Abstract
Since a three-dimensional (3D) printed drug was first approved by the Food and Drug Administration in 2015, there has been a growing interest in 3D printing for drug manufacturing. There are multiple 3D printing methods – including selective laser sintering, binder deposition, stereolithography, inkjet printing, extrusion-based printing, and fused deposition modeling – which are compatible with printing drug products, in addition to both polymer filaments and hydrogels as materials for drug carriers. We see the adaptability of 3D printing as a revolutionary force in the pharmaceutical industry. Release characteristics of drugs may be controlled by complex 3D printed geometries and architectures. Precise and unique doses can be engineered and fabricated via 3D printing according to individual prescriptions. On-demand printing of drug products can be implemented for drugs with limited shelf life or for patient-specific medications, offering an alternative to traditional compounding pharmacies. For these reasons, 3D printing for drug manufacturing is the future of pharmaceuticals, making personalized medicine possible while also transforming pharmacies.
Keywords: three-dimensional (3D) printing, drug dosing and delivery, drug release characteristics, hydrogels, personalized medicine
1. Introduction
Three-dimensional (3D) printing is an additive manufacturing method whereby successive layers of material are deposited/solidified to form a 3D structure. This technology has been applied in numerous fields, representing the large variety of possible applications, including the consumer goods industry[1], aerospace research[2,3], regenerative medicine[4–12], medical device development[13–19] and the automotive industry[20]. An emerging application of 3D printing is for drug manufacturing[21,22].
Interest in 3D printing of pharmaceutical products has been growing since the Food and Drug Administration (FDA) approved the first 3D printed drug in 2015[1,21]. Several methods and materials have since been investigated and demonstrated to serve this purpose[ 1,23–25]. Selective laser sintering (SLS) is the most analogous method to the common drug manufacturing process of powder pressing, in that it relies on loose powder that becomes joined into a solid object. Another powder-based method is binder deposition, in which a liquid binding solution is printed onto a bed of powder. Stereolithography, the selective solidification of a pool/ bed of photosensitive material, may also be used for drug manufacturing. Inkjet printing offers high resolution printing of viscous materials. Extrusion printing is another alternative method which is compatible with both viscous and solid materials. Furthermore, drug products can be printed using hydrogels or polymer-based filaments[ 1].
The rationales supporting the increasing research in 3D printing for drug manufacturing are noteworthy. In general, there is a demand for adaptability, a feature that is not often seen in pharmaceuticals[26]. This includes the ability to fabricate dosage forms with complex geometries and architectures, which directly correlates to increased complexity and control over release characteristics. The adaptability of 3D printing may also be applied for the precise and unique dosing of drugs, whereby drug doses can be printed with the safety of digital control. Additionally, multiple doses or multiple drugs can be printed together in a singular dosage form. Finally and importantly, 3D printing allows for drug products to be adapted for on-demand, prescriptionspecific production. The ability of on-the-spot drug fabrication will have major implications in emergency medicine and for medications with limited shelf-life[1]. Furthermore, 3D printing of drugs means that they can be manufactured for patients on an individualized basis. This capacity directly responds to the demand for individualized medicine and healthcare[1]. Patientspecific medicine entails the modification of drug dosing and combinations to meet the individual’s needs. Conventional drug manufacturing methods lack the ability to fulfill this necessity, as they focus on large-scale batches[26]. There is little flexibility in the typical manufacturing process, requiring several steps which would be too difficult to tailor for a small batch. Conversely, 3D printing-based fabrication of drug products can be changed between prescriptions, also showing promise to transform pharmacy compounding.
Herein, we provide a case for the exploration of 3D printing for drug manufacturing. We first review 3D printing methods and materials that are applicable to drug manufacturing. Then, we elaborate on the benefits of this developing approach in pharmaceuticals, justifying why the FDA has encouraged continued development of 3D printed products[1,21]. We see the 3D printing of drug products as the next imminent revolution in the medicine and healthcare industries, and aim to demonstrate it as such.
2. Applicable 3D Printing Methods
For the purposes of printing drug products, we are concerned with 3D printing technology which utilizes bio-compatible materials that incorporate pharmaceutical elements. There are numerous 3D printing methods, many of which have previously been reported as adapted for bioprinting and drug manufacturing needs[23–25]. In particular, SLS (Figure 1A), binder deposition (Figure 1B), stereolithography (Figure 1C), inkjet printing (Figure 1D), and extrusion-based printing (Figures 1E and F) are ideal approaches for printing for drug manufacturing.
Figure 1.
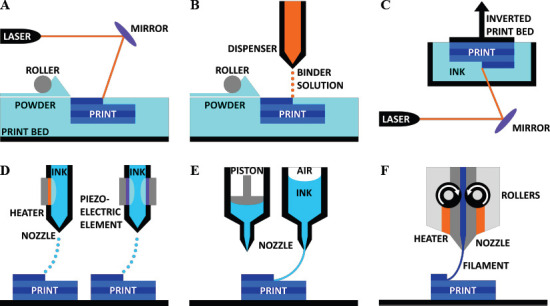
3D printing methods for drug manufacturing. (A) Selective laser sintering. A laser is directed towards a bed of powder which is refilled by a roller system; the laser solidifies the powder to form the desired print. (B) Binder deposition. A binding solution is spotted onto a bed of powder which is refilled by a roller system; upon contact, the binder causes the powder to dissolve and re-crystalize. (C) Stereolithography. A laser is directed towards an inverted print bed which is submerged in a pool of photosensitive ink; the ink is cured and solidified by the laser. (D) Inkjet printing. On the left, a thermal inkjet nozzle uses a heating element to create a bubble in the continuous flow of ink, which generates a droplet. On the right, a piezoelectric element uses electrical pulses to create an acoustic wave which causes the formation of an air bubble, thereby generating a droplet. (E) Extrusion-based printing (of viscous materials). On the left, a piston is used to apply mechanical pressure to the ink to extrude a continuous stream. On the right, pneumatic pressure is applied from above to extrude the ink. (F) Fused deposition modeling (for solid materials). Solid filament is fed through the nozzle by rollers, then melted by heating elements within the nozzle, and extruded on the print bed.
2.1 Powder-Based Printing
Powder-based printing methods are the most similar 3D printing method to the common drug manufacturing method of powder pressing, in which a bed of powder is pressed into a pre-fabricated mold. By the SLS method, a thermal-sensitive powder is spread over the build surface by a roller and pressed to form the appropriate layer thickness[27]. After the layer of powder is established, a laser supplies thermal energy to the powder to stimulate the melting and bonding of powder into the desired form[28]. This process of spreading powder followed by laser sintering is repeated for each successive layer. SLS also allows for partial sintering, and the encapsulation of non-sintered material within a sintered shell.
An additional powder-based method is binder deposition[ 29–31]. Following the same procedure for powder spreading, binder deposition is an additive method that builds from a bed of powder layers. Instead of a laser melting the powder as in SLS, a binder solution is spotted onto the powder. This binder solution dissolves the powder which then re-crystalizes to form the solid form. By this method, the drug ingredients may either be mixed in with the powder, or the drug may be mixed into the binder solution.
2.2 Stereolithography
Similar to SLS, stereolithography utilizes a laser or projector to solidify material while in a bulk setting. With stereolithography, also known more generally as photo-polymerization, the drug would be dissolved into a liquid pool of hydrogel or resin material[32,33]. The material of choice must be photosensitive. When the laser light shines onto the surface of the pool/bed of photosensitive, drug-loaded material, the material cures and solidifies. This method is extremely high resolution and considerably fast, but the nature of the pool of drug-loaded material has an inherent risk of crosscontamination between the fabrications of different drug products.
2.3 Inkjet Printing
Inkjet-based printing follows the same principles as a commercial inkjet printer for paper: ink is deposited onto a substrate by either a thermal-driven or piezoelectricdriven nozzle, offering high resolution printing capabilities. With the introduction of z-axis motion, 3D patterns may be fabricated by this method.
For the thermal inkjet printing approach, a thermal element within the print head generates droplets of ink. This heating element is electrically-controlled to cyclically produce brief spikes in thermal energy which is transferred to the ink[34]. The increase in thermal energy causes the formation of a small bubble, which provides a pulse of pressure to force ink out of the nozzle, thereby producing a droplet[4,35].
An alternative to thermal inkjet printing is the piezoelectric approach, which implements a piezoelectric actuator to form droplets. A piezoelectric crystal within the print head is stimulated when voltage is applied, which induces a rapid, reversible deformation[4]. This deformation propagates acoustic waves which supply the pulse of pressure needed to disrupt the flow of ink through the print head, thereby producing droplets.
The inkjet printing method can further be applied to microvalve-based 3D printing. Microvalve printing utilizes a motorized stage comprised of an array of microvalves which are capable of depositing droplets of material[36–38]. Each microvalve is connected to its own pressure regulator, allowing for individual control of each one. By controlling the stage and the pressure regulators in unison, various materials can be simultaneously deposited. This scheme has been previously applied to cell-laden bioprinting, whereby support material, growth media, and cell-laden material were printed together[37]. Microvalve-based 3D printing can be applied to drug fabrication by depositing various drug-loaded materials along with binders, scaffolds, and other biodegradable materials.
2.4 Extrusion-Based Printing
Extrusion-based printing entails the extrusion of a continuous stream of ink, as compared to the droplets which are formed via inkjet printing. By using this method, the substrate material is mixed with the drug of interest, and deposited by a nozzle or needle. The substrate may be a viscous liquid or a solid filament. Furthermore, advances in micro-extrusion allow for highly precise deposition of drug-loaded material for small-scale products[39].
3. Material Considerations
In discussing 3D printed pharmaceuticals, it is also important to consider the type of material – whether it be a powder, solid bulk, or liquefied substance – that is used to print the drug product[1]. SLS and binder deposition both require a powder substance. Compatible with extrusion-based printing, fused deposition modeling (FDM) relies on the extrusion of solid filaments loaded with the desired drug. Due to the reliance on solid polymer-based filaments, this method poses more challenges in making it appropriate for oral dosage medicines. Conversely, natural and synthetic hydrogels have a more viscous consistency that makes them more appropriate for oral drug products. Additionally, the viscous nature of hydrogels allows for extrusion-based or inkjet-based printing. Finally, various forms of smart materials are described for drug delivery applications.
3.1 Materials for Powder-Based Printing
Materials used for powder-based printing methods must meet certain criteria for printability. With respect to binder deposition, the requirements and necessary parameters of the materials used are relatively straightforward. Factors that impact the printability include particle size, binder viscosity, droplet size of the binder solution, the concentration of the binder solutions, and the thickness of each powder layer[40]. The powder size must not be too small as to cause low flowability, nor may it be too large such that high density printed parts are not feasible. Additionally, the binder solution must be of low enough viscosity and high surface tension to precisely form small droplets, while also being able to penetrate the layer of powder. This is interdependent on the requirement that the powder layer be thin enough for binder saturation, but thick enough to prevent excessive binding. In most cases of binder deposition printing, the binder solution acts as a solvent for the powder, whereby the powder is dissolved upon contact with the binder[41,42]. The binder-powder mixture may either dry to form the solid part, or the materials may react to cause localized polymerization, curing or bonding. Examples of such powders may include soluble polymers, plastics and starches, while binders include chloroform and water, among other solvents.
Selective laser sintering has more complexity involved with material selection. Powders that have been previously studied include polyamides, poly-ε- caprolactone (PCL), hydroxyapatite (HA), polyethylene (PE) and poly(lactic acid) (PLA)[43–46]. By nature of SLS, a laser applies localized heat to selectively melt the powder where the laser strikes. To facilitate this process, the entire powder bed may be maintained at a temperature just below the melting point of the powder[27]. The powder material must withstand the elevated temperature of the print bed without degrading or agglomerating[47]. Ideally, the powder possesses a high melting point and a relatively lower glass transition temperature, which is often seen if the material exists as a semi-crystalline polymer at room temperature[28]. These thermal properties make the material suitable for printing at high temperatures. In the case of semicrystalline polymers, the powder bed would be held at a temperature above the glass transition temperature, close to the melting point. At this state, the laser only needs to introduce enough energy to exceed the point of phase transition, while minimizing the temperature increase in the surrounding powder[47].
The powders used in SLS can range in particle size on the scale of microns to hundreds of microns[48]. For all particle sizes, the laser sintering causes the formation of necks between the particles; these necks, or linkages, remain small compared to the size of the particles themselves. This neck formation process is due to the surface heating of adjacent powder particles, and therefore is a function of particle size and shape, temperature, and the relative arrangement and density of particles. Particle size also contributes to another important material property, one which is shared with the binder deposition method: the flowability of the powder. Flowability refers to the ease of spreading precisely controlled layers of desired thickness; this parameter is directly related to the granulometry and morphology of the particles[47]. For instance, small particles at high density lead to lower flowability. On the other hand, high density powder is better suited for accuracy and strength of the sintered part. To balance these characteristics, particles are preferred to be nearspherical and flowability agents are often added[49].
3.2 Fused Deposition of Solid Materials
Fused deposition modeling (FDM) 3D printers are a specific category of extrusion-based printers which use a solid, polymer filament. The filament is fed through an electronically controlled nozzle which melts the filament and deposits it onto the print bed where the melted filament solidifies into the final 3D printed form. Such printers are simple and versatile, and are compatible with filaments such as poly(lactic acid) (PLA), poly(vinyl alcohol) (PVA), and ethylene vinyl acetate (EVA)[50–52]. Due to the polymer nature of the filaments, they exhibit considerable structural stability after printing and solidifying. These filaments are also largely watersoluble, and are capable of being loaded with a drug in solution. Filament can be loaded with varying concentrations of drugs for specified doses by dissolving the drug in an ethanolic solution and submerging the unprinted, solid filament in the solution[53,54]. Filament can also be loaded with drugs by melting the filament and re-solidifying it after the addition of the drugs[55]. Once the 3D printed drug product is placed in vivo, the drug itself will diffuse out of the print, while the biodegradable filament will dissolve over time.
3.3 Natural and Synthetic Hydrogels
As opposed to the solid nature of polymer-based filaments used in FDM printing, hydrogels are viscous and capable of being extruded or deposited as droplets via extrusion-based printing and inkjet-based printing, respectively. Implementing a controllable gelling hydrogel system, many layers of drug-loaded hydrogels can be printed into 3D structures, characterized by pores and channels which can be printed into the materials according to programmable patterns[35]. Following printing, curing and soaking, the hydrogel patterns develop into water-swollen networks formed by the deposited hydrogel material[56–58]. These networks exhibit considerable porosity and high diffusion rates for various substances, and of particular interest is the ability to carry and release loaded drugs. Additionally, some hydrogels even respond to pH, temperature, or enzymatic activity, enabling controlled and targeted release of drugs[59–61].
Hydrogels may be formed with either naturally-derived or synthetic materials, each having nuanced properties and applications. Natural hydrogel materials include alginate, gelatin, agarose, fibrin and chitosan[57,62]. Synthetic materials include poly (ethylene glycol) (PEG), oligo(poly(ethylene glycol) fumarate) (OPF) and poly (acrylic acid) derivatives (PAA). PEG is a commonly used hydrogel material for drug delivery due to its nontoxic and non-adhesive properties, in addition to its compatibility with crosslinking which allows for more durable internal bonds to finalize the printed shape.
A more recent hydrogel contender in the field is gelatin methacryloyl (GelMA), which is an inexpensive biomaterial naturally derived from denatured collagen and chemically modified by the addition of a methacrylate group[63]. Similar to PEG, GelMA can be photo-crosslinked; when exposed to light in the presence of a photoinitiator, the methacrylate groups of the GelMA crosslink with each other, forming a gel. GelMA also exhibits the benefit of a temperature-dependent viscosity transition which makes it ideal for 3D printing. Furthermore, GelMA has been demonstrated as a drug delivery hydrogel by combining it with PAA, whereby the relative concentration of PAA controls the degree of and timing of drug release[64].
Pertaining to the formulation of all hydrogel materials, various parameters must be considered to achieve material properties suitable for high-resolution drug manufacturing. The type of crosslinking directly impacts the degradability and mechanical properties of the printed hydrogel[65–67]. Hydrogels can be chemically crosslinked – radical polymerization, reaction with complementary or end groups, and enzymatic activity – or physically crosslinked – crystallization, ionic interactions, hydrogen bonds, and protein interactions. Each form of crosslinking has varying levels of rigidity and degradability; stronger and greater numbers of bonds are associated with stronger printed products, but at the expense of lower degradability. For drug delivery application, the crosslinking bonds must be strong and plentiful enough to maintain the hydrogel for a given time period, but must also be weak and few enough to breakdown and degrade. In addition to the crosslinking, the combination of materials is also an important factor: multiple materials can be combined into a copolymer, in which case the relative ratios of each individual material tunes the final material properties[56,68,69]. Copolymers can also be utilized to control the drug release by leveraging hydrophobic and hydrophilic properties of both the individual hydrogel materials and of the added drug(s) [70,71]. For instance, the release of a hydrophilic drug can be controlled and slowed by embedding it within a hydrophobic hydrogel. An alternative example, a nondegrading hydrophobic hydrogel that has excellent thermo-mechanical properties can be modified to be biodegradable by the addition of hydrophilic material. Another consideration is the viscosity, surface tension, and temperature-dependent properties of hydrogels. These factors are crucial for finding or synthesizing materials that are appropriate for 3D printing[62,72]. A final limitation of practically all hydrogels that should be considered is the geometric precision during the 3D printing process. When printing drugs, accuracy is of utmost importance, yet due to the low viscosity during printing and the gelatinous consistency post-printing, accurately printing corners or small designs can be very difficult.
As for the loading of the drug into the hydrogel, two general methods have been presented: the printed hydrogel may be placed into a liquid medium saturated with the drug, or the drug may be pre-mixed into the hydrogel material[59]. These methods have been reported as diffusion and entrapment, respectively. Diffusion relies on the porosity of the hydrogel in order to take up and store the drug. Entrapment is more suitable for drugs with larger molecule sizes or for more careful and specific drug loading. Alternatively, drugs can also be directly deposited into the middle of a print, thereby entrapping the drug inside a hydrogel drug carrier. With both diffusion and entrapment, once the 3D printed drug-loaded hydrogel is placed in vivo, similar to a FDM-fabricated drug, the drug will diffuse out of the hydrogel network. The concentration gradient of the drug formed between the 3D print and the surrounding environment may cause an initial burst release or a triphasic release profile – burst release due to swelling and drugs eluted from the surface, followed by zero order release, and finished by a second phase of rapid release as the hydrogel degrades – dependent on various factors[59,73]. These factors include the size of the drug particles relative to the pore size of the hydrogel (if the drug is larger than the pores, diffusion is restricted, thereby reducing the burst release effect), the distribution of drug particles within the print (if the surface of the printed hydrogel contains a large concentration of the drug, a burst release is more likely), and whether the drug is loaded by mixing or bonding[58,73]. Herein lies another advantage of hydrogels over solid materials: drugs can be covalently or physically linked to the hydrogel network to limit the drug’s release, whereas this is impossible in FDM[74,75]. Drug release would only occur as the bond between the drug and hydrogel, or the hydrogel itself, degrades. It is important to note, though, that degradation of hydrogels often occurs primarily by bulk degradation, as opposed to surface degradation, due to high diffusion; surface erosion would be made possible by further modification and careful selection of the hydrogel materials[76–78].
3.4 Smart Materials for Drug Delivery
Drug delivery can greatly benefit from smart materials which are compatible with 3D printing. More specifically, smart materials of interest include shape memory materials and environment-stimulated materials such as pH- and temperature-sensitive materials. In general terms, degradable shape memory polymer (SMP) are multifunctional materials which are designed to conform to their therapeutically relevant shape and mechanical properties after implantation[79]. SMPs are considered active polymers, as they change from a temporary shape to their original, permanent shape upon exposure to a stimulus. This controllable, conformable change can be leveraged for drug delivery. Most commonly, the stimulus is in the form of a temperature change, i.e. the internal temperature of the human body compared to the outside temperature. pH-sensitive materials are also very useful in designing SMPs: due to the dependence on the physiological environment of the drug, pH sensitivity can act as a reversible switch for drug release as the drug form migrates through the body or as the environment changes in acidity/alkalinity[80]. Other possible stimuli include light, pressure, or a magnetic or electric field[81].
When considering the printing of SMPs, it is often referred to as four-dimensional (4D) printing, wherein the fourth dimension is time[82–85]. After 3D printing the drug form, the printed drug can change its shape or functionality when the external stimulus is applied. For instance, a drug form can be printed that has high surface area in its permanent shape but a compact, low surface area shape in its temporary shape; the drug would be ingested or implanted while in its compact shape, and once in the targeted location, external stimulus would cause the transformation to its high surface area shape to allow for high drug release rates.
Importantly, researchers have shown that the incorporation of drugs does not have a significant impact on the thermo-mechanical behavior and shape memory properties of the SMPs[86,87]. Furthermore, SMPs exhibiting biodegradability and zero-order drug release have also been developed[79,88]. By leveraging the elastic properties of particular SMPs combined with hydrophobic materials, SMPs can be designed to release drugs without an initial burst followed by hydrolytic degradation. Another exemplary implementation of SMPs is for double layer delivery systems[89]. A multilayer approach allows for finer tuning of drug release, while still maintaining the mechanical properties of an elastic material ideal for implantable applications. With a focus on drug eluting implants, SMP stents have been designed to perform a double duty: provide the mechanical function of an implantable stent while simultaneously delivery drugs[90]. By adding the drug eluting feature to SMPs, implants and stents can be designed with anti-inflammatory agents built-in[91].
4. Benefits of 3D printing for Drug Manufacturing
Once an appropriate 3D printing method is determined and the material best suited for the pharmaceutical application is selected, it is a matter of developing and printing the drug itself. It is at this stage in the drug manufacturing process where 3D printing presents itself as the ideal approach, attributed to some noteworthy benefits[92]. One of the primary considerations in the delivery of drugs is the release characteristics. 3D printing enables increased geometric and architectural complexity, facile fabrication of multi-layer delivery systems, and the application of various controlled release mechanisms. Printing as an approach for drug manufacturing also introduces precise and unique dosing, and the ability to create multi-dose or multidrug pharmaceutical products. Dosing may also be tailored specifically for individual patients. Similarly, the printing of drugs makes point-of-care, pharmacybased drug production possible, without the risks and extensive fabrication time associated with compounding pharmacies. These benefits of 3D printing for drug manufacturing pave the way for the future of pharmaceuticals (Figure 2).
Figure 2.
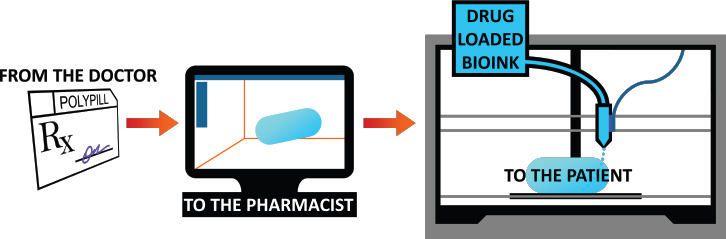
Theoretical scheme of 3D printing for drug manufacturing. Based on a patient’s specific prescription from his doctor, a custom medication is designed via computer-aided design. The dosage form may be composed of complex geometries, multiple doses, or even multiple drugs. Drug-loaded bioink (biocompatible material) is then 3D printed on-demand.
4.1 Release Characteristics of Drugs
The release characteristics of a drug refer to the quantity of the loaded drug that is emitted from the dosage form with respect to elapsed time. This has a significant impact on the application and relative effectiveness of the medication. One manner of manipulating the release characteristic of a drug is the geometric design and architectural complexity of the drug product. 3D printing is an ideal method for increasing the geometric and architectural complexity of dosage forms. 3D printing allows for custom-designed, discrete shapes to be fabricated, each with its own respective release timing. For instance, tablets of various shapes prepared with a constant surface area-to-volume ratio display different drug release rates, from fastest to slowest: sphere, cube, torus, cylinder and pyramid[93]. These findings are the result of drug diffusion and polymer dissolution[94,95]. The predominance of one of these factors over the other depends on the tablet shape and the solubility of the drug that is loaded in the tablet. This information can be leveraged with the capabilities of 3D printers to manufacture drugs with purposeful geometries for different release characteristics; in particular, by diverging away from typical spherical forms, 3D printing for drug manufacturing enables different and more precise drug delivery functions[96].
In addition to creating complex architectures of drug carriers, 3D printing is also capable of fabricating multilayer delivery systems. Bilayer tablets are common for controlled release delivery systems, by incorporating instant-release and slow-release layers in the same dosage form. While bilayer tablets are not a novel pharmaceutical product, the ability to fabricate them in essentially a single step by means of a 3D printer may revolutionize the process[97]. Furthermore, controlling the release timing by printing the carrier in different shapes and densities may also contribute to greater control over burst release, which is the phenomenon of excess drug being released upon initial contact with the dissolution media[98].
A final and important consideration for the release characteristics of drugs is the material of the drug carrier. As described above, hydrogel is a great candidate for a 3D printing medium, as well as for a drug carrier. Hydrogels also exhibit a diverse range of controlled release functions[99]. As a material property, many hydrogels are biodegradable over time, which lends itself to taking advantage of polymer dissolution as a form of controlled release. Additionally, due to the high diffusion of hydrogels, they may also be leveraged for diffusioncontrolled release and swelling-controlled release. Finally, hydrogels are also compatible with chemicallycontrolled release.
4.2 Precise and Unique Dosing
3D printing of drug products enables a newfound level of customization and personalization in drug dosing that is nearly impossible using typical commercial, mass-scale production methods. Presently, proper dosing is often imprecise, which can have costly results, in terms of both money and health[100]. While clinical pharmacology studies have been conducted to improve methods of drug dosing control and to help doctors prescribe the correct dose on a patient-to-patient basis, less work has focused on the production of the prescribed doses. 3D printing offers highly-precise fabrication, in addition to the capacity of unique dosing, all attributed to the free-form nature of 3D printing.
Exemplifying the application of 3D printing to provide precise dosing, a flexible-dose dispenser was developed that combines FDM 3D printing with hot melt extrusion (HME), which is a common pharmaceutical manufacturing process[101]. HME was implemented to produce drug-loaded filament by accurately mixing the drug with the filament material via a twin-screw compounder, thereby providing digital control over the concentration of drug melted into the filament. Following the HME process, the drug-loaded filament was used in a FDM printer to direct-write pharmaceutical tablets, which also introduces a high level of digital control through the design of the 3D printed tablets. Leveraging the linear relationship between the mass and volume of printed tablets, tablets of varying doses were able to be printed with high precision.
Digital control over drug dosing is not limited to FDM 3D printing; it has also been demonstrated with hydrogels which are compatible with inkjet printing. Following the deposition of drug-loaded hydrogel material, the hydrogel is cured by exposure to ultraviolet (UV) or near-UV light, which causes photo-initiator embedded within the material to crosslink. This photosensitive crosslinking may be utilized as a mean of digital drug dosing by using a projector to precisely cure a portion of the printed hydrogel[102]. The cured portion of the hydrogel forms the drug dose, while the uncured hydrogel can be washed away.
3D printing for drug manufacturing also advances drug dosing by making multi-drug and multi-dose carriers not only possible, but simple to fabricate. So called “polypills” can be created by combining different drugs, materials with varying concentrations of the same or different drugs, and various materials with differing release characteristics into the same pill. 3D printing is the most effective and efficient method for accomplishing this[103,104]. Multiple drugs can be combined in a pill by printing with multiple filaments or inks, each loaded with a different drug. Likewise, multiple materials can be printed simultaneously to form a single pill comprising multiple release characteristics. These capacities come together to produce a myriad of possibilities, including, but not limited to: multiple layers, self-contained compartments, and inner-toouter variation, each with either different doses or even different drugs. Such polypills made possible by 3D printing may very well be the future of precise and unique drug dosing.
4.3 On-Demand Capabilities
Point-of-care and pharmacy-based drug production may be the future of pharmaceuticals. 3D printing fulfills this niche portion of drug manufacturing. By implementing 3D printing as a fabrication method for pharmaceuticals, drugs can be on-demand in time-limited and resourcelimited settings[1,105]. The on-demand capabilities are applicable to settings such as disaster relief, emergency and operating rooms, on board first response vehicles, or medical facilities for the military. Time is also a critical factor when fabricating and delivering low-stability drugs, which is another instance of an application for ondemand printing of drugs[97].
The printing of pharmaceutical products can also be implemented to produce drugs on-the-spot and in accordance with specific, individualized prescriptions, thereby revolutionizing pharmacy compounding[106]. Currently, patient-centered pharmaceuticals are limited to compounding pharmacies. Traditionally, pharmacy compounding is reserved for patients with special medical needs, offering custom medications which are not commercially available. In particular, pharmacists prepare small-scale batches based on individual prescriptions. However, the individual-centric practices of compounding pharmacies have associated risks[107].
Pharmacy compounding is traditionally reserved for cases in which a patient requires a dosage form, strength, or medicine cocktail that is not commercially available. In such cases, the risk-benefit ratio of using the compounded medicine is favorable for the patient[ 107]. However, the risk-benefit scale tips in the opposite direction for medications that have other more commercialized options. The reason for the variance in the merit of compounding is the inherit variability and likely imprecision involved in the pharmaceutical making process. Solving these potential risks is the precision of custom 3D printing drugs. Leveraging 3D printing’s control over release characteristics of drugs and its ability to produce precise and unique doses, individualized medicine can provide prescription-based pharmaceutical products with the safety of digital control and the personalization of 3D printing.
5. Conclusion and Future Perspectives
Through this article, we have provided our perspective on the merits of 3D printing for drug manufacturing. Selective laser sintering, inkjet printing, and extrusionbased printing were presented as applicable 3D printing methods for drug manufacturing. Solid filament materials and natural and synthetic hydrogels were considered as possible materials for drug loading and printing. Various rationales for the 3D printing of drugs were also presented, including control over the release characteristics of drugs, the ability to print precise and unique doses, and the on-demand capabilities inherent with the printing approach.
3D printing for drug manufacturing represents the future of pharmaceuticals. While diverse industries across all of society are adopting 3D printing as a method for manufacturing, medicine and healthcare have yet to fully harness the capabilities of 3D printing for the direct-write fabrication of medications. With continued research, we believe personalized medicine will reach new levels of possibility, and pharmacies will be revolutionized by this particular application of 3D printing.
Conflict of Interest
No conflict of interest was reported by the authors.
Acknowledgments
S.T. acknowledges the American Heart Association Scientist Development Grant (15SDG25080056), Connecticut Innovations Biopipeline Award, and the University of Connecticut Research Excellence Program award for financial support of this research. S.T. is founder of, and have an equity interest in mBiotics, LLC, a company that is developing microfluidic technologies for point-of-care diagnostic solutions. S.T.’s interests were viewed and managed in accordance with the conflict of interest policies of the University of Connecticut. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Norman J, Madurawe R D, Moore C M V, et al. A new chapter in pharmaceutical manufacturing:3D-printed drug products. Adv Drug Deliv Rev. 2017;108(1):39–50. doi: 10.1016/j.addr.2016.03.001. http://doi.org/10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Wong J Y, Pfahnl A C. 3D printing of surgical instruments for long-duration space missions. Aviat Space Environ Med. 2014;85(7):758–763. doi: 10.3357/asem.3898.2014. http://doi.org/10.3357/ASEM.3898.2014. [DOI] [PubMed] [Google Scholar]
- 3.Cesaretti G, Dini E, De Kestelier X, et al. Building components for an outpost on the Lunar soil by means of a novel 3D printing technology. Acta Astronautica. 2014;93:430450. http://doi.org/10.1016/j.actaastro.2013.07.034. [Google Scholar]
- 4.Murphy S V, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. http://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 5.Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013;31(1):10–19. doi: 10.1016/j.tibtech.2012.10.005. http://doi.org/10.1016/j.tibtech.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J H, Jang J, Lee J S, et al. Three-dimensional printing of tissue/organ analogues containing living cells. Ann Biomed Eng. 2017;45(1):180–194. doi: 10.1007/s10439-016-1611-9. http://doi.org/10.1007/s10439-016-1611-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee V K, Dai G. Printing of three-dimensional tissue analogs for regenerative medicine. Ann Biomed Eng. 2017;45(1):115–131. doi: 10.1007/s10439-016-1613-7. http://doi.org/10.1007/s10439-016-1613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowlton S, Yenilmez B, Anand S, et al. Photocrosslinking-based bioprinting:Examining crosslinking schemes. Bioprinting. 2017;5:10–18. http://doi.org/10.1016/j.bprint.2017.03.001. [Google Scholar]
- 9.Knowlton S, Yenilmez B, Tasoglu S. Towards single-step biofabrication of organs on a chipvia3D printing. Trends Biotechnol. 2016;34(9):685–688. doi: 10.1016/j.tibtech.2016.06.005. http://doi.org/10.1016/j.tibtech.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton S, Joshi A, Yenilmez B, et al. Advancing cancer research using bioprinting for tumor-on-a-chip platforms. Int J Bioprint. 2016;2(2):3–8. http://doi.org/10.18063/IJB.2016.02.003. [Google Scholar]
- 11.Knowlton S, Yu C H, Ersoy F, et al. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication. 2016;8(2):025019. doi: 10.1088/1758-5090/8/2/025019. http://doi.org/10.1088/ 1758-5090/8/2/025019. [DOI] [PubMed] [Google Scholar]
- 12.Knowlton S, Onal S, Yu C H, et al. Bioprinting for cancer research. Trends Biotechnol. 2015;33(9):504–513. doi: 10.1016/j.tibtech.2015.06.007. http://doi.org/10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Knowlton S M, Sencan I, Aytar Y, et al. Sickle cell detection using a smartphone. Sci Rep. 2015;5:15022. doi: 10.1038/srep15022. http://doi.org/10.1038/srep15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowlton S, Yu C H, Jain N, et al. Smart-phone based magnetic levitation for measuring densities. PLoS ONE. 2015;10(8):e0134400. doi: 10.1371/journal.pone.0134400. http://doi.org/10.1371/journal.pone.0134400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin R, Knowlton S, Yenilmez B, et al. Smart-phone attachable, flow-assisted magnetic focusing device. RSC Adv. 2016;6(96):93922–93931. http://doi.org/10.1039/C6RA19483D. [Google Scholar]
- 16.Amin R, Knowlton S, Hart A, et al. 3D-printed microfluidic devices. Biofabrication. 2016;8(2):022001. doi: 10.1088/1758-5090/8/2/022001. http://doi.org/10.1088/1758-5090/8/2/022001. [DOI] [PubMed] [Google Scholar]
- 17.Yenilmez B, Knowlton S, Yu C H, et al. Label-free sickle cell disease diagnosis using a low-cost, handheld platform. Adv Mater Technol. 2016;1(5):1600100. http://doi.org/10.1002/admt.201600100. [Google Scholar]
- 18.Knowlton S, Joshi A, Syrrist P, et al. 3D-printed smartphone-based point of care tool for fluorescenceand magnetophoresis-based cytometry. Lab Chip. 2017;17(16):283951. doi: 10.1039/c7lc00706j. http://doi.org/10.1039/C7LC00706J. [DOI] [PubMed] [Google Scholar]
- 19.Yenilmez B, Knowlton S, Tasoglu S. Selfcontained handheld magnetic platform for point of care cytometry in biological samples. Adv Mater Technol. 2016;1(9):1600144. http://doi.org/10.1002/admt.201600144. [Google Scholar]
- 20.Giffi C A, Gangula B, Illinda P. 3D opportunity for the automotive industry. Deloitte University Press, New York 2014 [Google Scholar]
- 21.Katstra W E, Palazzolo R D, Rowe C W, et al. Oral dosage forms fabricated by Three Dimensional Printing™. J Control Release. 2000;66(1):1–9. doi: 10.1016/s0168-3659(99)00225-4. http://doi.org/10.1016/S0168-3659(99)00225-4. [DOI] [PubMed] [Google Scholar]
- 22.Ursan I D, Chiu L, Pierce A. Three-dimensional drug printing:A structured review. J Am Pharm Assoc. 2013;53(2):136–144. doi: 10.1331/JAPhA.2013.12217. http://doi.org/http://dx.doi.org/10.1331/JAPhA.2013.12217. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Erkal J L, Gross B C, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86(7):3240–3253. doi: 10.1021/ac403397r. http://doi.org/10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, Haverinen H M, Dhagat P, et al. Inkjet printing-process and its applications. Adv Mater. 2010;22(6):673685. doi: 10.1002/adma.200901141. http://doi.org/10.1002/adma.200901141. [DOI] [PubMed] [Google Scholar]
- 25.Scoutaris N, Alexander M R, Gellert P R, et al. Inkjet printing as a novel medicine formulation technique. J Control Release. 2011;156(2):179–185. doi: 10.1016/j.jconrel.2011.07.033. http://doi.org/10.1016/j.jconrel.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Alhnan M A, Okwuosa T C, Sadia M, et al. Emergence of 3D printed dosage forms:Opportunities and challenges. Pharm Res. 2016;33(8):1817–1832. doi: 10.1007/s11095-016-1933-1. http://doi.org/10.1007/s11095-016-1933-1. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoli A. Selective laser sintering in biomedical engineering. Med Biol Eng Comput. 2013;51(3):245–256. doi: 10.1007/s11517-012-1001-x. http://doi.org/10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 28.Tan K H, Chua C K, Leong K F, et al. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003;24(18):3115–3123. doi: 10.1016/s0142-9612(03)00131-5. http://doi.org/10.1016/S0142-9612(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 29.Pardeike J, Strohmeier D M, Schrödl N, et al. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int J Pharm. 2011;420(1):93–100. doi: 10.1016/j.ijpharm.2011.08.033. http://doi.org/10.1016/j.ijpharm.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Goole J, Amighi K. 3D printing in pharmaceutics:A new tool for designing customized drug delivery systems. Int J Pharm. 2016;499(1-2):376–394. doi: 10.1016/j.ijpharm.2015.12.071. http://doi.org/10.1016/j.ijpharm.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 31.Sokolsky-Papkov M, Agashi K, Olaye A, et al. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2014;59(4-5):187–206. doi: 10.1016/j.addr.2007.04.001. http://doi.org/10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Vehse M, Petersen S, Sternberg K, et al. Drug delivery from poly(ethylene glycol) diacrylate scaffolds produced by DLC based micro-stereolithography. Macromol Symp. 2014;346(1):43–47. http://doi.org/10.1002/masy.201400060. [Google Scholar]
- 33.Xing J-F, Zheng M-L, Duan X-M. Two-photon polymerization microfabrication of hydrogels:An advanced 3D printing technology for tissue engineering and drug delivery. Chem Soc Rev. 2015;44(15):5031–5039. doi: 10.1039/c5cs00278h. http://doi.org/10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, Jin J, Gregory C, et al. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–99. doi: 10.1016/j.biomaterials.2004.04.011. http://doi.org/10.1016/j.biomaterials.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Boland T, Xu T, Damon B, et al. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1(9):910–917. doi: 10.1002/biot.200600081. http://doi.org/10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 36.Horváth L, Umehara Y, Jud C, et al. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep. 2015;5(1):7974. doi: 10.1038/srep07974. http://doi.org/10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng W L, Wang S, Yeong W Y, et al. Skin bioprinting:Impending reality or fantasy? Trends Biotechnol. 2016;34(9):689–699. doi: 10.1016/j.tibtech.2016.04.006. http://doi.org/10.1016/j.tibtech.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Lee W, Debasitis J C, Lee V K, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30(8):1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. http://doi.org/10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Panwar A, Tan L P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules. 2016;21(6):685. doi: 10.3390/molecules21060685. http://doi.org/10.3390/molecules21060685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaezi M, Chua C K. Effects of layer thickness and binder saturation level parameters on 3D printing process. Int J Adv Manuf Technol. 2011;53(1-4):275–284. http://doi.org/10.1007/s00170-010-2821-1. [Google Scholar]
- 41.Lam C X F, Mo X M, Teoh S H, et al. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002;20(1-2):49–56. http://doi.org/10.1016/S0928-4931(02)00012-7. [Google Scholar]
- 42.Giordano R A, Wu B M, Borland S W, et al. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J Biomater Sci Polym Ed. 1997;8(1):63–75. doi: 10.1163/156856297x00588. http://doi.org/10.1163/156856297X00588. [DOI] [PubMed] [Google Scholar]
- 43.Antonov E N, Bagratashvili V N, Whitaker M J, et al. Three-dimensional bioactive and biodegradable scaffolds fabricated by surface-selective laser sintering. Adv Mater. 2005;17(3):327–330. doi: 10.1002/adma.200400838. http://doi.org/10.1002/adma.200400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rimell J T, Marquis P M. Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. J Biomed Mater Res A. 2000;53(4):414–420. doi: 10.1002/1097-4636(2000)53:4<414::aid-jbm16>3.0.co;2-m. http://doi.org/10.1002/1097-4636(2000)53:4<414::AID-JBM16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Wiria F E, Leong K F, Chua C K, et al. Poly-εcaprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007;3(1):1–12. doi: 10.1016/j.actbio.2006.07.008. http://doi.org/10.1016/j.actbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Verbelen L, Dadbakhsh S, Van Den Eynde M, et al. Characterization of polyamide powders for determination of laser sintering processability. Eur Polym J. 2016;75:163–174. http://doi.org/10.1016/j.eurpolymj.2015.12.014. [Google Scholar]
- 47.Drummer D, Rietzel D, Kühnlein F. Development of a characterization approach for the sintering behavior of new thermoplastics for selective laser sintering. Phys Procedia. 2010;5(PART B):533–542. http://doi.org/10.1016/j.phpro.2010.08.081. [Google Scholar]
- 48.Gusarov A V, Laoui T, Froyen L, et al. Contact thermal conductivity of a powder bed in selective laser sintering. Int J Heat Mass Transf. 2003;46(6):1103–9. http://doi.org/10.1016/S0017-9310(02)00370-8. [Google Scholar]
- 49.Dupin S, Lame O, Barrès C, et al. Microstructural origin of physical and mechanical properties of polyamide 12 processed by laser sintering. Eur Polym J. 2012;48(9):16111621. http://doi.org/10.1016/j.eurpolymj.2012.06.007. [Google Scholar]
- 50.Water J J, Bohr A, Boetker J, et al. Three-dimensional printing of drug-eluting implants:Preparation of an antimicrobial polylactide feedstock material. J Pharm Sc. 2015;104(3):1099–1107. doi: 10.1002/jps.24305. http://doi.org/10.1002/jps.24305. [DOI] [PubMed] [Google Scholar]
- 51.Skowyra J, Pietrzak K, Alhnan M A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17. doi: 10.1016/j.ejps.2014.11.009. http://doi.org/10.1016/j.ejps.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Genina N, Hollander J, Jukarainen H, et al. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2016;90:53–63. doi: 10.1016/j.ejps.2015.11.005. http://doi.org/10.1016/j.ejps.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Goyanes A, Buanz A B M, Basit A W, et al. Fusedfilament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476(1):88–92. doi: 10.1016/j.ijpharm.2014.09.044. http://doi.org/10.1016/j.ijpharm.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 54.Goyanes A, Buanz A B M, Hatton G B, et al. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–162. doi: 10.1016/j.ejpb.2014.12.003. http://doi.org/10.1016/j.ejpb.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Okwuosa T C, Stefaniak D, Arafat B, et al. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm Res. 2016;33(11):2704–2712. doi: 10.1007/s11095-016-1995-0. http://doi.org/10.1007/s11095-016-1995-0. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed E M. Hydrogel:Preparation, characterization, and applications:A review. J Adv Res. 2015;6(2):105–121. doi: 10.1016/j.jare.2013.07.006. http://doi.org/10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drotleff S, Lungwitz U, Breunig M, et al. Biomimetic polymers in pharmaceutical and biomedical sciences. Eur J Pharm Biopharm. 2004;58(2):385–407. doi: 10.1016/j.ejpb.2004.03.018. http://doi.org/10.1016/j.ejpb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Hoare T R, Kohane D S. Hydrogels in drug delivery:Progress and challenges. Polymer. 2008;49(8):19932007. http://doi.org/10.1016/j.polymer.2008.01.027. [Google Scholar]
- 59.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62(1):83–99. doi: 10.1016/j.addr.2009.07.019. http://doi.org/10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 60.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2012;64(Supplement):49–60. doi: 10.1016/s0169-409x(01)00203-4. http://doi.org/10.1016/j.addr.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Gupta P, Vermani K, Garg S. Hydrogels:From controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7(10):569–579. doi: 10.1016/s1359-6446(02)02255-9. http://doi.org/10.1016/S1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 62.Lee J M, Yeong W Y. Design and printing strategies in 3D bioprinting of cell-hydrogels:A review. Adv Healthc Mater. 2016;5(22):2856–2865. doi: 10.1002/adhm.201600435. http://doi.org/10.1002/adhm.201600435. [DOI] [PubMed] [Google Scholar]
- 63.Yue K, Trujillo-de Santiago G, Alvarez M M, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254271. doi: 10.1016/j.biomaterials.2015.08.045. http://doi.org/10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serafim A, Tucureanu C, Petre D-G, et al. Onepot synthesis of superabsorbent hybrid hydrogels based on methacrylamide gelatin and polyacrylamide Effortless control of hydrogel properties through composition design. New J Chem. 2014;38(7):3112–3126. http://doi.org/10.1039/c4nj00161c. [Google Scholar]
- 65.Hennink W E, van Nostrum C F. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2012;64(Supplement):223–236. doi: 10.1016/s0169-409x(01)00240-x. http://doi.org/10.1016/j.addr.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Berger J, Reist M, Mayer J M, et al. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57(1):19–34. doi: 10.1016/s0939-6411(03)00161-9. http://doi.org/10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 67.Akhtar M F, Hanif M, Ranjha N M. Methods of synthesis of hydrogels . A review. Saudi Pharm J. 2016;24(5):554–559. doi: 10.1016/j.jsps.2015.03.022. http://doi.org/10.1016/jjsps.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu L, Zhang Z, Zhang H, et al. Mixing a sol and a precipitate of block copolymers with different block ratios leads to an injectable hydrogel. Biomacromolecules. 2009;10(6):1547–1553. doi: 10.1021/bm900145g. http://doi.org/10.1021/bm900145g. [DOI] [PubMed] [Google Scholar]
- 69.Peppas N A, Bures P, Leobandung W, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. http://doi.org/10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 70.Qiao M, Chen D, Ma X, et al. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers:Synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm. 2005;294(1-2):103–112. doi: 10.1016/j.ijpharm.2005.01.017. http://doi.org/10.1016/j.ijpharm.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Molina I, Li S, Martinez M B, et al. Protein release from physically crosslinked hydrogels of the PLA/PEO/ PLA triblock copolymer-type. Biomaterials. 2001;22(4):363–369. doi: 10.1016/s0142-9612(00)00192-7. http://doi.org/10.1016/S0142-9612(00)00192-7. [DOI] [PubMed] [Google Scholar]
- 72.He Y, Yang F, Zhao H, et al. Research on the printability of hydrogels in 3D bioprinting. Sci Rep. 2016;6:29977. doi: 10.1038/srep29977. http://doi.org/10.1038/srep29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu Y, Kao W J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429–444. doi: 10.1517/17425241003602259. http://doi.org/10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duffy C V, David L, Crouzier T. Covalentlycrosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015;20:51–59. doi: 10.1016/j.actbio.2015.03.024. http://doi.org/10.1016/j.actbio.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Schoenmakers R G, van de Wetering P, Elbert D L, et al. The effect of the linker on the hydrolysis rate of druglinked ester bonds. J Control Release. 2004;95(2):291–300. doi: 10.1016/j.jconrel.2003.12.009. http://doi.org/10.1016/j.jconrel.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Shen W, Zhang K, Kornfield J A, et al. Tuning the erosion rate of artificial protein hydrogels through control of network topology. Nat Mater. 2006;5(2):153–158. doi: 10.1038/nmat1573. http://doi.org/10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 77.Metters A T, Bowman C N, Anseth K S. A statistical kinetic model for the bulk degradation of PLA -b- PEG-è-PLA hydrogel networks. J Phys Chem B. 2000;104(30):7043–7049. http://doi.org/10.1021/jp000523t. [Google Scholar]
- 78.Martens P, Metters A T, Anseth K S, et al. A generalized bulk-degradation model for hydrogel networks formed from multivinyl cross-linking molecules. J Phys Chem B. 2001;105(22):5131–5138. http://doi.org/10.1021/jp004102n. [Google Scholar]
- 79.Wischke C, Neffe A T, Steuer S, et al. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J Control Release. 2009;138(3):243–250. doi: 10.1016/j.jconrel.2009.05.027. http://doi.org/10.1016/jjconrel.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 80.Chen H, Li Y, Liu Y, et al. Highly pH-sensitive polyurethane exhibiting shape memory and drug release. Polym Chemi. 2014;5(17):5168–5174. http://doi.org/10.1039/C4PY00474D. [Google Scholar]
- 81.Wang K, Strandman S, Zhu X X. A mini review:Shape memory polymers for biomedical applications. Front Chem Sci Eng. 2017;11(2):1–11. http://doi.org/10.1007/s11705-017-1632-4. [Google Scholar]
- 82.Sydney Gladman A, Matsumoto E A, Nuzzo R G, et al. Biomimetic 4D printing. Nat Mater. 2016;15(4):413–418. doi: 10.1038/nmat4544. http://doi.org/10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- 83.Bakarich S E, et al. Gorkin R III in het Panhuis M. 4D printing with mechanically robust, thermally actuating hydrogels. Macromol Rapid Commun. 2015;36(12):1211–1217. doi: 10.1002/marc.201500079. http://doi.org/10.1002/marc.201500079. [DOI] [PubMed] [Google Scholar]
- 84.Ge Q, Sakhaei A H, Lee H, et al. Multimaterial 4D printing with tailorable shape memory polymers. Sci Rep. 2016;6:31110. doi: 10.1038/srep31110. http://doi.org/10.1038/srep31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao B, Yang Q, Zhao X, et al. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016;34(9):746–756. doi: 10.1016/j.tibtech.2016.03.004. http://doi.org/10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Neffe A T, Hanh B D, Steuer S, et al. Polymer networks combining controlled drug release, biodegradation, and shape memory capability. Adv Mater. 2009;21(32-33):33943398. doi: 10.1002/adma.200802333. http://doi.org/10.1002/adma.200802333. [DOI] [PubMed] [Google Scholar]
- 87.Nagahama K, Ueda Y, Ouchi T, et al. Biodegradable shape-memory polymers exhibiting sharp thermal transitions and controlled drug release. Biomacromolecules. 2009;10(7):1789–1794. doi: 10.1021/bm9002078. http://doi.org/10.1021/bm9002078. [DOI] [PubMed] [Google Scholar]
- 88.Kashif M, Yun B M, Lee K S, et al. Biodegradable shape-memory poly^-caprolactone)/polyhedral oligomeric silsequioxane nanocomposites:Sustained drug release and hydrolytic degradation. Mater Lett. 2016;166:125–128. http://doi.org/10.1016/j.matlet.2015.12.051. [Google Scholar]
- 89.Musial-Kulik M, Kasperczyk J, Smola A, et al. Double layer paclitaxel delivery systems based on bioresorbable terpolymer with shape memory properties. Int J Pharm. 2014;465(1-2):291–298. doi: 10.1016/j.ijpharm.2014.01.029. http://doi.org/10.1016/.ijpharm.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 90.Wache H M, Tartakowska D J, Hentrich A, et al. Development of a polymer stent with shape memory effect as a drug delivery system. J Mater Sci Mater Med. 2003;14(2):109–112. doi: 10.1023/a:1022007510352. http://doi.org/10.1023/A:1022007510352. [DOI] [PubMed] [Google Scholar]
- 91.Xiao Y, Zhou S, Wang L, et al. Crosslinked poly^caprolactone)/poly(sebacic anhydride) composites combining biodegradation, controlled drug release and shape memory effect. Compos B Eng. 2010;41(7):537–542. http://doi.org/10.1016/j.compositesb.2010.07.001. [Google Scholar]
- 92.Banks J. Adding value in additive manufacturing:Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse. 2013;4(6):22–26. doi: 10.1109/MPUL.2013.2279617. http://doi.org/10.1109/MPUL.2013.2279617. [DOI] [PubMed] [Google Scholar]
- 93.Goyanes A, Robles Martinez P, Buanz A, et al. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–663. doi: 10.1016/j.ijpharm.2015.04.069. http://doi.org/10.1016/.ijpharm.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 94.Reynolds T D, Mitchell S A, Balwinski K M. Investigation of the effect of tablet surface area/volume on drug release from hydroxypropylmethylcellulose controlledrelease matrix tablets. Drug Dev Ind Pharm. 2002;28(4):457–466. doi: 10.1081/ddc-120003007. http://doi.org/10.1081/DDC-120003007. [DOI] [PubMed] [Google Scholar]
- 95.Kamaly N, Yameen B, Wu J, et al. Degradable controlled-release polymers and polymeric nanoparticles:Mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. http://doi.org/10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee B K, Yun Y H, Choi J S, et al. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. Int J Pharm. 2012;427(2):305–310. doi: 10.1016/j.ijpharm.2012.02.011. http://doi.org/10.1016/j.ijpharm. 2012.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Khaled S A, Burley J C, Alexander M R, et al. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461(1-2):105–111. doi: 10.1016/j.ijpharm.2013.11.021. http://doi.org/10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 98.Huang X, Brazel C S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2-3):121–136. doi: 10.1016/s0168-3659(01)00248-6. http://doi.org/10.1016/S0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 99.Lin C C, Metters A T. Hydrogels in controlled release formulations:Network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58(12-13):1379–1408. doi: 10.1016/j.addr.2006.09.004. http://doi.org/10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Bailey J M, Haddad W M. Drug dosing control in clinical pharmacology. IEEE Control Syst Mag. 2005;25(2):3551. http://doi.org/10.1109/MCS.2005.1411383. [Google Scholar]
- 101.Pietrzak K, Isreb A, Alhnan M A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. 2015;96:380–387. doi: 10.1016/j.ejpb.2015.07.027. http://doi. org/10.1016/j.ejpb.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 102.Faralli A, Melander F, Larsen E K U, et al. Digital drug dosing:Dosing in drug assays by light-defined volumes of hydrogels with embedded drug-loaded nanoparticles. In Proceedings of the 2nd IEEE EMBS Micro and Nanotechnology in Medicine Conference 2014 [Google Scholar]
- 103.Khaled S A, Burley J C, Alexander M R, et al. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. http://doi.org/10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 104.Khaled S A, Burley J C, Alexander M R, et al. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–650. doi: 10.1016/j.ijpharm.2015.07.067. http://doi.org/10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 105.Srai J S, Badman C, Krumme M, et al. Future supply chains enabled by continuous processing-opportunities and challenges May 20-21, 2014 Continuous Manufacturing Symposium. J Pharm Sci. 2015;104(3):840–849. doi: 10.1002/jps.24343. http://doi. org/10.1002/jps.24343. [DOI] [PubMed] [Google Scholar]
- 106.Alomari M, Mohamed F H, Basit A W, et al. Personalised dosing:Printing a dose of one's own medicine. Int J Pharm. 2015;494(2):568–577. doi: 10.1016/j.ijpharm.2014.12.006. http://doi.org/10.1016/j.ijpharm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Gudeman J, Jozwiakowski M, Chollet J, et al. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1–8. doi: 10.1007/s40268-013-0005-9. http://doi.org/10.1007/s40268-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


