Abstract
The COVID-19 outbreak is now one of the most critical crises to manage for most of national healthcare systems in the world. The situation is complicated by the absence of vaccines and authorized pharmacological treatments, except for remdesivir. In this context, many medicaments, including different Ebola and HIV antivirals, are used off-label in the hospital wards as life-treating medicines for COVID-19 patients. Authorized medicaments manipulation is sometimes necessary because they are not always formulated to be administered to non-cooperative patients or they are in shortage. It is this the case of the fixed combination of lopinavir/ritonavir, which was extensively used in the first phase of the outbreak inducing a shortage of the oral solution available in the EU market. This work provides data on size distribution, osmolarity other than drug chemical stability of a lopinavir/ritonavir extemporaneous preparation made by using the solid dosage form (i.e., tablet) available on the market as drug source. The reported data indicate that such preparation is suitable to be delivered through a nasogastric tube, and enough stable for two weeks from the preparation at room temperature.
Keywords: COVID-19, Medicament manipulation, Nasogastric tube, Osmolarity, Particle size distribution, Lopinavir, Ritonavir
Specifications Table
| Subject | Pharmacology, Toxicology and Pharmaceutical Science |
| Specific subject area | Pharmaceutical Science |
| Type of data | Table, Figure, Text |
| How data were acquired | High-pressure liquid chromatography (HPLC) |
| Data format | Raw and analyzed |
| Parameters for data collection | Data on lopinavir/ritonavir stability in suspension through two-weeks days from the preparation after storage at 4° and room temperature |
| Description of data collection | The pH 4.2-buffered suspension was prepared in a hospital pharmacy by manipulating the lopinavir/ritonavir tablet. It was characterized in terms of particle size distribution, osmolarity and 15-day chemical stability. The drug stability in suspensions was tested at different storage conditions (4 °C, RT) for two weeks. |
| Data source location | Milan, Italy |
| Data accessibility | Analyzed data with the article. Raw data and chromatograms and preparation procedure with supplementary materials. |
Value of the Data
-
•
The data can be useful to healthcare professionals that need to manipulate lopinavir/ritonavir fixed combination for treating adults or children affected by COVID-19 or HIV.
-
•
The data are useful for the development of new dosage forms indicated for inpatients of intensive care units.
-
•
The data provide evidence on the physical stability of lopinavir/ritonavir suspended in a pH 4.2-buffered viscous vehicle.
-
•
The data provide evidence on the lopinavir/ritonavir chemical stability when they are suspended and stored for two weeks at different conditions.
1. Data Description
One of the possible pharmacological treatments has been investigated in the early stage of COVID-19 outbreak in the EU resides in the administration of antiretroviral medicaments [1,2]. In particular, the fixed combination of lopinavir, a second-generation antiretroviral that inhibits viral HIV protease, and ritonavir, an antiviral administered at a low dose as pharmacoenhancer for lopinavir, (LPV/r) had been considered a possible treatment option for COVID-19 infections, based on demonstrated efficacy against SARS-CoV [3] and some anecdotal cases [4]. This fixed combination is available in the EU market as an oral solution, soft capsules, and tablets [5]. Both oral solutions and tablets are generally available in hospitals. The former, designed for pediatric patients, is also preferred for inpatients since it can be easily administered with the enteral nutrition by nasogastric tube. Nevertheless, some criticisms limit the use of the oral solution in patients fed with enteral nutrition: it contains, as excipient, alcohol that is not indicated in the case of nasogastric administration; it is in shortage for the increased demand of the hospitals due to the use in intensive care units. Therefore, the unique possibility for ensuring the continuity of patient care resides in the use of solid dosage forms, which must be manipulated by the hospital pharmacist to allow their enteral administration by nasogastric tube [6].
However, the manipulation of industrial drug products may alter the medicine quality profile, with potential impact on the efficacy and safety of the pharmacological treatment [7]. For example, the bioavailability of LPV/r fixed combination can be altered by splitting or crushing of solid dosage forms [8]. The administration of suspension via nasogastric tube may also be affected by clogging due to the aggregation of particles of the grounded tablets [9]. Finally, the osmolarity of administered suspension can influence the bioavailability of drug substances characterized by a low water solubility or/and permeability (BCS Class III or IV compounds according to the biopharmaceutics classification system), such as LPV/r [10]. Indeed, the enteral administration of a hypertonic solution may change the drug solubility in the digestive tract in adults.
This work provides data on the main critical quality attributes of the lopinavir/ritonavir suspension (e.g., size distribution, osmolarity other than drug chemical stability) to support the establishment of a standardized manipulation procedure at the bedside based on the real-world experience of Italian hospital pharmacists. In the case of study, a citrate buffer pH 4.2 with 1% carboxymethyl cellulose (CMC) solution was used as vehicle.
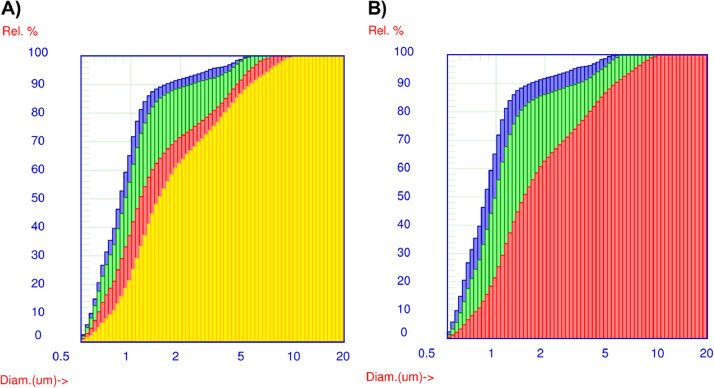
The preparation according to the procedure reported in Supplementary Materials led to a homogeneous whiteish suspension characterized by a very fine particulate. Data reported in the Supplementary Materials show that, after preparation, most of the solid particulate in suspension resulted in a size lower than 3 µm (d95 < 3.05) with a median value (d50) of about 0.88 µm. The suspension remained homogeneous in appearance up to 30 min of rest. Accordingly, the particle size distribution of the grounded powder did not change significantly, as shown in Fig. 1.
Fig. 1.
Exemplification of the particle size distribution of suspension. Panel A: immediately after preparation (blue) and after 30 min (green), 60 min (red) and 180 min of rest in the syringe (yellow); panel B: immediately after preparation (blue), after 180 min of rest (red) and after manual handshaking (green). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Afterwards, the particulate started to aggregate as it can be noted by progressive shift of the curves towards larger dimensions (Fig. 1A) and the increase of the d50-value (Supplementary Materials). This finding was not an issue for the clotting of the nasogastric tube during the administration since the greater particles remain in the range of few micrometres (d99 < 10 µm; Supplementary Materials). Still, the aggregation could affect dissolution of the drugs in the gastrointestinal tract. However, data reported in Fig. 1B show that manual handshaking of the suspension for around 1 min was enough to resuspend the precipitate, restoring the initial particle distribution (d50 = 1.00 µm; Supplementary Materials).
The osmolality of the suspension was 322 ± 14 mOsm/Kg, which fell in the range of isotonicity and, therefore, was compatible with the oral administration. Indeed, if the osmolality of the ileum is generally hypotonic in a fasted state (≈ 200 mOsm/Kg), it can physiologically tolerate values up to 300–400 mOsm/Kg in fed conditions [11]. Still, the suspension osmolality can increase the water amount retained in the different gastrointestinal sections [8], reducing the risk of LPV/r precipitation in the lumen. However, it is also possible that this phenomenon affect drug permeation and, therefore, bioavailability as observed in children [10].
Finally, the chemical stability of extemporaneous antiretroviral suspension was investigated through 15 days. The literature suggests that both drug substances are stable, although lopinavir seems more stable than ritonavir [12]. On the contrary, few data are available on their stability in suspension. Table 1 provides data to support the stability of both active substances at the two storage conditions. Despite stress conditions applied (acid/basic and heat treatment) on the suspension, no impurities due to chemical degradation were observed throughout the stability study. However, high variability of the drug assay (> 15%) was found (Table 1). These findings may be probably due to issues in sampling a uniform dose due to sedimentation and viscosity, and this appears more relevant in samples stored at low temperature. Low temperature is theoretically expected to reduce solubility of the drugs, as well as capability of the aqueous medium to solvate the particles, obtaining a homogenous suspension. Consequently, the suspension should be extemporaneously compounded as single-dose preparation and not as multi-dose ones, particularly if stored at low temperature.
Table 1.
Assay of the extemporaneous antiretroviral suspension during stability study at room temperature (RT) and 4 °C.
| Assay (%) | Sampling time (days) |
|||
|---|---|---|---|---|
| 0 | 1 | 4 | 15 | |
| At RT | ||||
| • Lopinavir | 100.0 ± 6.0 | 129.6 ± 6.9 | 113.8 ± 6.3 | 114.5 ± 17.9 |
| • Ritonavir | 100.0 ± 5.6 | 137.9 ± 7.7 | 113.2 ± 5.9 | 115.3 ± 14.3 |
| At 4 °C | ||||
| • Lopinavir | 100.0 ± 6.0 | 90.8 ± 5.1 | 110.5 ± 2.3 | 68.5 ± 3.3 |
| • Ritonavir | 100.0 ± 5.6 | 91.1 ± 4.9 | 109.3 ± 1.4 | 70.9 ± 1.9 |
Overall data suggest that the suspension is enough stable to be delivered through the nasogastric tube, and it can be pre-made and stored, until use, for two weeks from the preparation in the hospital pharmacy at room temperature. However, data suggest that single-dose preparations should be preferred instead of multi-dose preparations to reduce the risk of dosing errors.
2. Experimental Design, Materials, and Methods
2.1. Materials
Kaletra® Tablet 200 mg/50 mg (Abbvie Deutschland GMBH & Co, D). Tablet contents: copovidone, sorbitan laurate, colloidal anhydrous silica, sodium stearyl fumarate. Film-coating: hypromellose, titanium dioxide, macrogols type 400 (Polyethylene glycol 400), hydroxypropyl cellulose, talc, colloidal anhydrous silica, macrogols type 3350 (Polyethylene glycol 3350), yellow ferric oxide E172, polysorbate 80 [5].
Sodium carboxymethyl cellulose (CMC), trisodium citrate dihydrate, and citric acid were purchased by Farmalabor (I). All other chemicals/solvents used in the study were analytical grade and they were used without further purification.
2.2. Suspension preparation
The exact preparation procedure is reported in Supplementary Materials. Briefly, tablets of Kaletra® were crushed in a mortar to obtain a fine and homogenous powder. Then, the powder was precisely weighed and loaded in a 50-ml syringe. Using a female-female Luer-lock connector, the syringe was linked to another one containing 20-mL of the suspension vehicle. A 1% w/v CMC solution in pH 4.2 citrate buffer was used as vehicle. The vehicle volume was set up to obtain a final suspension containing 20 mg/ml of lopinavir and 5 mg/ml of ritonavir. Moving the syringe pluggers, the powder and the solution had mixed each other to reach a homogenous whiteish suspension (appx. 50 syringe complete movements).
2.3. Size distribution analysis
The size distribution of the solid residues in the compounded antiretroviral preparation was analyzed by the single-particle optical sensing (SPOS) technique, using an Accusizer 770 (PSS Inc., USA). In brief, 100 µL of the compounded antiretroviral preparation was analyzed. The area-weighted cumulative distributions were described as the median and the dispersion of the size distribution [Span, Eq. (1)].
| (1) |
where d10, d50 and d90 represent the diameters at 10, 50 and 90% of the size area distribution, respectively. The analyses were carried out at different times from the preparation, i.e. 30, 90, 180 min. For 180 min, the studies were carried out on samples left to rest or shaken by hand. The data were expressed as the value obtained by a single formulation batch.
2.4. Osmolarity determination
The osmolarity of the extemporaneous antiretroviral suspension was tested by using a K-7400S Semi-Micro Osmometer (Knauer, D). Briefly, a 150-µl aliquot of the extemporaneous antiretroviral suspension was withdrawn and analyzed. The data were expressed as mean ± st.dev. of three replicas.
2.5. Stability study
The suspension stability was tested at 4 °C and room temperature (RT) up to 15 days: fixed sampling timings were 0, 1, 4, and 15 days. Aliquots of the suspension were stored at both 4 °C and RT in 1.5 ml polypropylene tube. The sampling was carried out based on volume measurement to simulate sampling conditions in the real-life setting of an intensive care unit (from bottles to infusion pumps for instance). The aliquots of each suspension were heated to RT and mixed by a vortex at the time of each analysis. The samples were diluted 1:1 with acetonitrile/water 40/60 v/v, mixed by vortex strongly, then sonicated for 10 min. The sample (diluted 1:1) was divided into three replicates diluted 1:125 again with a mixture of acetonitrile/water 40/60 v/v. The obtained dilutions were sonicated for 10 min and shacked by mechanical agitator for 30 min until the analysis in HPLC. Preliminary stress tests were performed on aliquots of the obtained diluted suspensions to identified degradation compounds of both drugs. Aliquots of both suspensions were treated in the following conditions: at 96 °C, at RT and 96 °C after the addition of phosphoric acid (pH 2.5), at RT and 96 °C after the addition of ammonia (pH 10). The assay of both drugs in the preparation was calculated as Eq. (2).
| (2) |
Where t is the sampling point (0, 1, 4, 15 days), At and A0 are the area HPLC peaks of the drugs analyzed ad the sampling point t and at the time of the preparation, respectively. Data were expressed as mean ± st.dev. of three replicas.
2.6. Analytical method
A liquid chromatographer Waters 2695 HPLC system (Milan, Italy) coupled with a 2998 PDA detector was employed. HPLC-PDA system was controlled by Empower 2 Pro-software (version year 2005; Waters), based on a validated method previously reported [13]. A Luna® 5 m C18 (150 × 4.6 mm ID; Phenomenex, CA) chromatographic column, protected by a SecurityGuard® with C18 (4.0 × 3.0 mm ID; Phenomenex, CA) was used for chromatographic separation. Temperature Control Module II (Waters) was set at 45 °C. Runtime was 28 min, performed at 1 mL/min; mobile phase was composed of solvent A (KH2PO4 50 mM with orthophosphoric acid, pH = 3.23) and solvent B (acetonitrile). Lopinavir (LPV) and Ritonavir (RTV) were monitored at 260 nm and 241 nm, respectively (Supplementary Materials). The chromatographic gradient used was the same as reported by Zanon and coworkers [2]. Raw data and chromatograms are reported in Supplementary Materials.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2020.106445.
Appendix. Supplementary materials
References
- 1.WHO, Q&A on COVID-19, HIV and antiretrovirals. https://www.who.int/news-room/q-a-detail/q-a-on-covid-19-hiv-and-antiretrovirals, 2020 (accessed 21 March 2020).
- 2.Zanon D., Manca A., De Nicolò A., D'Avolio A., Musazzi U.M., Cilurzo F., Maximova N., Tomasello C., Minghetti P. Data on the stability of darunavir/cobicistat suspension after tablet manipulation. Data Brief. 2020 doi: 10.1016/j.dib.2020.105552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y., HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han W., Quan B., Guo Y., Zhang J., Lu Y., Feng G., Wu Q., Fang F., Cheng L., Jiao N., Li X., Chen Q. The course of clinical diagnosis and treatment of a case infected with coronavirus disease. J. Med. Virol. 2020;92:461–463. doi: 10.1002/jmv.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EMA, Kaletra®. https://www.ema.europa.eu/en/medicines/human/EPAR/kaletra, 2020 (accessed 21 March 2020).
- 6.Council of Europe, Resolution CM/res(2016)1 on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients. https://www.edqm.eu/en/Quality-Safety-Standards-Resolutions-1588.html, 2020 (accessed 3 November 2020). [DOI] [PMC free article] [PubMed]
- 7.Huesgen E., DeSear K.E., Egelund E.F., Smith R., Max B., Janelle J. A HAART-breaking review of alternative antiretroviral administration: practical considerations with crushing and enteral tube scenarios. Pharmacotherapy. 2016;36:1145–1165. doi: 10.1002/phar.1835. [DOI] [PubMed] [Google Scholar]
- 8.Best B.M., Capparelli E.V., Diep H., Rossi S.S., Farrell M.J., Williams E., Lee G., van den Anker J.N., Rakhmanina N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J. Acquir. Immune Defic. Syndr. 2011;58:385–391. doi: 10.1097/QAI.0b013e318232b057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruzsíková A., Součková L., Suk P., Opatřilová R., Kejdušová M., Šrámek V. Quantitative analysis of drug losses administered via nasogastric tube – in vitro study. Int. J. Pharm. 2015;478:368–371. doi: 10.1016/j.ijpharm.2014.11.065. [DOI] [PubMed] [Google Scholar]
- 10.Ichijo K., Oda R., Ishihara M., Okada R., Moteki Y., Funai Y., Horiuchi T., Kishimoto H., Shirasaka Y., Inoue K. Osmolality of orally administered solutions influences luminal water volume and drug absorption in intestine. J. Pharm. Sci. 2017;106:2889–2894. doi: 10.1016/j.xphs.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Kalantzi L., Goumas K., Kalioras V., Abrahamsson B., Dressman J.B., Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006;23:165–176. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- 12.Donato E.M., Dias C.L., Rossi R.C., Valente R.S., Fröehlich P.E., Bergold A.M. LC method for studies on the stability of lopinavir and ritonavir in soft gelatin capsules. Chromatographia. 2006;63:437–443. [Google Scholar]
- 13.D'Avolio A., Baietto L., Siccardi M., Sciandra M., Simiele M., Oddone V., Bonora S., Di Perri G. An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients. Ther. Drug Monit. 2008;30:662–669. doi: 10.1097/FTD.0b013e318189596d. 30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.