT™ (courtesy of Advanced Solutions Life Sciences, Kentucky, US) (K) Aether 1 (courtesy of Aether, San Francisco, US).
Figure 6.
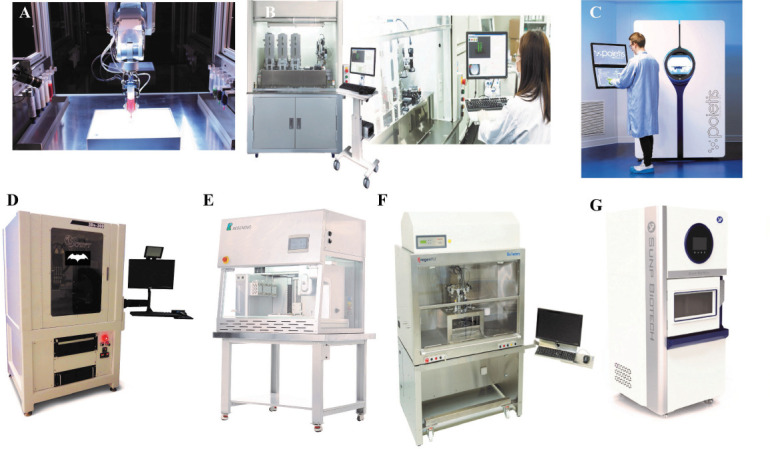
Large Bioprinters: (A) BIOASSEMBLYBOT® courtesy of Advanced Solutions Life Sciences, Kentucky, US); (B) REGENOVA (courtesy of Cyfuse Biomedical K.K., Tokyo, Japan); (C) NGB 17.03 (courtesy of Poietis, France); (D) 3Dn 300 TE Series (courtesy of nScrypt, Orlando, US) (E) Bio-Architect® (courtesy of Regenovo Biotechnology Co.Ltd., Hnagzhou, China); (F) BioFactory™ (courtesy of RegenHU, Fribourg, Switzerland); (G) ALPHA-CPT1 (courtesy of SunP Biotech International, NJ, US).
Figure 7.
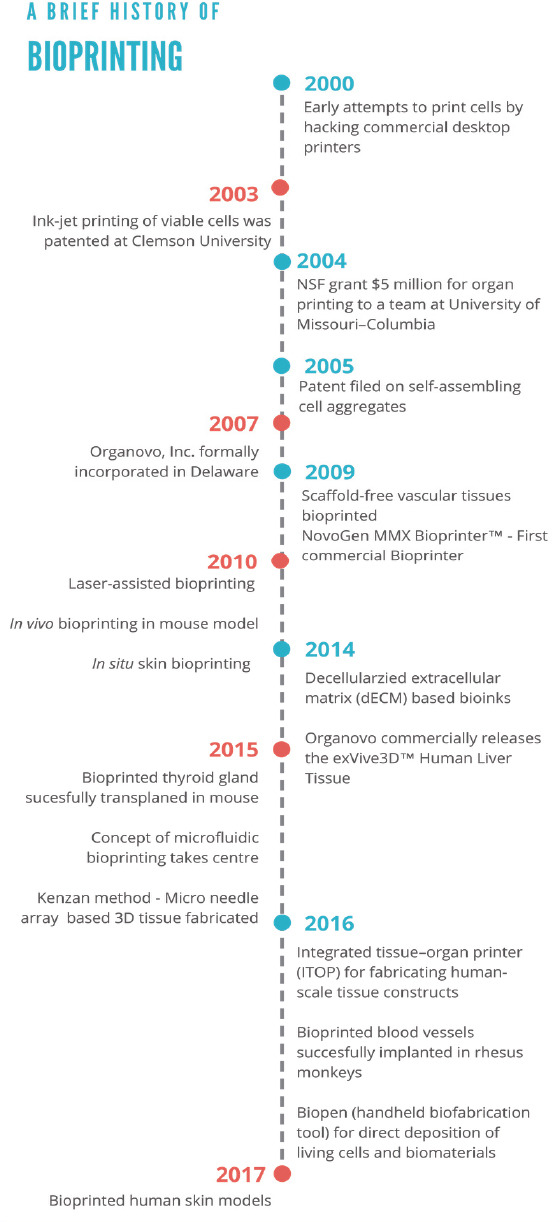
A brief history of bioprinting: Seminal events in bioprinting so far
2.1 The Case Study of the Industry Pioneer- Organovo
In 2003, Boland et al. patented the first ink-jet technology for printing viable cells. The team subsequently secured a US$ 5 million National Science Foundation (NSF) grant[59] which led to their first major patent on Novo Gen MMX Bioprinter™ (Figure 5B) platform soon after in 2005. The first 3D bioprinting company, Organovo came into existence in 2007[59]. Organovo partnered with Invetech (Australia) to launch their bioprinting system-Novo Gen MMX Bioprinter™ in 2009.
Figure 5.

Medium-sized Bioprinters: (A) 3DDiscovery™ (courtesy of RegenHU, Fribourg, Switzerland); (B) NovoGen MMX Bioprinter™ (courtesy of Organovo, San Diego, United States); (C) FABION (courtesy of 3D Bioprinting Solutions, Russia); (D) 3D-Bioplotter® (courtesy of Envision TEC, Gladbeck, Germany); (E) BioScaffolder (courtesy of GeSim, Radeberg, Germany); (F) RX1™ BIOPRINTER (courtesy of Aspect Biosystems, Vancouver, Canada); (G) BIO X (courtesy of CELLINK, Sweden); (H) INVIVO (courtesy of Rokit, Seoul, Korea); (I) Allevi 6 (courtesy of Allevi, Philadelphia, US).
Organovo had their first breakthrough in 2010 when working with a grant from National Institutes of Health (NIH); the company successfully bioprinted fully cellular blood vessels using only primary human cells[59]. Organovo’s ExVive™ 3D Bioprinted Human Liver Tissue Models debuted in 2014. The tissues demonstrate histological and functional similarity to the native liver with consistent expression of albumin, ATP and CYP3A4 activity up to 28 days[31]. The tissue model has been used to demonstrate the clinical effect of drugs such as Valproic acid and Monocrotaline[60]. In 2016, Organovo launched ExVive™ Human Kidney Tissue, a fully 3D bioprinted human tissue consisting of an apical layer of polarized primary renal proximal tubule epithelial cells (RPTECs), supported by a collagen IV-rich tubulointerstitial interface of primary renal fibroblasts and endothelial cells. ExVive™ Human Kidney Tissue exhibits in vivo like barrier function, expression of renal transporters and secretion of important enzyme gmmaglutamyltransferase (GGT)[43]. This bioprinted kidney tissue when exposed to the drug Cisplatin releases kidney injury biomarkers as well as depicts transporterdependent (OCT2) drug uptake.
Apart from commercial tissues, Organovo also offers two kinds of partnerships: custom tissue partnerships (co-develop in vitro tissues for drug screening, disease modelling and therapy) as well as technology access partnerships (access to their NovoGen Bioprinter™ to experts on a specific tissue/disease model). To date, Organovo has inked significant multi-year collaborations with Merck (drug screening)[61] and with world’s largest cosmetics company, L’Oréal (screening of cosmetics in printed dermal tissue)[62].
2.2 Commercial 3D Bioprinting Companies
Since the launch of Organovo, many other bioprinting companies have entered the market. Many of them offer bioprinting systems for research use with several them also selling proprietary bioinks while others such as Tevido have focussed on recreating human tissue. In this section, we highlight the technology and differentiating factor of each of these companies.
3D Bioprinting Solutions (3dbio) successfully 3D printed world’s first animal thyroid gland in March 2015[63] and transplanted into a living mouse[64]. With plans to fabricate artificial thyroid and kidney[64], 3dbio has also been collaborating with Russia’s national space agency, United Rocket and Space Corporation (URSC) to create a magnetic 3D bioprinter that can fabricate artificial tissues in the International Space Station[65]. 3dbio is a subsidiary of Vivax Bio, a global biotech company. Vivax Bio offers FABION (Figure 5C) bioprinter which 5 nozzles, 3 of which are specially designed to dispense bioink and spheroids of any type or size as well as cell suspensions or other biomaterials[66].
3Dynamic Systems (3DS), a UK-based 3D Bioprinting company offers two 3D bioprinters called 3Dynamic Alpha (Figure 4F) and Omega, with the focus of constructing 3D transplantable bone and complex tissue constructs for injured patients[67]. 3DS have partnered with Bioink Solutions to offer a new gelatin-based bioink (Gel4Cell®)[68].
Aether introduced its first bioprinter Aether 1 (Figure 4K) in March 2016. The system boasts a multitude of printing capabilities where 10+ different materials, including viscous pastes, gels, ceramics, filament and oils can be brought in use simultaneously[69]. The other optional features include high-resolution motors, a laser system for ultra-high-precision cutting and engraving, CNC milling machine, photocrosslinking UV LED, microscope, 3D electronics printer, and universal modular fabrication device[70].
Advanced Solutions Life Sciences (ASLS) offers two systems: BioAssemblyBot (3D bioprinter) (Figure 6A) and BioBot™ Basic (Figure 4J). The six-axis robotic arm of BioAssemblyBot is well complemented by the option to load up to ten independent delivery systems during a single print run, thus facilitating the fabrication of more versatile biological 3D scaffolds. The key highlight lies in the form of its software interface: Tissue Structure Information Modeling (TSIM). Both systems also offer features such as rotational stage movement and automated material change.
Allevi (previously called BioBots) has a range of printers (Figure 4A and Figure 5I) featuring pneumatically-controlled dual-extrusion print heads and visible light technology[71]. Allevi’s systems also employ an open concept for easy switching between bioinks and a temperature-controlled extruder head (25°C to 120°C). Allevi 6 is WiFi enabled and includes a sophisticated array of sensors and computer vision which automatically tunes the printing parameters for materials[72]. Allevi also offers a wide-range of commercial bioinks[73].
Aspect Biosystems have been using their proprietary Lab-on-a-Printer™ microfluidic technology in the latest RX1™ bioprinting platform (Figure 5F)[74]. Equipped with a coaxial flow-focusing technology, which ensures direct extrusion of biological fibres with varied diameters. The system was used to demonstrate the fabrication of an artificial airway, termed 3DBioRingTM. The airway consists of contractile smooth muscle tissue comprising of primary human airway smooth muscle cells. The airway tissue has shown appropriate and reproducible contractions to physiological stimuli (histamine) and dilations in response to pharmacological stimuli (B2-agonist)[75].
Bio3D Technologies launched its first modular bioprinter in August 2014. The key highlights of their technology include an anti-vibration levitating platform, multiple interchangeable printing heads, nozzle-toplatform auto-alignment and remote viewing and control. One of the models, Bio3D Explorer, stands out as being the first foldable bioprinter designed for easy transportation[76].
BIOLIFE4D is an upcoming biotech firm founded in 2015, with headquarters at Illinois (USA). The company aspires to 3D bioprint a fully functional, patient-specific heart for safe and affordable organ transplantation[77]. They are a strong team of business leaders and biomedical researchers, currently financed through equity crowdfunding in their scientific endeavour[78]. The BIOLIFE4D technology intends to use adult induced pluripotent stem cells (iPSCs) for 3D bioprinting a human heart, after conducting a thorough MRI scan to obtain the specific dimensions for its construction.
CELLINK markets both bioprinters and a widerange of bioinks[79,80]. In fact, CELLINK was the first to commercialize bioinks for bioprinting applications. INKREDIBLE (Figure 4D) is a pneumatic-based extrusion bioprinter with dual print heads and a UV LED system. In 2017, CELLINK launched BIO X (Figure 5G). Some of the key highlights of BIO X include exchangeable print heads, UV curing tool head, cool pneumatic head, HD camera tool head and printbed temperature control (4–60 °C). Bio X is a fully standalone product which offers two different ways of ensuring clean printing chambers: (a) The HEPA H14 filter positive air system which retains germs and other dust particles and (b) UVC germicidal lamps to sterilize the printing chamber.
Cyfuse Biomedical K. K developed their bioprinter Regenova® (Figure 6B) in partnership with Kanazawabased Japanese medical-device manufacturer, Shibuya Kogyo Co. Ltd. In 2016, the company also collaborated with San Diego-based Cell Applications Inc. allowing them to create scaffold-free, 3D-engineered tissues using the novel Kenzan bioprinting method[81]. Regenova applies a novel robotic approach that enables the fabrication of three-dimensional cellular structures by placing cellular spheroids in a temporary array of fine needles; a methodology has been termed as “Kenzan”. The company in collaboration with Cell Applications Inc. also provides a print service, which allows researchers to order their own scaffold-free 3D tissue constructs[81].
EnvisionTEC has been working at the frontier of additive manufacturing with sales of over 5000 3D printers across 66 countries in the last 15 years. EnvisionTEC 3D-Bioplotters (Figure 5D) can be used in sterile biosafety cabinets and feature automated nozzle cleaning process, external temperature sensor ports and layer by layer photographic log[82].
GeSiM is a spin-off from a publicly funded German research laboratory, Helmholtz-Zentrum Dresden- Rossendorf (HZDR). BioScaffolder 3.1 (Figure 5E) features a piezoelectric micro-pipetting system, on the fourth z-axis allowing non-contact dispensing of small drops in the pico- and nanoliter scale[83]. Other add-on alternatives on BioScaffolder include melt electrospinning and a camera to measure strut dimensions.
nScrypt has a patented Micro Dispense Direct Write technology (MDDW)[84]. nScrypt highlights 3Dn 300 TE Series (Figure 6D) uses a positive pressure pump, SmartPump™ to achieve resolutions as small as 15 microns. Along with nTtip™ comes in a wide range of standard sizes from 12.5 microns (inner diameter) 125 microns, the company has demonstrated the possibility to print living cells with near 100% cell viability[85].
Ourobotics’ 8 materials with options to incorporate additional features such as UV, laser and drill for construction of more intricate and challenging architectures[86].
Pensées designed their Vitarix™ Bioprinter (Figure 4G) to work on a patented articulation-based approach instead of Cartesian coordinates[87]. Pensées has also been developing alginate-based bioinks.
Poietis utilises a technology portfolio from INSERM and University of Bordeaux. The company specializes in 4D laser-assisted bioprinting technology and has collaborations with L’Oréal for developing bioprinting hair follicle[88] and BASF for bioprinted skin models[89]. Equipped with an eight-axis motion, their NGB 17.03 (Figure 6C) bioprinting platform is capable of 3D printing with a resolution of a single cell[90]. In early 2018, Poietis has commercially launched the first bioprinted human full skin model Poieskin® fabricated using their NGB bioprinter[91].
REGEMAT 3D successfully bioprint osteochondral tissue using their REGEMAT 3D V1 (Figure 4E) bioprinting system which incorporates three printing technologies: Individually Pore Filling (IPF), Injection Filling (IF) and Fused Deposition Modelling (FDM)[92]. IPF enhances the viability and survival of the cells when working with high temperature thermoplastics whereas IF fills the different printed volumes when working with small injury sites. It offers users to fully configure their bioprinters.
regenHU offers two different models of commercial bioprinters: BioFactory™ (Figure 6F) and 3DDiscovery (Figure 5A)[93]. Key highlights of the systems include a high degree of customizability and a dispensing resolution of nanolitre. Both printers can also be further customized to incorporate modified laser or photocrosslinking devices, multiple dispensing units and a wide variety of software suites. RegenHU has also launched biomaterials for 3D tissue printing: BioInk™, OsteoInk™ and Stark™[94].
Regenovo Biotechnology Co., Ltd. markets Bio- Architect® (Figure 6E)[95]. The ability to print in a sterilized environment at temperatures ranging from -5 °C to 260 °C is undoubtedly a key highlight of the Regenovo 3D bioprinter.
Revotek’s T-Series™ 3D Bio Printers come with proprietary injection nozzles and specialized, modular Rollovesselar™ platforms which print scaffold-free 3D bio-vascular structures using Biosynsphere™ ink , a proprietary formulation[96]. The Biosynsphere™ ink itself is created using another encapsulation machine made by Revotek. The system comes complete with in-house software, RevoCloud™ which can convert 2D images such as CT scans and MRIs into 3D representations. Working with researchers from West China Hospital at Sichuan University, Revotek has been successful in embedding 3D printed blood vessels into simian test subjects[97]. A 2-centimetre segment of the abdominal artery was replaced with a 3D printed blood vessel in 30 rhesus monkey, where the stem cell bioink was prepared from the autologous adipose mesenchymal stem cells (ADSCs) of the monkeys[98].The printer currently employs a print head with two nozzles and boasts of printing ten-centimetre blood vessels in just two minutes[99].
ROKIT received funding from Korean government[100] and partnered with top Korean Universities to develop their bioprinter ROKIT INVIVO (Figure 5H). Major highlights of the system include the interactive user interface (through Android OS system and WiFi connectivity) along with the customised nozzle setup where the user can select from- extrusion, mechanical dispensing and hot melt pneumatic dispensing. INVIVO can print a wide array of biomaterials ranging from PLGA, PCL, PLLA to collagen or gelatin-based hydrogels with cell mixtures.
Seraph Robotics was born out of Cornell University. Scientist™ 3D printer (Figure 4B), the latest model of Seraph Robotics, is based on Fab@Home Model 3 research platform which allows detailed customisation[101]. The printer also comes with UV LED accessory at various wavelengths (365 or 385 nm) for cross-linking. Scientist™ 3D printer is programmed with XDFL instead of the usual G-Code.
SE3D is funded by National Science Foundation (US). The company has been collaborating with high schools to offer hands-on 3D printing curriculum while providing bioprinting tools and training to research labs working in tissue engineering[102]. The key highlight of SE3D is its r3bEL 3D bioprinter (Figure 4H). The printer is based on open-source programs and includes a swappable multi-tool printhead, comprising of a hydrogel extruder (20–100°C) and a fused filament extruder (up to 260°C). SE3D also supplies a wide range of ready-touse BioKits, with biomaterials and reagents required for initiating educational bioprinting in classrooms[103].
SunP Biotech International offers several categories of bioprinters (ALPHA-BP11, tower-style ALPHA-CPT1 (Figure 6G), desktop ALPHA-CPD1 and mini ALPHACPM1)[ 104]. They have developed a unique low-temp printing technology in ALPHA-BP11 which integrates lyophilisation in 3D printed microfilaments. This results in controlled macro pores (a few hundred microns) for tissue scaffolds and micro pores (10–50 microns) in the printed microfilament. Apart from regular printable scaffold materials, the company also provides personalized bioinks for various in vitro models such as tumours, iPSCs and hepatocytes[105].
Te Vido biodevices utilise patented technology from Clemson University[106]. TeVido’s first product on offer is a bioprinted nipple-areola graft for breast reconstruction. The clinical trials are predicted in 2–3 years’ time[107]. TeVido’s second offering is targeted for Vitiligo patients where they want to print skin tissues which would potentially reduce the contrast in colours[108]. Te Vido biodevices currently does not offer any commercial bioprinters in the market.
2.3 Analysis of Commercial Bioprinting Companies
Bioprinting as a technology has a global presence, and it is clearly demonstrated by the locations of those companies (Figure 8). The majority (almost 40%) of the bioprinting companies are based in North America, and rest are split in between Europe and Asia. Almost three-quarters of all the bioprinting companies have been established within the last 6 years. In fact, 20% of all the companies have been in operation for less than 3 years, and this shows the dynamism of this sector. Organovo, the first bioprinting-only company started operating only in the last 10 years, while much older companies like EnvisionTEC, GeSiM and nScrypt (who have been in business for more than 15 years) had their core applications focus on non-bioprinting related technologies. Extrusion-based printing mode is undoubtedly the most popular mode (Figure 9) in the current bioprinters owing to their ease of operation and lower set-up and maintenance costs. The most common form a biomaterial gets printed is in the hydrogel form, and microextrusion is ideally suited for this. More than half of the printing heads in the commercially available bioprinters are microextrusion-based, and the majority are pneumatically controlled. The number of extrusionbased print heads could vary from 1 to about 10 across various bioprinters.
Figure 8.
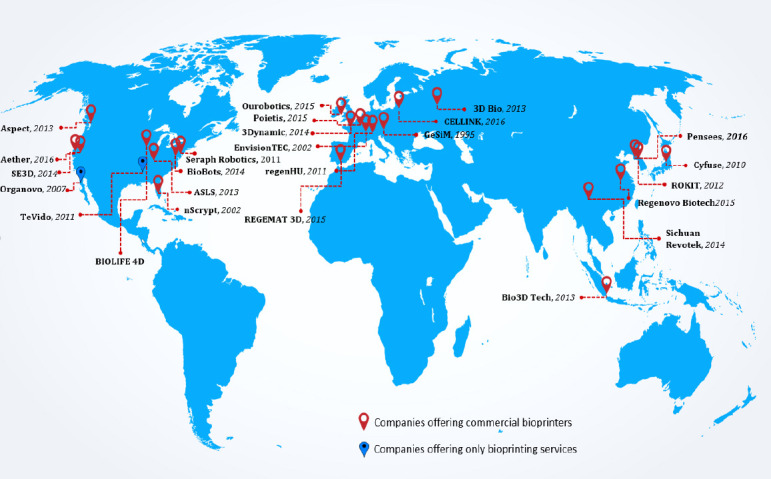
Bioprinting companies around the world
Figure 9.
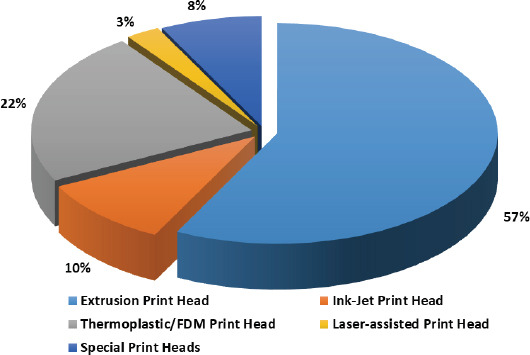
Various print heads in current commercial bioprinters
The second most popular print head in bioprinters is FDM-based where they can extrude biocompatible thermoplastics mainly for fabricating scaffolds of good mechanical strength. Only a handful of companies offer ink-jet printing (Figure 9) of cells owing to the technical challenge of getting uniform droplets as well as the practical challenge of getting higher cell densities with them. Although laser-assisted printing ensures the best printing resolution as well as the viability of printed cells, it is the least common print head in the market owing to the cost associated with the laser itself with only one company focussing on laser-assisted bioprinters. Apart from the regular print heads, there has been the development of special print heads/printing technologies in bioprinting. RX1™ Bioprinter (Aspect Biosystems) offers microfluidic print head which ensures higher resolution as well as flexible co-axial flow focusing technology. Regenova Bioprinter (Cyfuse Biomedical) uses the proprietary Kenzan method to generate cellular spheroids. T-Series 3D Bio Printers (Revotek) prints scaffold-free vascular constructs.
A majority of the bioprinters follow the usual 3D printers cartesian-based coordinate system which helps the system to decide where and how to move. They usually have a square print bed which runs along the Y-axis, while the X-axis carries the print head. The Z-axis is for upward or downward motion of the print bed. Ideally if each of the axes is controlled independently then the assembly is very intuitive, easy to operate and would ensure reliable operation. However many of the bioprinters operate on a Cartesian system which utilizes sliding rails; this in itself is prone to contamination from particles caused by friction.
BioBot™ Basic (ASLS) is a polar coordinate based bioprinting system which offers a rotational stage movement apart from the usual 3-axis motion. Vitarix™ Bioprinter (Pensées) offers a third kind of system called as delta coordinate system with a circular print bed. The extruders are suspended in air above the print bed by three arms in a triangular configuration (thus the name “delta”). This suspension arguably eliminates any contamination from particles caused by friction during its motion.
The nozzle diameters across the extrusion print-heads vary from 50–200 microns. The printing resolution of the bioprinters primarily depends on the printing mode as well the reliability of the associated motor system, but ideally it can go to as low as 1 um across all the 3 axes. The regular extrusion-based print heads have also been made possible to operate at lower temperatures (sub-zero, e.g., 4 deg C) and are being sold as cooled pneumatic heads. Many of the bioprinters are now offering a high-definition (HD) camera tool head to document the work, as well to keep track of the printing process. Many of the companies also sell bioinks which could be natural-based, synthetic-based, sacrificial, biocompatible thermoplastics, or tissue/in vitro model specific formulations.
2.4 Potential Improvements to the Current Bioprinters-Future Design Considerations
Although there has been many new bioprinting technologies and innovations in the field, there remain key challenges which need to be overcome in order to realize the tissue manufacturing process. Ozbolat et al.[109] have articulated many of such limitations with different bioprinting technologies. This section discusses additional features which may be incorporated into a bioprinter to make the printing process faster, flexible, efficient and more predictable.
Flexibility in the direction of printing: Vertical printing is not always desirable. For example, complex neural tissues demand more skewed architecture[110]. The proposed solution would be engineering bioprinters with multiple robotic arms. In this regard, it is worthwhile to note that BioAssemblyBot (ASLS), which is a 6-axis robot arm that can carry out not only additive printing but also contour printing. However, significant progress is still needed in terms of the ability to directly fabricate using biologically relevant hydrogels, with tighter control of the microstructure and anisotropy in 3D. A newly lab-developed 3D bioprinting technique called freeform reversible embedding of suspended hydrogels (FRESH) can ensure printing of hydrogels in complex 3D structures[111].
Incorporation of co-axial extrusion system: Co-axial extrusion system will facilitate simultaneous chemical crosslinking while 3D printing.
Scaffold-free tissues: In recent years, the notion of scaffold-free tissue engineering has gained traction[112], utilizing the self-organization and self-assembly based approaches. Spheroid printing technologies (for example, Kenzan) are expected to be the future of tissue engineering. Kenzan method does not use any gel (ECM) however in case of some tissues, external biochemical and mechanical cues may be crucial for their development and maturation.
Tissue maturation: Cell-material constructs need optimal culturing conditions in terms of the physiochemical environment in order to become functional tissue. Currently, none of the bioprinters has any bioreactors associated with them which can support the printed tissue construct for further maturation. For successful tissue maturation and large-scale implantations, this needs to be given serious thought.
Integration of inline sensors/modules: Sensors could be integrated with the bioprinters for enhanced data gathering and analysis: During the actual printing process, it would be of real value to gauge real-time measurements of parameters such as temperature of printing chamber, humidity in printing chamber, pressure in extruder, speed and fidelity of the printing process etc. Since printing process usually takes hours, dynamic readouts using sensors can ensure remote observation and control too.
In situ/in vivo bioprinting modules: An in vitro bioprinting approach may pose many inherent limitations regarding its clinical applicability in some cases like (a) ethical issues (b) fixation of fragile living constructs inside body (c) mismatch between the shape of the prefabricated construct and the actual defect and (d) maturation of the construct in a bioreactor before implantation. A possible solution is in vivo bioprinting (used interchangeably with in situ bioprinting), which will involve the de novo tissues/organs to be directly printed and positioned at the wound/defect site. This method would be highly effective in the treatment of tissues/organs that can be easily arrested and immobilized during bioprinting, e.g., the musculoskeletal system, craniofacial skeletal defects etc. For this to come true, portable bioprinters need to be designed and tested. There have already been some efforts in this direction, including the development of a hand-held bioprinting device called BioPen[113,114] and the concept of Robotic arm based printer[115].
3. Conclusion
The advent of commercial bioprinting has revolutionized the field of tissue engineering. Within a short span we have at least 26 companies who are working in bioprinting business and out of which at least 23 are selling their bioprinters in the market. The industry now has a worldwide reach with companies based in almost all the major markets. Some of the companies also offer their bioprinters as services and others offer avenues for collaborations/partnerships. Hence a researcher has a multitude of choices, either to purchase bioprinting system (based on his/her applications requirements and budgetary constraints) or to enter into collaborations with the company of choice or to contract out the printing process. The fact that a simple and reasonably reliable extrusion-based bioprinting system is available for under five thousand US dollars makes it an attractive option for most of the small academic labs or individual researchers.
While this is a young industry with most groundbreaking developments happening only in the last 10 years, there has been tremendous growth. Extrusion bioprinting has emerged as the most popular choice owing to its simplicity and relatively lesser costs as compared to other modes. There is much potential in SLA bioprinting for manufacturing of tissues, but no commercial bioprinter can do it yet; although many of the printers do have UV light-source for curing of hydrogels. Currently, many labs around the world are trying to develop non-toxic cocktails of photoinitiators and novel biomaterials/composites which could be cured around visible light wavelengths for SLA bioprinting. The cost barrier for laser-assisted bioprinting is very high, and hence so far only one company is offering it. The development of first 6-axis robotic bioprinter, as well as the desktop polar coordinate-based system, has only demonstrated how much flexibility and sophistication are still possible. The incorporation of IOT elements and possibly AI (artificial intelligence) in future could only make the systems smarter. Integration of a bioreactor with a bioprinter would be key for natural maturation of printed tissue constructs.
The most important component in the bioprinting apart from the bioprinter is the bioink itself which supports the cell growth, differentiation and proliferation leading to maturation of the printed tissue. Several bioprinter companies have expanded their portfolio by selling bioinks; some of these inks are well-known, wellresearched biomaterials while others are proprietary materials/formulations. This has enabled the companies to offer a complete solution to potential clients. Another crucial element for the optimum function of bioprinting hardware is its harmony with the respective controlling software. So far some of the low-cost bioprinters have been using open source software (e.g. Repetier-Host), and this could have reliability issues. Going forward, the printer having the most dedicated hardware-software integration would have the edge over the rest. With so many companies already jostling for space in this bioprinting business, coming decade is bound to see more intense competitions with the launch of upgraded and smarter bioprinters as well as the development of more biomimetic bioinks. Whether these commercial bioprinters get upgraded into high-throughput tissue manufacturing systems, only time will tell. Whether there will be a clear winner who is going to take the lion share of the bioprinting device market is to be seen. The regulatory aspect also needs to keep up with the pace of the device and material development in this field.
Author contributions
DC and MWN conceptualized the manuscript and DC, MWN and SA wrote the manuscript. DC and SA made the figures and tables. MWN edited the manuscript.
Conflict of Interest
All authors reported no conflict of interest.
Funding
The authors would like to thank the funding support to Bio-Manufacturing Programme (BMP) given by A*STAR, Singapore. DC and MWN are A*STAR employees.
Acknowledgments
The authors would like to sincerely thank the bioprinting companies for providing high-resolution pictures of their bioprinters.
References
- 1.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33(7):395–400. doi: 10.1016/j.tibtech.2015.04.005. http://dx.doi.org/10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Murphy S, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. http://dx.doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 3.Gungor-Ozkerim P S, Inci I, Zhang Y S, et al. Bioinks for 3D bioprinting:An overview. Biomater Sci. 2018;6(5):915946. doi: 10.1039/c7bm00765e. http://dx.doi.org/10.1039/C7BM00765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mironov V, Boland T, Trusk T, et al. Organ printing:Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. https://dx.doi.org/10.1016/ S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 5.Holzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3):032002. doi: 10.1088/1758-5090/8/3/032002. https://doi.org/10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury D, Tun H W, Wang T, et al. Organderived decellularized extracellular matrix:A game changer for bioink manufacturing? Trends Biotechnol. 2018 doi: 10.1016/j.tibtech.2018.03.003. https://dx.doi. org/10.1016/j.tibtech.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Moroni L, Boland T, Burdick J A, et al. Biofabrication:A guide to technology and terminology. Trends Biotechnol. 2018;36(4):384–402. doi: 10.1016/j.tibtech.2017.10.015. https://dx.doi.org/10.1016/ j.tibtech.2018.03.00310.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Atala A, Yoo J J. Essentials of 3D biofabrication and translation. 1st edition. Academic Press; 2015. [Google Scholar]
- 9.Chua C K, Yeong W Y. Bioprinting:Principles and applications. World Scientific Publishing Co Inc. 2014:1. https:// dx.doi.org/10.1142/9193. [Google Scholar]
- 10.Bergin J. Bioprinting:Technologies and global markets. BCC Research, USA 2016 [Google Scholar]
- 11.3D Bioprinting market size, share &trends analysis report by technology (Magnetic levitation, inkjet based, syringe based, laser based), by application, and segment forecasts 20182024 2018. Avaliable from: https://www.grandviewresearch. com/industry-analysis/3d-bioprinting-market .
- 12.Hornick J F, Rajan K. The 3D bioprinting patent landscape takes shape as IP leaders emerge. 2016 Avaliable from: http://3dprintingindustry.com/news/3d-bioprinting-patent-landscape-takes-shape-ip-leaders-emerge-84541/
- 13.Rodríguez-Salvador M, Rio-Belver R M, Garechana-Anacabe G. Scientometric and patentometric analyses to determine the knowledge landscape in innovative technologies:The case of 3D bioprinting. PLOS ONE. 2017;12(6):e0180375. doi: 10.1371/journal.pone.0180375. https://dx.doi.org/10.1371/journal.pone.0180375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozbolat I T, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. https://dx.doi.org/10.1016/ j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 15.Knowlton S, Yenilmez B, Anand S, et al. Photocrosslinking-based bioprinting:Examining crosslinking schemes. Bioprinting. 2017;5:10–18. https://dx.doi.org/10.1016/ j.bprint.2017.03.001. [Google Scholar]
- 16.de Gans B J, Duineveld P C, Schubert U S. Inkjet printing of polymers:State of the art and future developments. Adv Mater. 2004;16(3):203–213. http://dx.doi.org/10.1002/adma.200300385. [Google Scholar]
- 17.Koch L, Gruene M, Unger C, et al. Laser assisted cell printing. Curr Pharm Biotechnol. 2013;14(1):91–97. https:// dx.doi.org/10.2174/138920113804805368. [PubMed] [Google Scholar]
- 18.Nahmias Y, Schwartz R E, Verfaillie C M, et al. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–136. doi: 10.1002/bit.20585. http://dx.doi.org/10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 19.Guillotin B, Souquet A, Catros S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. https://dx.doi.org/10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Cooke M N, Fisher J P, Dean D, et al. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B Appl Biomater. 2003;64(2):65–69. doi: 10.1002/jbm.b.10485. https://dx.doi. org/10.1002/jbm.b.10485. [DOI] [PubMed] [Google Scholar]
- 21.Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10(9-10):1316–1322. doi: 10.1089/ten.2004.10.1316. https://dx.doi.org/10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 22.Ozbolat I T, Yu Y. Bioprinting toward organ fabrication:Challenges and future trends. IEEE T BioOMed Eng. 2013;60(3):691–699. doi: 10.1109/TBME.2013.2243912. https://dx.doi.org/10.1109/ TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 23.Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109(7):1855–1863. doi: 10.1002/bit.24455. https://dx.doi.org/10.1002/bit.24455. [DOI] [PubMed] [Google Scholar]
- 24.Michael S, Sorg H, Peck C T, et al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLOS ONE. 2013;8(3):e57741. doi: 10.1371/journal.pone.0057741. https://dx.doi.org/10.1371/journal. pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee V, Singh G, Trasatti J P, et al. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods. 2014;20(6):473–484. doi: 10.1089/ten.tec.2013.0335. https://dx.doi. org/10.1089/ten.TEC.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng W L, Qi J T Z, Yeong W Y, et al. Proofof-concept:3D bioprinting of pigmented human skin constructs. Biofabrication. 2018;10(2):025005. doi: 10.1088/1758-5090/aa9e1e. https://dx.doi. org/10.1088/1758-5090/aa9e1e. [DOI] [PubMed] [Google Scholar]
- 27.Gao G, Schilling A F, Hubbell K, et al. Improved properties of bone and cartilage tissue from 3D inkjetbioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett. 2015;37(11):2349–2355. doi: 10.1007/s10529-015-1921-2. https://dx.doi.org/10.1007/s10529015-1921-2. [DOI] [PubMed] [Google Scholar]
- 28.Apelgren P, Amoroso M, Lindahl A, et al. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLOS ONE. 2017;12(12):e0189428. doi: 10.1371/journal.pone.0189428. https://dx.doi.org/10.1371/journal.pone.0189428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler K M, Morgan B, Young-Joon S, et al. A 3D bioprinted complex structure for engineering the muscletendon unit. Biofabrication. 2015;7(3):035003. doi: 10.1088/1758-5090/7/3/035003. https://dx.doi. org/10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 30.Horváth L, Umehara Y, Jud C, et al. Engineering an in vitro air-blood barrier by 3D bioprinting. Scientific Reports. 2015;5:7974. doi: 10.1038/srep07974. https://dx.doi.org/10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen D G, Funk J, Robbins J B, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLOS ONE. 2016;11(7):e0158674. doi: 10.1371/journal.pone.0158674. https://dx.doi.org/10.1371/journal. pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alan F-J, Catherine F, Dirk-Jan C, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7(4):044102. doi: 10.1088/1758-5090/7/4/044102. https:// dx.doi.org/10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- 33.Jang J, Park H J, Kim S W, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–274. doi: 10.1016/j.biomaterials.2016.10.026. http://dx.doi.org/10.1016/j.biomaterials.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y S, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. https://dx.doi.org/10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han H W, Hsu S H. Using 3D bioprinting to produce mini-brain. Neural Regeneration Research. 2017;12(10):15951596. doi: 10.4103/1673-5374.217325. https://dx.doi.org/10.4103/1673-5374.217325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang H-W Lee, S J, Ko I K, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotech. 2016;34(3):312–319. doi: 10.1038/nbt.3413. https:// dx.doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 37.Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnology Advances. 2016;34(4):422–434. doi: 10.1016/j.biotechadv.2015.12.011. http://dx.doi.org/10.1016/j.biotechadv. 2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng W, Datta P, Ayan B, et al. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017;57:26–46. doi: 10.1016/j.actbio.2017.05.025. http://dx.doi.org/10.1016/j.actbio.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Poldervaart M T, Gremmels H, van Deventer K, et al. Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J Control Release. 184:58–66. doi: 10.1016/j.jconrel.2014.04.007. http://dx.doi.org/10.1016/j.jconrel.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Knowlton S, Onal S, Yu C H, et al. Bioprinting for cancer research. Trends Biotechnol. 2015;33(9):504–513. doi: 10.1016/j.tibtech.2015.06.007. http://dx.doi.org/10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen D G, Pentoney S L. Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discov Today. 2017;23:37–44. doi: 10.1016/j.ddtec.2017.03.001. http://dx.doi.org/10.1016/j.ddtec.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Nupura S B, Vijayan M, Solange M, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(1):014101. doi: 10.1088/1758-5090/8/1/014101. http://dx.doi.org/10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 43.King S M, Higgins J W, Nino C R, et al. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol. 2017;8:123. doi: 10.3389/fphys.2017.00123. http://dx.doi.org/10.3389/fphys.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Nero P, Song Y H, Fischbach C. Microengineered tumor models:Insights &opportunities from a physical sciences-oncology perspective. Biomed Microdevices. 2013;15(4):583–593. doi: 10.1007/s10544-013-9763-y. http://dx.doi.org/10.1007/s10544-013-9763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y S, Duchamp M, Oklu R, et al. Bioprinting the cancer microenvironment. ACS Biomater Sci Eng. 2016;2(10):1710–1721. doi: 10.1021/acsbiomaterials.6b00246. http://dx.doi.org/10.1021/acsbiomaterials.6b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang T Q, Qu X, Liu J, et al. 3D printing of biomimetic microstructures for cancer cell migration. Biomed Microdevices. 2014;16(1):127–132. doi: 10.1007/s10544-013-9812-6. http://dx.doi.org/10.1007/s10544-013-9812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hribar K C, Finlay D, Ma X, et al. Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture. Lab on a Chip. 2015;15(11):2412–2418. doi: 10.1039/c5lc00159e. http://dx.doi.org/10.1039/C5LC00159E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Memic A, Navaei A, Mirani B, et al. Bioprinting technologies for disease modeling. Biotechnol Lett. 2017;39(9):1279–1290. doi: 10.1007/s10529-017-2360-z. http://dx.doi.org/10.1007/s10529-017-2360-z. [DOI] [PubMed] [Google Scholar]
- 49.Majidi C. Soft Robotics:A perspective:Current trends and prospects for the future. Soft Robot. 2013;1(1):5–11. https:// dx.doi.org/10.1089/soro.2013.0001. [Google Scholar]
- 50.Kim S, Laschi C, Trimmer B. Soft robotics:A bioinspired evolution in robotics. Trends Biotechnol. 2013;31(5):287–294. doi: 10.1016/j.tibtech.2013.03.002. https://dx.doi.org/10.1016/j.tibtech.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Patino T, Mestre R, Sanchez S. Miniaturized soft biohybrid robotics:A step forward into healthcare applications. Lab Chip. 2016;16(19):3626–3630. doi: 10.1039/c6lc90088g. http://dx.doi.org/10.1039/C6LC90088G. [DOI] [PubMed] [Google Scholar]
- 52.Chan V, Park K, Collens M B, et al. Development of miniaturized walking biological machines. Sci Rep. 2012;2:857. doi: 10.1038/srep00857. http://dx.doi.org/10.1038/srep00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godoi F C, Prakash S, Bhandari B R. 3d printing technologies applied for food design:Status and prospects. J Food Eng. 2016;179:44–54. https://dx.doi.org/10.1016/ j.jfoodeng.2016.01.025. [Google Scholar]
- 54.Forgacs G, Marga F, Jakab K R. The curators of the university of missouri (assignee) 2014. Engineered comestible meat patent [Google Scholar]
- 55.Marga F S. inventor;Modern Meadow Inc. (assignee) Dried food products formed from cultured muscle cells patent. 2016 [Google Scholar]
- 56.Schroeder T B H, Guha A, Lamoureux A, et al. An electric-eel-inspired soft power source from stacked hydrogels. Nature. 2017;552:214. doi: 10.1038/nature24670. http://dx.doi.org/10.1038/nature24670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Julia S, Tilman A, Max A, et al. Green bioprinting:Extrusion-based fabrication of plant cell-laden biopolymer hydrogel scaffolds. Biofabrication. 2017;9(4):045011. doi: 10.1088/1758-5090/aa8854. http://dx.doi.org/10.1088/1758-5090/aa8854. [DOI] [PubMed] [Google Scholar]
- 58.Wicaksono A, da Silva J A T. Plant bioprinint novel perspective for plant biotechnology. J Plant Develop. 2015;22(1):135–141. [Google Scholar]
- 59.Organovo, History. 2018 Avaliable from: http://organovo.com/about/history/
- 60.Candance Grundy R S, Jeff Nickel Rhiannon N, Hardwick Deborah G, Nguyen Utilization of exVive3D™human liver tissues for the evaluation of valproic acid-induced liver injury Society of Toxicology Meeting. New Orleans 2016 [Google Scholar]
- 61.Stanton D. Avaliable from: https://wwwoutsourcingpharma.com/Article/2015/04/23/MSD-bio-inks-deal-to-use3D-printed-liver-in-toxicology-studies 2015
- 62.L'Oreal USA announces research partnership with organovo to develop 3-D bioprinted skin tissue. 2015 Avaliable from: http://ir.organovo.com/phoenix.zhtml?c=254194&p=irol-newsArticle&ID=2129344 .
- 63.Bulanova E A, Koudan E V, Degosserie J, et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication. 2017;9(3):034105. doi: 10.1088/1758-5090/aa7fdd. https://dx.doi. org/10.1088/1758-5090/aa7fdd. [DOI] [PubMed] [Google Scholar]
- 64.Scott C. 3D bioprinting solutions succeeds in performing the first 3D printed thyroid transplant. 2015 Avaliable from: https://3dprint.com/103721/3dbioprintingsolutionsthyroid/
- 65.3dbio 2016 URSC. Cooperation agreement with 3d bioprinting solutions the development of a bioprinter for space applications. Avaliable from: http://www.bioprinting.ru/en/press-center/publications/orch-the-agreement-withthe-company-3d-bioprinting-solutions-on-cooperation-anddevelopment-of-bio-p/
- 66.Bioprinter FABION. 2018 Avaliable from: http://www.vivaxbio.com/products-services/bioprinter-fabion/
- 67.3Dynamic Systems. 2018 Avaliable from: http://www.bioprintingsystems.com/about.html .
- 68.3DYNAMIC SYSTEMS-NANO-CELLULOSE BIOINK. 2018 Avaliable from: http://www.bioprintingsystems.com/bioinks.html .
- 69.Aether. 2017 Avaliable from: http://bioprinting.aether1.com .
- 70.PRNewswire, 2016. Aether Redefines 3D Printing With Aether 1-World's Most Advanced 3D Bioprinter @PRNewswire, San Francisco. Avaliable from: http://www.prnewswire.com/news-releases/aether-redefines3d-printing-with-aether-1---worlds-most-advanced-3dbioprinter-30023↣.html .
- 71.Allevi. 2018 Avaliable from: https://allevi3d.com .
- 72.Allevi 6. 2018 Avaliable from: https://allevi3d.com/allevi6bioprinter .
- 73.Allevi Bioinks. 2018 Avaliable from: https://allevi3d.com/ shop/?category=Bioinks .
- 74.RX1™BIOPRINTER. 2018 Avaliable from: https://www. aspectbiosystems.com/product-categories/rx1-bioprinter .
- 75.Tran J L. To Bioprint or not to bioprint. NCJ L &TECH. 2015;17(1) [Google Scholar]
- 76.Bio3D Modules. Avaliable from: http://bio3d.tech/modules.html .
- 77.Biolife4D. About BIOLIFE4D. 2018 Avaliable from: https:// biolife4d.com/about/
- 78.BIOLIFE4D launches regulation A+(Mini-IPO) offering;Plans to raise $50M, 2018. Avaliable from: https://www. cnbc.com/2018/02/01/globe-newswire-biolife4d-launchesregulatio n-a-mini-ipo-offering-plans-to-raise-50m.html .
- 79.BIO X 3D BIOPRINTER. 2018 Avaliable from: https:// cellink.com/bioprinter/
- 80.CELLINK-Bioinks. 2018 Avaliable from: https://cellink. com/bioink/
- 81.Scott C. Cyfuse biomedical partners with cell applications Inc. for the First Use of Regenova 3D Bioprinter Outside Japan. 2016 Avaliable from: https://3dprint. com/119161/cyfuse-cell-applications/
- 82.3D-Bioplotter. 2018 Avaliable from: https://envisiontec. com/3d-printers/3d-bioplotter/
- 83.BioScaffolder. 2018 Avaliable from: https://gesimbioinstruments-microfluidics.com/category/bioscaffolder-en/ bioscaffolder-basic-features-en/
- 84.nScrypt. 2018 Avaliable from: https://www.nscrypt.com/
- 85.nScrypt, Bioprinting. 2018 Avaliable from: https://www. nscrypt.com/solutions/bioprinting/
- 86.Ourobotics. Revolution multi material 3D bioprinter. 2018 Avaliable from: https://www.weare3dbioprintinghumans.org/
- 87.VitarixTM Bio Printer. 2018 Avaliable from: http://www.pensees.co.kr/bio_printer .
- 88.L'Oréal and Poietis sign an exclusive research partnership to develop bioprinting of hair. 2016 Avaliable from: https:// www.poietis.com/en/post-release.php?id=6&view=0 .
- 89.BASF and poietis continue work toward 3D printed skin in renewed bioprinting agreement. 2018 Avaliable from: https://3dprint.com/192132/basf-poietis-bioprinting/
- 90.Poietis. 2018 Avaliable from: https://www.poietis.com/en/ platform.php .
- 91.Poieskin. 2018 Avaliable from: http://poietis.com/fr/poieskin/welcome.php .
- 92.REGEMAT 3D. 2018 Avaliable from: http://www.regemat3d.com/versions .
- 93.regenHU Biosystem Architects. 2017 Avaliable from: https://www.regenhu.com/
- 94.regenHU Bioinks. 2018 Avaliable from: https://www. regenhu.com/biomaterials/#BioInk .
- 95.Hangzhou Regenovo Biotechnology Co., Ltd. 2017 Avaliable from: http://regenovo.com/english/
- 96.Biosynsphere™ink. 2018 Avaliable from: http://www.revotekhealth.com/technology.aspx?t=1 .
- 97.Millsaps B B. China's Sichuan revotek announces creation of stem cell bio-ink technology, 3D bio-printer, software. 2015 Avaliable from: https://3dprint.com/102431/ sichuan-revotek-stem-cell/
- 98.Wang S, Hunt K. Chinese company implants 3-D printed blood vessels into monkeys CNN, Hong Kong. 2017 Avaliable from: http://www.cnn.com/2017/01/10/health/china-3d-printed-blood-vessels/index.html .
- 99.Davies S. Chinese medical researchers create natural blood vessels using 3D bio-printer. 2016 Avaliable from: https:// www.tctmagazine.com/api/content/55948f80-c07b-11e6b59b-0aea2a882f79/
- 100.Alec. ROKIT sheds more light on upcoming Edison Invivo 3D bioprinter, announces multi-material Stealth 300 3D printer. 2016 Avaliable from: http://www.3ders.org/articles/20160406-rokit-edison-invivo-3d-bioprinterannounces-multi-material-stealth-300-3d-printer.html .
- 101.Scientist 3D Printers. 2018 Avaliable from: http://www.scientist3d.com/
- 102.Bioprintng in high schools. 2018 Avaliable from: https:// www.se3d.com/high-school .
- 103.SE3D-Biokits. 2018 Avaliable from: https://www.se3d.com/ biokits .
- 104.SunP Biotech Bioprinters. 2018 Avaliable from: http://sunpbiotech.com/productss/3d-bioprinters/
- 105.SunP Biotech Bioinks. 2018 Avaliable from: http://sunpbiotech.com/productss/bio-inks/
- 106.How 3-D printing body parts will revolutionize medicine. 2013 Avaliable from: https://www.popsci.com/science/ article/2013-07/how-3-d-printing-body-parts-willrevolutionize-medicine#page-2 .
- 107.Graboyes R F. 3D printing transplantable organs:Laura bosworth and TeVido biodevices, 2016. 2016 Avaliable from: http://www.insidesources.com/3d-printing-transplantableorgans-laura-bosworth-and-tevido-biodevices/
- 108.TeVido Vitiligo 2018 Avaliable from: http://tevidobiodevices.com/vitiligo/
- 109.Ozbolat I T, Moncal K K, Gudapati H. Evaluation of bioprinter technologies. Addit Manuf. 2017;13:179–200. https:// dx.doi.org/10.1016/j.addma.2016.10.003. [Google Scholar]
- 110.Knowlton S, Anand S, Shah T, et al. Bioprinting for neural tissue engineering. Trends Neurosci. 2018;41(1):31–46. doi: 10.1016/j.tins.2017.11.001. https://dx.doi.org/10.1016/j.tins.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Hinton T J, Jallerat Q, Palchesko R N, et al. Threedimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9) doi: 10.1126/sciadv.1500758. http://dx.doi.org/10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hospodiuk M, Dey M, Sosnoski D, et al. The bioink:A comprehensive review on bioprintable materials. Biotechnol Adv. 2016;35(12):217–235. doi: 10.1016/j.biotechadv.2016.12.006. http://dx.doi.org/10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 113.Cathal DOC, Claudia Di B, Fletcher T, et al. Development of the biopen:A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication. 2016;8(1):015019. doi: 10.1088/1758-5090/8/1/015019. http://dx.doi.org/10.1088/1758-5090/8/1/015019. [DOI] [PubMed] [Google Scholar]
- 114.Duchi S, Onofrillo C, O'Connell C D, et al. Handheld co-axial bioprinting:Application to in situ surgical cartilage repair. Sci Rep. 2017;7(1):5837. doi: 10.1038/s41598-017-05699-x. http://dx.doi.org/10.1038/s41598-017-05699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li X, Lian Q, Li D, et al. Development of a robotic arm based hydrogel additive manufacturing system for in-situ printing. Appl Sci. 2017;7(1):73. http://dx.doi.org/10.3390/app7010073. [Google Scholar]


