Abstract
Objectives
Coronavirus disease 19 (COVID-19) is a major cause of hospital admission and represents a challenge for patient management during intensive care unit (ICU) stay. We aimed to describe the clinical course and outcomes of COVID-19 pneumonia in critically ill patients.
Methods
We performed a systematic search of peer-reviewed publications in MEDLINE, EMBASE and the Cochrane Library up to 15th August 2020. Preprints and reports were also included if they met the inclusion criteria. Study eligibility criteria were full-text prospective, retrospective or registry-based publications describing outcomes in patients admitted to the ICU for COVID-19, using a validated test. Participants were critically ill patients admitted in the ICU with COVID-19 infection.
Results
From 32 articles included, a total of 69 093 patients were admitted to the ICU and were evaluated. Most patients included in the studies were male (76 165/128 168, 59%, 26 studies) and the mean patient age was 56 (95%CI 48.5–59.8) years. Studies described high ICU mortality (21 145/65 383, 32.3%, 15 studies). The median length of ICU stay was 9.0 (95%CI 6.5–11.2) days, described in five studies. More than half the patients admitted to the ICU required mechanical ventilation (31 213/53 465, 58%, 23 studies) and among them mortality was very high (27 972/47 632, 59%, six studies). The duration of mechanical ventilation was 8.4 (95%CI 1.6–13.7) days. The main interventions described were the use of non-invasive ventilation, extracorporeal membrane oxygenation, renal replacement therapy and vasopressors.
Conclusions
This systematic review, including approximately 69 000 ICU patients, demonstrates that COVID-19 infection in critically ill patients is associated with great need for life-sustaining interventions, high mortality, and prolonged length of ICU stay.
Keywords: COVID-19, Critically ill, ICU, Outcomes, Resource, SARS-CoV-2
Introduction
Since the first case of coronavirus disease 19 (COVID-19), identified in December 2019 and caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the disease has spread rapidly, infecting millions of people worldwide and causing a major challenge for healthcare systems [1]. Although in most cases the disease is mild or asymptomatic, a subset of patients develop moderate to severe COVID-19 pneumonia requiring intensive care unit (ICU) admission [[2], [3], [4]]. As an exceptionally high number of cases have required hospitalization, emergency departments and ICUs have been strained and, in several countries, ICUs have been unable to deliver enough beds and ventilators for patients with respiratory distress [1,5].
However, despite the numerous reports of critically ill patients in the literature [2,6], the clinical course, outcomes and interventions of patients admitted to the ICU are unclear. Owing to the differences in design, patient population, and geographies, there is a large variation among studies in the ICU admission rate (from 4.0% [7] to 32% [8]) and mortality rate (from 0.7% [9] to 52.4% [10]) in patients with COVID-19. Moreover, as patients with COVID-19 often have severe presentations and multiorgan failure, and require life-sustaining interventions for basic care (e.g. personal protective equipment and laboratory analysis), there is a need for highly skilled staff and more sophisticated and expensive interventions such as invasive mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and renal replacement therapy (RRT) [2,6].
A more comprehensive knowledge of ICU utilization and outcomes could potentially help healthcare professionals and managers to estimate the need for ventilators, ICU beds, and dialysis monitors, and to manage more adequately their staffing patterns. These findings have significant implications for a better understanding of the epidemiology of COVID-19, and for better planning and organization of hospitals and ICUs to ensure better preparation and optimization of delivery of care under the pandemic circumstances.
In the present study we performed a systematic review of the current literature with the aim of describing the clinical course, interventions used and short-term outcomes of COVID-19 pneumonia requiring ICU admission.
Methods
Data sources and study selection
We conducted a systematic review of the literature according to the recommendations of the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group [11] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12]. We searched MEDLINE, EMBASE and the Cochrane Library.
The following primary search terms were used in MEDLINE: ((“covid 19"[Title/Abstract]) OR (“sars cov"[Title/Abstract])) OR (“coronavirus"[Title/Abstract]). These terms were cross-referenced to the following terms: ((((“icu"[Title/Abstract]) OR (“intensive care"[Title/Abstract])) OR (“critically ill"[Title/Abstract])) OR (“ards"[Title/Abstract])) OR (“severe acute respiratory syndrome"[Title/Abstract]). There was no language restriction. The literature search was performed from 1st December 2019 to 15th August 2020. Preprints were searched on the site preprints.org using the following terms: COVID-19 or coronavirus. Reports were also searched manually and were included if they meet the inclusion criteria.
The most recent search was performed on 20th August 2020. The reference lists of retrieved articles and relevant review articles, as well as personal files, were manually searched. We considered the following criteria for study inclusion: (a) full-text prospective, retrospective or registry-based publications in patients admitted to the ICU for COVID-19, (b) studies including patients diagnosed with COVID-19 using a validated test, and (c) studies reporting an association between COVID-19 and at least one of the following outcomes: death at any time, length of stay in the ICU or in hospital, duration of mechanical ventilation (MV), and occurrence of acute respiratory distress syndrome (ARDS). Letters to the editor, individual case reports or reviews, and studies not reporting the number of confirmed cases were excluded.
Two investigators (RBS, JIFS) performed the study selection process, including the initial search for the identification of references, the selection of potentially relevant titles for review of abstracts and, among them, of those chosen for review of the full-length reports. All selections were decided by consensus. This report was prospectively registered with the PROSPERO database of systematic reviews (CRD42020180850).
Data extraction and study quality assessment
Data extraction from the selected articles was independently performed by two authors (RBS, JIFS). The following data were recorded (when available): study characteristics (such as type of study, selection of patients, number of patients enrolled, publication date), patient characteristics (such as age, sex, patient setting), and outcomes (ARDS incidence, need for MV, vasopressor used, death in the ICU/hospital, and all patients length of hospital/ICU stay). We considered that the centre had an ICU according to the description of each author; data were not available to allow another classification or to allow further characterization. The ICNARC report described the ICU and high dependency units together in the same analysis [6].
To assess the methodological quality of the studies, we adapted the Newcastle–Ottawa Quality Assessment Scale (NOS) [13] to better describe the risk of bias in our systematic review (Supplementary Material Table S1).
Analytical approach
We evaluated COVID-19 patient characteristics and main outcomes described.
We had especial interest in describing mortality, length of hospital stay, and interventions used (MV, non-invasive ventilation (NIV), RRT and use of vasopressors). For continuous variables, we described the mean and 95% confidence interval (CI) based on the reported data. Proportions were described with the numerator/denominator and percentages. We used the SPSS v21 to analyse the data. Owing to the differences in design of the studies, we were not able to perform a meta-analysis.
Results
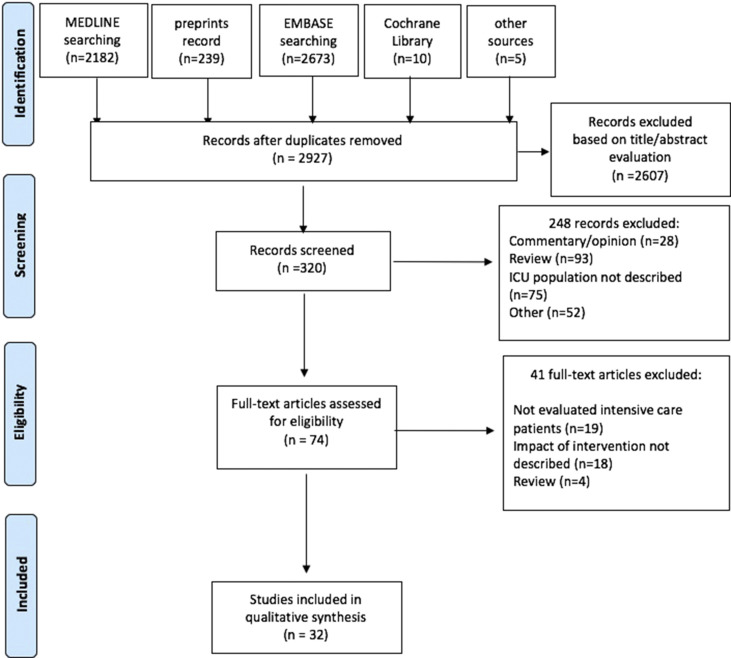
The literature search yielded 2927 studies. Of the 320 potentially relevant abstracts screened, 74 articles were available for detailed analysis. Finally, 32 articles that met the inclusion criteria were included in this systematic review. A flow diagram of the search and selection of the studies is depicted in Fig. 1 .
Fig. 1.
Flow diagram of study inclusion.
Study characteristics
The characteristics of the 32 included studies are shown in Table 1 . Twenty-eight studies [[3], [4], [5],[8], [9], [10],[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] and five non-peer-reviewed reports from national registries or research groups [2,6,[36], [37], [38]] were included. A total of 69 093 patients were admitted in the ICU and were evaluated. Most studies were performed in China, but they represent only 1.35% (932/69 093) of the ICU patients described [3,4,9,[16], [17], [18], [19], [20],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Reports from national ICU registries and research databases included the largest number of patients in analysis (64 979 of ICU patients). The follow-up period of studies ranged from late December 2019 to 15th August 2020.
Table 1.
Characteristics of included studies
| Author | Sample considered | Date of publication | Region | Follow-up | Sample size study | Sample size (ICU pts)) | Age (years) | Male |
|---|---|---|---|---|---|---|---|---|
| Arentz et al. [10] | Only ICU patients | March 19, 2020 | Evergreen Hospital, Snohomish countries in Washington State, USA | February 20 to March 5, 2020 | 21 | 21 | 70 | 11 (52%) |
| Auld et al. [14] | Only ICU patients | April 26, 2020 | Three Emory Healthcare acute-care hospitals in Atlanta, Georgia, USA | March 6 to April 17, 2020 | 217 | 217 | 64 | 119 (55%) |
| Bhatraju et al. [15] | Only ICU patients | March 30, 2020 | Nine Seattle-area hospitals, USA | Before March 23, 2020 | 24 | 24 | 64 | 15 (63%) |
| CDC Report [36] | Hospitalized patients | March 18, 2020 | USA | February 12 to March 16, 2020 | 508 | 121 (22.30%) | 68 | NA |
| Chen et al. [17] | Hospitalized patients | January 29, 2020 | Wuhan Jinyintan Hospital, China | January 1 to January 20, 2020 | 99 | 23 (23%) | 55.5 | 67 (68%) |
| Chen et al. [16] | Hospitalized patients | March 2, 2020 | Shanghai Public Health Clinical Centre (SPHCC), Shanghai, China | January 20 to February 6, 2020 | 249 | 22 (8%) | 56 | 187 (75%) |
| Epimed report [36] | Only ICU patients | October 15, 2020 | Hospitals in Brazil | March 1 to October 15, 2020 | 41 858 | 41 858 | 61 | 24 738 (59.1%) |
| Grasselli et al. [5] | Only ICU patients | April 6, 2020 | Lombardy, a region of northern Italy | February 20 to March 18, 2020 | 1591 | 1591 | 63 | 1304 (82%) |
| Guan et al. [18] | Hospitalized patients | February 28, 2020 | National Health, Commission of China | Up to February 25, 2020 | 1099 | 55 (5%) | 47 | 637 (59%) |
| Huang et al. [8] | Hospitalized patients | January 24, 2020 | The Central Hospital of Wuhan, China | Up to December 31, 2019 | 41 | 13 (32%) | 30 | NA |
| Huang et al. [29] | Only ICU patients | February 24, 2020 | Zhongnan Hospital of Wuhan University, China | Up to February 1, 2020 | 34 | 34 | 49 | 30 (95%) |
| ICNARC report [6] | Only ICU patients | July 24, 2020 | Critical care units in England, Wales and Northern Ireland | Up to July 23, 2020 | 10 547 | 10 547 | 60 | 7409 (70.2%) |
| ISARIC report [2] | Hospitalized patients | July 13, 2020 | 25 countries | Up to July 13, 2020 | 60 430 | 9754 (16%) | 72 | 34 422 (57%) |
| Lin et al. [30] | Hospitalized patients | March 3, 2020 | Wuhan Jinyintan Hospital, Chine | January 1 to January 20, 2020 | 20 | 1 (5%) | NA | NA |
| Mo et al. [20] | Hospitalized patients | March 16, 2020 | Zhongnan Hospital of Wuhan University, China | January 1 to February 5, 2020 | 155 | 155 (23%) | NA | NA |
| NICE report Netherland [35] | Only ICU patients | April 29, 2020 | The Netherlands | Up to April, 2020 | 2699 | 2699 | 63.4 | NA |
| Petrilli et al. [33] | Hospitalized patients | May 22, 2020 | Four acute-care hospitals in New York City and Long Island, USA | March 1 to April 8, 2020 | 2741 | 990 (36%) | 54 | 1678 (61.2%) |
| Qian et al. [20] | Hospitalized patients | March 10, 2020 | Five hospitals in east of Zhejiang province, China | January 20 to February 11, 2020 | 91 | 9 (9.89%) | NA | NA |
| Richardson et al. [21] | Hospitalized patients | April 22, 2020 | Hospitals in New York City, Long Island, and Westchester County, New York, USA | March 1 to April 4, 2020 | 5700 | 373 (6.54%) | 63 | 3437 (60.3%) |
| Wan et al. [9] | Hospitalized patients | March 21, 2020 | Chongqing University Three Gorges Hospital, USA | January 23 to February 8, 2020 | 135 | 40 (29.6%) | 47 | 72 (53%) |
| Wang et al. [23] | Only ICU patients | April 30, 2020 | Zhongnan Hospital of Wuhan University in Wuhan and Xishui Hospital, Hubei Province, China | Up to February 10, 2020 | 107 | 107 | 51 | 57 (53.3%) |
| Wang et al. [22] | Hospitalized patients | February 7, 2020 | Zhongnan Hospital of Wuhan University in Wuhan, China | From January 1 to January 28, 2020 | 138 | 36 (26%) | 56 | 75 (54.3%) |
| Liang et al. [32] | Hospitalized patients | April 9, 2020 | 575 hospitals in 31 provincial administrative regions of China | Up to Jan 31, 2020 | 1590 | 99 (6.23%) | 48.9 | 911 (57.3%) |
| Wu et al. [3] | Hospitalized patients | March 13, 2020 | Wuhan Jinyintan Hospital in China | December 25 to January 26, 2020 | 201 | 53 (24.4%) | 51 | 128 (63.7%) |
| Xu et al. [24] | Hospitalized patients | February 13, 2020 | Zhejiang province, China | January 10, 2020 to January 26, 2020 | 62 | 1 (0.02%) | 35 | 36 (58%) |
| Xu et al. [25] | Hospitalized patients | March 18, 2020 | Suzhou, China | January 2020 to February 18, 2020 | 87 | 4 (4.06%) | NA | 46 (53%) |
| Yang et al. [31] | Hospitalized patients | February 21, 2020 | Wuhan Jin Yin-tan hospital in Wuhan, China | Late December 2019, and Jan 26, 2020 | 710 | 52 (7.32%) | 59.7 | 475 (67%) |
| Young et al. [4] | Hospitalized patients | March 3, 2020 | Four hospitals in Singapore | January 23 to February 3, 2020 | 18 | 2 (4%) | 47 | 9 (50%) |
| Zangrillo et al. [34] | ICU patients | April 23, 2020 | Large tertiary hospital in Milan | February 20 to April 2, 2020 | 73 | 73 | 61 | 61(83,6%) |
| Zhang et al. [26] | Hospitalized patients | March 6, 2020 | Zhongnan Hospital of Wuhan University, Wuhan, China | January 2 to February 10, 2020 | 221 | 44 (19%) | 55 | 108 (49.8%) |
| Zheng et al. [27] | Hospitalized patients | March 24, 2020 | Ten hospitals across Hubei province. China | February 1 to February 10, 2020 | 25 | 25 | 3 | 14 (70%) |
| Zhou et al. [28] | Hospitalized patients | March 9, 2020 | 135 from Jinyintan Hospital and 56 from Wuhan Pulmonary Hospital, China | Up to Jan 31, 2020 | 191 | 50 (26%) | 56 | 119 (62%) |
| Overall | 131 682 | 69 093 | 56 (95%CI 48.5–59.8) | 76 165/128 168 (59%) |
NA, not available; CI, confident interval.
Quality assessment of studies
Seventeen cohort studies evaluated hospitalized patients and described patients who needed ICU admission (median of 122 patients, 95%CI 32–962) [9,[16], [17], [18], [19], [20], [21], [22], [23],[25], [26], [27], [28],[30], [31], [32],34]. Nine cohort studies included only ICU patients (median of 155 patients, 95%CI 146–3713) [5,8,10,14,15,18,23,29,36]. Only one study described a 28-day mortality [31] and no studies described long-term outcomes after hospital discharge. Studies reported different results regarding symptoms, time to critical illness, clinical course, development of organ failure, intervention used, and short-term outcomes. The NOS score of each study is shown in the Supplementary Material Table S2.
Characteristics of patients admitted to the ICU
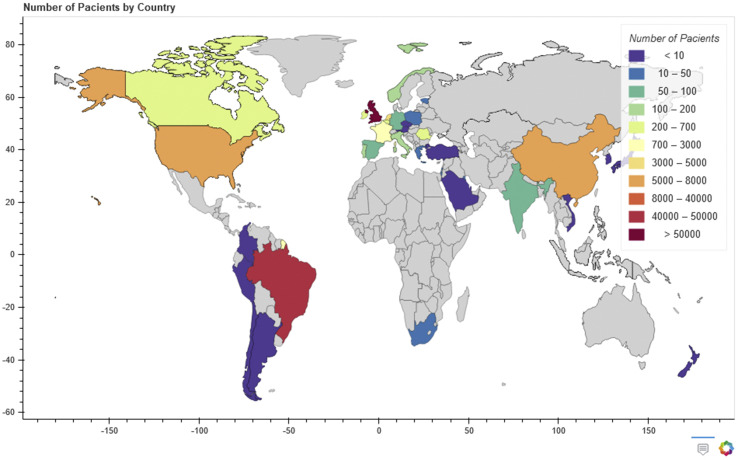
The design, sample size and outcomes in all the studies were highly variable. Fig. 2 shows the number of patients included from each country. Most ICU patients were males (76 165/128 168, 59%, 26 studies) [[2], [3], [4], [5], [6],9,10,14,15,17,18,[21], [22], [23], [24], [25], [26], [27],29,[31], [32], [33], [34],36] and the mean patient age was 56 years (95%CI 48.5–59.8, 11 studies) [5,6,10,14,15,23,27,29,34,36,37]. Nine studies [3,[8], [9], [10],18,22,26,28,31] described the diagnosis of ARDS in patients admitted to the ICU (316/365, 85%). Most patients with ARDS were male (240/316, 76.2%, nine studies), and the median age was 53 (95%CI 48.2–62.8) [3,[8], [9], [10],18,22,26,28,31].
Fig. 2.
Patients included from each country.
Main outcomes
Regarding short-term outcomes, most studies described ICU mortality and length of hospital stay. Studies described a high ICU mortality (21 145/65 383, 32.3%, 15 studies) [2,5,6,8,10,14,15,20,23,27,31,[34], [35], [36]] and the median ICU length of stay was 9.0 (95%CI 6.5–11.2) days [6,2,9,25,26,28,18,[34], [35], [36], [37]] (Table 2 ). The mortality in patients requiring invasive MV was described in six studies (27 972/47 632, 59%) [6,21,28,31,33,36]. Only two studies described the mortality in patients with ARDS in the ICU: 93% (50/59) [29] and 71% (26/35) [32]. Only three studies described the duration of MV, and the median was 8.4 (95%CI 1.6–13.7) days [2,6,34].
Table 2.
Interventions, life-sustaining therapies and outcomes described in the included studies
| Author | NIV | HFNO | MV | ECMO | RRT | Vasopressors | ICU LOS (days) | ICU mortality | Hospital mortality |
|---|---|---|---|---|---|---|---|---|---|
| Arentz et al. [10] | 4 (19.5%) | 1 (4.8%) | 15 (71%) | NA | NA | 14 (67%) | NA | 11 (52.4%) | NA |
| Auld et al. [14] | NA | NA | 165 | NA | NA | NA | NA | 52 (23.9%) | NA |
| Bhatraju et al. [15] | NA | 10 (42%) | 18 (75%) | NA | NA | NA | NA | 12 (50%) | NA |
| CDC Report [35] | NA | NA | NA | NA | NA | NA | 6.1 | 103 (20.4%) | NA |
| Chen et al. [17] | 13 (13%) | NA | 4 (4%) | 3 (3%) | 8 (8.6%) | NA | NA | NA | NA |
| Chen et al. [16] | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Epimed report [36] | 8179 (22.5%) | NA | 15 921 (43,8%) | NA | 5525 (15.2%) | 12 577 (34.6%) | 11.9 | 12 432 (34.2%) | 12 868 (35.4%) |
| Grasseli et al. [5] | 137 (11%) | NA | 1150 (88%) | 5 (1%) | NA | NA | NA | 405 (26%) | NA |
| Guan et al. [19] | 56 (5.1%) | NA | 25 (2.3%) | 5 (0.5%) | 9 (0.8%) | NA | 12.8 | NA | 15 (1.4%) |
| Huang et al. [8] | 10 (24%)a | 2 (5%) | 2 (5%) | NA | NA | NA | 6 (15%) | NA | |
| Huang et al. [29] | 2 (5.1%)a | 3 (8.80%) | NA | NA | NA | NA | NA | NA | |
| ICNARC report [6] | NA | NA | 7355 (72.2%)c | NA | 2707 (27%) | 1583 (20.6%) | 12 & | 4023 (40%) | NA |
| ISARIC report [2] | 5070 (56.7%) | 1928 (53%) | 5375 (14.3%) | 221 (2.73%) | 1262 (16%) | 3406 (43.8%) | 9.0 | 3348 (30%) | 17 031 (28%) |
| Liang et al. [32] | NA | NA | 50 (3.14%) | NA | NA | NA | NA | NA | 50 (3.14%) |
| Lin et al. [30] | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mo et al. [19] | NA | NA | 36 (23.3%) | NA | NA | NA | NA | NA | NA |
| NICE report Netherland [37] | NA | NA | NA | NA | NA | NA | NA | 633 (23%) | NA |
| Petrilli et al. [33] | NA | NA | 647 (23.6%) | NA | NA | NA | 7 | NA | NA |
| Qian et al. [20] | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Richardson et al. [21] | NA | NA | 320 (12.2%) | NA | NA | NA | 4.1 | NA | 553 (21%) |
| Wan et al. [9] | 34 (25.2%) | NA | 1 (0.7%) | 0 (0%) | 5 (3.7%) | NA | NA | 1 (0.7%) | NA |
| Wang et al. [23] | NA | NA | NA | NA | NA | NA | 11.0 | NA | NA |
| Wang et al. [22] | 15 (10.9%) | NA | 17 (12.32%) | 4 (2.9%) | NA | NA | NA | NA | NA |
| Wu et al. [3] | 61 (30.3%) | NA | 5 (2.5%) | 1 (0.5) | NA | NA | NA | NA | 44 (21.9%) |
| Xu et al. [24] | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Xu et al. [25] | NA | NA | NA | NA | NA | NA | NA | 0 (0%) | NA |
| Yang et al. [31] | 29 (4%) | 33 (63.5%) | 22 (42%) | 6 (11·5%) | 9 (17%) | 18 (35%) | NA | NA | 32 (61·5%)b |
| Young et al. [4] | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Zangrillo et al. [34] | NA | NA | 33 (45.2%) | NA | NA | NA | NA | 17 (23.3%) | NA |
| Zhang et al. [26] | 27 (12.2%) | NA | 16 (7.2%) | 10 (4.5%) | NA | NA | NA | 48 (21.8%) | NA |
| Zheng et al. [27] | NA | NA | 1 (4%) | NA | NA | NA | NA | NA | NA |
| Zhou et al. [28] | NA | 41 (21%) | 32 (17%) | 3 (2%) | 10 (5%) | NA | 8 | 54 (28%) | NA |
| Overall | 13 637/53 574 (25,5%) | 2013/9948 (20,5%) | 31 213/53 465 (58%) | 265/11 385 (2.3%) | 2184/13 187 (16.6%) | 17 580/62 232 (28%) | 9.0 (95% CI 6.5 – 11.2) | 21 145/65 383(32.3%) | 30 593/102 355 (29,87%) |
NA, not available; ARDS, acute respiratory distress syndrome; NIV, non-invasive ventilation; MV, mechanical ventilation; ECMO, extracorporeal membrane oxygenation; HFOT, high-flow oxygen therapy.
The studies described together the number of NIVs and HFOTs.
This study described the 28-day mortality.
This study described the use of MV only in the first 24 h after admission, and in this study we considered length of ICU stay of survivors.
Support measures and life-sustaining therapies in the ICU
The use of MV was described in 23 studies, and 58% (31 213/53 465) of patients admitted to the ICU were ventilated [2,3,5,6,[8], [9], [10],[15], [16], [17],19,21,22,[26], [27], [28], [29],[31], [32], [33],36]. Eight studies described the use of high-flow oxygen therapy (HFOT), and it was employed in 2013/9948 patients (20.5%) in the ICU [8,10,15,28,29,31]. Thirteen studies described the use of NIV in 13 637/53 574 patients (25.5%) in the ICU [2,3,5,[8], [9], [10],16,18,22,26,29,31,36]. The use of ECMO was described in 11 studies representing 265/11 385 (2.3%) patients in the ICU [2,3,5,8,9,16,21,25,27,30].
The use of RRT was described in seven studies, representing 2184/13 187 patients (16.6%) in the ICU [2,6,17,19,29,32,37]. Finally, the use of vasopressors was described in five studies representing 17 580/62 232 patients (28%) in the ICU [2,6,10,31,36].
Discussion
This systematic review represents a synthesis of currently available data on the patients admitted to the ICU for COVID-19, with a focus on the main clinical outcomes, interventions used, and advanced life support measures. Our systematic review identified 32 studies and reports. We described a significant number of patients (n = 69 093) who required critical care. ARDS was present in 85% of patients admitted to the ICU [3,[8], [9], [10],18,22,26,28,31].
The surge in patients with COVID-19 requiring hospitalization, ICU admission, and ventilatory support has represented an unparalleled challenge to physicians, nurses, hospital managers and healthcare systems. However, despite the rapid response of the medical community, the burden of COVID-19 on ICU facilities is unclear. Despite the lack of studies, this systematic review was able to provide relevant data on ICU utilization from peer-reviewed articles [4,5,[8], [9], [10],[14], [15], [16], [17], [18], [19], [20], [21], [22], [23],[25], [26], [27], [28], [29],[31], [32], [33], [34],36,38], three non-peer-reviewed preprints [14,27,30] and also five large databases of scientific institutions and national registries of intensive care [2,6,[35], [36], [37]]. This review provides a unique international perspective on the interventions used and outcomes in patients with COVID-19 requiring ICU admission, thus increasing the current epidemiological knowledge and potentially providing useful information to help care for these patients.
Our study shows the high burden of COVID-19 on ICUs, which was demonstrated by the exceedingly high mortality and length of stay even when compared to other infectious diseases requiring ICU admission. The ICU mortality rate among patients with COVID-19 was 30.6%, which is relevant even when compared to the usually described mortality rates for community-acquired pneumonia (CAP) of about 16.6–18% [39,40] and for sepsis of 24.2–55.7% [41,42]. Moreover, when considering only mechanically ventilated patients, the mortality was exceptionally high (27 972/47 632, 59%, six studies) [6,21,28,31,33,36], especially in ARDS patients (up to 93%), which is more than the typical mortality rate from ARDS of about 35–45% [43,44]. We also described an elevated ICU length of stay for COVID-19 (8.0, 95%CI 5.1–11.0) as compared to that described in patients with severe CAP [39,45], representing a challenge in ICU bed management.
Our study found that almost half the patients admitted to the ICU needed invasive MV (58%), and a lower percentage (25.5%) required non-invasive ventilation. Studies in CAP described a much higher proportion of patients (up to 56%) using NIV in acute respiratory failure [45,46]. Indeed, the role of NIV in COVID-19 remains unclear. The uncertainty around the treatment of acute hypoxaemic respiratory failure with NIV [47] plus the purported risks of aerosol generation and delayed intubation have led to varying recommendations between authorities [7,48], potentially contributing to its limited use.
The use of ECMO as a salvage therapy for critically ill COVID-19 patients was limited and described in a small number of patients (272/11 494, 2.4%, 11 studies) [2,3,5,8,9,16,18,22,26,28,31]. Mortality in ECMO was described in only three studies and was very high (9/10, 90%) [3,28,31]. It remains unclear whether ECMO therapy is associated with improved outcomes. A recent study using data from the Extracorporeal Life Support Organization (ELSO) included 1035 patients with COVID-19 who received ECMO and also described a high mortality (380/968, 39%) and a significant percentage of patients (101/1035, 10%) discharged to a long-term acute-care centre [38]. Published studies from different countries describing the use of ECMO as rescue therapy in the 2009 H1N1 pandemic have reported lower mortality rates than that reported during the current COVID-19 pandemic (14–41%) [[49], [50], [51], [52]].
This study has several limitations. First, owing to the urgency of publishing preliminary information on patients with COVID-19 during the current pandemic, studies have presented very heterogeneous data on clinical characteristics, interventions used, and outcomes of ICU patients. Significant differences in the design of the studies also contributed to the lack of specific and core clinical information in several reports. This limited our ability to pool and meta-analyse data on specific subgroups. In Supplementary Material Table S3 we have suggested a list of outcomes that should be assessed in future studies describing critically ill patients with COVID-19 [53,54]. Second, the sample sizes varied significantly, ranging from 20 patients in small cohort studies up to 64 979 patients in reports from national ICU registries. Third, the classification of ICU patients was performed according to the definition of each centre. Details regarding intensive care support were not described; hence, further stratification was not possible. This could have led to overestimation of the surge capacity. Fourth, the overlap of reported cases from the same centres may cause some bias; however, we believe that the large sample size and similarity of patients can minimize this effect. Fifth, only one study assessed the 28-day mortality, and no study reported long-term outcomes after hospital discharge [31]. Finally, owing to several aspects—including the temporal and geographic development of the pandemic—available data from low-income countries are limited; they would be essentially the report from Brazil and the international report from ISARIC that included developing and developed countries [2,5,6,10,14,15,20,[34], [35], [36]].
The present study does have several strengths. As far as we are aware, this is the first systematic review to describe the interventions used and main clinical outcomes of COVID-19 patients admitted to the ICU. It describes a large number of ICU patients (n = 69 093) in 37 countries over five continents. The addition of reports from national registries, although not peer-reviewed, adds relevant and insightful epidemiological data. Our main findings reflect a substantial use of ICU beds when a COVID-19 patient is hospitalized. It also shows that nearly half of ICU patients (57%) required mechanical ventilation [2,3,5,6,[8], [9], [10],[15], [16], [17],19,21,22,[26], [27], [28], [29],[31], [32], [33],36] and had a high mortality (59%) [6,21,28,31,33,36].
Conclusions
This systematic review provides relevant data on ICU utilization of a high number of patients with COVID-19 during a time of scarce resources. Patients with COVID-19 admitted to the ICU have great need for invasive support, high mortality, and prolonged length of stay in the ICU.
Author contributions
RBS and JIFS were responsible for data collection, data input, study design, data analysis, and drafting of the manuscript. RBS was responsible for statistical analysis. RBS, PP, VSD, AK, and JIFS were responsible for critical analysis, manuscript revisions, and editorial assistance. All authors have read and approved the final manuscript.
Transparency declaration
The authors state that they have no competing interests. The study was performed with institutional departmental funding. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Editor: Mical Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.10.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Coronavirus disease (COVID-19): situation report 111. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200510covid-19-sitrep-111.pdf?sfvrsn=1896976f_2. Accessed July 23 2020.
- 2.ISARIC (International Severe Acute Respiratory and emerging Infection Consorti Database) 2020. [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ICNARC report on COVID-19 in critical care ICNARC case mix programme database. 2020. [Google Scholar]
- 7.ANZICS Australian and New Zealand Intensive Care Society . 2020. COVID-19 guidelines. [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M. Ottawa Health Research Institute Web; 2014. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 14.Auld S., Caridi-Scheible M., Blum J.M., Robichaux C.J., Kraft C.S., Jacob J.T. ICU and ventilator mortality among critically ill adults with COVID-19. medRxiv. 2020 doi: 10.1101/2020.04.23.20076737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region—case series. New Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian G.Q., Yang N.B., Ding F., Ma A.H.Y., Wang Z.Y., Shen Y.F. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020 doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W., Qu S., Xing M., Zhang M., Lu G., Liao Z. Epidemiologic features and clinical findings of COVID-19-infected patients in Suzhou. Lancet. 2020 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3551352 (preprint) [Google Scholar]
- 25.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. Br Med J. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. Lancet. 2020 doi: 10.1016/j.jcv.2020.104364. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546095 (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng F., Liao C., Fan Q.H., Chen H.B., Zhao X.G., Xie Z.G. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y., Tu M., Wang S., Chen S., Zhou W., Chen D. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Trav Med Infect Dis. 2020:101606. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin B., Lei Z., Cao H., Peng L., Jie Y., Gao Z. Comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China- manuscript draft. Lancet Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Resp Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang W.H., Guan W.J., Li C.C., Li Y.M., Liang H.R., Zhao Y. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a Nationwide Analysis of China. Eur Respir J. 2020 doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zangrillo A., Beretta L., Scandroglio A.M., Monti G., Fominskiy E., Colombo S. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020 doi: 10.1016/S1441-2772(23)00387-3. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epimed report. http://www.utisbrasileiras.com.br/sari-covid-19/benchmarking-covid-19/.
- 37.NICE—national Intensive Care Evaluation COVID-19 op de Nederlandse Intensive Cares; Patiëntkarakteristieken en uitkomsten vergeleken met pneumonie patiënten op de IC in 2017-2019 (COVID-19 on the Dutch Intensive Cares; Patient characteristics and outcomes compared to pneumonia patients in the ICU in 2017-2019). https://www.demedischspecialist.nl/nieuws/kwaliteitsregistratie-nice-publiceert-covid-19-rapport.
- 38.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G., Cook D.J., Thabane L., Friedrich J.O., Crozier T.M., Muscedere J. Risk factors for mortality in patients admitted to intensive care units with pneumonia. Respir Res. 2016;17:80. doi: 10.1186/s12931-016-0397-5. [published correction appears in Respir Res 2016 Oct 7;17 (1):128] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cillóniz C., Liapikou A., Martin-Loeches I., Garcia-Vidal C., Gabarrús A., Ceccato A. Twenty-year trend in mortality among hospitalized patients with pneumococcal community-acquired pneumonia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaukonen K.M., Bailey M., Suzuki S., Pilcher D., Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 42.Machado F.R., Cavalcanti A.B., Bozza F.A., Ferreira E.M., Angotti Carrara F.S., Sousa J.L. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17:1180–1189. doi: 10.1016/S1473-3099(17)30322-5. [DOI] [PubMed] [Google Scholar]
- 43.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 44.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A. LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291.pmid:26903337. [DOI] [PubMed] [Google Scholar]
- 45.Daniel P., Woodhead M., Welham S., McKeever T.M., Lim W.S. British Thoracic S. Mortality reduction in adult community-acquired pneumonia in the UK (2009–2014): results from the British Thoracic Society audit programme. Thorax. 2016;71:1061–1063. doi: 10.1136/thoraxjnl-2016-208937. [DOI] [PubMed] [Google Scholar]
- 46.Murad A., Li P.Z., Dial S., Shahin J. The role of noninvasive positive pressure ventilation in community-acquired pneumonia. J Crit Care. 2015;30:49–54. doi: 10.1016/j.jcrc.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Bourke S.C., Piraino T., Pisani L., Brochard L., Elliott M.W. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: implications for practice. Lancet Resp Med. 2018;6:935–947. doi: 10.1016/S2213-2600(18)30388-6. [DOI] [PubMed] [Google Scholar]
- 48.NHS . 2020. Guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected)https://amhp.org.uk/app/uploads/2020/03/Guidance-Respiratory-Support.pdf [Google Scholar]
- 49.Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I. Davies A., Jones D., Bailey M., Beca J., Bellomo R. Blackwell N et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 50.Dominguez-Cherit G., Lapinsky S.E., Macias A.E., Pinto R., Espinosa-Perez L., de la Torre A. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A., Zarychanski R., Pinto R., Cook D.J., Marshall J., Lacroix J. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 52.Patroniti N., Zangrillo A., Pappalardo F., Peris A., Cianchi G., Braschi A. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intens Care Med. 2011;37:1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [published correction appears in Lancet Infect Dis. 2020 Aug 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong A., Elliott J.H., Azevedo L.C. Core outcomes set for trials in people with coronavirus disease. Crit Care Med. 2019 doi: 10.1097/CCM.0000000000004585. [published online ahead of print, 2020 Aug 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.